Hydrothermal Synthesis of Zinc Oxide Nanoparticles Using Different Chemical Reaction Stimulation Methods and Their Influence on Process Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Zinc chloride–ZnCl2 (99.9%, anhydrous)–Sigma-Aldrich
- Potassium hydroxide–KOH (pure)–Chempur, Poland
2.2. Methods
2.2.1. Microwave Reactor
2.2.2. Autoclave
2.2.3. Impulse Reactor
2.2.4. ERTEC Reactor with Meander Heater and Volume Type Joule
2.2.5. XRD Analysis
2.2.6. Density and Specific Surface Area (BET)
2.2.7. SEM Analysis
3. Experiments and Results
4. Discussion
- The product has high purity and density close to the theoretical value-only traces of an unidentified phase were found, which should be minimized by the choice of optimal electrode material;
- The relatively high specific surface area of the powder allows obtaining zinc oxide nanoparticles at an unprecedentedly low temperature of approx. 25 °C.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obregon, O.; Barba, D.; Dominguez, M.A. Modeling of the Density of States in Field-Effect Zinc Oxide Semiconductor Devices Fabricated by Ultrasonic Spray Pyrolysis on Plastic Substrates. Eng. Proc. 2021, 4, 12. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Review. Sensors 2020, 20, 5118. [Google Scholar] [CrossRef]
- Parvez Ahmad, M.D.; Venkateswara Rao, A.; Suresh Babuy, K.; Narsinga Rao, G. Effect of carbon-doping on structural and dielectric properties of zinc oxide. J. Adv. Dielectr. 2020, 10, 2050017. [Google Scholar] [CrossRef]
- Maia, D.B.; Raimundo, R.A.; Passos, R.A.; Torquato, T.A. Analysis of structural, morphological and magnetic properties of diluted magnetic semiconductor ZnO:Eu obtained by combustion reaction. Cerâmica 2020, 66, 262–268. [Google Scholar] [CrossRef]
- Kim, I.; Yun, J.; Badloe, T.; Park, H.; Seo, T.; Yang, Y.; Kim, J.; Chung, Y.; Rho, J. Structural color switching with a doped indium-gallium-zinc-oxide semiconductor. Photonics Res. 2020, 8, 1409. [Google Scholar] [CrossRef]
- Liu, X. Simulation of the Conductive Process of Nano ZnO Varistors Based on Animation Plane Form. Hindawi J. Chem. 2020, 2020, 9726173. [Google Scholar] [CrossRef]
- Xie, P.; Wang, Z.; Wu, K. Evolution of Intrinsic and Extrinsic Electron Traps at Grain Boundary during Sintering ZnO Based Varistor Ceramics. Materials 2022, 15, 1098. [Google Scholar] [CrossRef]
- Loncar, B.; Vujisic, M.; Stankovic, K.; Osmokrovic, P. Stability of Metal–Oxide Varistor Characteristics in Exploitation Conditions. Acta Physica Polonica A 2009, 116, 1081–1084. [Google Scholar] [CrossRef]
- Aini Ismail, N.Q.; Saat, N.K.; Mohd Zaid, M.H. Effect of soda lime silica glass doping on ZnO varistor ceramics: Dry milling method. J. Asian Ceram. Soc. 2020, 8, 909–914. [Google Scholar] [CrossRef]
- Gutknecht, T.; Gustafsson, A.; Forsgren, C.; Ekberg, C.; Steenari, B.-M. Investigations into Recycling Zinc from Used Metal Oxide Varistors via pH Selective Leaching: Haracterization, Leaching, and Residue Analysis. Hindawi Publ. Corp. Sci. World J. 2015, 2015, 653219. [Google Scholar] [CrossRef]
- Frigura-Iliasa, F.M.; Musuroi, S.; Sorandaru, C.; Vatau, D. New Technical Parameters and Operational Improvements of the Metal Oxide Varistors Manufacturing Process. Processes 2019, 7, 18. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.; Ji, C. Simulation research on space charge characteristics of zinc oxide varistor. Energy Rep. 2020, 6, 462–468. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Olanrewaju Alade, I.; Qamar, M.; Akbar, S. Zinc Oxide-Based Acetone Gas Sensors for Breath Analysis: A Review. Chem. Asian J. 2021, 16, 1519–1538. [Google Scholar] [CrossRef]
- Mohd Ramzuz, I.N.; Zakaria, N.; Mohd Zain, Z. Synthesis and characterization of zinc oxide nanostructures in biosensor application. Int. J. Biosen. Bioelectron. 2020, 6, 48–54. [Google Scholar] [CrossRef]
- Ocola, L.E.; Wang, Y.; Divan, R.; Chen, J. Multifunctional UV and Gas Sensors Based on Vertically Nanostructured Zinc Oxide: Volume Versus Surface Effect. Sensors 2019, 19, 2061. [Google Scholar] [CrossRef]
- Ren, Q.; Cao, Y.-Q.; Arulraj, D.; Liu, C.; Wu, D.; Li, W.-M.; Liz, A.-D. Review—Resistive-Type Hydrogen Sensors Based on Zinc Oxide Nanostructures. J. Electrochem. Soc. 2020, 167, 067528. [Google Scholar] [CrossRef]
- Kanaparthi, S.; Govind Singh, S. Chemiresistive Sensor Based on Zinc Oxide Nanoflakes for CO2 Detection. ACS Appl. Nano. Mater. 2019, 2, 700–706. [Google Scholar] [CrossRef]
- Kumar Aliyana, A.; Naveen Kumar, S.K.; Marimuthu, P.; Baburaj, A.; Adetunji, M.; Frederick, T.; Sekhar, P.; Fernandez, R.E. Machine learning-assisted ammonium detection using zinc oxide/multi-walled carbon nanotube composite based impedance sensors. Sci. Rep. 2021, 11, 24321. [Google Scholar] [CrossRef]
- Narayana, A.; Bhat, S.A.; Fathima, A.; Lokesh, S.V.; Suryad, S.G.; Yelamaggad, C.V. Green and low-cost synthesis of zinc oxide nanoparticles and their application in transistor based carbon monoxide sensing. RSC Adv. 2020, 10, 13532. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Ghazali, N.A.B.; Chong, H.M.H.; Cobley, R.J.; Li, L.; Kalna, K. Channel Mobility and Contact Resistance in Scaled ZnO Thin-Film Transistors. Solid-State Electron. 2020, 172, 107867. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Cheng, C.-Y.; Lee, C.-T. Bottom gate thin-film transistors using parallelly lateral ZnO nanorods grown by hydrothermal method. Mater. Sci. Semicond. Process. 2020, 119, 105223. [Google Scholar] [CrossRef]
- Vyas, S. A Short Review on Properties and Applications of Zinc Oxide Based Thin Films and Devices. Johns. Matthey Technol. Rev. 2020, 64, 202–218. [Google Scholar] [CrossRef]
- Ohya, Y.; Niwa, T.; Ban, T.; Takahashi, Y. Thin Film Transistor of ZnO Fabricated by Chemical Solution Deposition. Jpn. J. Appl. Phys. 2001, 40, 297–298. [Google Scholar] [CrossRef]
- Kumer Saha, J.; Naik Bukke, R.; Naik Mude, N.; Jang, J. Significant improvement of spray pyrolyzed ZnO thin film by precursor optimization for high mobility thin film transistors. Sci. Rep. 2020, 10, 8999. [Google Scholar] [CrossRef]
- Oluwaseun, A.; Adetunji, A.J.; Ibironke, B.D.; Olufunke Lydia, A.; Ismaila Taiwo, B. On the Structural, Optical and Electrical Characterization of Zinc Oxide and Aluminium doped Zinc Oxide for Optoelectronic Applications. J. At. Mol. Condens. Nano Phys. 2020, 7, 25–33. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Leontie, L.; Galca, A.C.; Enculescu, M.; Carlescu, A.; Guerboub, M.; Mothudi, B.M.; Jorio, A.; et al. Graphene Oxide Concentration Effect on the Optoelectronic Properties of ZnO/GO Nanocomposites. Nanomaterials 2020, 10, 1532. [Google Scholar] [CrossRef]
- Strachowski, T.; Grzanka, E.; Mizeracki, J.; Chlanda, A.; Baran, M.; Małek, M.; Onyszko, K.; Januszewski, B.; Przybysz, M. Luminescence Properties of Nano Zinc Oxide Doped with Al(III) Ions Obtained in Microwave-Assisted Hydrothermal Synthesis. Materials 2022, 15, 1403. [Google Scholar] [CrossRef]
- Millers, D.; Grigorjeva, L.; Łojkowski, W.; Strachowski, T. Luminescence of ZnO nanopowders. Radiat. Meas. 2004, 38, 589–591. [Google Scholar] [CrossRef]
- Adhikari, A.; Przezdziecka, E.; Mishra, S.; Sybilski, P.; Sajkowski, J.; Guziewicz, E. Optical Properties of ZnO Deposited by Atomic Layer Deposition on Sapphire: A Comparison of Thin and Thick Films. Phys. Status Solidi. A 2021, 218, 2000669. [Google Scholar] [CrossRef]
- Bindua, P.; Thomas, S. Optical Properties of ZnO Nanoparticles Synthesised from a Polysaccharide and ZnCl2. Acta Phys. Pol. A 2017, 131, 1474–1478. [Google Scholar] [CrossRef]
- Pronti, L.; Candida Felici, A.; Ménager, M.; Vieillescazes, C.; Piacentini, M. Spectral Behavior of White Pigment Mixtures Using Reflectance, Ultraviolet-Fluorescence Spectroscopy, and Multispectral Imaging, Applied Spectroscopy. Soc. Appl. Spectrosc. 2017, 71, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.; Mekuria, M.; Getachew, A. Incorporation of zinc oxide nanoparticles in cotton textiles for ultraviolet light protection and antibacterial activities. Nanomater. Nanotechnol. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Becheri, A.; Durr, M.; Nostro, P.L. Synthesis and characterizations of zinc oxide nanoparticles: Applications to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Bueno, C.; Pacio, A.; Osorio, E.; Alvarado, J.A.; Maestre, D.; Cremades, A.; García, J.A.; Flores-Carrasco, G.; Juárez, H. Growth Mechanism and Optical Properties of Nano and Microstructures of ZnO Obtained by Thermal Oxidation of Zinc Powders at Atmospheric Pressure. Solid State Phenom. 2019, 286, 33–39. [Google Scholar] [CrossRef]
- Reza Khanlary, M.; Vahedi, V.; Reyhani, A. Synthesis and Characterization of ZnO Nanowires by Thermal Oxidation of Zn Thin Films at Various Temperatures. Molecules 2012, 17, 5021–5029. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, L.Z.; Wang, Z.Y.; Zhao, Y.; Liang, X.P.; Wang, M.T. High-quality ZnO thin films prepared by low temperature oxidation of metallic Zn. Appl. Surf. Sci. 2004, 229, 311–315. [Google Scholar] [CrossRef]
- Kumar, A. Sol Gel Synthesis of Zinc Oxide Nanoparticles and their application as Nano-composite Electrode Material for Supercapacitor. J. Mol. Struct. 2020, 1220, 128654. [Google Scholar] [CrossRef]
- Omri, K.; Najeh, I.; Dhahri, R.; El Ghoul, J.; El Mir, L. Effects of temperature on the optical and electrical properties of ZnO nanoparticles synthesized by sol–gel method. Microelectron. Eng. 2014, 128, 53–58. [Google Scholar] [CrossRef]
- Amakali, T.; Daniel, L.S.; Uahengo, V.; Dzade, N.Y.; de Leeuw, N.H. Structural and Optical Properties of ZnO Thin Films Prepared by Molecular Precursor and Sol–Gel Methods. Crystals 2020, 10, 132. [Google Scholar] [CrossRef]
- Hakim, A.A.N.; Rashid, A.R.A.; Arsad, N.; Surani, A.H. Zinc Oxide Thin Film Synthesized by Sol-Gel Method. Solid State Phenom. 2019, 307, 51–57. [Google Scholar] [CrossRef]
- Mukhtar, M.; Munisa, L.; Saleh, R. Co-Precipitation Synthesis and Characterization of Nanocrystalline Zinc Oxide Particles Doped with Cu2+ Ions. Mater. Sci. Appl. 2012, 3, 543–551. [Google Scholar] [CrossRef]
- Jeyachitra, R.; Kalpana, S.; Senthil, T.S.; Kang, M. Electrical behavior and enhanced photocatalytic activity of (Ag, Ni) co-doped ZnO nanoparticles synthesized from co-precipitation technique. Water Sci. Technol. 2020, 81, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Basyar Baharudin, K.; Abdullah, N.; Derawi, D. Synthesis of raspberry-like structure zinc oxide nanoparticles via glycol-solvothermal, low-temperature solvothermal and coprecipitation methods. Comptes Rendus Chim. 2021, 24, 33–42. [Google Scholar] [CrossRef]
- Purwaningsih, S.Y.; Pratapa, S.; Darminto, T. Synthesis of Nano-sized ZnO Particles by Co-precipitation Method with Variation of Heating Time. AIP Conf. Proc. 2016, 1710, 030040. [Google Scholar] [CrossRef]
- Polarz, S.; Roy, A.; Merz, M.; Halm, S.; Schrçder, D.; Schneider, L.; Bacher, G.; Kruis, F.E.; Driess, M. Chemical Vapor Synthesis of Size-Selected Zinc Oxide Nanoparticles. Small 2005, 1, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Reuge, N.; Bacsa, R.; Serp, P.; Caussat, B. Chemical Vapor Synthesis of Zinc Oxide Nanoparticles: Experimental and Preliminary Modeling Studies. J. Phys. Chem. C 2009, 113, 19845–19852. [Google Scholar] [CrossRef]
- Tani, T.; Madler, L.; Pratsinis, S.E. Homogeneous ZnO nanoparticles by flame spray pyrolysis. J. Nanoparticle Res. 2002, 4, 337–343. [Google Scholar] [CrossRef]
- Chang, H.; Tsai, M.-H. Synthesis And Characterization Of ZnO Nanoparticles Having Prism Shape By A Novel Gas Condensation Process. Rev. Adv. Mater. Sci. 2008, 18, 734–743. [Google Scholar]
- Zhou, Z.; Wang, J.; Jhun, C.G. ZnO Nanospheres Fabricated by Mechanochemical Method with Photocatalytic Properties. Catalysts 2021, 11, 572. [Google Scholar] [CrossRef]
- Moballegh, A.; Shahverdi, H.R.; Aghababazadeh, R.; Mirhabibi, A.R. ZnO nanoparticles obtained by mechanochemical technique and the optical properties. Surf. Sci. 2007, 601, 2850–2854. [Google Scholar] [CrossRef]
- Tsuzuki, T.; McCormick, P.G. ZnO Nanoparticles Synthesised By Mechanochemical Processing. Scr. Mater 2001, 44, 1731–1734. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, B.C. A mechanochemical synthesis of nanostructured zinc oxide via acetate route for LPG sensing. J. Exp. Nanosci. 2014, 9, 501–511. [Google Scholar] [CrossRef]
- Navas, D.; Ibanez, A.; Gonzalez, I.; Palma, J.L.; Dreyse, P. Controlled dispersion of ZnO nanoparticles produced by basic precipitation in solvothermal processes. Heliyon 2020, 6, e05821. [Google Scholar] [CrossRef] [PubMed]
- Lojkowski, W.; Gedanken, A.; Grzanka, E.; Opalinska, A.; Strachowski, T.; Pielaszek, R.; Tomaszewska-Grzeda, A.; Yatsunenko, S.; Godlewski, M.; Matysiak, H.; et al. Solvothermal synthesis of nanocrystalline zinc oxide doped with Mn2+, Ni2+, Co2+ and Cr3+ ions. Nanopart. Res. 2009, 11, 1991–2002. [Google Scholar] [CrossRef]
- Lemos de Peres, M.; de Avila Delucis, R.; Amico, S.C.; Gatto, D.A. Zinc oxide nanoparticles from microwave-assisted solvothermal process: Photocatalytic performance and use for wood protection against xylophagous fungus. Nanomater. Nanotechnol. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Motelica, L.; Marinof, L.; Holban, A.; Vasile, B.S.; Ficai, A. Optical, Photocatalytic And Antibacterial Properties Of Zinc Oxide Nanoparticles Obtained By A Solvothermal Method. UPB Sci. Bull. Ser. B 2020, 82, 59–70. [Google Scholar]
- Strachowski, T.; Grzanka, E.; Pałosz, B.; Presz, A.; Slusarski, L.; Łojkowski, W. Microwave Driven Hydrothermal Synthesis of Ninc Oxide Nanopowders. Solid State Phenom. 2003, 94, 189–192. [Google Scholar] [CrossRef]
- Łojkowski, W.; Opalińska, A.; Strachowski, T.; Presz, A.; Gierlotka, S.; Grzanka, E.; Pałosz, B.; Stręk, W.; Hreniak, D.; Grigorjeva, L.; et al. The Nan-Micro Interface: Bringing the Micro and Nano Worlds Together; Fecht, H.-J., Werner, M., Eds.; Wiley-VCH: Weinheim, Germania, 2004; pp. 163–179. [Google Scholar]
- Cao, Z.; Wang, Y.; Li, Z.; Naisen, Y. Hydrothermal Synthesis of ZnO Structures Formed by High-Aspect-Ratio Nanowires for Acetone Detection. Nanoscale Res. Lett. 2016, 11, 347. [Google Scholar] [CrossRef][Green Version]
- Aneesh, P.M.; Vanaja, K.A.; Jayaraj, M.K. Synthesis of ZnO nanoparticles by hydrothermal method. Proc. SPIE 2007, 6639, 66390J. [Google Scholar] [CrossRef]
- Kwabena Droepenu, E.; Siong Wee, B.; Fun Chin, S.; Ying Kok, K.; Firdaus Maligan, M. Zinc Oxide Nanoparticles Synthesis Methods and its Effect on Morphology: A Review. Biointerface Res. Appl. Chem. 2022, 12, 4261–4292. [Google Scholar] [CrossRef]
- Mohan, S.; Vellakkat, M.; Aravind, A.R.U. Hydrothermal synthesis and characterization of Zinc Oxide nanoparticles of various shapes under different reaction conditions. Nano Express 2020, 1, 030028. [Google Scholar] [CrossRef]
- Baruwati, B.; Kishore Kumar, D.; Manorama, S.V. Hydrothermal synthesis of highly crystalline ZnO nanoparticles: A competitive sensor for LPG and EtOH. Sens. Actuators B 2006, 119, 676–682. [Google Scholar] [CrossRef]
- Gerbreders, V.; Krasovska, M.; Sledevskis, E.; Gerbreders, A.; Mihailova, I.; Tamanis, E.; Ogurcovs, A. Hydrothermal synthesis of ZnO nanostructures with controllable morphology change. CrystEngComm 2020, 22, 1346–1358. [Google Scholar] [CrossRef]
- Bulcha, B.; Leta Tesfaye, J.; Anatol, D.; Shanmugam, R.; Dwarampudi, L.P.; Nagaprasad, N.; Nirmal Bhargavi, V.L.; Krishnaraj, R. Synthesis of Zinc Oxide Nanoparticles by Hydrothermal Methods and Spectroscopic Investigation of Ultraviolet Radiation Protective Properties. Hindawi J. Nanomater. 2021, 10, 8617290. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Chou, Y.-H.; Liu, C.-M.; Liu, Y.-M.; Ger, M.-D.; Shu, Y.-Y. Microwave-assisted hydrothermal synthesis of zinc oxide particles starting from chloride precursor. Mater. Res. Bull. 2012, 47, 96–100. [Google Scholar] [CrossRef]
- Barreto, G.P.; Morales, G.; Luisa López Quintanilla, M. Microwave Assisted Synthesis of ZnO Nanoparticles: Effect of Precursor Reagents, Temperature, Irradiation Time, and Additives on Nano-ZnO Morphology Development. Hindawi Publ. Corp. J. Mater. 2013, 2013, 478681. [Google Scholar] [CrossRef]
- Klapiszewska, I.; Kubiak, A.; Parus, A.; Janczarek, M.; Slosarczyk, A. The In Situ Hydrothermal and Microwave Syntheses of Zinc Oxides for Functional Cement Composites. Materials 2022, 15, 1069. [Google Scholar] [CrossRef]
- Hasanpoor, M.; Aliofkhazraei, M.; Delavari, H. Microwave-assisted synthesis of zinc oxide nanoparticles. Procedia Mater. Sci. 2015, 11, 320–325. [Google Scholar] [CrossRef]
- Institute of High Pressure Physics PAS. Reaction Method and Chemical Reactor. Polish Patent UPRP P 377451, 3 January 2006. [Google Scholar]
- Karam, S.T.; Abdulrahman, A.F. Green Synthesis and Characterization of ZnO Nanoparticles by Using Thyme Plant Leaf Extract. Photonics 2022, 9, 594. [Google Scholar] [CrossRef]
- Al-She’irey, A.Y.; Balouch, A.; Mawarnis, E.R.; Roza, L.; Rahman, M.Y.A.; Mahar, A.M. Effect of ZnO seed layer annealing temperature on the growth of ZnO nanorods and its catalytic application. Opt. Mater. 2022, 131, 112652. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Miralrio, A.; Hernández-Rodríguez, Y.M.; Cruz-Martínez, H.; Pérez-Pérez, R.; Cigarroa-Mayorga, O.E. Needle-and cross-linked ZnO microstructures and their photocatalytic activity using experimental and DFT approach. Mater. Lett. 2021, 291, 129474. [Google Scholar] [CrossRef]
- New Website in Construction. Available online: www.ertec.pl (accessed on 27 October 2022).
- Albinati, A.; Willis, B.T.M. The Rietveld Method in Neutron and X-ray Powder Diffraction. J. Appl. Cryst. 1982, 15, 361–374. [Google Scholar] [CrossRef]
- Lutterotti, L.; Scardi, P. Simultaneous structure and size–strain refinement by the Rietveld method. J. Appl. Cryst. 1990, 23, 246–252. [Google Scholar] [CrossRef]
- Tsubota, M.; Kitagawa, J. A necessary criterion for obtaining accurate lattice parameters by Rietveld method. Sci. Rep. 2017, 7, 15381. [Google Scholar] [CrossRef] [PubMed]
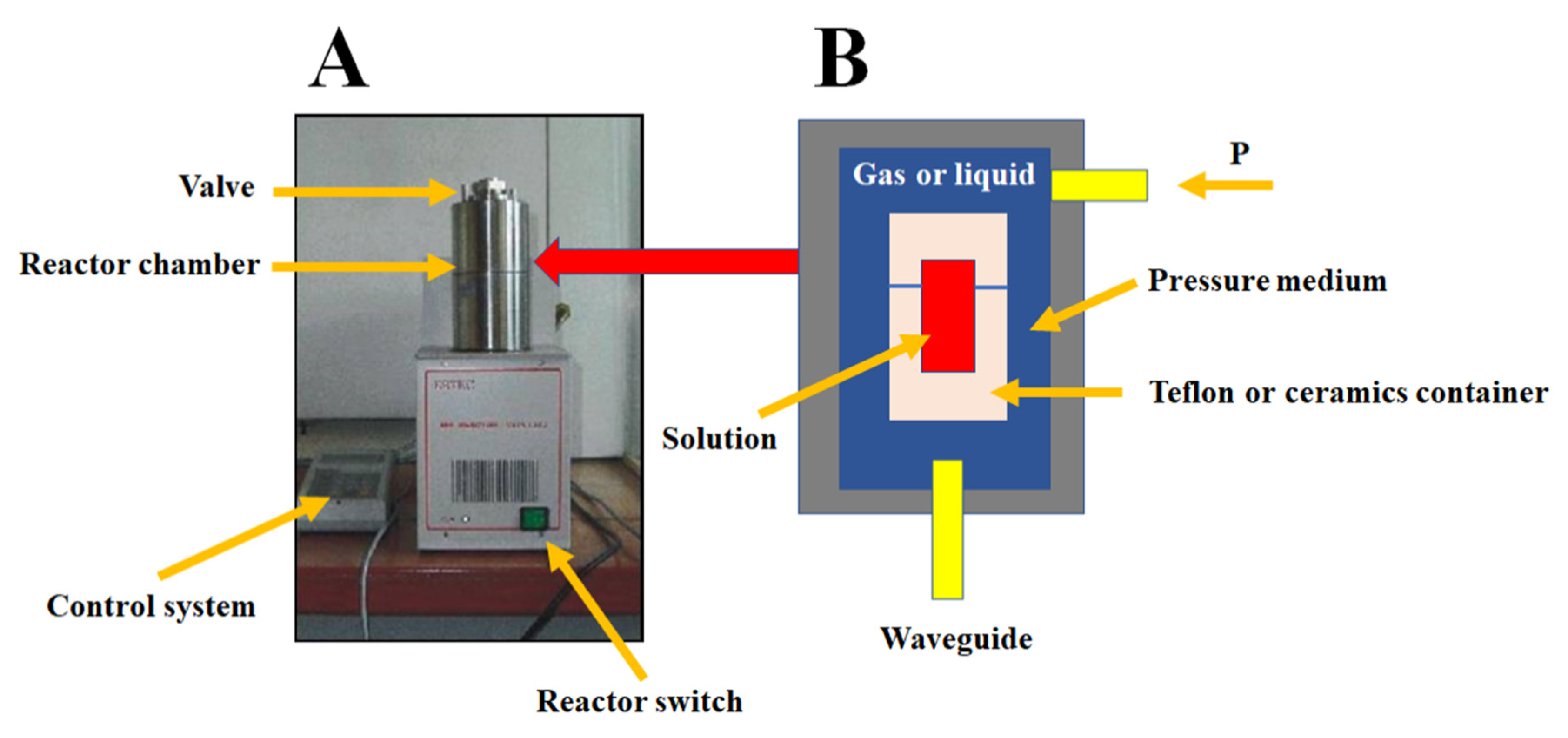
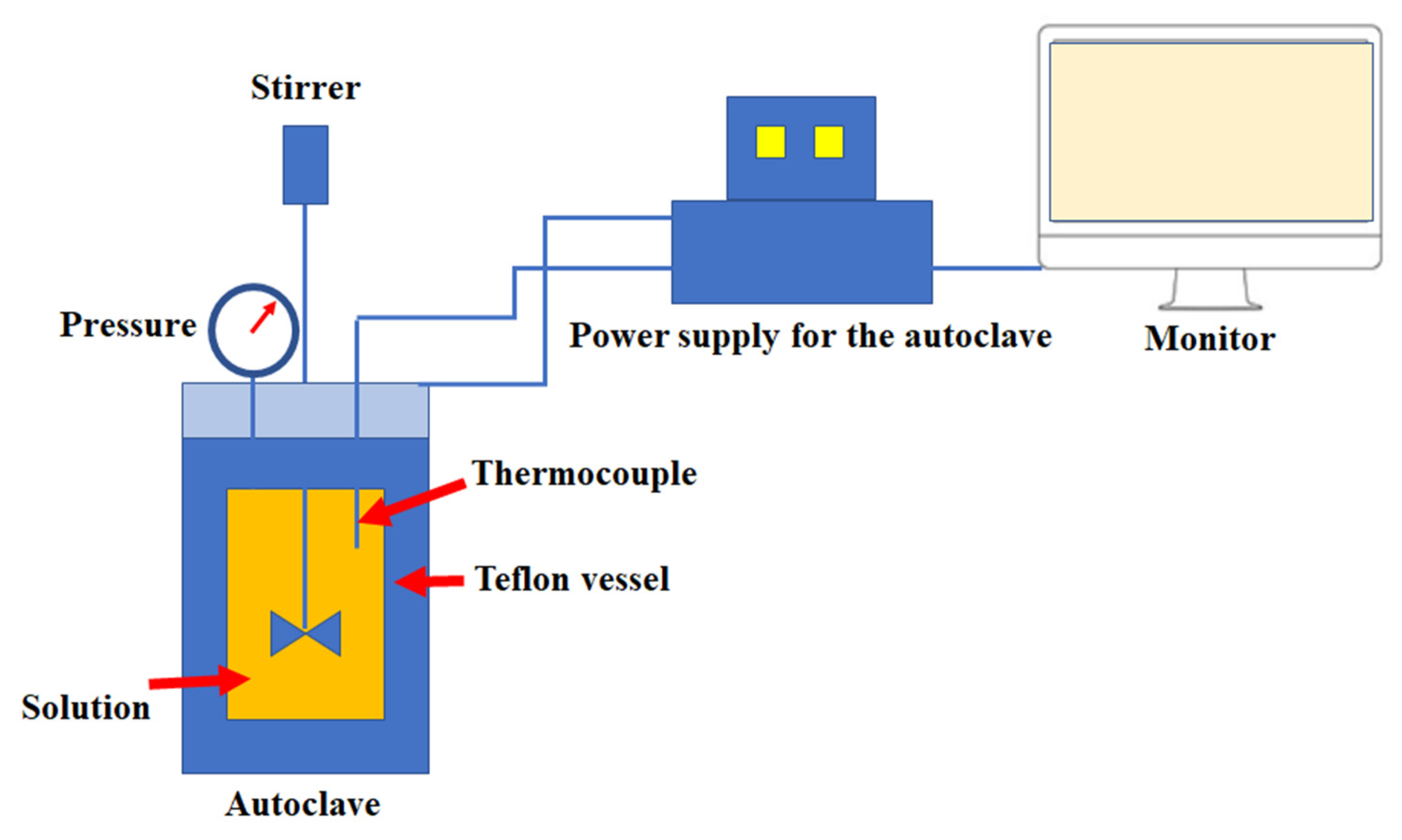
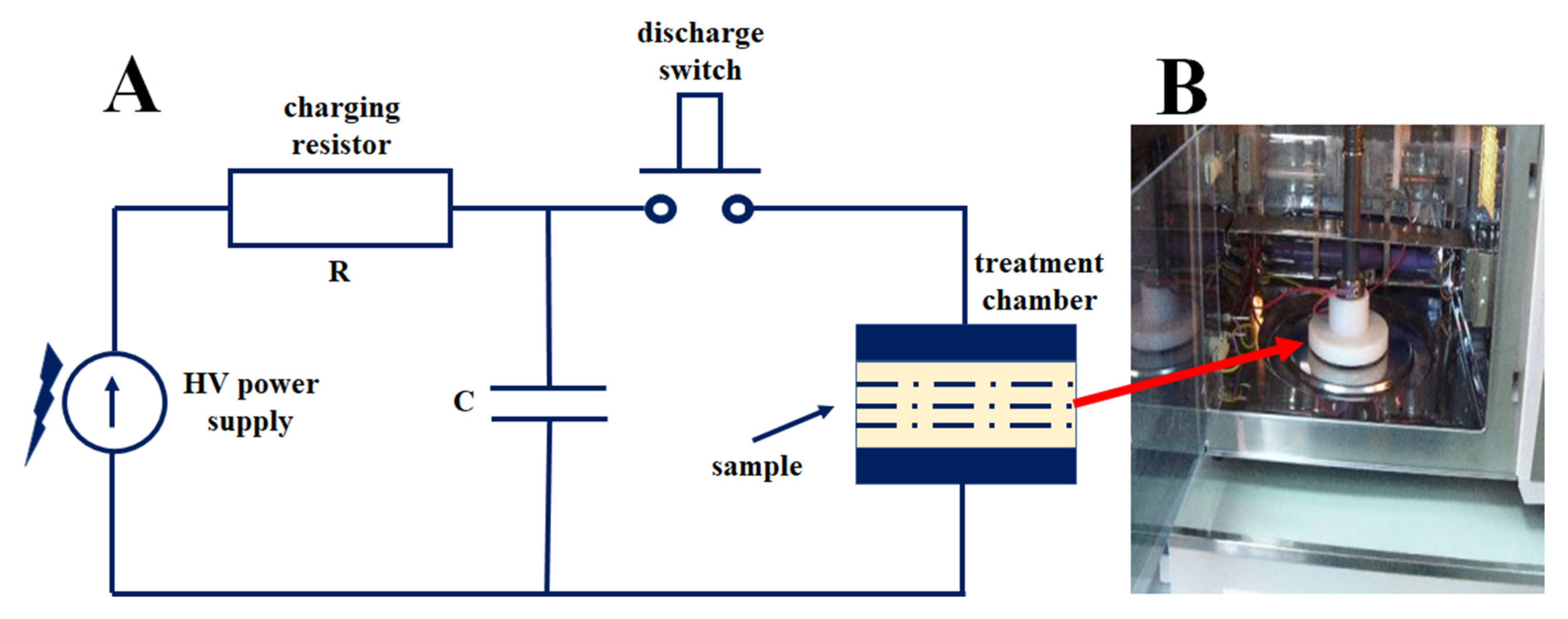

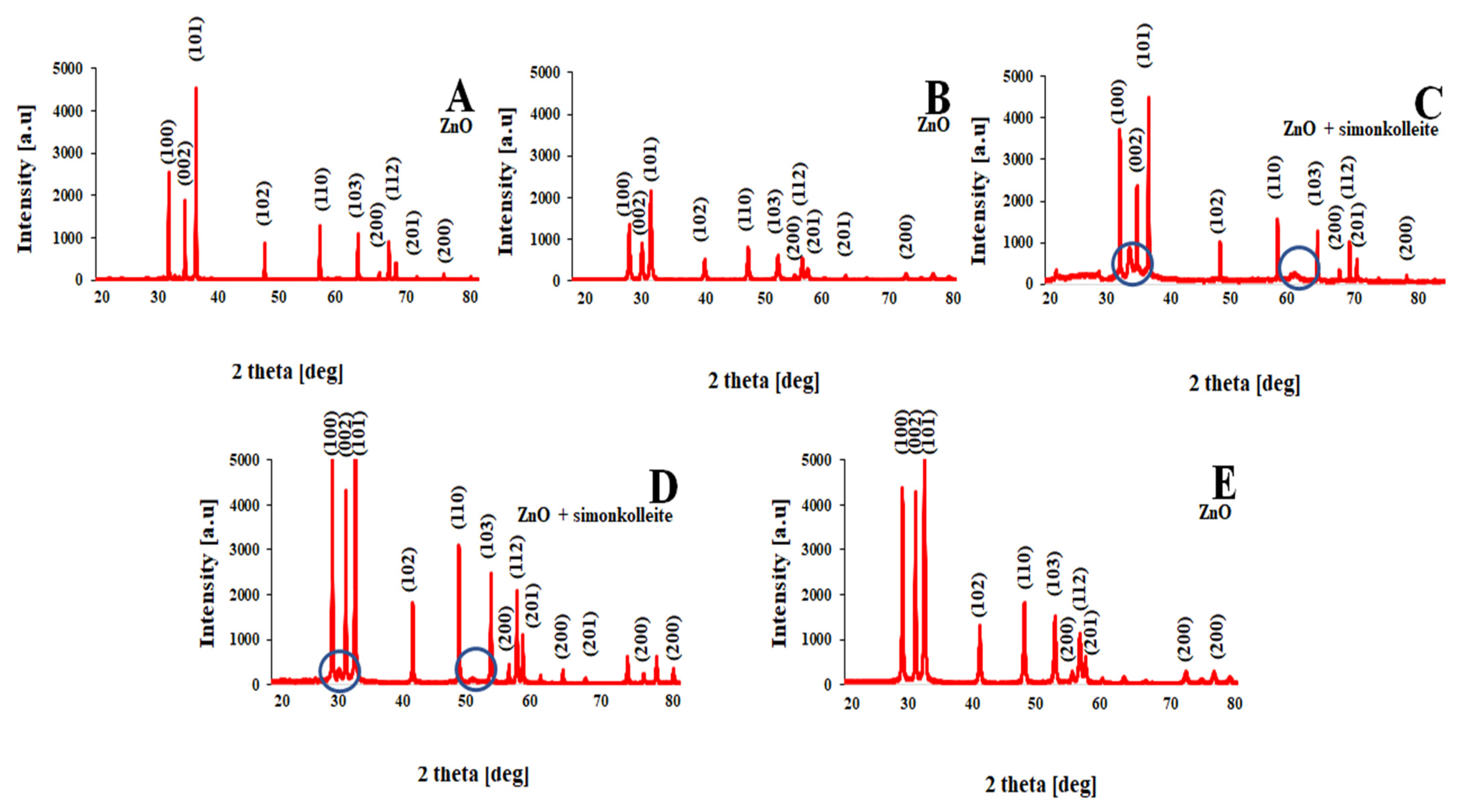
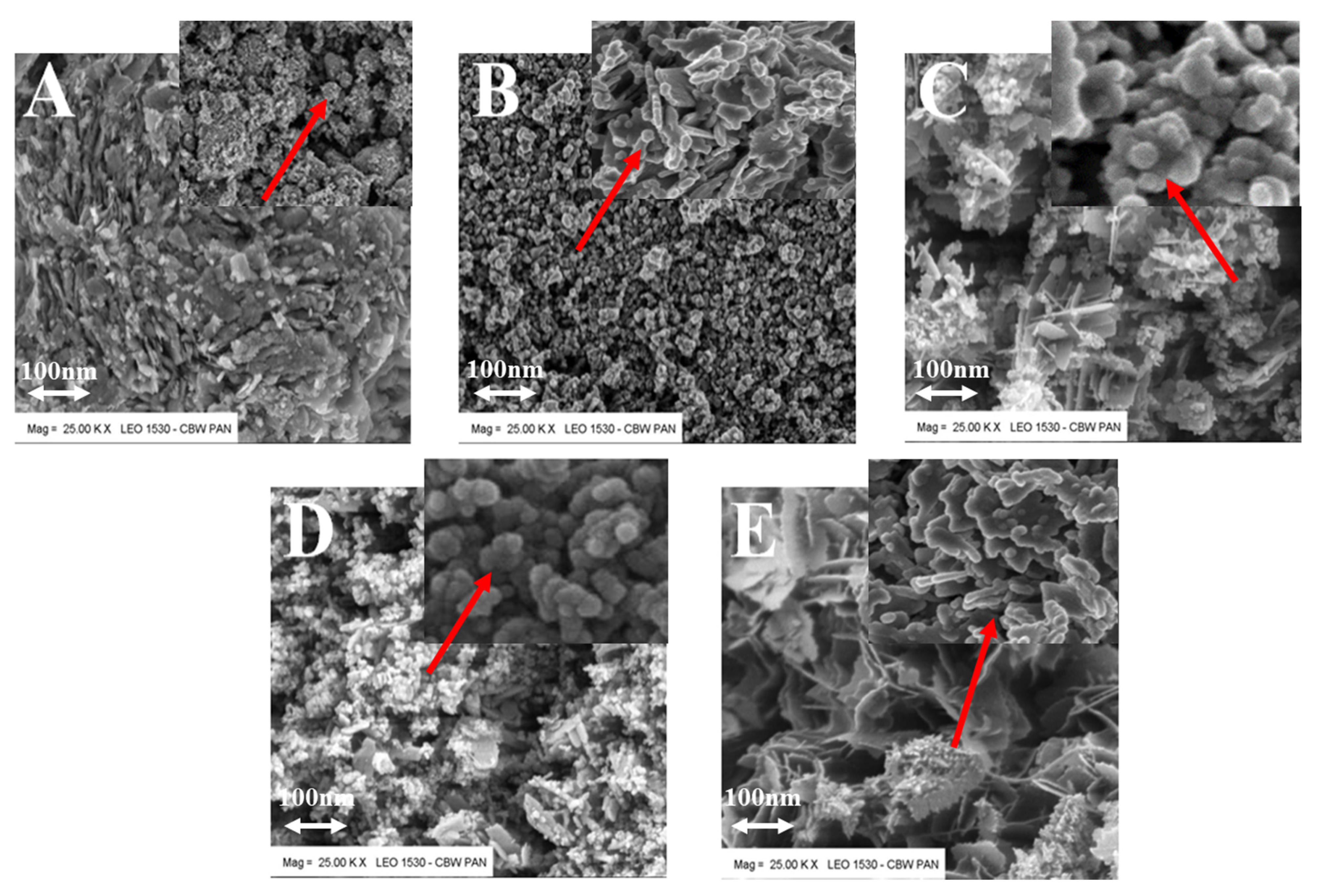
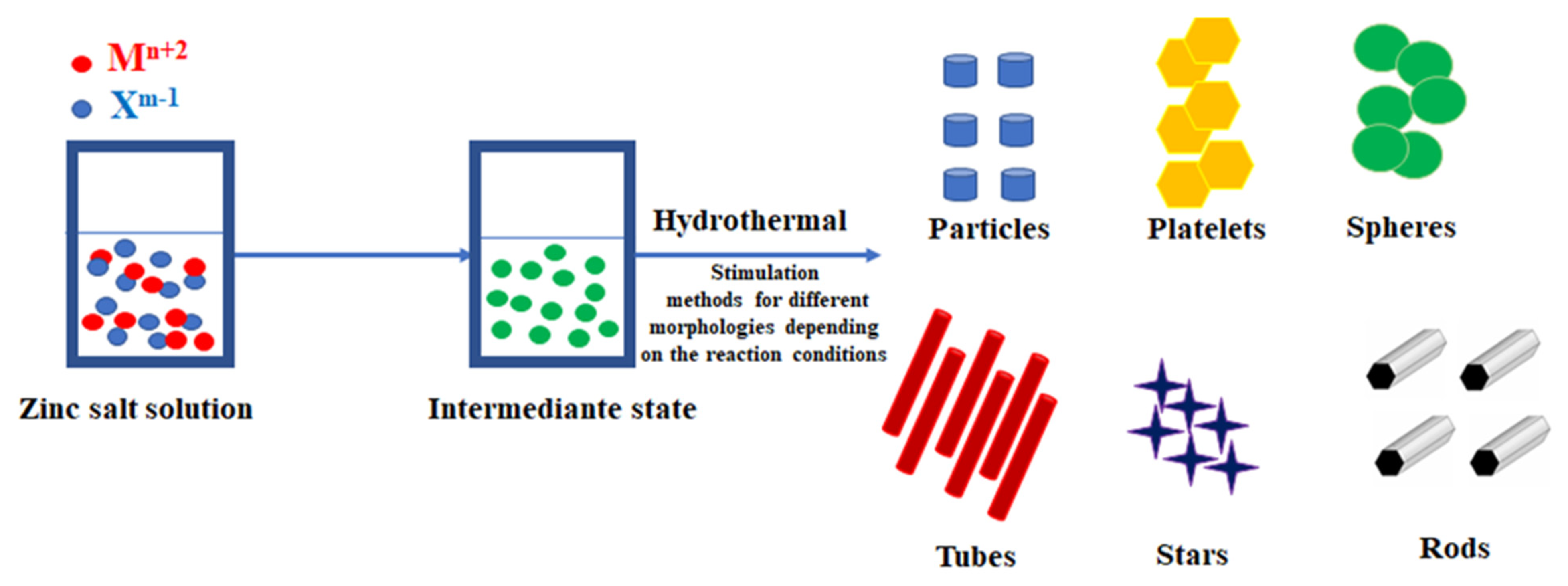
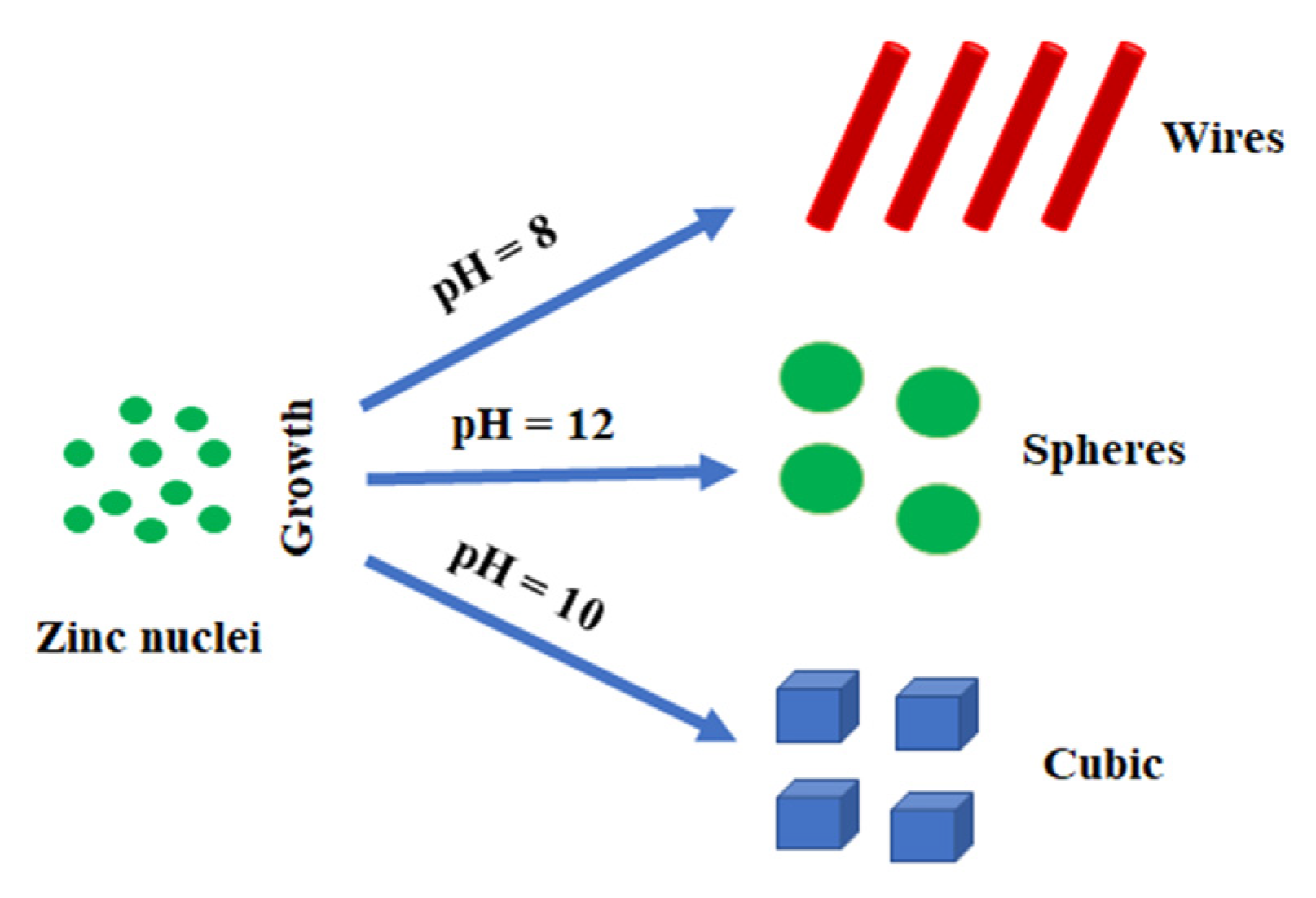
| Stimulaton Methods | Time [min] | Pressure [MPa] | Temperature Ts/Te [°C] | Shape | Color | BET [nm] | Average Crystallite Size [nm] | Density [g/cm3] | Phases |
|---|---|---|---|---|---|---|---|---|---|
| Microwave reactor | 3 | 1 | 25/80 | Cubic | White | 37 | 25 | 5.60 ± 0.02 | ZnO |
| Electric autoclave | 15 | 20 | 25/200 | Plates | White | 39 | 24 | 5.60 ± 0.03 | ZnO |
| Meander-type heater | 3 | 1 | 25/78 | Needles/plates | Gray | 32 | 20 | 5.52 ± 0.02 | ZnO + simonkolleite |
| Joule-type heater | 4 | 2 | 26/88 | Plates/spheres | Beige | 40 | 32 | 5.50 ± 0.04 | ZnO + simonkolleite |
| Impulse reactor | 0.3 | 1 | 25/50 | Flowers | White | 46 | 40 | 5.60 ± 0.05 | ZnO + unknown phase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strachowski, T.; Baran, M.; Małek, M.; Kosturek, R.; Grzanka, E.; Mizeracki, J.; Romanowska, A.; Marynowicz, S. Hydrothermal Synthesis of Zinc Oxide Nanoparticles Using Different Chemical Reaction Stimulation Methods and Their Influence on Process Kinetics. Materials 2022, 15, 7661. https://doi.org/10.3390/ma15217661
Strachowski T, Baran M, Małek M, Kosturek R, Grzanka E, Mizeracki J, Romanowska A, Marynowicz S. Hydrothermal Synthesis of Zinc Oxide Nanoparticles Using Different Chemical Reaction Stimulation Methods and Their Influence on Process Kinetics. Materials. 2022; 15(21):7661. https://doi.org/10.3390/ma15217661
Chicago/Turabian StyleStrachowski, Tomasz, Magdalena Baran, Marcin Małek, Robert Kosturek, Ewa Grzanka, Jan Mizeracki, Agata Romanowska, and Stefan Marynowicz. 2022. "Hydrothermal Synthesis of Zinc Oxide Nanoparticles Using Different Chemical Reaction Stimulation Methods and Their Influence on Process Kinetics" Materials 15, no. 21: 7661. https://doi.org/10.3390/ma15217661
APA StyleStrachowski, T., Baran, M., Małek, M., Kosturek, R., Grzanka, E., Mizeracki, J., Romanowska, A., & Marynowicz, S. (2022). Hydrothermal Synthesis of Zinc Oxide Nanoparticles Using Different Chemical Reaction Stimulation Methods and Their Influence on Process Kinetics. Materials, 15(21), 7661. https://doi.org/10.3390/ma15217661









