From Seaweeds to Hydrogels: Recent Progress in Kappa-2 Carrageenans
Abstract
1. Introduction
2. From Seaweeds to Hybrid Carrageenans
2.1. Seaweeds Used for Kappa-2 Carrageenan Production: The Carrageenophytes
| Seaweed Species | Carrageenans in Seaweeds | Extraction | K Content in K2 | Ref. |
|---|---|---|---|---|
| Ahnfeltiopsis devoniensis | K, I, M | water | 50–55 | [26] |
| alkali | 17–35 a,b | [27] | ||
| K, I, M, N | alkali | 30–50 c | [28] | |
| K, I, M, N | water | 42–50 c | [28] | |
| Chondracanthus acicularis | K, I | alkali | 76 | [10,22] |
| K, I, M, N, L, T | [23] | |||
| T | [24] | |||
| water/alkali | 60 a | [27] | ||
| Chondracanthus canaliculatus | K, I c | alkali | 78 | [10,22] |
| Chondracanthus chamissoi | K, I, T | alkali | 82 | [10,22] |
| water | 35–100 b | [29] | ||
| water | 59 | [30] | ||
| alkali | 64 | [30] | ||
| Chondracanthus corymbiferus | K, I | alkali | 74 | [10,22] |
| Chondracanthus teedei | K, I, M, N, T | alkali | 50 | [24,25] |
| water/alkali | 53–58 a | [27] | ||
| K, I, N | alkali | 50–58 c | [10,22] | |
| Chondrus canaliculata | K, L, T | alkali | 69 | [10,22] |
| Chondrus crispus | K, I, M, L, T c | alkali | 30–81 c | [10,22] |
| K, I, M, L | [24] | |||
| K, I, M | water | 60–70 | [26] | |
| alkali | 70 a | [27] | ||
| alkali | 64 a | [31] | ||
| alkali | 71–72 | [32] | ||
| K, M, N | alkali | 65–84 c | [28] | |
| K, M, N | water | 65–75 c | [28] | |
| Chondrus ocellatus | K, I, L | 85 | [10,22] | |
| Eucheuma isiforme | I | alkali | 60 | [10,22] |
| Eucheuma platycladum | K, I | alkali | 83 | [10,22] |
| Gigartina alveata | K, L | alkali | 64 | [10,22] |
| Gigartina bracteata | K, L | [10] | ||
| Gigartina chamissoi | alkali | 54 | [22] | |
| alkali | 57 | [32] | ||
| Gigartina clathrata | alkali | 80 | [22] | |
| Gigartina pistillata | K, I, M, N, L | [24] | ||
| K, I | 41–45 | [10,22] | ||
| KIM, L, T | [23] | |||
| alkali | 35–49 a | [27] | ||
| Gigartina radula | K, L | alkali | 50 | [10,22] |
| Gigartina skottsbergii d | K, I, M, N, L c | alkali | 59 | [10,22] |
| alkali | 57 | [32] | ||
| water | 62 | [33] | ||
| water | 70 | [34] | ||
| Gymnogongrus crenulatus | K, I, L | alkali | 64 c | [10,22] |
| alkali | 60–64 | [27] | ||
| Gymnogongrus humilis | K, I | alkali | 68 | [10,22] |
| Gymnogongrus tenuis | water | 14–51 b,c | [35] | |
| Gymnogongrus torulosus | alkali | 45 | [10] | |
| Gymnogongrus vermicularis | alkali | 71 | [10] | |
| Iridaea undulosa | alkali | 58–62 | [10] | |
| Iridaea cordata | water | 42 a,b,c–66 a | [36] | |
| Mastocarpus pacificus | water | >50 | [37] | |
| Mastocarpus stellatus | K, I, M, N, L c | alkali | 74 | [10,22] |
| K, I, M, N | [23,24] | |||
| K, I, M, N | water | 53–67 | [26] | |
| alkali | 62 a | [27] | ||
| water | 55–64 a | [38] | ||
| K, M, N | alkali | 62–80 a | [39] | |
| K, M, N | water | 62–65 a | [39] | |
| Mazzaella laminarioides | I, L | alkali | 55–75 c | [10,22] |
| K, I, M, N | [40] | |||
| water | 46 b | [30] | ||
| alkali | 54 | [30] | ||
| Mazzaella parksii | water | 63–79 c | [41] | |
| Mazzaella splendens | K, I, L, T | alkali | 72 | [10,22] |
| Sarcothalia crispata | K, I, M, N | [24] | ||
| K, I, M, N, L, T c | alkali | 40–63 | [10,22] | |
| water | 51 | [30] | ||
| alkali | 56 | [30] | ||
| water | 56 | [34] | ||
| Sarcothalia radula | water | 45 | [30] | |
| alkali | 53 | [30] | ||
| Turnerella mertensiana | water | 65 c | [42] | |
| alkali | 82 | [42] |
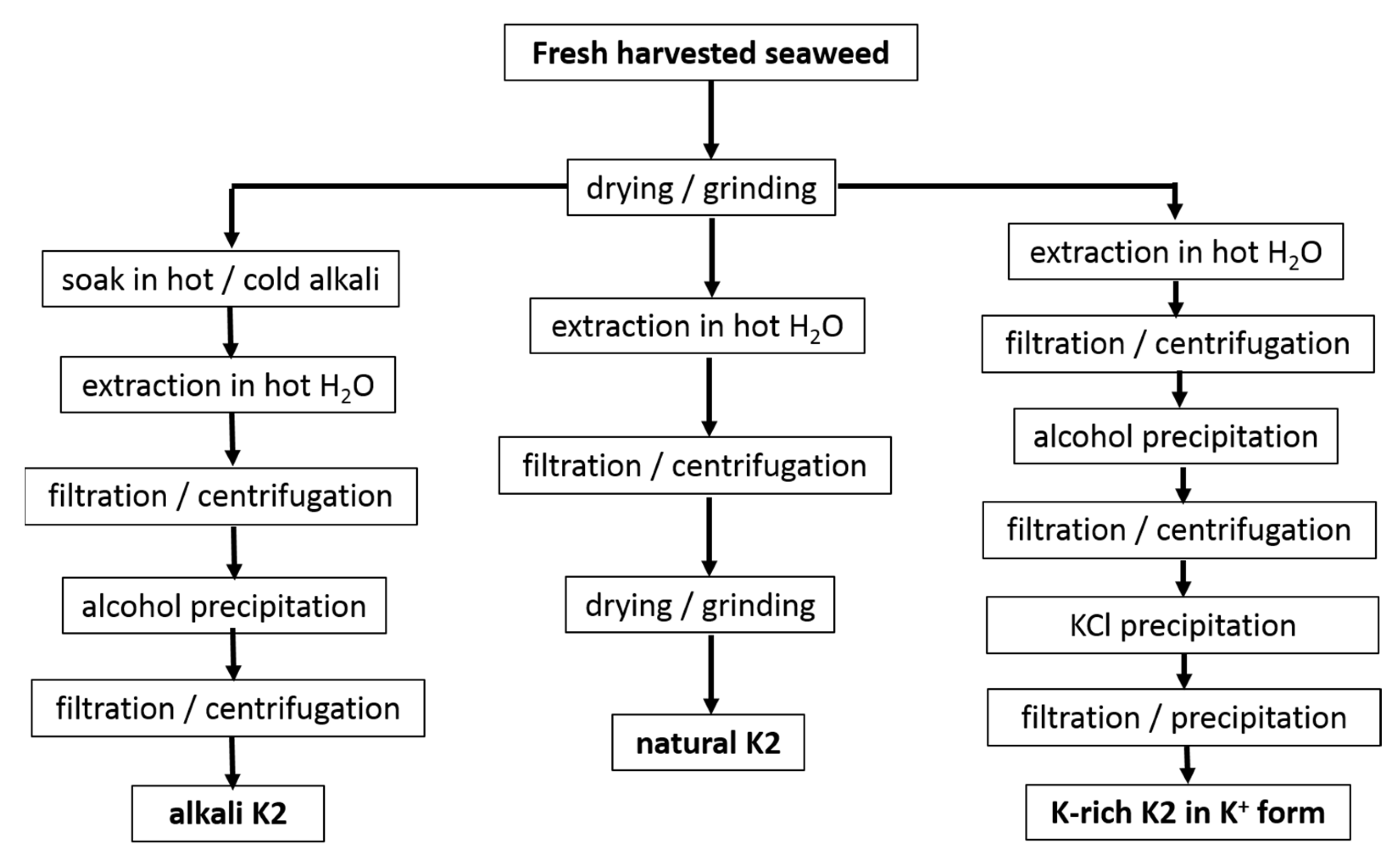
2.2. Extraction of Hybrid Carrageenans
2.3. Statistical Block Copolymer Structure of Gelling Hybrid Carrageenans

3. Gel Formation
4. Linear Viscoelastic Properties of K2 Gels
4.1. Impact of K2 Chemical Composition on the Gel Elastic Modulus
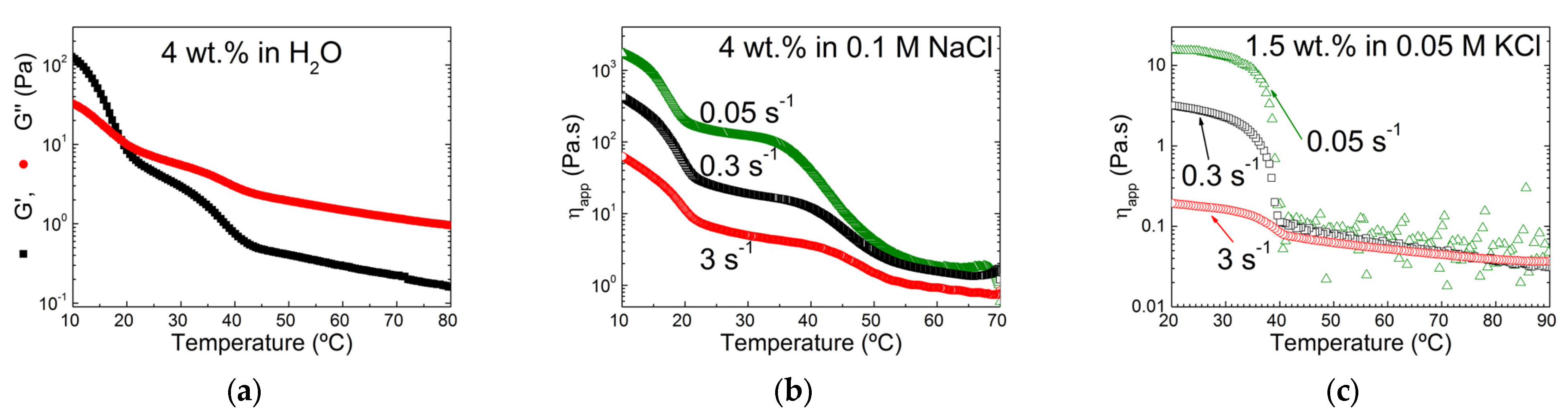
4.2. Effect of Salt Type and Ionic Strength
4.3. Concentration Scaling of the Gel Elasticity
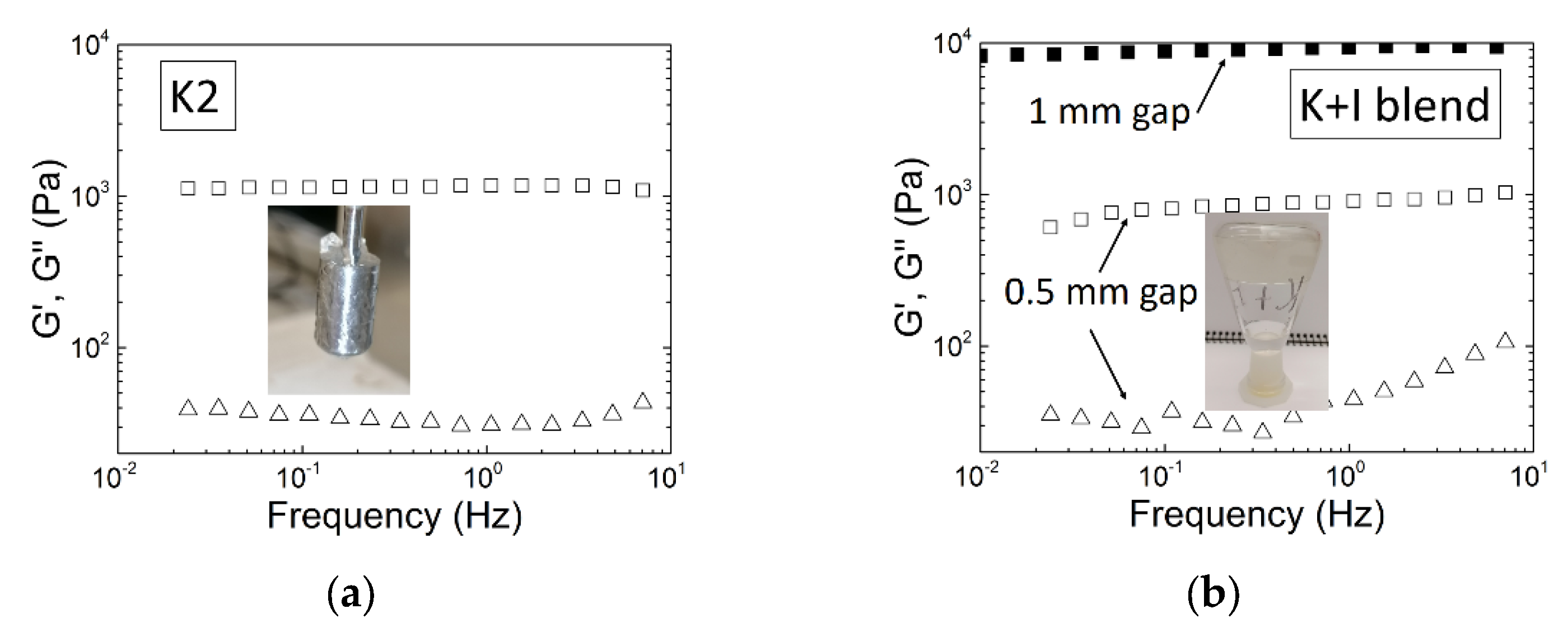
5. Gel Properties under Large Deformation

6. Conclusions and Perspectives
- -
- Focus on the study of K2 in the Na+ form and gel in the presence of NaCl. This is because the use of specific cations to K blocks smear-out the elasticity of I blocks in the copolymer. As a result, only the “weak kappa” character is unveiled, whereas in NaCl the elastic contribution of I blocks to the network are at least as strong as the K blocks contributions, resulting, for example, in the two-step gel setting;
- -
- Expand the study by van de Velde et al. [55] with more K2 with added complexity in the chemical composition. In the industry, many hybrid carrageenans are produced with significant levels of sulfated biological precursors and yet find application. Their texturing properties (as thickeners, not limited to gelling agents) also need to be understood based on their chemical attributes;
- -
- Focus on the effect of the molecular mass, Mw, on the K2 gel properties. Since each seaweed produces copolymers with specific distributions of K and I blocks, it seems natural that different Mw produced from the same parent K2 will have different effects on the gel properties unless Mw reduction does not change the blocks distribution. So far, this topic has not been studied, but it should also contribute to the above point: establish the relationship between K2′s chemical composition and the gel elasticity without perturbation from Mw effects;
- -
- Systematic comparative studies between K2 gels and K + I gels with similar G4S-DA composition and Mw could be very instructive in understanding the block copolymer effect on the gel properties and, thus, contribute to settling the debate on K + I’s microstructure. This is because in K2, I cannot be separated from K;
- -
- Rheological and structural studies of carrageenan gels submitted to large deformation. As mentioned in Section 5, this topic has received nearly no attention, but it should bring an overwhelming amount of information on the elastically relevant structures in the gel, since theories are available to connect structures to the gel strain hardening. In addition, many applications involve the strong and fast deformation of gels and gel-forming solutions. There is, thus, an industrial need for such studies.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piculell, L. Gelling Carrageenans. In Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 239–287. [Google Scholar]
- Porse, H.; Rudolph, B. The seaweed hydrocolloid industry: 2016 updates, requirements and outlook. J. Appl. Phycol. 2017, 29, 2187–2200. [Google Scholar] [CrossRef]
- Van de Velde, F.; Lourenço, N.D.; Pinheiro, H.M.; Bakker, M. Carrageenan: A food-grade and biocompatible support for immobilisation techniques. Adv. Synth. Catal. 2002, 344, 815–835. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Xue, C. Recent advances in carrageenan-based delivery systems for bioactive ingredients: A review. Trends Food Sci. Technol. 2021, 112, 348–361. [Google Scholar] [CrossRef]
- Liu, F.; Hou, P.F.; Zhang, H.; Tang, Q.J.; Xue, C.H. Food-grade carrageenans and their implications in health and disease. Compr. Rev. Food Sci. Food. 2021, 20, 3918–3936. [Google Scholar] [CrossRef] [PubMed]
- Bixler, H.J. The carrageenan controversy. J. Appl. Phycol. 2017, 29, 2201–2207. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug delivery systems and other biomedical applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Froeba, M.; Grosse, M.; Setz, C.; Rauch, P.; Auth, J.; Spanaus, L.; Muench, J.; Ruetalo, N.; Schindler, M.; Morokutti-Kurz, M.; et al. Iota-carrageenan inhibits replication of SARS-CoV-2 and the respective variants of concern Alpha, Beta, Gamma and Delta. Int. J. Mol. Sci. 2021, 22, 13202. [Google Scholar] [CrossRef]
- Bixler, H.J. Recent developments in manufacturing and marketing carrageenan. Hydrobiologia 1996, 326/327, 35–57. [Google Scholar] [CrossRef]
- Van de Velde, F. Structure and function of hybrid carrageenans. Food Hydrocoll. 2008, 22, 727–734. [Google Scholar] [CrossRef]
- Hilliou, L. Hybrid carrageenans: Isolation, Chemical Structure, and Gel Properties. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Oxford, UK, 2014; Volume 72, pp. 17–43. [Google Scholar] [CrossRef]
- Bixler, H.J.; Johndro, K.; Falshaw, R. Kappa-2 carrageenan: Structure and performance of commercial extracts: II. Performance in two simulated dairy applications. Food Hydrocoll. 2001, 15, 619–630. [Google Scholar] [CrossRef]
- Knutsen, S.H.; Myslabodski, D.E.; Larsen, B.; Usov, A.I.I. A modified system of nomenclature for red algal galactans. Bot. Mar. 1994, 37, 163–169. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Ma, Y.; Huan, L.; Wang, Y.; Xia, B.; Wang, G. Economically important red algae resources along the Chinese coast: History, status, and prospects for their utilization. Algal Res. 2020, 46, 101817. [Google Scholar] [CrossRef]
- Ward, G.M.; Faisan, J.P., Jr.; Cottier-Cook, E.J.; Gachon, C.; Hurtado, A.Q.; Lim, P.E.; Matoju, I.; Msuya, F.E.; Bass, D.; Brodie, J. A review of reported seaweed diseases and pests in aquaculture in Asia. J. World Aquac. Soc. 2020, 51, 815–828. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Mendoza, W.G.; Rodrigueza, M.R.C.; Romero, J.B.; Montaño, M.N.E. Structure and functional performance of gigartinacean kappa-iota hybrid carrageenan and solieriacean kappa-iota carrageenan blends. Food Hydrocoll. 2004, 18, 283–292. [Google Scholar] [CrossRef]
- Agoda-Tandjawa, G.; Mazoyer, J.; (Cargill Starches Sweeteners & Texturizers, Prod & Proc Dev Ctr, Carentan, France). Personal communication, 2022.
- Ciancia, M.; Matulewicz, M.C.; Tuvikene, R. Structural diversity of galactans from red seaweeds and its influence on rheological properties. Front. Plant. Sci. 2020, 11, 559986. [Google Scholar] [CrossRef] [PubMed]
- Ficko-Blean, E.; Hervé, C.; Michel, G. Sweet and sour sugars from the sea: The biosynthesis and remodeling of sulfated cell wall polysaccharides from marine macroalgae. Perspect. Phycol. 2015, 2, 51–64. [Google Scholar] [CrossRef]
- Genicot-Joncour, S.; Poinas, A.; Richard, O.; Potin, P.; Rudolph, B.; Kloareg, B.; Helbert, W. The cyclization of the 3,6-anhydro-galactose ring of iota-carrageenan is catalyzed by two D-galactose-2,6-sulfurylases in the red alga Chondrus crispus. Plant Physiol. 2009, 151, 1609–1616. [Google Scholar] [CrossRef]
- Chopin, T.; Whalen, E. A new and rapid method for carrageenan identification by FT IR diffuse reflectance spectroscopy directly on dried, ground algal material. Carbohydr. Res. 1993, 246, 51–59. [Google Scholar] [CrossRef]
- Chopin, T.; Kerin, B.F.; Mazerolle, R. Phycocolloid chemistry as a taxonomic indicator of phylogeny in the Gigartinales, Rhodophyceae, a review and current developments using Fourier transform infrared diffuse reflectance spectroscopy. Phycol. Res. 1999, 47, 167–188. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Pereira, L.; Critchley, A.T.; Amado, A.M.; Ribeiro-Claro, P.J.A. A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carrageenophytes (Gigartinales, Rhodophyta). J. Appl. Phycol. 2009, 21, 599–605. [Google Scholar] [CrossRef]
- Pereira, L.; Amado, A.M.; Critchley, A.T.; van de Velde, F.; Ribeiro-Claro, J.A. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll. 2009, 23, 1903–1909. [Google Scholar] [CrossRef]
- Azevedo, G.; Torres, M.D.; Almeida, P.L.; Hilliou, L. Exploring relationships between seaweeds carrageenan contents and extracted hybrid carrageenan properties in wild and cultivated Mastocarpus stellatus, Chondrus crispus and Ahnfeltiopsis devoniensis. Algal Res. 2022, 67, 102840. [Google Scholar] [CrossRef]
- Pereira, L.; van de Velde, F. Portuguese carrageenophytes: Carrageenan composition and geographic distribution of eight species (Gigartinales, Rhodophyta). Carbohydr. Polym. 2011, 84, 614–623. [Google Scholar] [CrossRef]
- Azevedo, G.; Torres, M.D.; Sousa-Pinto, I.; Hilliou, L. Effect of pre-extraction alkali treatment on the chemical structure and gelling properties of extracted hybrid carrageenan from Chondrus crispus and Ahnfeltiopsis devoniensis. Food Hydrocoll. 2015, 50, 150–158. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, X.; Lv, Y.; Li, M.; Liu, X.; Li, G.; Yu, G. Structural and compositional characteristics of hybrid carrageenans from red algae Chondracanthus chamissoi. Carbohydr. Polym. 2012, 89, 914–919. [Google Scholar] [CrossRef]
- Jouanneau, D.; Boulenguer, P.; Mazoyer, J.; Helbert, W. Hybridity of carrageenans water- and alkali-extracted from Chondracanthus chamissoi, Mazzaella laminarioides, Sarcothalia crispata, and Sarcothalia radula. J. Appl. Phycol. 2011, 23, 105–114. [Google Scholar] [CrossRef]
- Tasende, M.G.; Cid, M.; Fraga, M.I. Spatial and temporal variations of Chondrus crispus (Gigartinaceae, Rhodophyta) carrageenan content in natural populations from Galicia (NW Spain). J. Appl. Phycol. 2012, 24, 941–951. [Google Scholar] [CrossRef]
- Guibet, M.; Boulenguer, P.; Mazoyer, J.; Kervarec, N.; Antonopoulos, A.; Lafosse, M.; Helbert, W. Composition and distribution of carrabiose moieties in hybrid κ-/ι-Carrageenans using carrageenases. Biomacromolecules 2008, 9, 408–415. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Impact of counterions on the thermo-rheological features of hybrid carrageenan systems isolated from red seaweed Gigartina skottsbergii. Food Hydrocoll. 2018, 84, 321–329. [Google Scholar] [CrossRef]
- Hughes, M.H.; Prado, H.J.; Rodríguez, M.C.; Michetti, K.; Leonardi, P.I.; Matulewicz, M.C. Carrageenans from Sarcothalia crispata and Gigartina skottsbergii: Structural Analysis and Interpolyelectrolyte Complex Formation for Drug Controlled Release. Mar. Biotechnol. 2018, 20, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Perez Recalde, M.; Canelón, D.J.; Compagnone, R.S.; Matulewicz, M.C.; Cerezo, A.S.; Ciancia, M. Carrageenan and agaran structures from the red seaweed Gymnogongrus tenuis. Carbohydr. Polym. 2016, 136, 1370–1378. [Google Scholar] [CrossRef]
- Barahona, T.; Rodríguez Sánchez, R.O.; Noseda, M.D.; Mansilla, A.; Matsuhiro, B.; Prado, H.J.; Matulewicz, M.C. Characterization of polysaccharides from cystocarpic and tetrasporic stages of Sub-Antarctic Iridaea cordata. Algal Res. 2021, 60, 102503. [Google Scholar] [CrossRef]
- Kravchenko, A.O.; Anastyuk, S.D.; Glazunov, V.P.; Sokolova, E.V.; Isakov, V.V.; Yermak, I.M. Structural characteristics of carrageenans of red alga Mastocarpus pacificus from sea of Japan. Carbohydr. Polym. 2020, 229, 115518. [Google Scholar] [CrossRef] [PubMed]
- Tasende, M.G.; Cid, M.; Fraga, M.I. Qualitative and quantitative analysis of carrageenan content in gametophytes of Mastocarpus stellatus (Stackhouse) Guiry along Galician coast (NW Spain). J. Appl. Phycol. 2013, 25, 587–596. [Google Scholar] [CrossRef]
- Azevedo, G.; Hilliou, L.; Bernardo, G.; Sousa-Pinto, I.; Adams, R.W.; Nilsson, M.; Villanueva, R. Tailoring kappa/iota-hybrid carrageenan from Mastocarpus stellatus with desired gel quality through pre-extraction alkali treatment. Food Hydrocoll. 2013, 31, 94–102. [Google Scholar] [CrossRef]
- Arias, F.; Mansilla, A.; Matsuhiro, B.; Pavez, J.; Torres, R.; Yáñez-Sánchez, M. Carrageenans from nuclear phases of subantartic Mazzaella laminarioides (Gigartinales, Rhodophyta) and graft copolymerization of alkali-modified carrageenan with acrylamide. J. Appl. Phyc. 2016, 28, 1275–1286. [Google Scholar] [CrossRef]
- Kravchenko, A.; Anastyuk, S.; Glazunov, V.; Sokolova, E.; Isakov, V.; Yermak, I. Structural peculiarities of carrageenans from Far Eastern red seaweed Mazzaella parksii (Gigartinaceae). Int. J. Biol. Macromol. 2023, 228, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Vaskovsky, V.E.; Smirnova, G.P.; Shashkov, A.S.; Usov, A.I. Polysaccharides of algae 67. Carrageenan from Pacific red alga Turnerella mertensiana (Gigartinales, Rhodophyta). Russ. Chem. Bull. 2015, 64, 1163–1167. [Google Scholar] [CrossRef]
- Rochas, C.; Lahaye, M. Solid state 13C NMR spectroscopy of red seaweeds, agars and carrageenans. Carbohydr. Polym. 1989, 10, 189–204. [Google Scholar] [CrossRef]
- Gordon-Mills, E.; Tate, M.; Hounslow, A. Use of solid and gel state 13C NMR spectroscopy for differentiation between agarophytes and carrageenophytes. Hydrobiologia 1990, 204/205, 629–636. [Google Scholar] [CrossRef]
- Véliz, K.; Chandía, N.; Rivadeneira, M.; Thiel, M. Seasonal variation of carrageenans from Chondracanthus chamissoi with a review of variation in the carrageenan contents produced by Gigartinales. J. Appl. Phycol. 2017, 29, 3139–3150. [Google Scholar] [CrossRef]
- Hilliou, L.; Larotonda, F.D.S.; Abreu, P.; Abreu, M.H.; Sereno, A.M.; Gonçalves, M.P. The impact of seaweed life phase and postharvest storage duration on the chemical and rheological properties of hybrid carrageenans isolated from Portuguese Mastocarpus stellatus. Carbohydr. Polym. 2012, 87, 2655–2663. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D. Gelling characteristics and rheology of kappa/iota-hybrid carrageenans extracted from Mastocarpus stellatus dried at different temperatures. Appl. Phycol. 2016, 28, 3635–3644. [Google Scholar] [CrossRef]
- Torres, M.D.; Chenlo, F.; Moreira, R. Rheology of κ/ι-hybrid carrageenan from Mastocarpus stellatus: Critical parameters for the gel formation. Int. J. Biol. Macromol. 2016, 86, 418–424. [Google Scholar] [CrossRef]
- Bahari, A.; Moelants, K.; Kloeck, M.; Wallecan, J.; Mangiante, G.; Mazoyer, J.; Hendrickx, M.; Grauwet, T. Mechanical disintegration and particle size sieving of Chondrus crispus (Irish Moss) gametophytes and their effect on carrageenan and phycoerythrin extraction. Foods 2022, 10, 2982. [Google Scholar] [CrossRef]
- Villanueva, R.; Hilliou, L.; Sousa-Pinto, I. Postharvest culture in the dark: An eco-friendly alternative to alkali treatment for enhancing the gel quality of κ/ι-hybrid carrageenan from Chondrus crispus (Gigartinales, Rhodophyta). Bioresour. Technol. 2009, 100, 2633–2638. [Google Scholar] [CrossRef]
- Song, H.-I.; Han, S.; Park, J.-S.; Kim, Y.-J.; Jeong, C.-B.; Yarish, C.; Kim, J.K. Dark treatment effect on the carrageenan characterization in a red alga, Chondrus crispus. Algal Res. 2022, 68, 102889. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Montaño, M.N.E. Enhancement of carrageenan gel quality in the commercially important tropical seaweed Eucheuma denticulatum (Rhodophyta), with postharvest treatment in low-nutrient conditions. Bot. Mar. 2014, 57, 217–223. [Google Scholar] [CrossRef]
- Bahari, A.; Moelants, K.; Wallecan, J.; Mangiante, G.; Mazoyer, J.; Hendrickx, M.; Grauwet, T. Understanding the effect of time, temperature and salts on carrageenan extraction from Chondrus crispus. Algal Res. 2021, 58, 102371. [Google Scholar] [CrossRef]
- Souza, H.K.S.; Hilliou, L.; Bastos, M.; Gonçalves, M.P. Effect of molecular weight and chemical structure on thermal and rheological properties of gelling κ/ι-hybrid carrageenan solutions. Carbohydr. Polym. 2011, 85, 429–438. [Google Scholar] [CrossRef]
- Van de Velde, F.; Antipoca, A.S.; Rollema, H.S.; Burova, T.V.; Grinberg, N.V.; Pereira, L.; Gilseman, P.M.; Tromp, R.H.; Rudolph, B.; Grinberg, V.Y. The structure of κ/ι-hybrid carrageenans II. Coil–helix transition as a function of chain composition. Carbohydr. Res. 2005, 340, 1113–1129. [Google Scholar] [CrossRef]
- Ciancia, M.; Noseda, M.D.; Matulewicz, M.C.; Cerezo, A.S. Alkali-modification of carrageenans: Mechanism and kinetics in the kappa/iota-, mu/nu- and lambda-series. Carbohydr. Polym. 1993, 20, 95–98. [Google Scholar] [CrossRef]
- Robal, M.; Brenner, T.; Matsukawa, S.; Ogawa, H.; Truus, K.; Rudolph, B.; Tuvikene, R. Monocationic salts of carrageenans: Preparation and physico-chemical properties. Food Hydrocoll. 2017, 63, 656–667. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Rivas, S.; Torres, M.D.; Domínguez, H. Microwave-Assisted Extraction of Carrageenan from Sarcopeltis skottsbergii. Mar. Drugs 2023, 21, 83. [Google Scholar] [CrossRef]
- Ponthier, E.; Domínguez, H.; Torres, M.D. The microwave assisted extraction sway on the features of antioxidant compounds and gelling biopolymers from Mastocarpus stellatus. Algal Res. 2020, 51, 102081. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Chondrus crispus treated with ultrasound as a polysaccharides source with improved antitumoral potential. Carbohydr. Polym. 2021, 273, 118588. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Falqué, E.; Domínguez, H.; Torres, M.D. Green Extraction of Carrageenans from Mastocarpus stellatus. Polymers 2022, 14, 554. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Sanz, V.; Domínguez, H.; Torres, M.D. Valorisation of the industrial hybrid carrageenan extraction wastes using eco-friendly treatments. Food Hydrocoll. 2022, 122, 107070. [Google Scholar] [CrossRef]
- Stancioff, D.J. Reflections on the Interrelationships between Red Seaweed Source, Chemistry and Use. In Proceedings of the International Seaweed Symposium 10, Göteborg, Sweden, 11–15 August 1980; Levrig, T., Ed.; De Gruyter: Berlin, Germany, 1981; pp. 113–121. [Google Scholar]
- Bellion, C.; Hamer, G.K.; Yaphe, W. The degradation of Eucheuma spinosum and Eucheuma cottonii carrageenans by ι-carrageenases and κ-carrageenases from marine bacteria. Can. J. Microbio. 1982, 28, 784–880. [Google Scholar] [CrossRef]
- Rochas, C.; Rinaudo, M.; Landry, S. Relation between the molecular structure and mechanical properties of carrageenan gels. Carbohydr. Polym. 1989, 10, 115–127. [Google Scholar] [CrossRef]
- Van de Velde, F.; Peppelman, H.A.; Rollema, H.S.; Tromp, R.H. On the structure of k/i-hybrid carrageenans. Carbohy. Res. 2001, 331, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Schefer, L.; Adamcik, J.; Diener, M.; Mezzenga, R. Supramolecular chiral self-assembly and supercoiling behavior of carrageenans at varying salt conditions. Nanoscale 2015, 7, 16182. [Google Scholar] [CrossRef]
- Diener, M.; Adamcik, J.; Sánchez-Ferrer, A.; Jaedig, F.; Schefer, L.; Mezzenga, R. Primary, secondary, tertiary and quaternary structure levels in linear polysaccharides: From random coil, to single helix to supramolecular assembly. Biomacromolecules 2019, 20, 1731–1739. [Google Scholar] [CrossRef]
- Azevedo, G.; Bernardo, G.; Hilliou, L. NaCl and KCl phase diagrams of kappa/iota-hybrid carrageenans extracted from Mastocarpus stellatus. Food Hydrocoll. 2014, 37, 116–123. [Google Scholar] [CrossRef]
- Torres, M.D.; Azevedo, G.; Hilliou, L. Phase diagrams of hybrid carrageenans extracted from Ahnfeltiopsis devoniensis and Chondrus crispus. Carbohydr. Polym. 2016, 136, 449–458. [Google Scholar] [CrossRef]
- Geonzon, L.E.; Descallar, F.B.A.; Du, L.; Bacabac, R.G.; Matsukawa, S. Gelation mechanism and network structure in gels of carrageenans and their mixtures viewed at different length scales—A review. Food Hydrocoll. 2020, 108, 106039. [Google Scholar] [CrossRef]
- Hilliou, L. Structure-elastic properties relationships in gelling carrageenans. Polymers 2021, 13, 4120. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-forming algae polysaccharides: From seaweeds to biomedical applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Colby, R.H.; Rubinstein, M. Scaling theory of polyelectrolyte solutions. Macromolecules 1995, 28, 1859–1871. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Faizullin, D.A.; Zuev, Y.F. Interplay between secondary structure and ion binding upon thermoreversible gelation of k-carrageenan. Carbohydr. Polym. 2020, 227, 115342. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, E.V.; Chusovitin, E.A.; Barabanova, A.O.; Balagan, S.A.; Galkin, N.G.; Yermak, I.M. Atomic force microscopy imaging of carrageenans from red algae of Gigartinaceae and Tichocarpaceae families. Carbohyudr. Polym. 2013, 93, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Hilliou, L.; Sereno, A.M.; Gonçalves, M.P. Gel setting of hybrid carrageenan solutions under steady shear. Food Hydrocoll. 2014, 35, 531–538. [Google Scholar] [CrossRef]
- Hilliou, L.; Gonçalves, M.P. Gelling properties of a κ/ι hybrid carrageenan: Effect of concentration and steady shear. Int. J. Food Sci. Technol. 2007, 42, 678–685. [Google Scholar] [CrossRef]
- Torres, M.D.; Chenlo, F.; Moreira, R. Thermal reversibility of kappa/iota hybrid carrageenan gels extracted from Mastocarpus stellatus at different ionic strengths. J. Taiwan Inst. Chem. Eng. 2017, 71, 414–420. [Google Scholar] [CrossRef]
- Chanvrier, H.; Durand, S.; Garnier, C.; Sworn, G.; Bourriot, S.; Doublier, J.L. Gelation Behaviour and Rheological Properties of κ/ι Hybrid Carrageenans. In Gums and Stabilisers for the Food Industry; Williams, P.A., Phillips, G.O., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2004; Volume 12, pp. 139–145. [Google Scholar]
- Hilliou, L.; Larotonda, F.D.S.; Sereno, A.M.; Gonçalves, M.P. Thermal and viscoelastic properties of κ/ι–hybrid carrageenan gels obtained from the Portuguese seaweed Mastocarpus stellatus. J. Agric. Food Chem. 2006, 54, 7870–7878. [Google Scholar] [CrossRef]
- Bahari, A.; Moelants, K.; Huc-Mathis, D.; Wallecan, J.; Mangiante, G.; Mazoyer, J.; Hendrickx, M.; Grauwet, T. Compositional and rheological analysis of carrageenan from the gametophyte phase of the red seaweed Chondrus crispus neutrally extracted at varying temperatures and times. Food Hydrocoll. 2022, 133, 107995. [Google Scholar] [CrossRef]
- Hughes, M.H.; Leonardi, P.I.; Genovese, D.B. Native hybrid carrageenans from Sarcopeltis skottsbergii and Sarcothalia crispata: The role of counterions on their gelling properties. Algal Res. 2023, 73, 103159. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Fissore, E.N.; Rojas, A.M.; Stortz, C.A. Chemical and rheological characterization of the carrageenans from Hypnea musciformis (Wulfen) Lamoroux. Carbohydr. Polym. 2014, 102, 780–789. [Google Scholar] [CrossRef]
- Piculell, L.; Nilsson, S.; Muhrbeck, P. Effects of small amounts of kappa-carrageenan on the rheology of aqueous iota-carrageenan. Carbohydr. Polym. 1992, 18, 199–208. [Google Scholar] [CrossRef]
- Parker, A.; Brigand, G.; Miniou, C.; Trespoey, A.; Vallée, P. Rheology and fracture of mixed ι– and κ–carrageenan gels: Two step gelation. Carbohydr. Polym. 1993, 20, 253–262. [Google Scholar] [CrossRef]
- Brenner, T.; Tuvikene, R.; Parker, A.; Matsukawa, S.; Nishinari, K. Rheology and structure of mixed kappa-carrageenan/iota-carrageenan gels. Food Hydrocoll. 2014, 39, 272–279. [Google Scholar] [CrossRef]
- Du, L.; Brenner, T.; Xie, J.; Matsukawa, S. A study on phase separation behavior in kappa/iota carrageenan mixtures by micro DSC, rheological measurements and simulating water and cations migration between phases. Food Hydrocoll. 2016, 55, 81–88. [Google Scholar] [CrossRef]
- Hu, B.; Du, L.; Matsukawa, S. NMR study on the network structure of a mixed gel of kappa and iota carrageenans. Carbohydr. Polym. 2016, 150, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Geonzon, L.C.; Bacabac, R.G.; Matsukawa, S. Microscopic characterization of phase separation in mixed carrageenan gels using particle tracking. J. Electrochem. Soc. 2019, 166, B3228. [Google Scholar] [CrossRef]
- Bui, V.T.N.T.; Nguyen, B.T.; Renou, F.; Nicolai, T. Rheology and microstructure of mixtures of iota and kappa-carrageenan. Food Hydrocoll. 2019, 89, 180–187. [Google Scholar] [CrossRef]
- Hatzikiriakos, S.G. Wall slip of molten polymers. Prog. Polym. Sci. 2012, 37, 624–643. [Google Scholar] [CrossRef]
- Rochas, C.; Rinaudo, M.; Landry, S. Role of the molecular weight on the mechanical properties of kappa carrageenan gels. Carbohydr. Polym. 1990, 12, 255–266. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Nicolai, T.; Benyahia, L.; Chassenieux, C. Synergistic effects of mixed salt on the gelation of k-carrageenan. Carbohydr. Polym. 2014, 112, 10–15. [Google Scholar] [CrossRef]
- Meng, F.; Terentjev, E.M. Nonlinear elasticity of semiflexible filament networks. Soft Matter 2016, 12, 6749–6756. [Google Scholar] [CrossRef]
- Jones, J.L.; Marques, C.M. Rigid polymer network models. J. Phys. 1990, 51, 1113–1127. [Google Scholar] [CrossRef]
- Carrillo, J.-M.Y.; MacKintosh, F.C.; Dobrynin, A.V. Nonlinear elasticity: From single chain to networks and gels. Macromolecules 2013, 46, 3679–3692. [Google Scholar] [CrossRef]
- Doi, M.; Kuzuu, N.Y. Nonlinear elasticity of rodlike macromolecules in condensed state. J. Polym. Sci. 1980, 18, 409–419. [Google Scholar] [CrossRef]
- Yang, D.; Yang, H. Effects of ethanol on gelation of iota-carrageenan. LWT Food Sci. Technol. 2020, 126, 109281. [Google Scholar] [CrossRef]
- Flores, S.L.; Descallar, F.B.A.; Matsukawa, S.; Bacabac, R.G. Dynamic rheological properties of mixed carrageenan gels under large strains. J. Biorheol. 2017, 31, 35–39. [Google Scholar] [CrossRef][Green Version]
- Hilliou, L.; Wilhelm, M.; Yamanoi, M.; Gonçalves, M.P. Structural and mechanical characterization of kappa/iota-hybrid carrageenan gels in potassium salt using Fourier Transform rheology. Food Hydrocoll. 2009, 23, 2322–2330. [Google Scholar] [CrossRef]
- Erk, K.A.; Henderson, K.J.; Shull, K.R. Strain stiffening in synthetic and biopolymer networks. Biomacromolecules 2010, 11, 1358–1363. [Google Scholar] [CrossRef] [PubMed]


| Sample | n | Ref. | ||
|---|---|---|---|---|
| G4S-DA (mol.%) | Salt | Comment | ||
| 100 | 0.1 M NaCl | Commercial K—used as received | 3.5 ± 0.4 | [72] |
| 100 | 0.02 M KCl | Commercial K—used as received | 2.08 ± 0.13 | [78] |
| 78 | 0.01 M KCl 0.1 M KCl 0.5 M KCl 0.5 M NaCl 1 M NaCl | No mixed cations | 1.42 ± 0.05 2.25 ± 0.07 2.98 ± 0.01 1.05 ± 0.01 1.23 ± 0.03 | [70] |
| 69 | 0.01 M KCl 0.1 M KCl 1 M NaCl | No mixed cations | 1.37 ± 0.08 2.2 ± 0.1 1.18 ± 0.02 | [69] |
| 51.2 | 0.1 M KCl | With 31.7 mol.% of G4S-DA2S | 3.1 ± 0.1 | [72] |
| 51.2 | 0.05 M KCl | With 31.7 mol.% of G4S-DA2S | 3.18 ± 0.05 | [78] |
| 48 | 0.1 M KCl 0.5 M KCl 1 M KCl 1 M NaCl | No mixed cations | 2.51 ± 0.04 2.14 ± 0.05 2.46 ± 0.08 1.01 ± 0.02 | [70] |
| 8 | 0.1 M NaCl | Commercial I—used as received | 2.01 ± 0.08 | [72] |
| 8 | 0.05 M KCl | Commercial I—used as received | 1.76 ± 0.05 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, H.K.S.; Kraiem, W.; Ben Yahia, A.; Aschi, A.; Hilliou, L. From Seaweeds to Hydrogels: Recent Progress in Kappa-2 Carrageenans. Materials 2023, 16, 5387. https://doi.org/10.3390/ma16155387
Souza HKS, Kraiem W, Ben Yahia A, Aschi A, Hilliou L. From Seaweeds to Hydrogels: Recent Progress in Kappa-2 Carrageenans. Materials. 2023; 16(15):5387. https://doi.org/10.3390/ma16155387
Chicago/Turabian StyleSouza, Hiléia K. S., Wala Kraiem, Amine Ben Yahia, Adel Aschi, and Loïc Hilliou. 2023. "From Seaweeds to Hydrogels: Recent Progress in Kappa-2 Carrageenans" Materials 16, no. 15: 5387. https://doi.org/10.3390/ma16155387
APA StyleSouza, H. K. S., Kraiem, W., Ben Yahia, A., Aschi, A., & Hilliou, L. (2023). From Seaweeds to Hydrogels: Recent Progress in Kappa-2 Carrageenans. Materials, 16(15), 5387. https://doi.org/10.3390/ma16155387






