Pd-Based Nano-Catalysts Promote Biomass Lignin Conversion into Value-Added Chemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Catalytic Depolymerization of Lignin
2.3. Characterizations
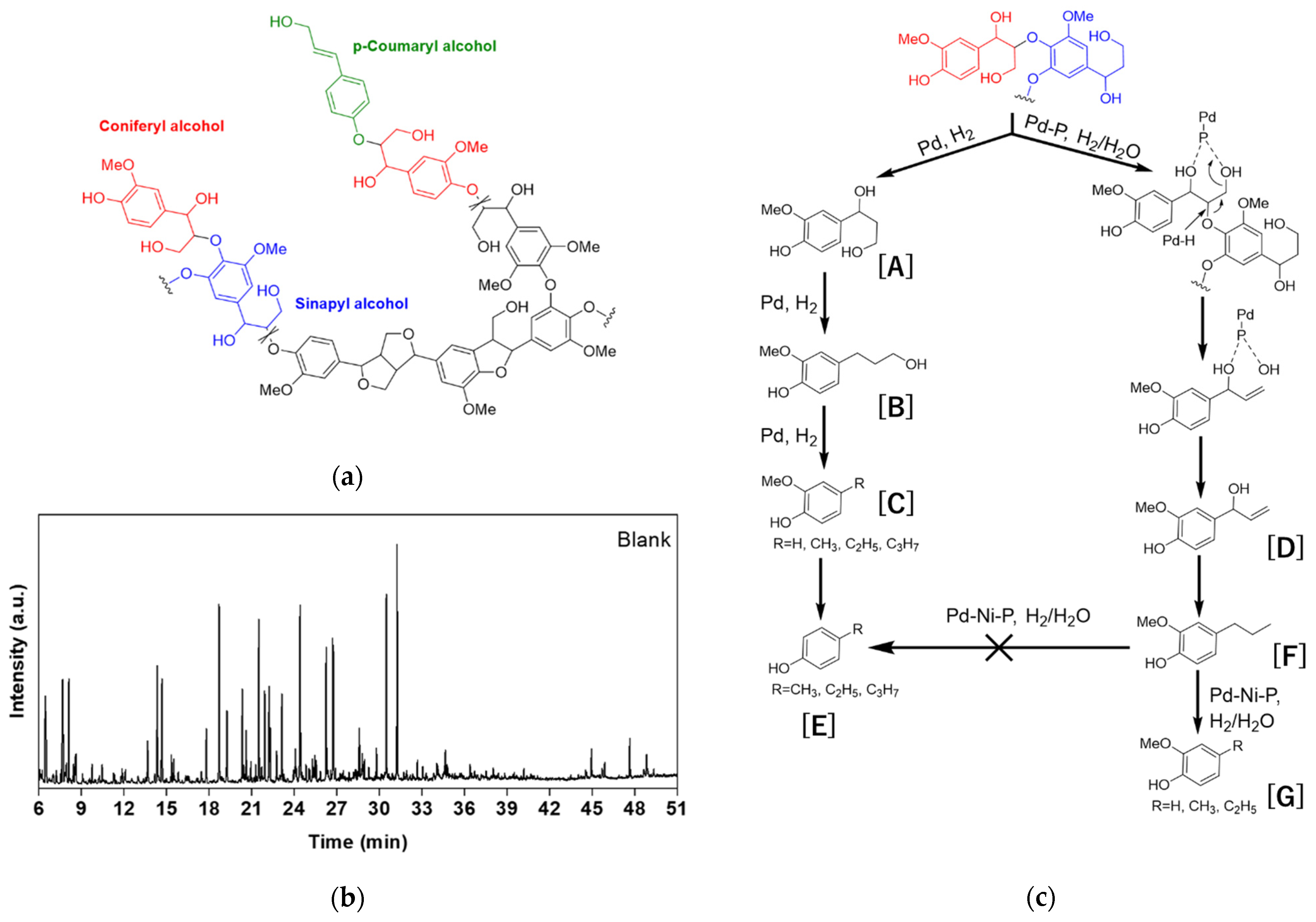
3. Results
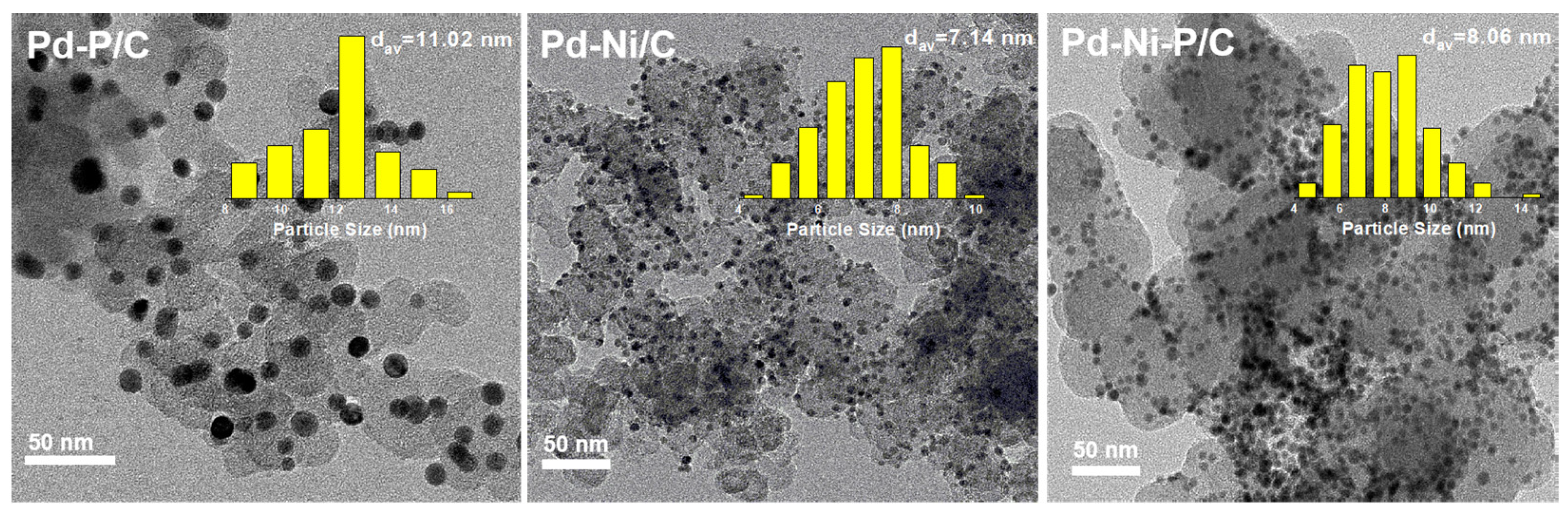
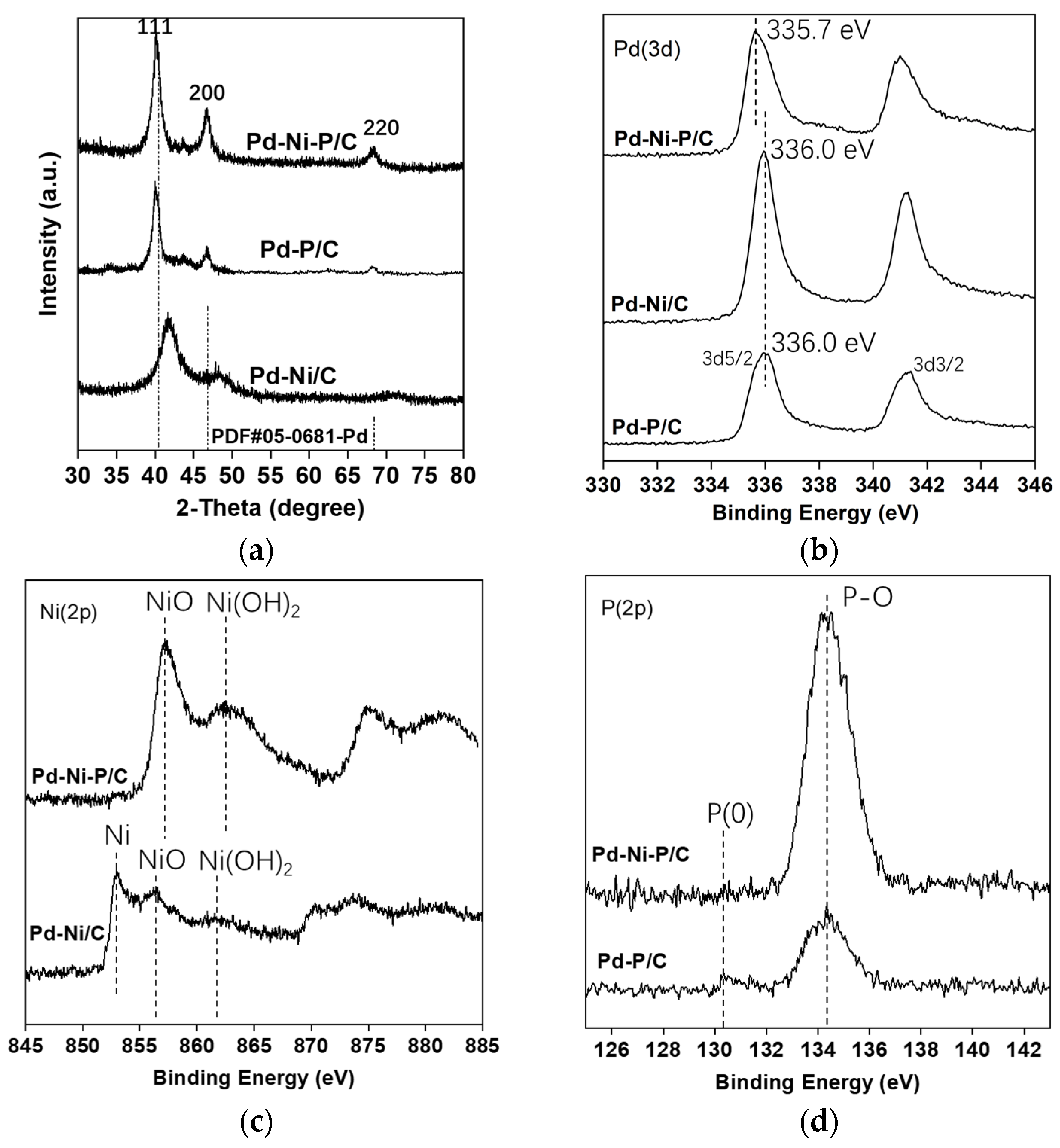
3.1. Catalyst Preparation and Characterizations
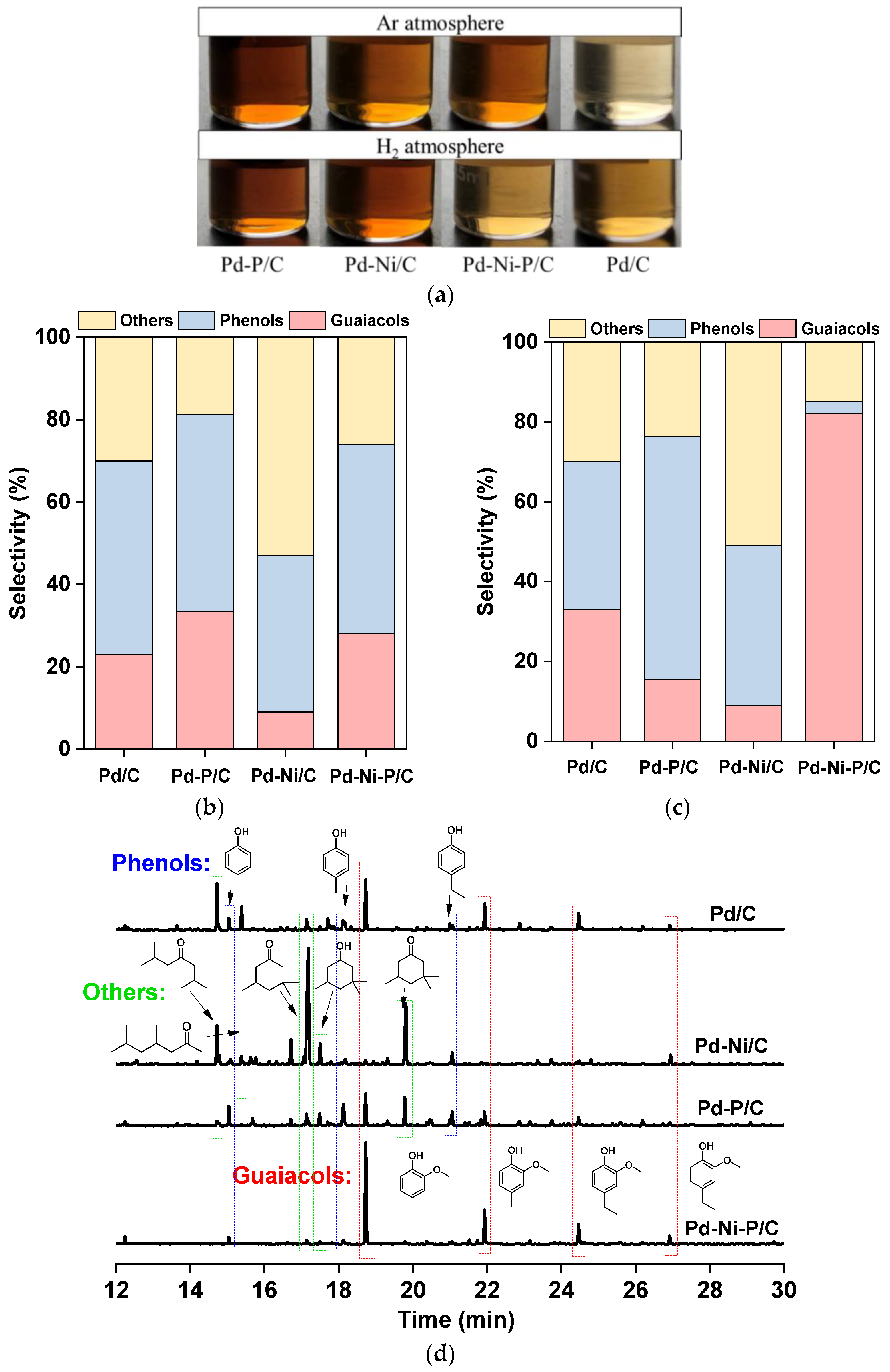
3.2. H2-Free Lignin Depolymerization
3.3. Lignin Depolymerization in H2 Atmosphere
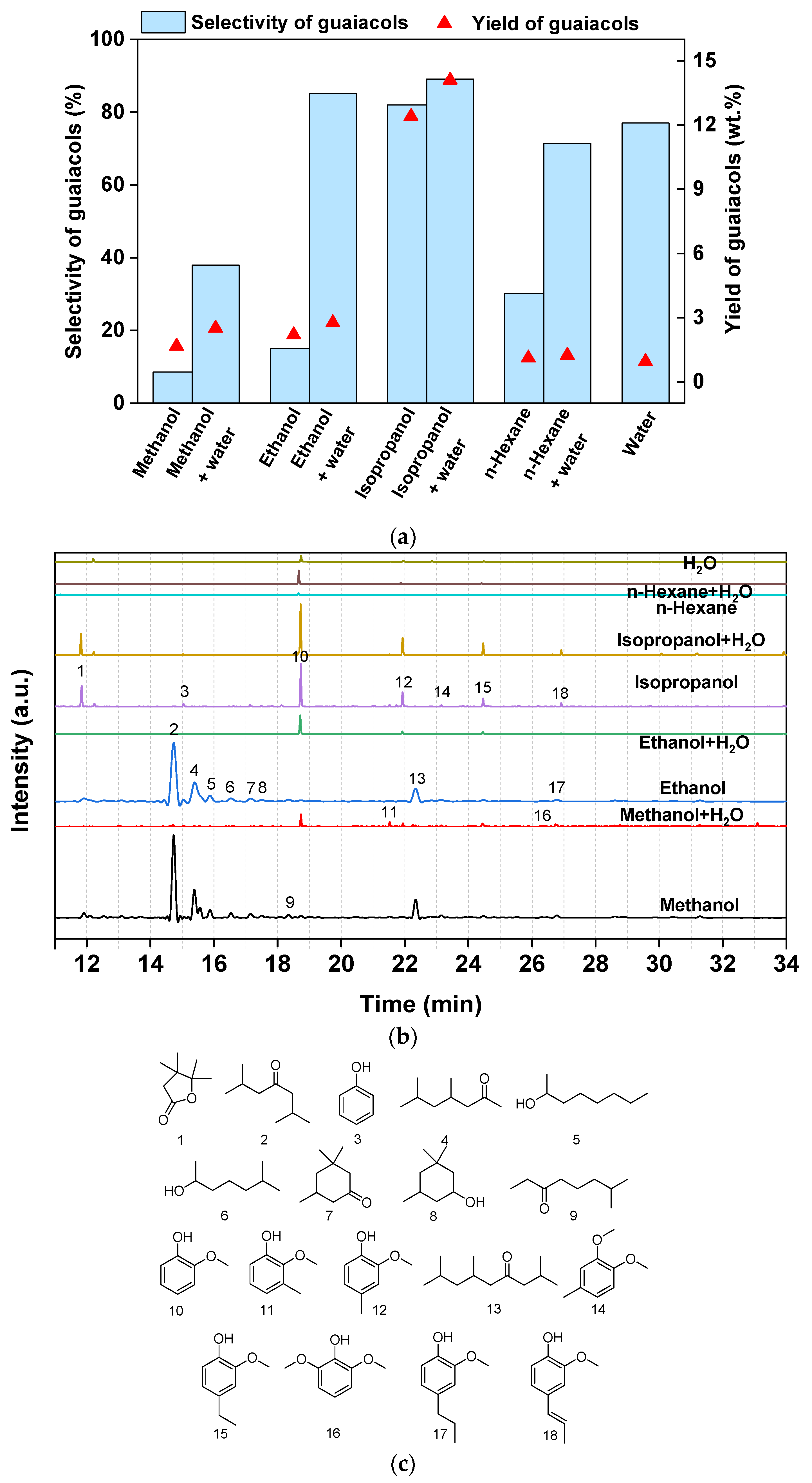
3.4. Lignin Depolymerization in Different Solvents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Zhao, L. Catalytic Conversion of Biomass into Value-Added Chemicals; Abstract book of KRIS 2023; The National Institute of Technology (NIT): Tokyo, Japan, 2023; p. 20. [Google Scholar]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Chen, H.; Fu, X. Industrial technologies for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2016, 57, 468–478. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Zhang, H.; Xiao, R. Catalytic oxidation of lignin to valuable biomass-based platform chemicals: A review. Fuel Process. Technol. 2019, 191, 181–201. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Park, J.H. Advancement in technologies for the depolymerization of lignin. Fuel Process. Technol. 2018, 181, 115–132. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef]
- Tang, D.; Huang, X.; Tang, W.; Jin, Y. Lignin-to-chemicals: Application of catalytic hydrogenolysis of lignin to produce phenols and terephthalic acid via metal-based catalysts. Int. J. Biol. Macromol. 2021, 190, 72–85. [Google Scholar] [CrossRef]
- Zakzeski, J.; Jongerius, A.L.; Weckhuysen, B.M. Transition metal catalyzed oxidation of Alcell lignin, soda lignin, and lignin model compounds in ionic liquids. Green Chem. 2010, 12, 1225–1236. [Google Scholar] [CrossRef]
- Chen, X.; Guan, W.; Tsang, C.W.; Hu, H.; Liang, C. Lignin valorizations with Ni catalysts for renewable chemicals and fuels productions. Catalysts 2019, 9, 488. [Google Scholar] [CrossRef]
- Hossain, M.A.; Phung, T.K.; Rahaman, M.S.; Tulaphol, S.; Jasinski, J.B.; Sathitsuksanoh, N. Catalytic cleavage of the β-O-4 aryl ether bonds of lignin model compounds by Ru/C catalyst. Appl. Catal. A-Gen. 2019, 582, 117100. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Catalytic depolymerization of alkali lignin in subcritical water: Influence of formic acid and Pd/C catalyst on the yields of liquid monomeric aromatic products. Green Chem. 2014, 16, 4740–4748. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.; Kim, U.J.; Choi, J.W. Conversion of lignin to phenol-rich oil fraction under supercritical alcohols in the presence of metal catalysts. Energy Fuels 2015, 29, 5154–5163. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Huš, M.; Likozar, B. Hydrogenation and hydrodeoxygenation of aromatic lignin monomers over Cu/C, Ni/C, Pd/C, Pt/C, Rh/C and Ru/C catalysts: Mechanisms, reaction micro-kinetic modelling and quantitative structure-activity relationships. Chem. Eng. J. 2019, 359, 305–320. [Google Scholar] [CrossRef]
- Shu, R.; Xu, Y.; Ma, L.; Zhang, Q.; Wang, C.; Chen, Y. Controllable production of guaiacols and phenols from lignin depolymerization using Pd/C catalyst cooperated with metal chloride. Chem. Eng. J. 2018, 338, 457–464. [Google Scholar] [CrossRef]
- Li, T.; Ji, N.; Jia, Z.; Diao, X.; Wang, Z.; Liu, Q.; Song, C.; Lu, X. Effects of metal promoters in bimetallic catalysts in hydrogenolysis of lignin derivatives into value-added chemicals. ChemCatChem 2020, 12, 5288–5302. [Google Scholar] [CrossRef]
- Shu, R.; Li, R.; Liu, Y.; Wang, C.; Liu, P.F.; Chen, Y. Enhanced adsorption properties of bimetallic RuCo catalyst for the hydrodeoxygenation of phenolic compounds and raw lignin-oil. Chem. Eng. Sci. 2020, 227, 115920. [Google Scholar] [CrossRef]
- Klein, I.; Marcum, C.; Kenttämaa, H.; Abu-Omar, M.M. Mechanistic investigation of the Zn/Pd/C catalyzed cleavage and hydrodeoxygenation of lignin. Green Chem. 2016, 18, 2399–2405. [Google Scholar] [CrossRef]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z. In Situ Preparation of Ru@N-Doped Carbon Catalyst for the Hydrogenolysis of Lignin To Produce Aromatic Monomers. ACS Catal. 2019, 9, 5828–5836. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Chen, M.; Zhang, J.; Shi, J.; Wang, C.; Yang, Z.; Wang, J. Effect of Mo content in Mo/Sepiolite catalyst on catalytic depolymerization of Kraft lignin under supercritical ethanol. Energy Convers. Manag. 2020, 222, 113227. [Google Scholar] [CrossRef]
- Zhao, M.; Abe, K.; Yamaura, S.; Yamamoto, Y.; Asao, N. Fabrication of Pd–Ni–P metallic glass nanoparticles and their application as highly durable catalysts in methanol electro-oxidation. Chem. Mater. 2014, 26, 1056–1061. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, L.; Cao, J.P.; Jiang, W.; Xie, J.X.; Zhu, C.; Wang, S.Y.; Wei, Y.L.; Zhao, X.Y.; Bai, H.C. Water-involved tandem conversion of aryl ethers to alcohols over metal phosphide catalyst. Chem. Eng. J. 2022, 435, 134911. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, J.P.; Wei, Y.L.; Jiang, W.; Xie, J.X.; Zhang, C.; Zhao, X.Y.; Zhao, M.; Bai, H.C. Rational synthesis of palladium nanoparticles modified by phosphorous for the conversion of diphenyl ether to KA oil. Appl. Catal. A-Gen. 2022, 630, 118464. [Google Scholar] [CrossRef]
- Raikwar, D.; Majumdar, S.; Shee, D. Effects of solvents in the depolymerization of lignin into value-added products: A review. Biomass Conv. Biorefin. 2021, 1–34. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, S.; Leitch, M.; Xu, C.C. Hydrolytic degradation of alkaline lignin in hot-compressed water and ethanol. Bioresour. Technol. 2010, 101, 9308–9313. [Google Scholar] [CrossRef]
- Song, G. The development of catalytic fractionation and conversion of lignocellulosic biomass under lignin-first strategy. J. For. Eng. 2019, 4, 1–10. [Google Scholar]
- Dyson, P.J.; Jessop, P.G. Solvent effects in catalysis: Rational improvements of catalysts via manipulation of solvent interactions. Catal. Sci. Technol. 2016, 6, 3302–3316. [Google Scholar] [CrossRef]
- Behling, R.; Valange, S.; Chatel, G. Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: What results? what limitations? what trends? Green Chem. 2016, 18, 1839–1854. [Google Scholar] [CrossRef]
- Gerrard, N.; Mistry, K.; Darling, G.R.; Hodgson, A. Water dissociation and hydroxyl formation on Ni(110). J. Phys. Chem. C 2020, 124, 23815–23822. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Wang, Y. Recent advances in the selective catalytic hydrodeoxygenation of lignin-derived oxygenates to arenes. Green Chem. 2020, 22, 1072–1098. [Google Scholar] [CrossRef]
- Morreel, K.; Dima, O.; Kim, H.; Lu, F.; Niculaes, C.; Vanholme, R.; Dauwe, R.; Goeminne, G.; Inze, D.; Messens, E.; et al. Mass spectrometry-based sequencing of lignin oligomers. Plant Physiol. 2010, 153, 1464–1478. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.R.; Beach, E.S.; Anastas, P.T. Depolymerization of organosolv lignin to aromatic compounds over Cu-doped porous metal oxides. Green Chem. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Gao, F.; Webb, J.D.; Sorek, H.; Wemmer, D.E.; Hartwig, J.F. Fragmentation of lignin samples with commercial Pd/C under ambient pressure of hydrogen. ACS Catal. 2016, 6, 7385–7392. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Tang, X.; Sun, Y.; Zeng, X.; Liu, S.; Lin, L. Depolymerization of cellulolytic enzyme lignin for the production of monomeric phenols over raney Ni and acidic zeolite catalysts. Energy Fuels 2015, 29, 1662–1668. [Google Scholar] [CrossRef]
- Konnerth, H.; Zhang, J.; Ma, D.; Prechtl, M.H.G.; Yan, N. Base promoted hydrogenolysis of lignin model compounds and organosolv lignin over metal catalysts in water. Chem. Eng. Sci. 2015, 123, 155–163. [Google Scholar] [CrossRef]
- Si, X.; Lu, F.; Chen, J.; Lu, R.; Huang, Q.; Jiang, H.; Taarning, E.; Xu, J. A strategy for generating high-quality cellulose and lignin simultaneously from woody biomass. Green Chem. 2017, 19, 4849–4857. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Wang, S.; Li, H.; Li, Z.; Shi, Z.-J.; Xiao, L.; Sun, R.-C.; Fang, Y.; Song, G. Catalytic hydrogenolysis of lignins into phenolic compounds over carbon nanotube supported molybdenum oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Feghali, E.; Carrot, G.; Thuéry, P.; Genre, C.; Cantat, T. Convergent reductive depolymerization of wood lignin to isolated phenol derivatives by metal-free catalytic hydrosilylation. Energy Environ. Sci. 2015, 8, 2734–2743. [Google Scholar] [CrossRef]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Cheng, Y.; Daemen, L.L.; Ramirez-Cuesta, A.J.; Yang, S.; et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104. [Google Scholar] [CrossRef] [PubMed]
| Composition (wt.%, daf 1) | ||||
|---|---|---|---|---|
| C | O 2 | H | N | S 3 |
| 69.12 | 23.37 | 4.69 | 0.31 | 2.51 |
| Catalyst | Atmosphere | Product Yields (wt.%) | |||
|---|---|---|---|---|---|
| Guaiacols | Phenols | Others | Total | ||
| Pd/C | Ar | 4.4 | 9.1 | 5.7 | 19.2 |
| Pd-P/C | 3.3 | 4.8 | 1.8 | 9.7 | |
| Pd-Ni/C | 0.7 | 2.9 | 4.1 | 7.7 | |
| Pd-Ni-P/C | 3.8 | 6.2 | 3.5 | 13.5 | |
| Pd/C | H2 | 4.4 | 4.8 | 3.9 | 13.2 |
| Pd-P/C | 2.7 | 9.6 | 4.0 | 16.3 | |
| Pd-Ni/C | 1.0 | 4.0 | 5.1 | 10.1 | |
| Pd-Ni-P/C | 10.1 | 0.4 | 1.9 | 12.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zhao, L.; Zhao, X.-Y.; Cao, J.-P.; Maruyama, K.-i. Pd-Based Nano-Catalysts Promote Biomass Lignin Conversion into Value-Added Chemicals. Materials 2023, 16, 5198. https://doi.org/10.3390/ma16145198
Zhao M, Zhao L, Zhao X-Y, Cao J-P, Maruyama K-i. Pd-Based Nano-Catalysts Promote Biomass Lignin Conversion into Value-Added Chemicals. Materials. 2023; 16(14):5198. https://doi.org/10.3390/ma16145198
Chicago/Turabian StyleZhao, Ming, Liang Zhao, Xiao-Yan Zhao, Jing-Pei Cao, and Koh-ichi Maruyama. 2023. "Pd-Based Nano-Catalysts Promote Biomass Lignin Conversion into Value-Added Chemicals" Materials 16, no. 14: 5198. https://doi.org/10.3390/ma16145198
APA StyleZhao, M., Zhao, L., Zhao, X.-Y., Cao, J.-P., & Maruyama, K.-i. (2023). Pd-Based Nano-Catalysts Promote Biomass Lignin Conversion into Value-Added Chemicals. Materials, 16(14), 5198. https://doi.org/10.3390/ma16145198






