Phase Structure, Bond Features, and Microwave Dielectric Characteristics of Ruddlesden–Popper Type Sr2TiO4 Ceramics
Abstract
1. Introduction
2. Materials and Methods
3. Results
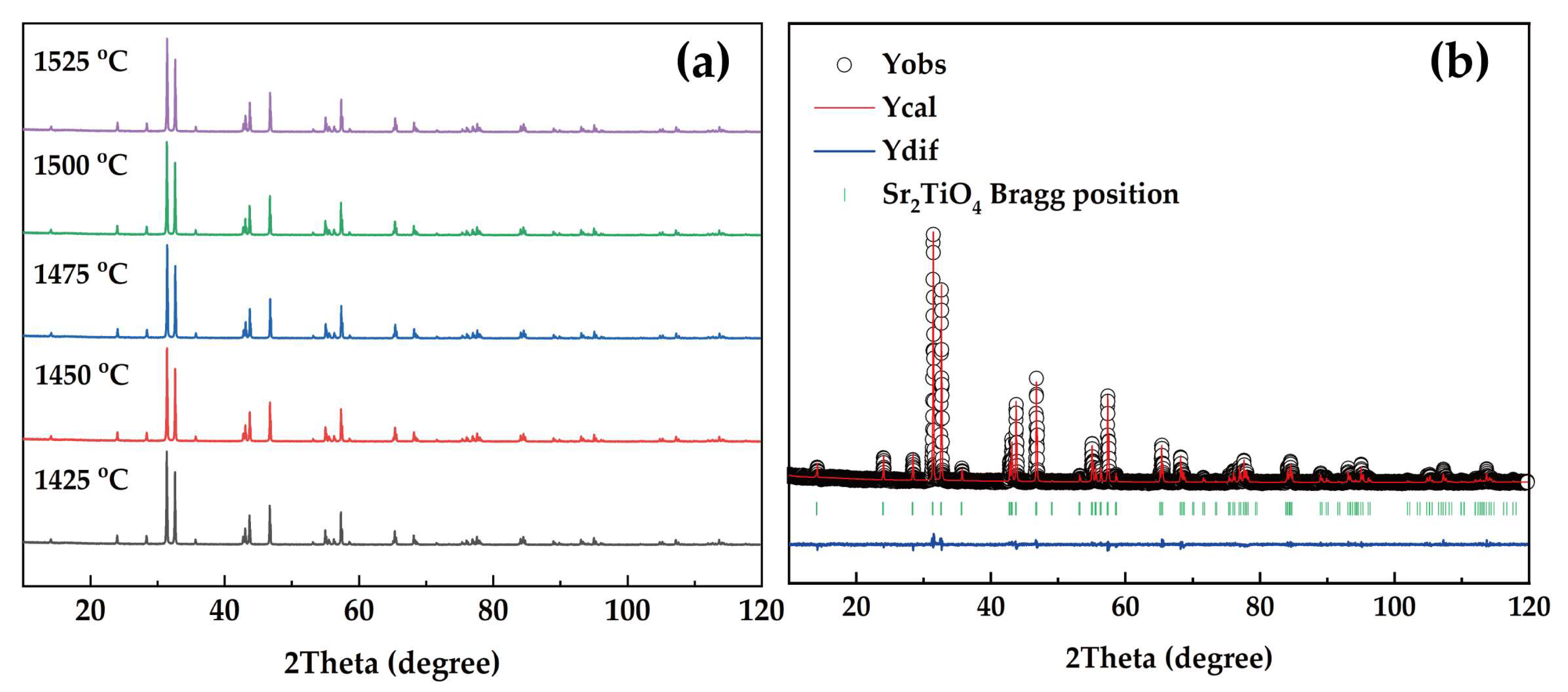
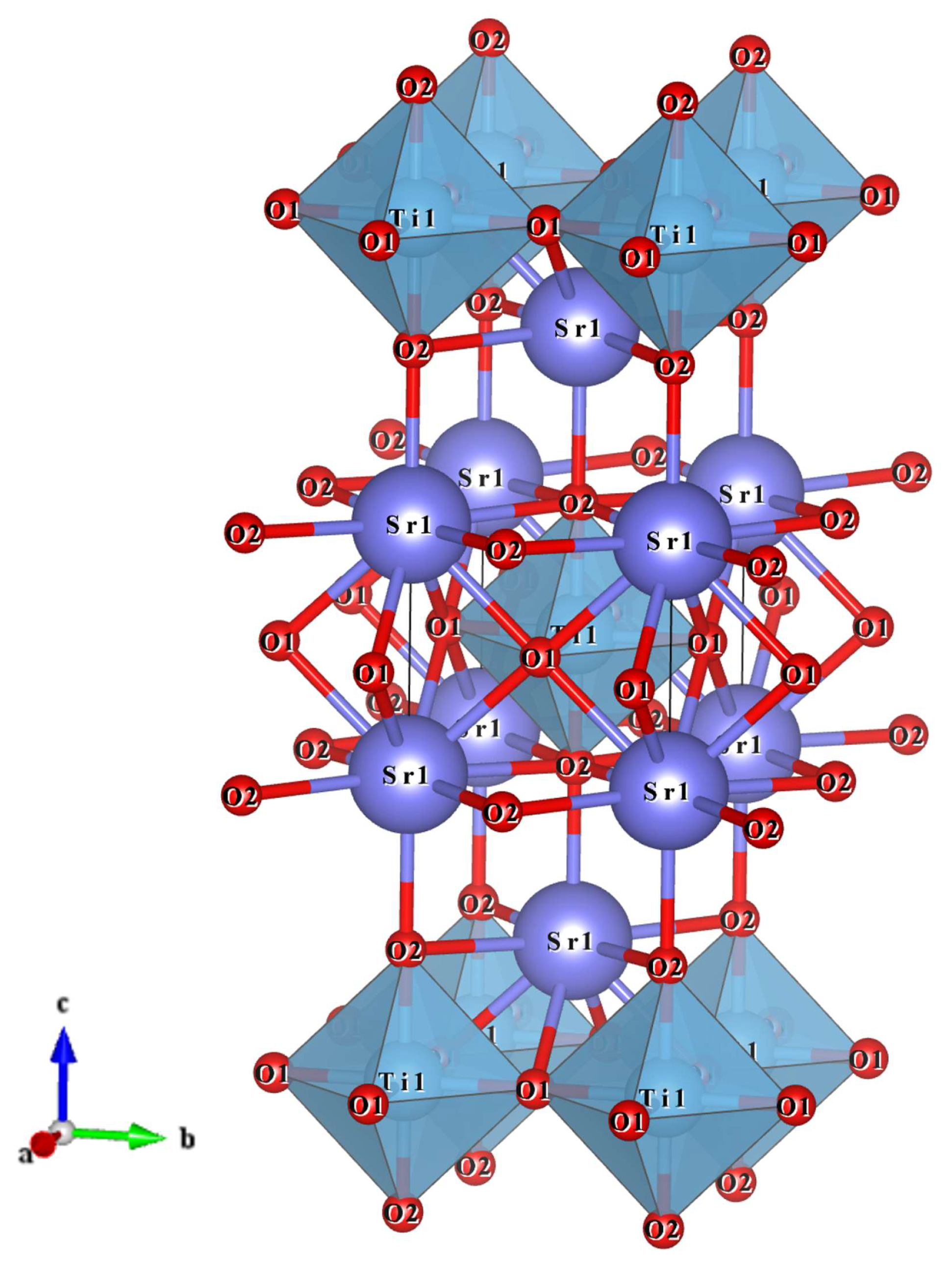
3.1. Phase Structure Investigation of Sr2TiO4 Ceramic
3.2. Microstructure Investigation of Sr2TiO4 Ceramic
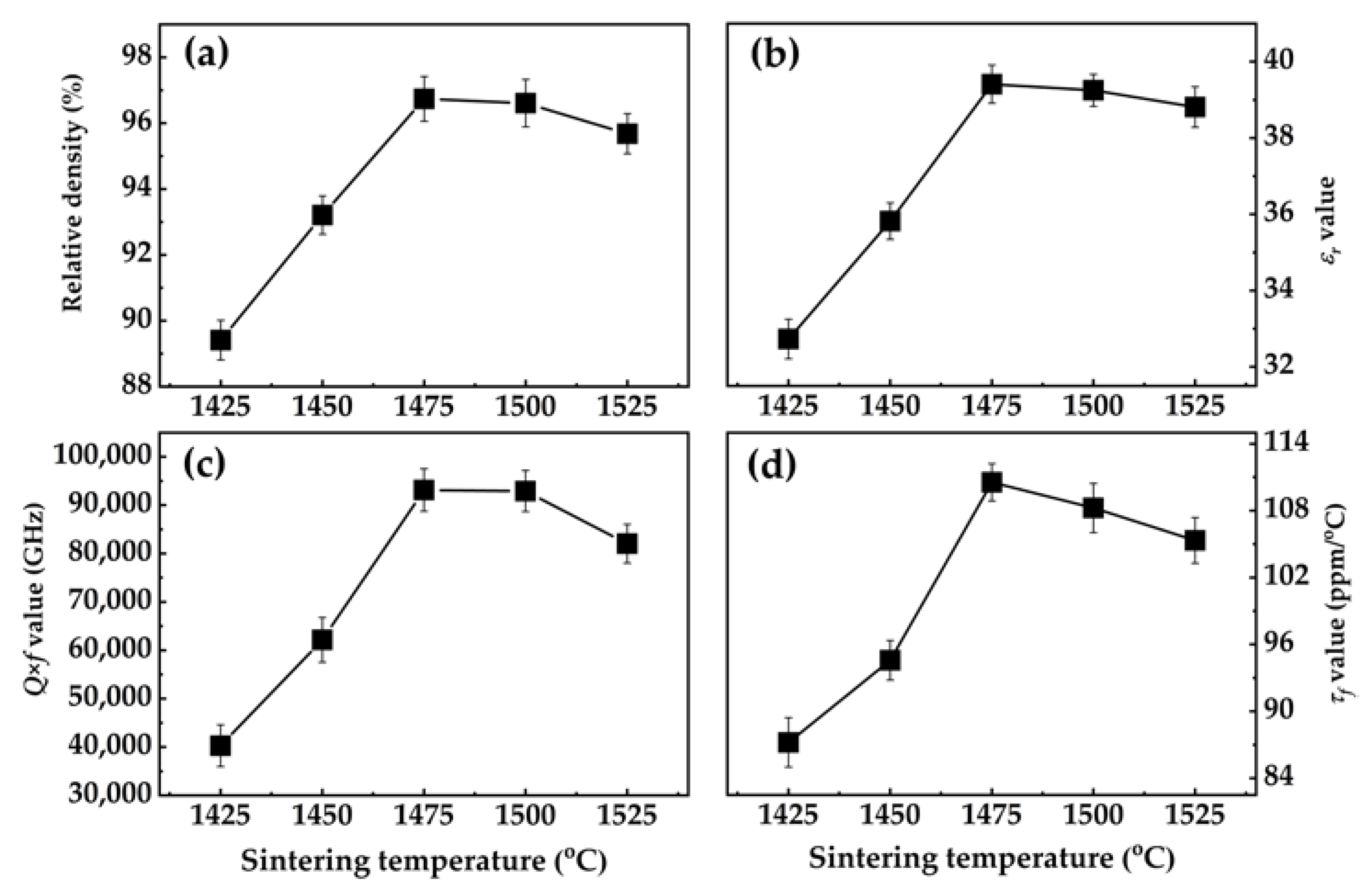
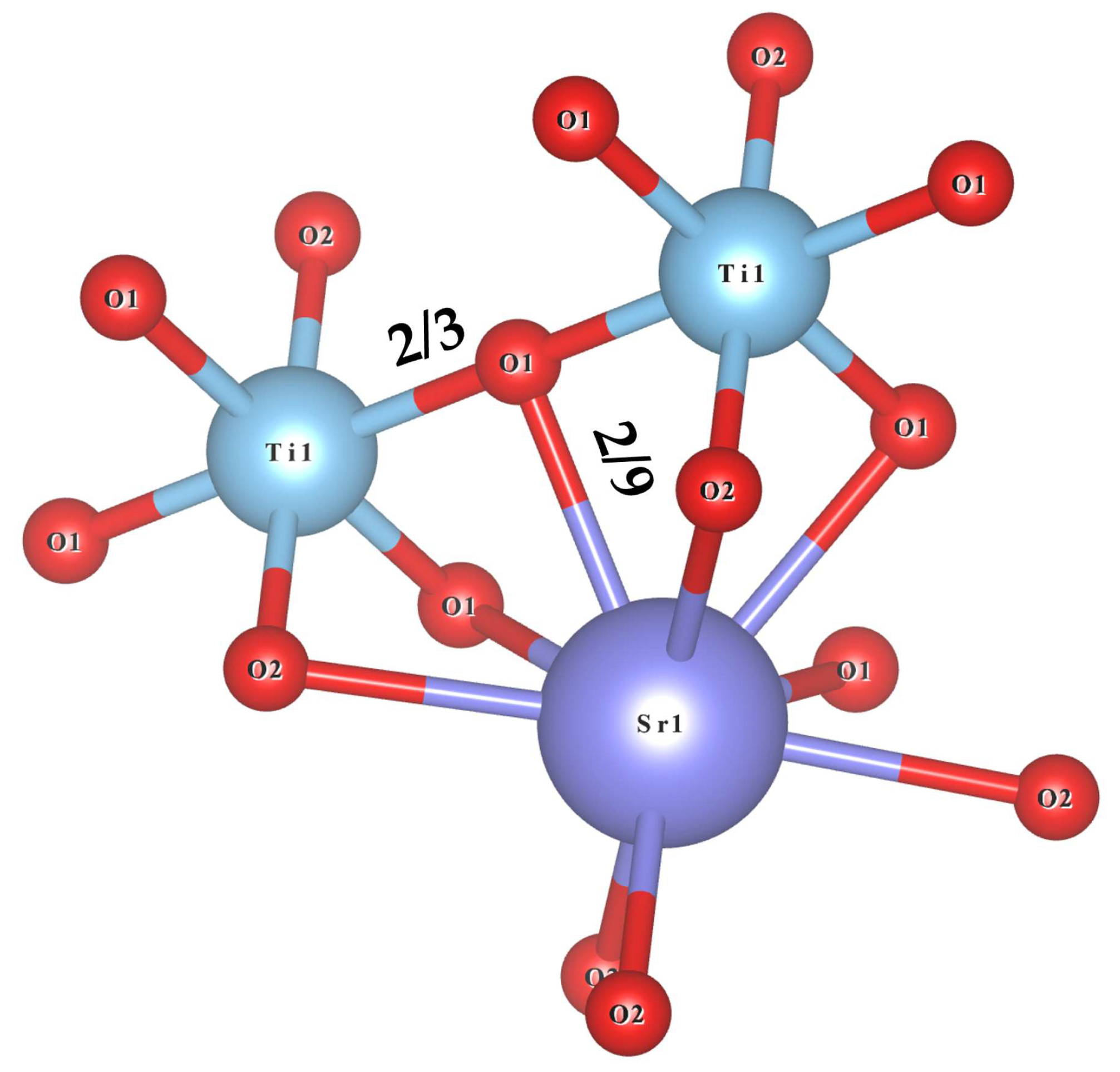
3.3. Bond Traits and Microwave Dielectric Performances Investigation of Sr2TiO4 Ceramic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, F.-F.; Zhou, D.; Du, C.; Jin, B.-B.; Li, C.; Qi, Z.-M.; Sun, S.; Zhou, T.; Li, Q.; Zhang, X.-Q. Design of a Sub-6 GHz Dielectric Resonator Antenna with Novel Temperature-Stabilized (Sm1–xBix)NbO4 (x = 0–0.15) Microwave Dielectric Ceramics. ACS Appl. Mater. Interfaces 2022, 14, 7030–7038. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chai, L.; Liang, G.; Xing, M.; Fang, Z.; Zhang, X.; Qin, T.; Li, E. Structure, far-infrared spectroscopy, microwave dielectric properties, and improved low-temperature sintering characteristics of tri-rutile Mg0.5Ti0.5TaO4 ceramics. J. Adv. Ceram. 2023, 12, 296–308. [Google Scholar] [CrossRef]
- Sebastian, M.T.; Jantunen, H. Low loss dielectric materials for LTCC applications: A review. Int. Mater. Rev. 2008, 53, 57–90. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, S.; Yang, H.; Li, E. Usage of P–V–L bond theory in studying the structural/property regulation of microwave dielectric ceramics: A review. Inorg. Chem. Front. 2020, 7, 4711–4753. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Liu, J.; Song, Z.; Xiao, M.; Wang, X. Correlation of crystal structure and microwave dielectric properties of Nd1.02(Nb1-xTax)0.988O4 ceramic. Dalton Trans. 2015, 44, 5053–5057. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, Y.; Yue, Z.; Li, L. High-Q and temperature-stable microwave dielectrics in layer cofired Zn1.01Nb2O6/TiO2/Zn1.01Nb2O6 ceramic architectures. J. Am. Ceram. Soc. 2019, 102, 342–350. [Google Scholar] [CrossRef]
- Luo, W.; Li, L.; Yu, S.; Sun, Z.; Zhang, B.; Xia, F. Structural, Raman spectroscopic and microwave dielectric studies on high-Q materials in Ge-doped ZnTiNb2O8 systems. J. Alloys Compd. 2018, 741, 969–974. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, S.; Yang, H.; Zhang, X.; Li, E. Structural Evolution and Microwave Dielectric Properties of xZn0.5Ti0.5NbO4-(1–x)Zn0.15Nb0.3Ti0.55O2 Ceramics. Inorg. Chem. 2018, 57, 8264–8275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Xiang, M. Crystal structure and microwave dielectric characteristics of Zr-substituted CoTiNb2O8 ceramics. J. Eur. Ceram. Soc. 2016, 36, 1945–1951. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.; Liu, X.Q.; Chen, X.M. Srn+1TinO3n+1 (n = 1, 2) microwave dielectric ceramics with medium dielectric constant and ultra-low dielectric loss. J. Am. Ceram. Soc. 2017, 100, 496–500. [Google Scholar] [CrossRef]
- Tseng, C.-F. Microwave dielectric properties of a new Cu0.5Ti0.5NbO4 ceramics. J. Eur. Ceram. Soc. 2015, 35, 383–387. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.Q.; Chen, X.M. Sr2LaAlTiO7: A New Ruddlesden-Popper Compound with Excellent Microwave Dielectric Properties. J. Mater. Chem. C 2016, 4, 1720–1726. [Google Scholar] [CrossRef]
- Mao, M.M.; Chen, X.M. Infrared Reflectivity Spectra and Microwave Dielectric Properties of (Sr1−xCax)SmAlO4 (0 ≤ x ≤ 1) Ceramics. Int. J. Appl. Ceram. Technol. 2011, 8, 1023–1030. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, X.; Jiang, T.; Du, J.; Wu, H.; Lu, Y.; Zhou, Y.Y.; Yue, Z. Bond characteristics and microwave dielectric properties of (Mn1/3Sb2/3)4+ doped molybdate based low-temperature sintering ceramics. J. Alloys Compd. 2022, 906, 164333. [Google Scholar] [CrossRef]
- Toby, B. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2004.
- Takada, T.; Wang, S.F.; Yoshikawa, S.; Jang, S.-J.; Newnham, R.E. Effect of Glass Additions on BaO–TiO2–WO3 Microwave Ceramics. J. Am. Ceram. Soc. 1994, 77, 1909–1916. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y. Structure, bond characteristics and microwave dielectric properties of new A0.75Ti0.75Ta1.5O6 (A = Ni, Co, Zn and Mg) ceramics based on complex chemical bond theory. J. Eur. Ceram. Soc. 2020, 40, 1181–1185. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Huang, X.; Rao, Y.; Yang, Y.; Gan, G.; Lai, Y.; Xu, F.; Li, J.; Liao, Y.; et al. Crystal structure and enhanced microwave dielectric properties of Ta5+ substituted Li3Mg2NbO6 ceramics. J. Am. Ceram. Soc. 2020, 103, 214–223. [Google Scholar] [CrossRef]
- Fang, Z.; Tang, B.; Yuan, Y.; Zhang, X.; Zhang, S. Structure and microwave dielectric properties of the Li2/3(1−x)Sn1/3(1−x)MgxO systems (x = 0-4/7). J. Am. Ceram. Soc. 2018, 101, 252–264. [Google Scholar] [CrossRef]
- Phillips, J.C. Ionicity of the Chemical Bond in Crystals. Rev. Modern Phys. 1970, 42, 317–356. [Google Scholar] [CrossRef]
- Van Vechten, J.A. Quantum Dielectric Theory of Electronegativity in Covalent Systems. I. Electronic Dielectric Constant. Phys. Rev. 1969, 182, 891–905. [Google Scholar] [CrossRef]
- Levine, B.F. Bond susceptibilities and ionicities in complex crystal structures. J. Chem. Phys. 1973, 59, 1463–1486. [Google Scholar] [CrossRef]
- Tian, H.; Zheng, J.; Liu, L.; Wu, H.; Kimura, H.; Lu, Y.; Yue, Z. Structure characteristics and microwave dielectric properties of Pr2(Zr1−xTix)3(MoO4)9 solid solution ceramic with a stable temperature coefficient. J. Mater. Sci. Technol. 2022, 116, 121–129. [Google Scholar] [CrossRef]
- Wu, Z.J.; Meng, Q.B.; Zhang, S.Y. Semiempirical study on the valences of Cu and bond covalency in Y1-xCaxBa2Cu3O6-y. Phys. Rev. B 1998, 58, 958–962. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Sun, J.; Zhang, N.; Sun, H.; Wu, H.; Tao, W. Effects of (Mg1/3Sb2/3)4+ substitution on the structure and microwave dielectric properties of Ce2Zr3(MoO4)9 ceramics. J. Adv. Ceram. 2021, 10, 778–789. [Google Scholar] [CrossRef]
- Kim, E.S.; Chun, B.S.; Freer, R.; Cernik, R.J. Effects of packing fraction and bond valence on microwave dielectric properties of A2+B6+O4 (A2+: Ca, Pb, Ba; B6+: Mo, W) ceramics. J. Eur. Ceram. Soc. 2010, 30, 1731–1736. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Wu, Z. Lattice energy estimation for inorganic ionic crystals. Inorg. Chem. 2003, 42, 2465–2469. [Google Scholar] [CrossRef]
- Xia, W.-S.; Li, L.-X.; Ning, P.-F.; Liao, Q.-W. Relationship Between Bond Ionicity, Lattice Energy, and Microwave Dielectric Properties of Zn(Ta1−xNbx)2O6 Ceramics. J. Am. Ceram. Soc. 2012, 95, 2587–2592. [Google Scholar] [CrossRef]
- Bosman, A.J.; Havinga, E.E. Temperature Dependence of Dielectric Constants of Cubic Ionic Compounds. Phys. Rev. 1963, 129, 1593–1600. [Google Scholar] [CrossRef]
- Ma, P.P.; Liu, X.Q.; Zhang, F.Q.; Xing, J.J.; Chen, X.M. Sr(Ga0.5Nb0.5)1−xTixO3 Low-Loss Microwave Dielectric Ceramics with Medium Dielectric Constant. J. Am. Ceram. Soc. 2015, 98, 2534–2540. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Zhou, S.; Pan, T. Estimation Thermal Expansion Coefficient from Lattice Energy for Inorganic Crystals. Jpn. J. Appl. Phys. 2006, 45, 8801–8804. [Google Scholar] [CrossRef]

| System | ST (°C) | εr | Q × f (GHz) | τf (ppm/°C) | Ref. |
|---|---|---|---|---|---|
| Nd1.02(Nb0.94Ta0.06)0.988O4 | 1275 | 21.7 | 51,000 | −45.7 | [5] |
| Zn1.01Nb2O6/TiO2/Zn1.01Nb2O6 | 1200 | 26.8 | 99,500 | 0.5 | [6] |
| ZnTi0.7Ge0.3Nb2O8 | 1120 | 35.6 | 62,700 | −58.0 | [7] |
| 0.516ZTN–0.484ZNT | 1100 | 46.1 | 27,031 | −1.5 | [8] |
| Co(Ti0.8Zr0.2)Nb2O8 | 1250 | 55.0 | 41,541 | 59.8 | [9] |
| Sr3Ti2O7 | 1500 | 63.0 | 84,000 | 293.0 | [10] |
| Cu0.5Ti0.5NbO4 | 960 | 71.2 | 11,000 | 49.2 | [11] |
| Atom | Position | Fractional Coordinates | Occ 1 | Uiso 2 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Sr | 4c | 0.000 | 0.000 | 0.355 (4) | 1 | 0.00988 (5) |
| Ti | 8d | 0.000 | 0.000 | 0.000 | 1 | 0.00387 (6) |
| O1 | 8d | 0.000 | 0.500 | 0.000 | 1 | 0.02071 (8) |
| O2 | 8d | 0.000 | 0.000 | 0.152 (3) | 1 | 0.02512 (4) |
| Bond lengths | Sr–O1 × 4: 2.66932 (2) Å Ti–O1 × 4: 1.94490 (10) Å Sr–O2(1) × 4: 2.75192 (4) Å Ti–O2 × 2: 1.91655 (3) Å Sr–O2(2) × 1: 2.55960 (10) Å | |||||
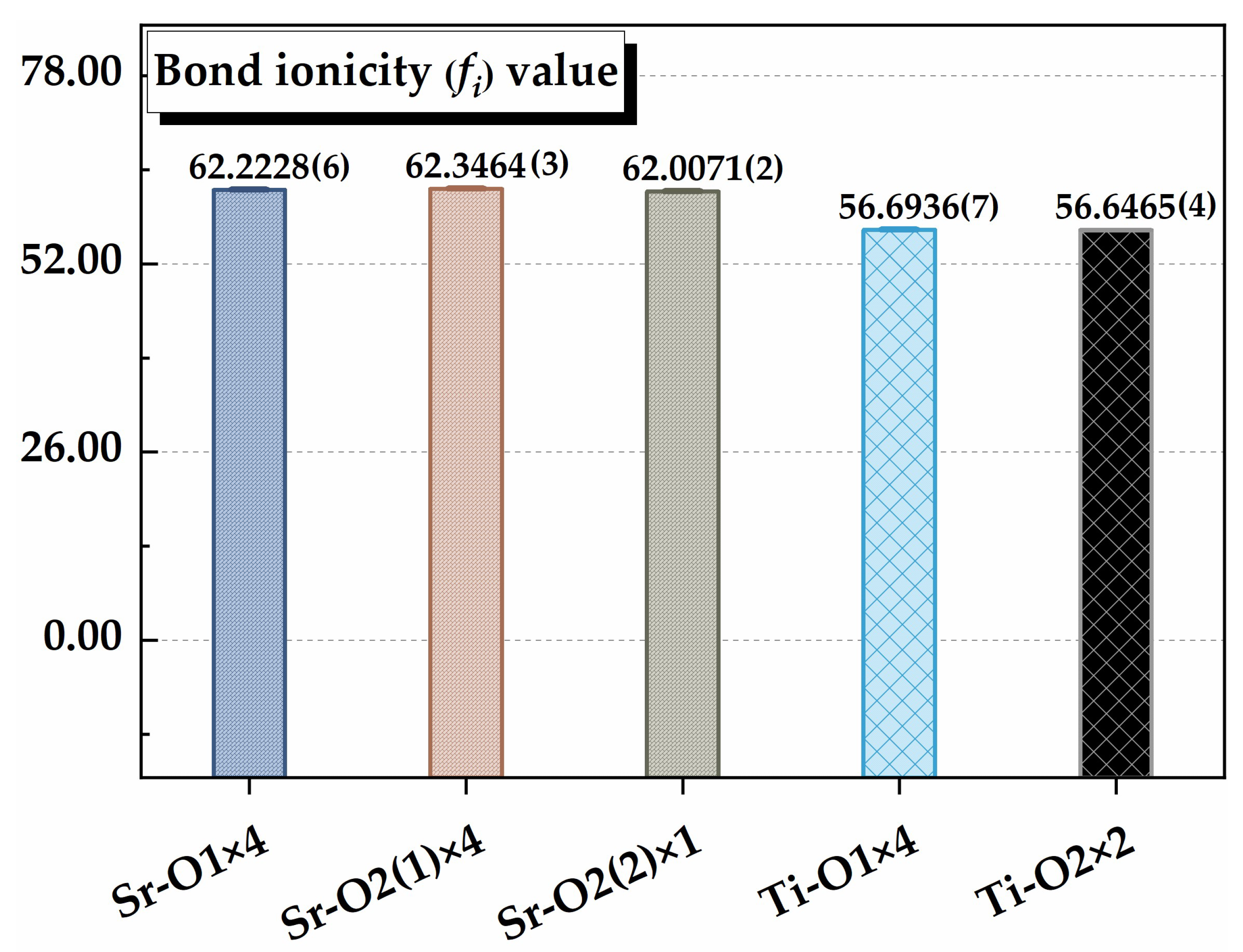
| Bond Type | ZOμ | χμ | Fμ | χμ/χ (%) | ||
|---|---|---|---|---|---|---|
| Sr–O1 × 4 | 0.193981 (7) | 5.66421 (4) | 4 | 7.415 (8) | 0.333 | 27.12 (3) |
| Sr–O2(1) × 4 | 0.177033 (5) | 5.26030 (6) | 4 | 7.859 (3) | 0.333 | 28.74 (8) |
| Sr–O2(2) × 1 | 0.220011 (8) | 6.26772 (3) | 4 | 6.854 (1) | 0.083 | 6.27 (2) |
| Ti–O1 × 4 | 1.504495 (3) | 11.6008 (2) | 12 | 13.933 (5) | 0.167 | 25.48 (2) |
| Ti–O2 × 2 | 1.572252 (4) | 12.0245 (5) | 12 | 13.547 (7) | 0.083 | 12.39 (3) |
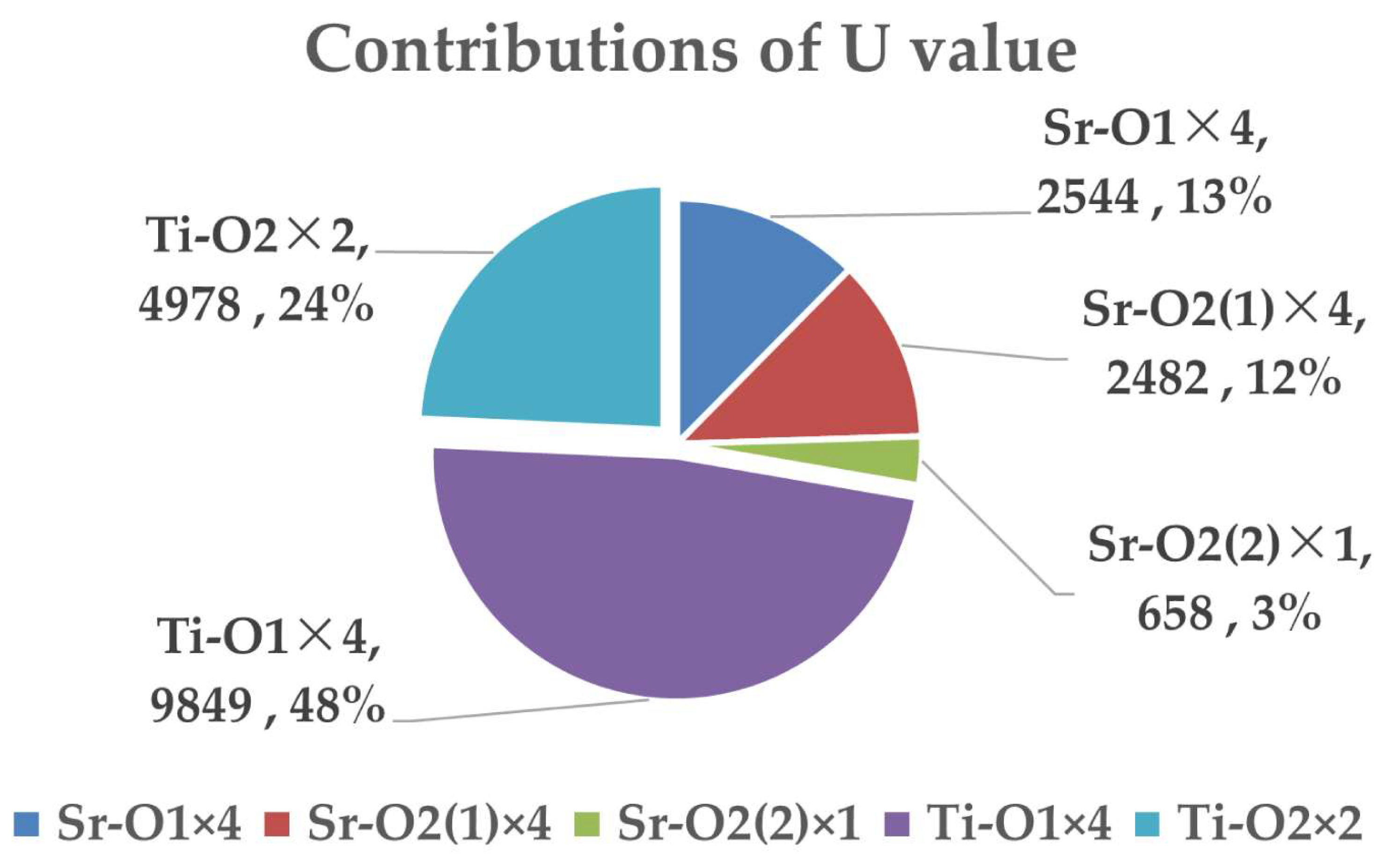
| Bond Type | U (kJ/mol) | α (10−6 K−1) | ||
|---|---|---|---|---|
| Sr–O1 × 4 | 0.333 | 2544 (8) | 20.23 (7) | 6.74 (1) |
| Sr–O2(1) × 4 | 0.333 | 2482 (3) | 20.81 (2) | 6.94 (3) |
| Sr–O2(2) × 1 | 0.083 | 658 (6) | 19.46 (5) | 1.62 (2) |
| Ti–O1 × 4 | 0.167 | 9849 (5) | 4.63 (4) | 0.77 (4) |
| Ti–O2 × 2 | 0.083 | 4978 (4) | 4.55 (1) | 0.38 (1) |
| αtotal (10−6 K−1) | 16.45 (11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Pang, J.; Luo, X.; Ao, L.; Xie, Q.; Wang, X.; Yang, H.; Tang, X. Phase Structure, Bond Features, and Microwave Dielectric Characteristics of Ruddlesden–Popper Type Sr2TiO4 Ceramics. Materials 2023, 16, 5195. https://doi.org/10.3390/ma16145195
Yang J, Pang J, Luo X, Ao L, Xie Q, Wang X, Yang H, Tang X. Phase Structure, Bond Features, and Microwave Dielectric Characteristics of Ruddlesden–Popper Type Sr2TiO4 Ceramics. Materials. 2023; 16(14):5195. https://doi.org/10.3390/ma16145195
Chicago/Turabian StyleYang, Jun, Jinbiao Pang, Xiaofang Luo, Laiyuan Ao, Qiang Xie, Xing Wang, Hongyu Yang, and Xianzhong Tang. 2023. "Phase Structure, Bond Features, and Microwave Dielectric Characteristics of Ruddlesden–Popper Type Sr2TiO4 Ceramics" Materials 16, no. 14: 5195. https://doi.org/10.3390/ma16145195
APA StyleYang, J., Pang, J., Luo, X., Ao, L., Xie, Q., Wang, X., Yang, H., & Tang, X. (2023). Phase Structure, Bond Features, and Microwave Dielectric Characteristics of Ruddlesden–Popper Type Sr2TiO4 Ceramics. Materials, 16(14), 5195. https://doi.org/10.3390/ma16145195






