Silicon Nitride Ceramics: Structure, Synthesis, Properties, and Biomedical Applications
Abstract
1. Introduction
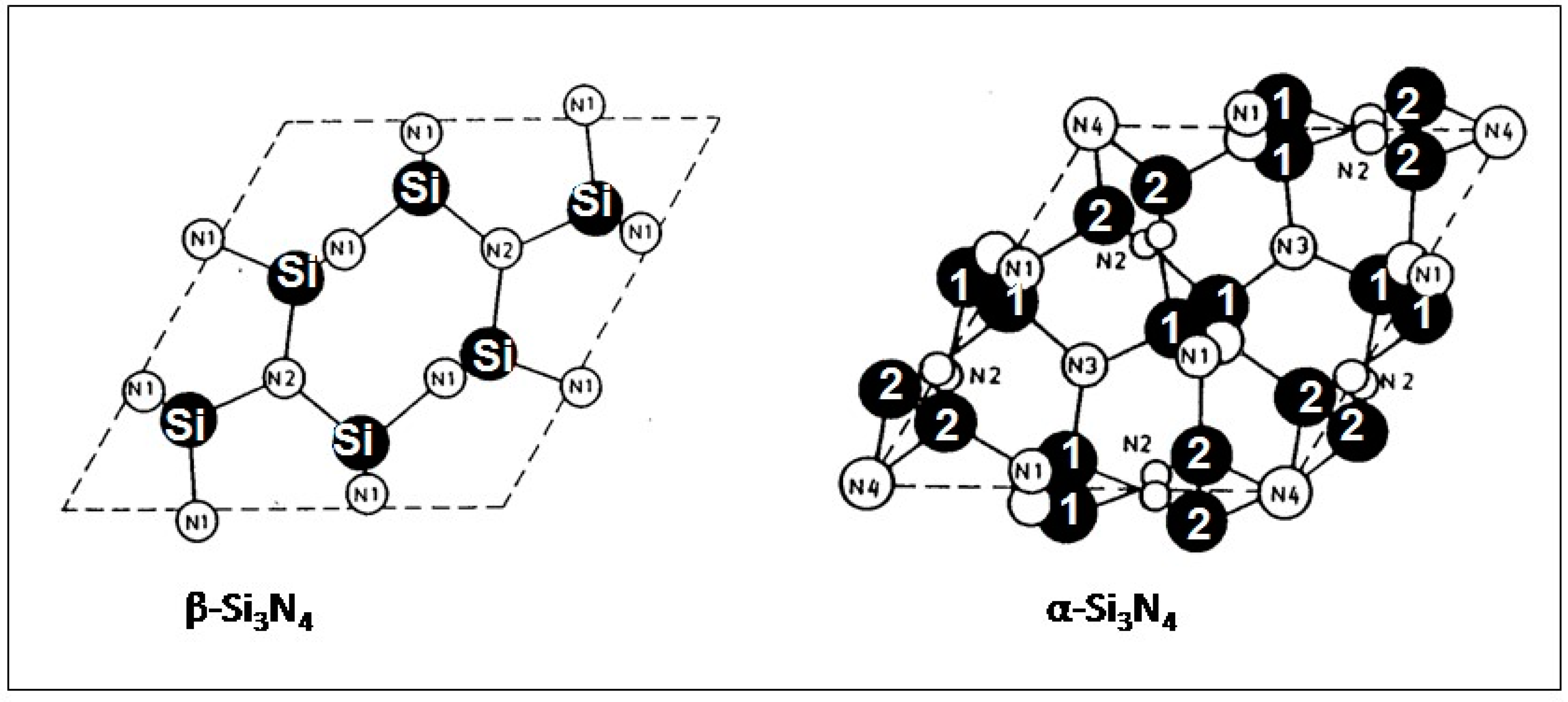
2. Crystallographic Structure
3. Synthesis, Processing, and Properties of Silicon Nitride
3.1. Silicon Nitride Powders
- -
- Direct nitriding of silicon powder (see Figure 2);
- -
- Carbothermal nitriding of silicon dioxide;
- -
- Diimide synthesis by reacting silicon tetrachloride with ammonia;
- -
- Chemical vapor deposition synthesis using silicon tetrachloride or monosilane;
- -
- Plasma chemical synthesis, such as plasma-enhanced chemical vapor deposition;
- -
- Excimer laser-induced reactions of silane–ammonia mixtures;
- -
- Pyrolysis of organo-silicon compounds;
- -
- Sol-gel processing of polymeric precursors;
- -
- Self-propagating high-temperature synthesis.
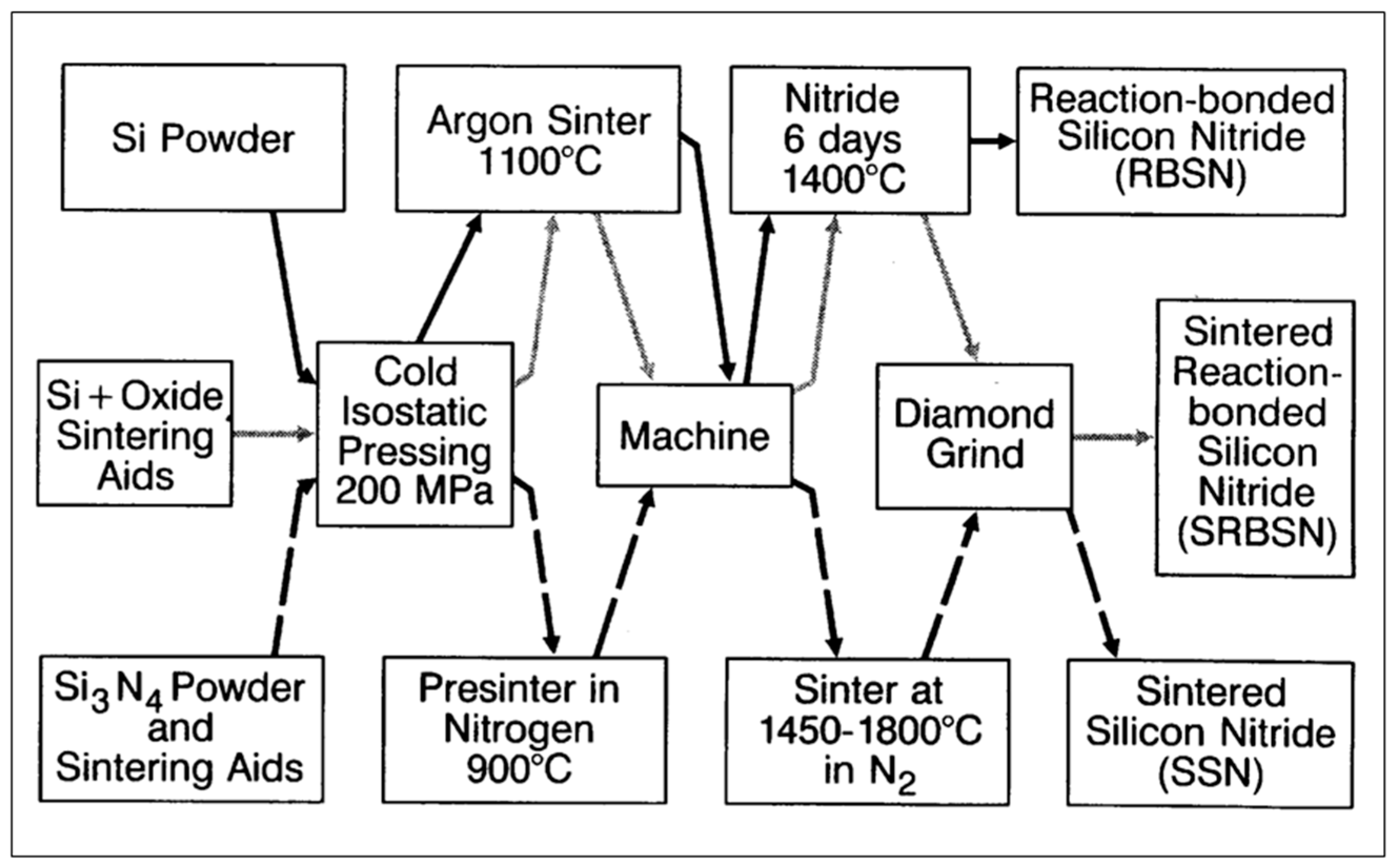
3.2. Silicon Nitride Compacts
3.2.1. Reaction-Bonded Silicon Nitride (RBSN)
3.2.2. Sintered Silicon Nitride (SSN)
3.2.3. Sintered Reaction-Bonded Silicon Nitride (SRBSN)
3.2.4. Textured Sintered Silicon Nitride (TSSN)
3.3. Silicon Nitride Foams
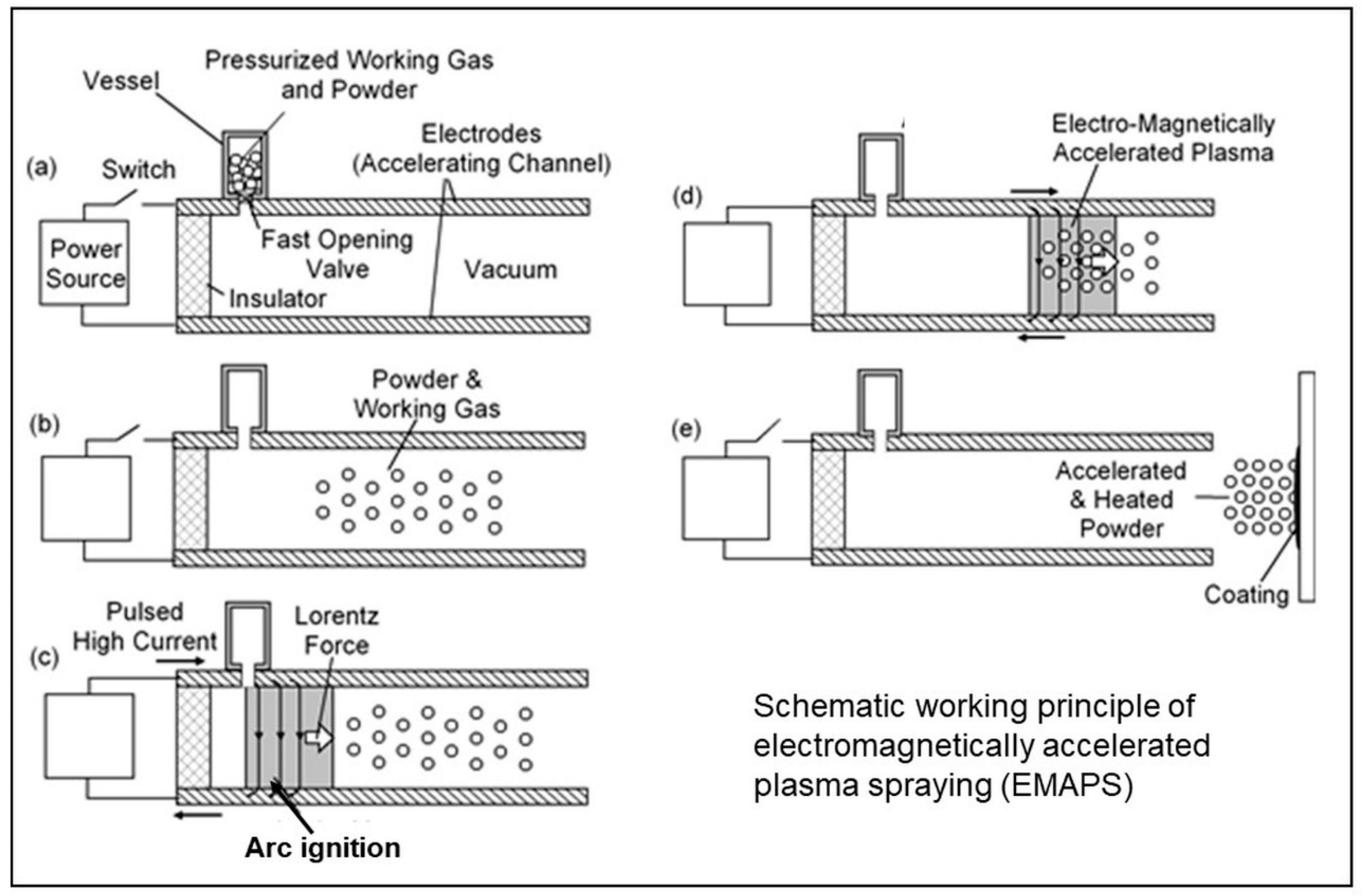
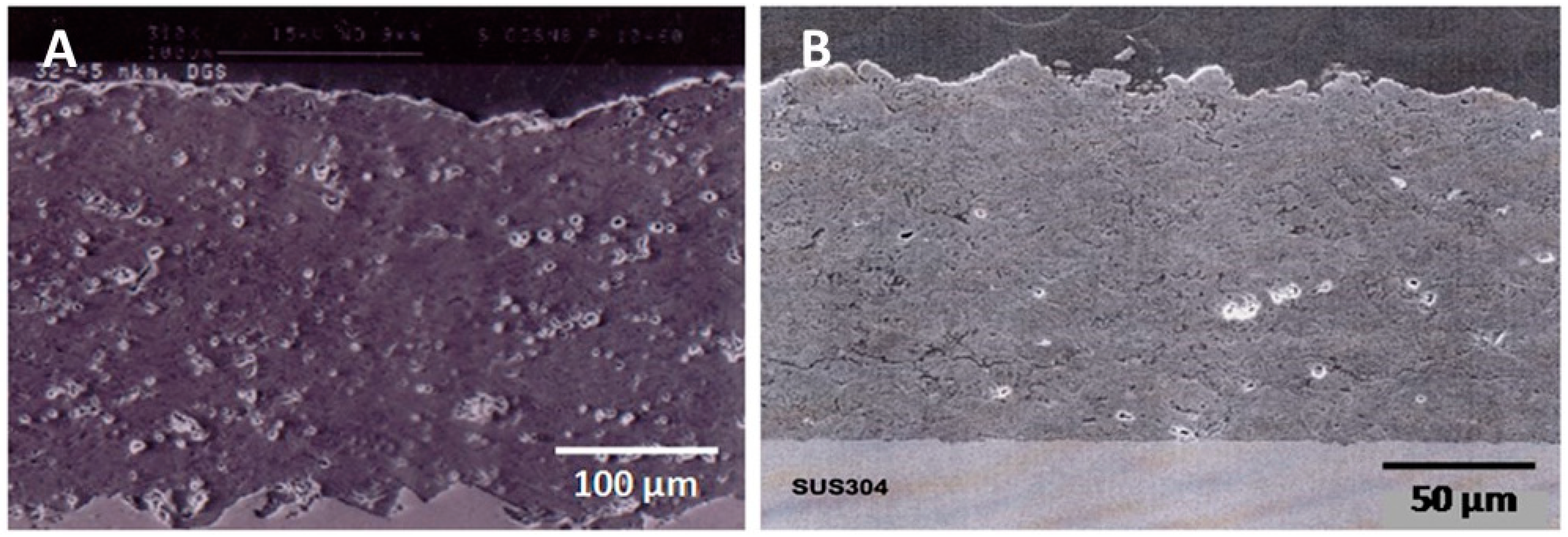
3.4. Silicon Nitride Coatings
4. Biomedical Applications
4.1. Intervertebral Spacers
4.2. Orthopedic Applications
4.3. Craniofacial Reconstruction
4.4. Dental Implants
4.5. Antibacterial and Antiviral Applications
4.6. Photonic Application in Medical Diagnostics
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klemm, H. Silicon nitride for high temperature applications. J. Am. Ceram. Soc. 2010, 93, 1501–1522. [Google Scholar] [CrossRef]
- Riley, F.L. Applications of silicon nitride ceramics. Key Eng. Mater. 1996, 122–124, 479–488. [Google Scholar] [CrossRef]
- Riley, F.L. Silicon nitride and related materials. J. Am. Ceram. Soc. 2000, 83, 145–265. [Google Scholar] [CrossRef]
- Petzow, G.; Herrmann, M. Silicon nitride ceramics. In High Performance Non-Oxide Ceramics II. Structure and Bonding; Jansen, M., Mingos, D.M.P., Haubner, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 102, pp. 47–168. [Google Scholar]
- Morosanu, C.E. The properties, characterization and applications of silicon nitride thin films. Thin Solid Films 1980, 65, 171–208. [Google Scholar] [CrossRef]
- Rahim, A.; Ryckeboer, E.; Subramanian, A.Z.; Clemmen, S.; Kuyken, B.; Dhakal, A.; Raza, A.; Hermans, A.; Muneeb, M.; Dhoore, S.; et al. Expanding the silicon photonics portfolio with silicon nitride photonic integrated circuits. J. Lightwave Technol. 2017, 35, 639–649. [Google Scholar] [CrossRef]
- Kunzig, R. Geoengineering: How to cool earth-at a price. Sci. Am. 2008, 299, 46–55. [Google Scholar] [CrossRef]
- Heimann, R.B. Silicon nitride, a close to ideal ceramic material for medical application. Ceramics 2021, 4, 208–223. [Google Scholar] [CrossRef]
- Bal, B.S.; Rahaman, M.N. Orthopedic applications of silicon nitride ceramics. Acta Biomater. 2012, 8, 2889–2898. [Google Scholar] [CrossRef]
- Zhang, W.; Titze, M.; Cappi, B.; Wirtz, D.C.; Telle, R. Improved mechanical long-term reliability of hip resurfacing prostheses by using silicon nitride. J. Mater. Sci. Mater. Med. 2010, 21, 3049–3057. [Google Scholar] [CrossRef]
- Raza, S.M.; Khurshid, Z.; Zafar, M.S.; Najeeb, S.; Ul Yaqin, S.A. Silicon nitride (SiN): An emerging material for dental implant applications. In Dental Implants: Materials, Coatings, Surface Modifications and Interfaces with Oral Tissue; Zafar, M.S., Khurshid, Z., Khan, A.S., Najeeb, S., Sefat, F., Eds.; Series in Biomaterials; Woodhead Publishing Ltd.: Sawston, UK, 2020; pp. 287–299. [Google Scholar]
- Wu, J.; Liu, Y.; Zhang, H.; Wu, Y.; Chu, Z.; Wu, Q.; Lu, M. Silicon nitride as a potential candidate for dental implants: Osteogenic activities and antibacterial properties. J. Mater. Res. 2021, 36, 1866–1882. [Google Scholar] [CrossRef]
- Katsaros, I.; Zhou, Y.; Welch, K.; Xia, W.; Persson, C.; Engqvist, H. Bioactive silicon nitride implant surfaces with maintained antibacterial properties. J. Funct. Biomater. 2022, 13, 129. [Google Scholar] [CrossRef]
- Pezzotti, G.; Boschetto, F.; Ohgitani, E.; Fujita, Y.; Zhu, W.; Marin, E.; McEntire, B.J.; Bal, B.S.; Mazda, O. Silicon nitride: A potent solid-state bioceramic inactivator of ssRNA viruses. Sci. Rep. 2021, 11, 2977. [Google Scholar] [CrossRef]
- Lee, M.R.; Russell, S.S.; Arden, J.W.; Pillinger, C.T. Nierite (Si3N4), a new mineral from ordinary and enstatite chondrites. Meteoritics 1995, 30, 387–398. [Google Scholar] [CrossRef]
- Leitch, S.; Moewes, A.; Ouyang, L.; Ching, W.Y.; Sekine, T. Properties of nonequivalent sites and band gap of spinel-phase silicon nitride. J. Phys. Condes. Mater. 2004, 16, 6469–6476. [Google Scholar] [CrossRef]
- Toraya, H. Crystal structure refinement of α-Si3N4 using synchrotron radiation powder diffraction data: Unbiased refinement strategy. J. Appl. Crystallogr. 2000, 33, 95–102. [Google Scholar] [CrossRef]
- Heimann, R.B. Classic and Advanced Ceramics. From Fundamentals to Applications; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Hector, A.L. Synthesis and processing of silicon nitride and related materials using preceramic polymer and non-oxide sol-gel approaches. Coord. Chem. Rev. 2016, 323, 120–137. [Google Scholar] [CrossRef]
- Waychunas, G.A.; Chang, H.Z. Structure, chemistry, and properties of mineral nanoparticles. Elements 2008, 4, 381–387. [Google Scholar] [CrossRef]
- Zha, H.K.; Yu, W.Q.; Li, J.W. Progress in preparation and properties of porous silicon nitride ceramics. Silicon 2023, 1–23. [Google Scholar] [CrossRef]
- Perevislov, S.N. Sintering behavior and properties of reaction-bonded silicon nitride. Russ. J. Appl. Chem. 2021, 94, 143–151. [Google Scholar] [CrossRef]
- Zhou, Y.; Hyuga, H.; Kusano, D.; Yoshizawa, Y.-I.; Ohji, T.; Hirao, K. Development of high-thermal conductivity silicon nitride ceramics. J. Asian Ceram. Soc. 2015, 3, 221–229. [Google Scholar] [CrossRef]
- Park, Y.-J.; Park, M.-J.; Kim, J.-M.; Lee, J.-W.; Ko, J.-W.; Kim, H.-D. Sintered reaction-bonded silicon nitride with high thermal conductivity: The effect of the starting Si powder and Si3N4 diluents. J. Eur. Ceram. Soc. 2014, 34, 1105–1113. [Google Scholar] [CrossRef]
- Zhu, X.; Sakka, Y. Textured silicon nitride: Processing and anisotropic properties. Sci. Technol. Adv. Mater. 2008, 9, 033001. [Google Scholar] [CrossRef]
- Hirao, K.; Nagaoka, T.; Brito, M.E.; Kanzaki, S. Microstructure control of silicon nitride by seeding with rodlike ß-silicon nitride particles. J. Am. Ceram. Soc. 1994, 77, 1857–1862. [Google Scholar] [CrossRef]
- Chen, F.; Shen, Q.; Zheng, L.M. Pressureless sintering of silicon nitride porous ceramics with high porosity and bimodal pore structure. Key Eng. Mater. 2012, 512/515, 873–877. [Google Scholar] [CrossRef]
- Xiao, C.F.; Bing, H. Preparation of porous silicon nitride by freeze drying. J. Mater. Res. Technol. 2019, 8, 6202–6208. [Google Scholar] [CrossRef]
- Shen, M.Y.; Zhao, H.F.; Feng, W.W.; Luo, Y.; Chen, H.; Zheng, Y.; Ge, L.; Guo, L. Porous silicon nitride for scaffold material by direct forming with protective gelling. Ceram. Intern. 2021, 47, 29342–29354. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; He, G.; Li, J.; Wang, C.-A. Microstructure and properties of porous Si3N4 ceramics by gelcasting-self-propagating high-temperature synthesis (SHS). J. Adv. Ceram. 2022, 11, 172–183. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, J.F.; Chen, Z.L.; Du, P.H. Fabrication of porous silicon nitride with high porosity by diatomite. Proc. Eng. 2012, 27, 787–792. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Fan, J.-P.; Sun, G.-L.; Zhang, J.-Y.; Liu, X.-M.; Zhang, D.-H.; Wang, H.-J. Preparation and properties of porous silicon nitride ceramics with uniform spherical pores by improved pore-forming agent method. J. Alloy Comp. 2015, 632, 655–660. [Google Scholar] [CrossRef]
- Man, Y.; Ding, G.; Xudong, L.; Xue, K.; Qu, D.; Xie, Z. A review on porous ceramics with hierarchical pore structure by 3D printing-based combined route. J. Asian Ceram. Soc. 2021, 9, 1377–1389. [Google Scholar] [CrossRef]
- Yu, F.L.; Wang, H.R.; Bai, Y.; Yang, J.F. Preparation and characterization of porous Si3N4 ceramics prepared by compression molding and slip casting methods. Bull. Mater. Sci. 2010, 33, 619–624. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, J.G. Tape casting of silicon nitride. MRS Online Proc. Library 1992, 287, 265–270. [Google Scholar] [CrossRef]
- Xiao, C.F.; Han, B. Preparation of porous silicon nitride ceramics by microwave sintering and its performance evaluation. J. Mater. Res. Technol. 2019, 8, 5984–5995. [Google Scholar] [CrossRef]
- Alem, A.; Drew, R.A.L.; Pugh, M.D. Sintered reaction-bonded silicon nitride foams with a high level of interconnected porosity. J. Mater. Sci. 2015, 50, 570–576. [Google Scholar] [CrossRef]
- Samudrala, S.C.; Das, S.; Lee, K.J.; Abdallah, M.G.; Wenner, B.R.; Allen, J.W.; Allen, M.S.; Magnusson, R.; Vasilyev, M. Silicon nitride micro-ring resonators for nonlinear optical and biosensing applications. Appl. Opt. 2021, 60, G132–G138. [Google Scholar] [CrossRef]
- Signore, M.A.; Sytchkova, A.; Dimaio, D.; Cappello, A.; Rizzo, a. Deposition of silicon nitride thin films by RF magnetron sputtering: A material and growth study. Opt. Mater. 2012, 34, 632–638. [Google Scholar] [CrossRef]
- Lugscheider, E.; Limbach, R. Plasma spraying of agglomerated powders on the basis of Si3N4. DVS Berichte 1990, 130, 224–225. (In German) [Google Scholar]
- Kucuk, A.; Lima, R.S.; Berndt, C.C. Composite coatings of Si3N4-soda lime silicate produced by the thermal spray process. J. Mater. Eng. Perform. 2000, 9, 603–608. [Google Scholar] [CrossRef]
- Eckardt, T.; Malléner, W.; Stöver, D. Reactive plasma spraying of silicon in controlled nitrogen atmosphere. In Thermal Spray Industrial Applications; Berndt, C.C., Sampath, S., Eds.; ASM International: Materials Park, OH, USA, 1994; pp. 515–519. [Google Scholar]
- Eckardt, T.; Malléner, W.; Stöver, D. Development of plasma-sprayed silicon/silicon nitride coatings by in-situ nitridation. In Thermische Spritzkonferenz: TS96; Lugscheider, E., Ed.; DVS-Berichte: Aachen, Germany, 1996; Volume 175, pp. 309–312. (In German) [Google Scholar]
- Sodeoka, S.; Ueno, K.; Hagiwara, Y.; Kose, S. Structure and properties of plasma-sprayed SiAlON coatings. J. Thermal Spray Technol. 1992, 1, 153–159. [Google Scholar] [CrossRef]
- Tomota, T.; Miyamoto, N.; Koyama, H. Formation of Thermal Spraying Ceramic Layer. Patent JP 63 169371, 24 November 1988. (In Japanese). [Google Scholar]
- Berger, L.-M.; Herrmann, M.; Nebelung, M.; Thiele, S.; Heimann, R.B.; Schnick, T.; Wielage, B.; Vuoristo, P. Investigations on thermal spraying of silicon nitride-based powders. In Thermal Spray: Meeting the Challenges of the 21st Century; Coddet, C., Ed.; ASM International: Materials Park, OH, USA, 1998; pp. 1149–1154. [Google Scholar]
- Thiele, S.; Heimann, R.B.; Berger, L.-M.; Nebelung, M.; Heimann, R.B.; Schnick, T.; Wielage, B.; Vuoristo, P. Microstructure and properties of thermally sprayed silicon nitride-based coatings. J. Thermal Spray Technol. 2002, 11, 218–225. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, T.; Gawne, D.T.; Mason, P. Quantitative model for the viscous flow and composition of two-phase silicon nitride-based particles in plasma-spray deposition. J. Eur. Ceram. Soc. 2008, 29, 1521–1528. [Google Scholar] [CrossRef]
- Berger, L.-M.; Herrmann, M.; Nebelung, M.; Heimann, R.B.; Wielage, B. Modified Composite Silicon Nitride Powders for Thermal Coatings and Process for their Production. U.S. Patent 6,110,853, 23 August 2000. [Google Scholar]
- Tahara, H.; Moriyama, M.; Fujiuchi, K.; Ando, Y. Large applications of electromagnetically accelerated plasma to material spraying. Thin Solid Films 2006, 506/507, 140–144. [Google Scholar] [CrossRef]
- Usuba, S.; Heimann, R.B. Dense Si3N4 coatings with high friction coefficient deposited by high-velocity pulsed plasma spraying. J. Thermal Spray Technol. 2006, 15, 356–363. [Google Scholar] [CrossRef]
- Robin, L.G.; Raghukandan, K.; Saravanan, S. Studies on wire-mesh and silicon carbide particle reinforcements in explosive cladding. J. Manufact. Proc. A 2020, 56, 887–897. [Google Scholar] [CrossRef]
- Heimann, R.B.; Kleiman, J. Shock-induced growth of superhard materials. In Superhard Materials, Convection, and Optical Devices; Freyhardt, H.C., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1988; pp. 1–73. [Google Scholar]
- Mazzocchi, M.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part I: Processing, microstructure, mechanical properties, cytotoxicity. J. Mater. Sci. Mater. Med. 2008, 19, 2881–2887. [Google Scholar] [CrossRef]
- Pezzotti, G.; McEntire, B.J.; Bock, R.; Boffelli, M.; Zhu, W.; Vitale, E.; Puppulin, L.; Adachi, T.; Yamamoto, T.; Kanamura, N.; et al. Silicon nitride: A synthetic mineral for vertebrate biology. Sci. Rep. 2016, 6, 31717. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Herrmann, M.; Schilm, J.; Michael, G.; Meinhardt, J.; Flegler, R. Corrosion of silicon nitride materials in acidic and basic solutions and under hydrothermal conditions. J. Eur. Ceram. Soc. 2003, 23, 585–594. [Google Scholar] [CrossRef]
- Heimann, R.B. Bioceramics-an overview. In Silicon Nitride Bioceramics; Pezzotti, G., Bal, B.S., McEntire, B., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2023; in press. [Google Scholar]
- Kersten, R.F.M.R.; Wu, G.; Pouran, B.; van der Veen, A.J.; Weinans, H.H.; de Gast, A.; Öner, F.C.; van Gaalen, S.M. Comparison of polyetheretherketone versus silicon nitride intervertebral spinal spacers in a caprine model. J. Biomed. Mater. Res. 2019, 107, 688–699. [Google Scholar] [CrossRef]
- Sorrell, C.; Hardcastle, P.H.; Druitt, R.K.; Howlett, C.R.; McCartney, E.R. Results of 15-years clinical study of reaction bonded silicon nitride intervertebral spacers. In Proceedings of the 7th World Biomaterial Congress, Sydney, Australia, 17–21 May 2004; Australian Society for Biomaterials: Sydney, Australia, 2004; p. 1872.
- Guedes e Silva, C.C.; König, B., Jr.; Carbonari, M.J.; Yoshimoto, M.; Allegrini, S., Jr.; Bressiani, J.C. Bone growth around silicon nitride implants—An evaluation by scanning electron microscopy. Mater. Character. 2008, 59, 1339–1341. [Google Scholar] [CrossRef]
- Anderson, M.C.; Olsen, R. Bone ingrowth into porous silicon nitride. J. Biomed. Mater. Res. A 2010, 92, 1598–1605. [Google Scholar] [CrossRef]
- Calvert, G.C.; Huffmon, G.V., III; Rambo, W.M., Jr.; Smith, M.W.; McEntire, B.J.; Sonny, B. Balcorresponding author5. Clinical outcomes for lumbar fusion using silicon nitride versus other biomaterials. J. Spine Surg. 2020, 6, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, R.J.; Rao, P.J.; Phan, K.; Hardcastle, P.; Choy, W.J.; McCartney, E.R.; Druitt, R.K.; Mouatt, C.A.; Sorrell, C.C. Anterior lumbar interbody fusion using reaction-bonded silicon nitride implants: Long-term case series of the first synthetic anterior lumbar interbody fusion spacer implanted in humans. World Neurosurg. 2018, 120, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Caseley, E.A.; Hall, R.M.; Tipper, J.L. Biological impact of silicon nitride for orthopaedic applications: Role of particle size, surface composition and donor variations. Sci. Rep. 2018, 8, 9109. [Google Scholar] [CrossRef]
- Olofsson, J.; Pettersson, M.; Teuscher, N.; Heilmann, A.; Larsson, K.; Grandfield, K.; Persson, C.; Jacobson, S.; Engqvist, H. Fabrication and evaluation of SixNy coatings for total joint replacements. J. Mater. Sci. Mater. Med. 2012, 23, 1879–1889. [Google Scholar] [CrossRef]
- Webster, T.J.; Patel, A.A.; Rahaman, M.N.; Sonny Bal, B. Anti-infective and osteointegration properties of silicon nitride, poly(etheretherketone), and titanium implants. Acta Biomater. 2012, 8, 4447–4454. [Google Scholar] [CrossRef] [PubMed]
- Guedes e Silva, C.C. Silicon nitride as biomaterial. In Silicon Nitride: Synthesis, Properties and Applications; Hierra, E.J., Salazar, J.A., Eds.; Nova Science Publication Inc.: New York, NY, USA, 2012; pp. 149–156. [Google Scholar]
- Filho, L.C.; Fu, I.; Engqvist, H.; Xia, W.; Persson, C. Wear Performance of a New Biocompatible Silicon Nitride for Biomedical Applications. 2020, European Union, Grant Number FP7-NMP-2012-310477 (Life Long Joints project) EBW+ Project Erasmus Mundus Programme, Action 2—STRAND 1, Lot 9 (Latin America), Brazil, Grant number 2014–0982. in press.
- Neumann, A.; Reske, T.; Held, M.; Jahnke, K.; Ragoß, C.; Maier, H.R. Comparative investigation of the biocompatibility of various silicon nitride ceramic qualities in vitro. J. Mater. Sci. Mater. Med. 2004, 15, 1135–1140. [Google Scholar] [CrossRef]
- Heimann, R.B. (Ed.) Materials for Medical Application; De Gruyter: Berlin, Germany, 2020; pp. 161–165. [Google Scholar]
- Jahanmir, S.; Ozmen, Y.; Ives, L.K. Water lubrication in silicon nitride in sliding. Tribol. Lett. 2004, 17, 409–417. [Google Scholar] [CrossRef]
- Ely, K.S.; Khandkar, A.C.; Lakshminarayanan, R.; Hofmann, A.A. Hip Prosthesis with Monoblock Ceramic Acetabular Cup. U.S. Patent 8,133,284, 13 March 2012. [Google Scholar]
- Filho, L.; Schmidt, S.; Leifer, K.; Engqvist, H.; Högberg, H.; Persson, C. Towards functional silicon nitride coatings for joint replacement. Coatings 2019, 9, 73. [Google Scholar] [CrossRef]
- Franklin Issac, R.; Devaraju, A. Effect of laser treatment on surface and interface of silicon nitride coated Ti6Al7Nb alloy—A statistical analysis. Silicon 2023, 15, 1545–1561. [Google Scholar]
- Awad, K.R.; Ahuja, N.; Shah, A.; Tran, H.; Aswath, P.B.; Brotto, M.; Varanasi, V. Silicon nitride enhances osteoprogenitor cell growth and formation of amide and nanocrystalline HA for craniofacial reconstruction. Med. Devices Sens. 2019, 2, e10032. [Google Scholar] [CrossRef]
- Ilyas, A.; Lavrik, N.; Kim, H.K.W.; Aswath, P.; Varanasi, V. Enhanced interfacial adhesion and osteogenesis for rapid ‘bone-like’ biomineralization by PECVD-based silicon oxynitride overlays. ACS Appl. Mater. Interfaces 2015, 7, 15368–15379. [Google Scholar] [CrossRef]
- Badran, Z.; Struillou, X.; Hughes, F.J.; Soueidan, A.; Hoornaert, A.; Ide, M. Silicon nitride (Si3N4) implants: The future of dental implantology? J. Oral Implantol. 2017, 43, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Özdogan, M.S.; Güngörmüş, M.; Çelik, A.; Topateş, G. Silicon nitride ceramic for all-ceramic dental restauration. Dent. Mater. J. 2020, 39, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Zanocco, M.; Boschetto, F.; Santini, M.; Zhu, W.; Adachi, T.; Ohgitani, E.; McEntire, B.J.; Bal, B.S.; Pezzotti, G. Silicon nitride laser cladding: A feasible technique to improve the biological response of zirconia. Mater. Des. 2020, 191, 108649. [Google Scholar] [CrossRef]
- Cappi, B.; Neuss, S.; Salber, J.; Telle, R.; Knüchel, R.; Fischer, H. Cytocompatibility of high strength non-oxide ceramics. J. Biomed. Mater. Res. A 2010, 93, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Bock, R.M.; McEntire, B.J.; Jones, E.; Boffelli, M.; Zhu, W.; Baggio, G.; Boschetto, F.; Puppulin, L.; Adachi, T.; et al. Silicon nitride bioceramics induce chemically driven lysis in Porphyromonas gingivalis. Langmuir 2016, 32, 3024–3035. [Google Scholar] [CrossRef] [PubMed]
- Boschetto, F.; Adachi, T.; Horiguchi, S.; Marin, E.; Paccotti, N.; Asai, T.; Zhu, W.; McEntire, B.J.; Yamamoto, T.; Kanamura, N.; et al. In situ molecular vibration insights into the antibacterial behavior of silicon nitride bioceramic versus gram-negative Escherichia coli. Spectr. Acta A 2019, 223, 117299. [Google Scholar] [CrossRef]
- Pezzotti, G.; Boschetto, F.; Ohgitani, E.; Fujita, Y.; Shin-Ya, M.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Marin, E.; Zhu, W.; et al. Mechanism of instantaneous inactivation of SARS-CoV-2 by silicon nitride bioceramics. Mater. Today Bio 2021, 12, 100144. [Google Scholar] [CrossRef]
- Gannot, I.; Ben-David, M. Optical fibers and waveguides for medical applications. In Biomedical Photonics Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 229–251. [Google Scholar]
- Hainberger, R.; Muellner, P.; Melnik, E.; Mutinati, G.; Eggeling, M.; Maese-Novo, A.; Vogelbacher, F.; Kraft, J. Silicon nitride waveguide integration platform for medical diagnostic application. In Proceedings of the 2016 Progress in Electromagnetic Research Symposium, Shanghai, China, 8–11 August 2016; p. 781. [Google Scholar]
- Carter, E.A.; Rayner, B.S.; McLeod, A.I.; Wu, L.E.; Marshall, C.P.; Levina, A.; Aitken, J.B.; Witting, P.K.; Lai, B.; Cai, Z.; et al. Silicon nitride as a versatile growth substrate for microspectroscopic imaging and mapping of individual cells. Mol. Biosyst. 2010, 6, 1316–1322. [Google Scholar] [CrossRef]

| Nanoscale Particle Properties | Desired Characteristics |
|---|---|
| Average particle size | 1–250 nm |
| Grain size distribution | Narrow or broad, depending on application |
| Particle shape | Spheres, needles, platelets, and wires |
| Degree of agglomeration | None or controlled |
| Purity level | Very high |
| Dispersability | High, in water and solvents 1 |
| Production scale | Several tons/year |
| Reproducibility | Very high (6σ) |
| Safety | Inherent, contained |
| Economy | 5 to 250 EUR/kg |
| Patents | Well established |
| Property | RBSN | SSN | SRBSN | TSSN |
|---|---|---|---|---|
| Density (Mg∙m−3) (% of theoretical density) | 70–88 | 95–100 | ~99 | 96–100 |
| Flexural strength (4-point, 25 °C) (MPa) | 150–350 | 500–1000 | 850 | 1400 |
| Fracture toughness (25 °C) (MPa∙√m) | 1.5–3 | 5–8 | 11 | >14 |
| Fracture energy (J∙m−2) | 4–10 | ~60 | ||
| Modulus of elasticity (25 °C) (GPa) | 120–220 | 300–330 | 280–540 | |
| Thermal conductivity (25 °C) (W/mK) | 4–30 | 15–50 | >170 | |
| Thermal shock resistance R (K) | 220–580 | 300–780 | ||
| Thermal shock fracture toughness R’ (W∙m−1) | 500–10,000 | 7000–32,000 | ||
| Coefficient of thermal expansion (10−6 K−1) | 3.2 | 3.2 | >3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heimann, R.B. Silicon Nitride Ceramics: Structure, Synthesis, Properties, and Biomedical Applications. Materials 2023, 16, 5142. https://doi.org/10.3390/ma16145142
Heimann RB. Silicon Nitride Ceramics: Structure, Synthesis, Properties, and Biomedical Applications. Materials. 2023; 16(14):5142. https://doi.org/10.3390/ma16145142
Chicago/Turabian StyleHeimann, Robert B. 2023. "Silicon Nitride Ceramics: Structure, Synthesis, Properties, and Biomedical Applications" Materials 16, no. 14: 5142. https://doi.org/10.3390/ma16145142
APA StyleHeimann, R. B. (2023). Silicon Nitride Ceramics: Structure, Synthesis, Properties, and Biomedical Applications. Materials, 16(14), 5142. https://doi.org/10.3390/ma16145142







