The Corrosion Properties of Bronze Alloys in NaCl Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
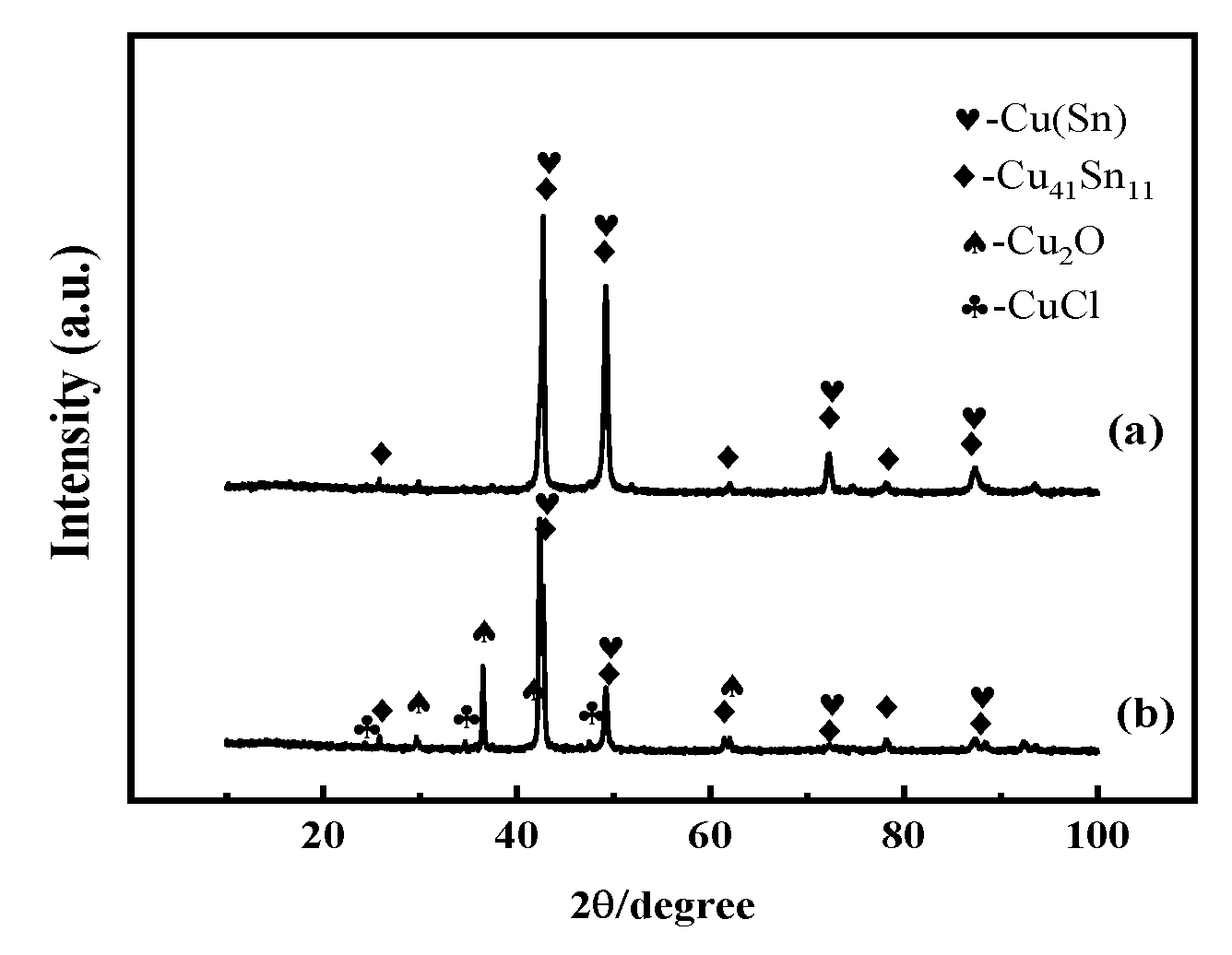
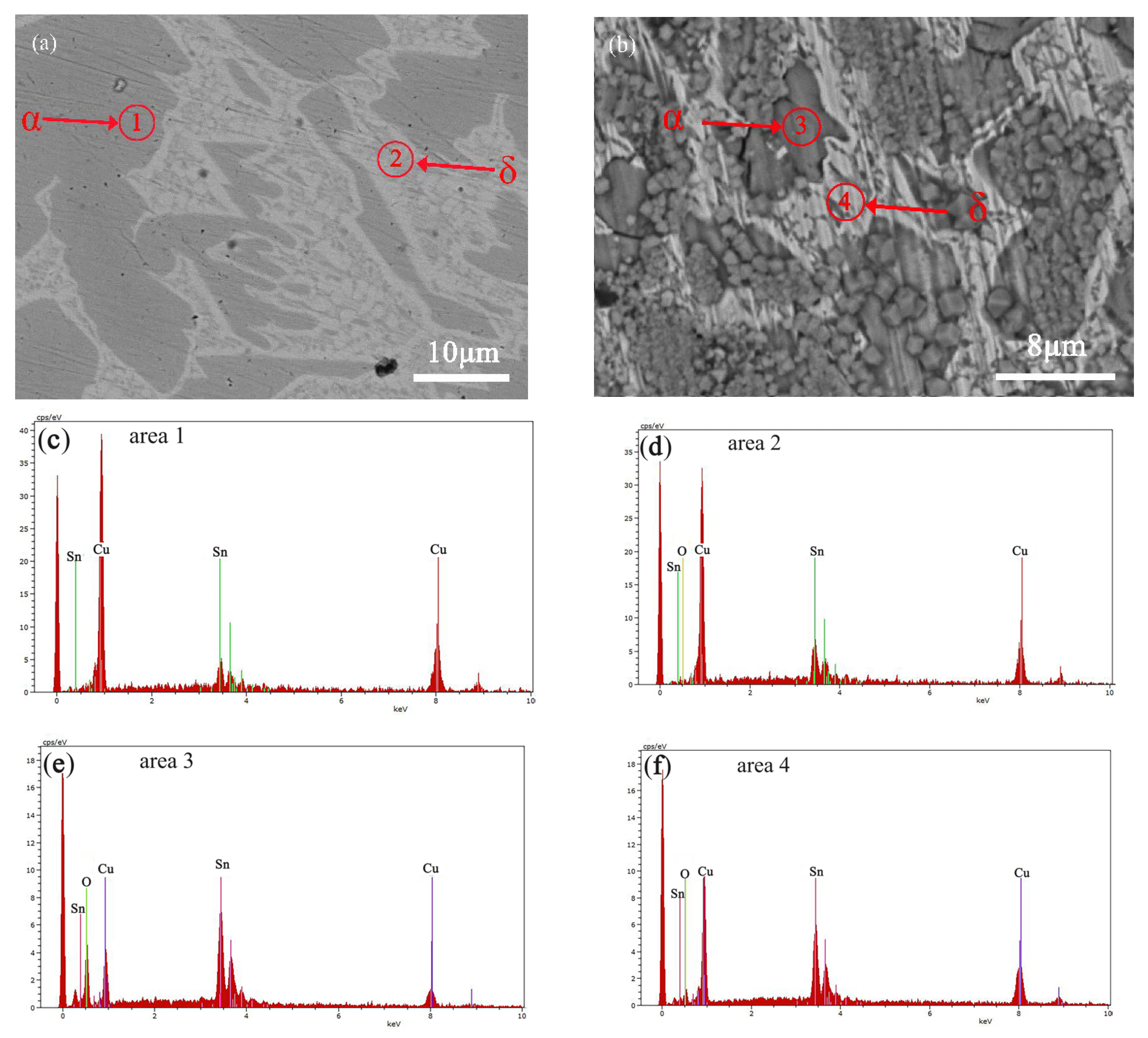
3.1. Structure and Composition of the Bronze Alloys
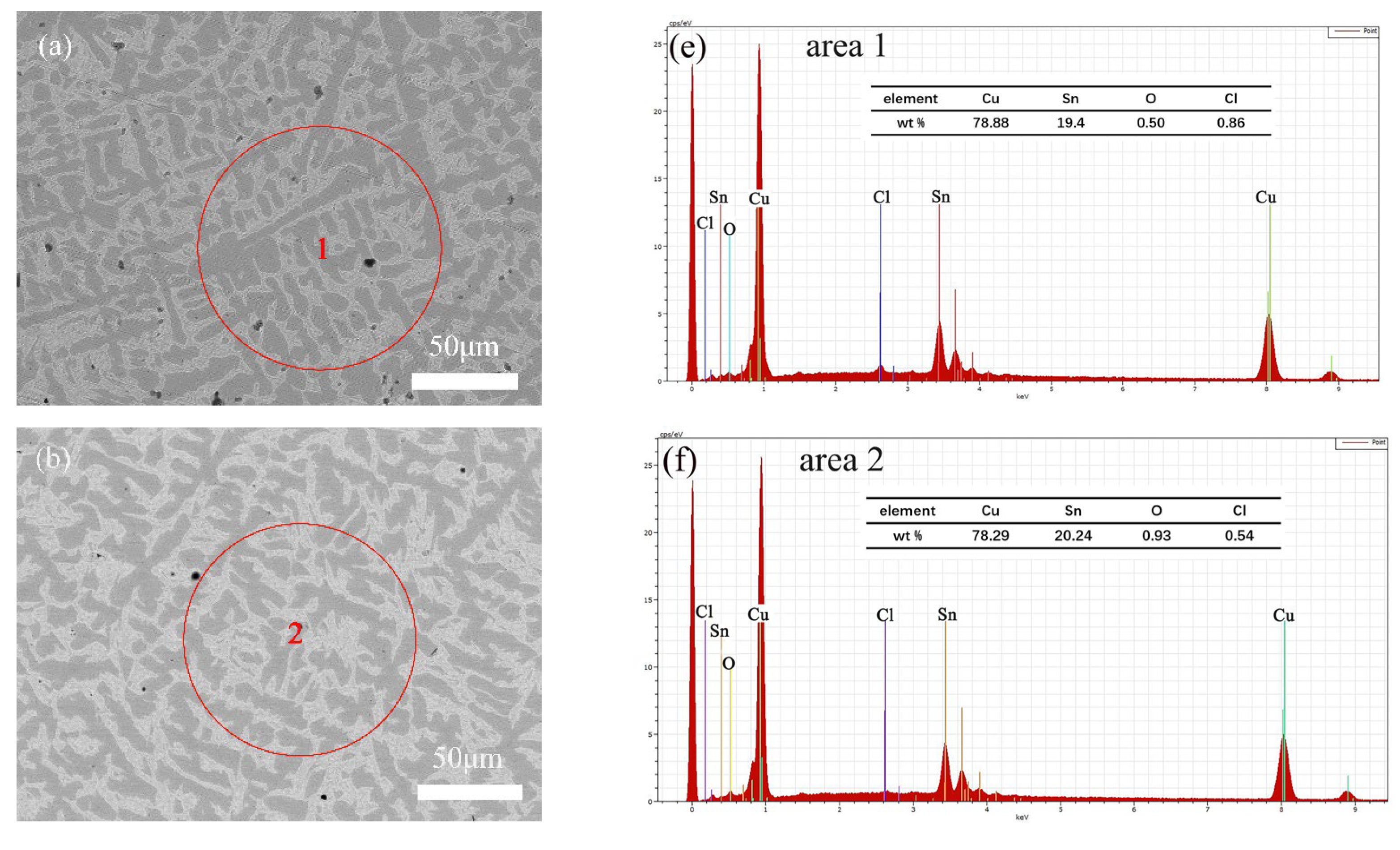
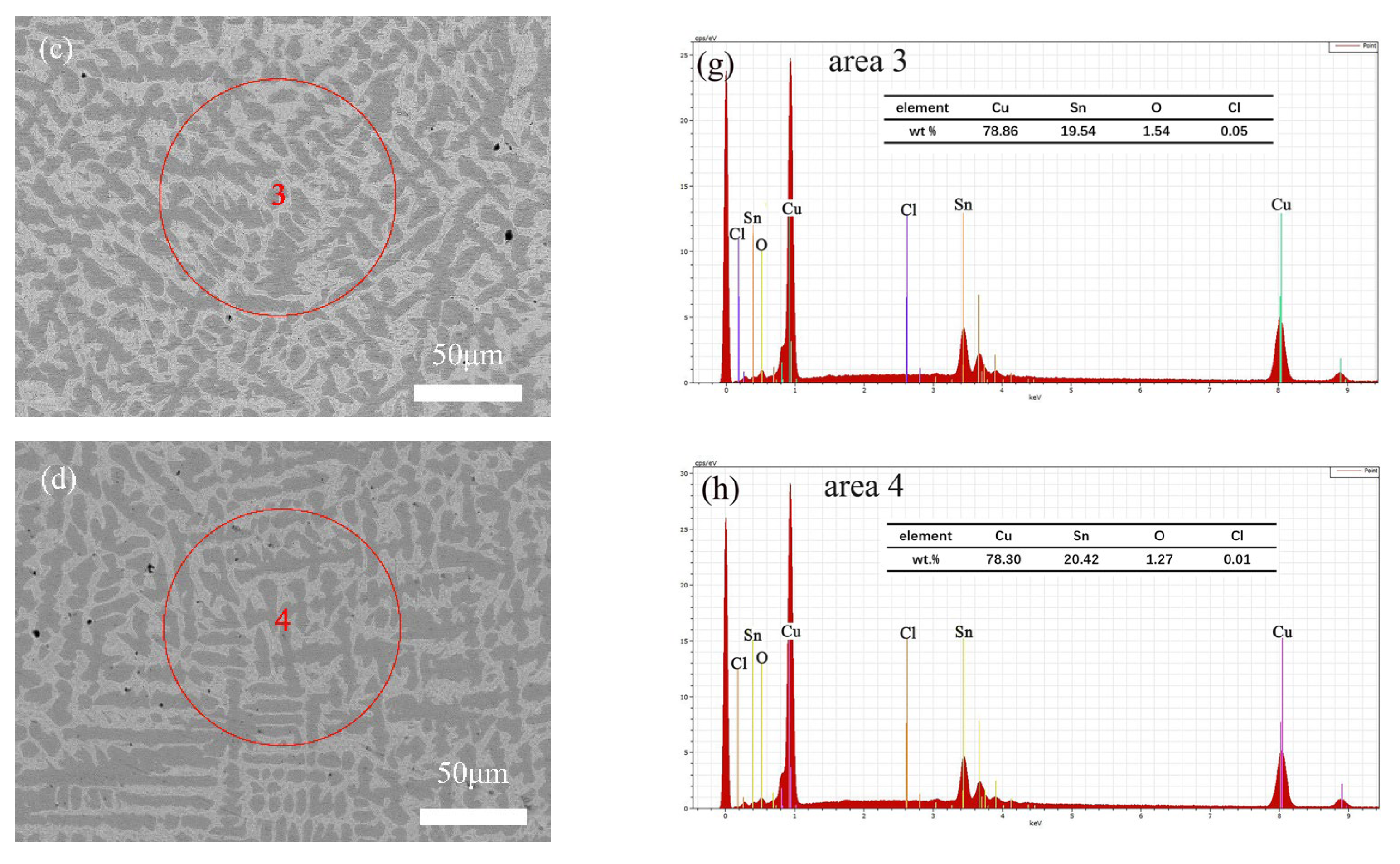
3.2. Microscopic Corrosion Morphology and Products
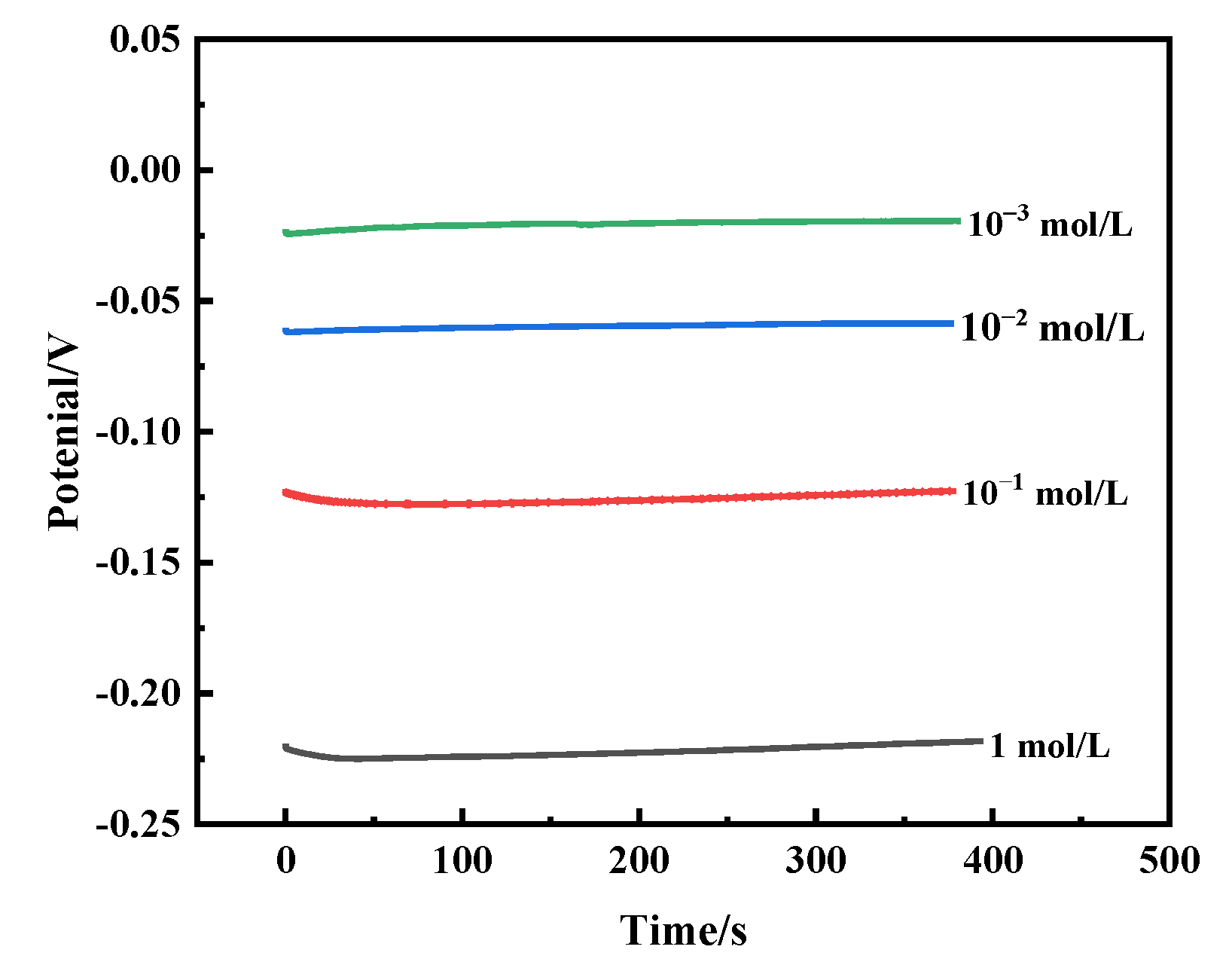
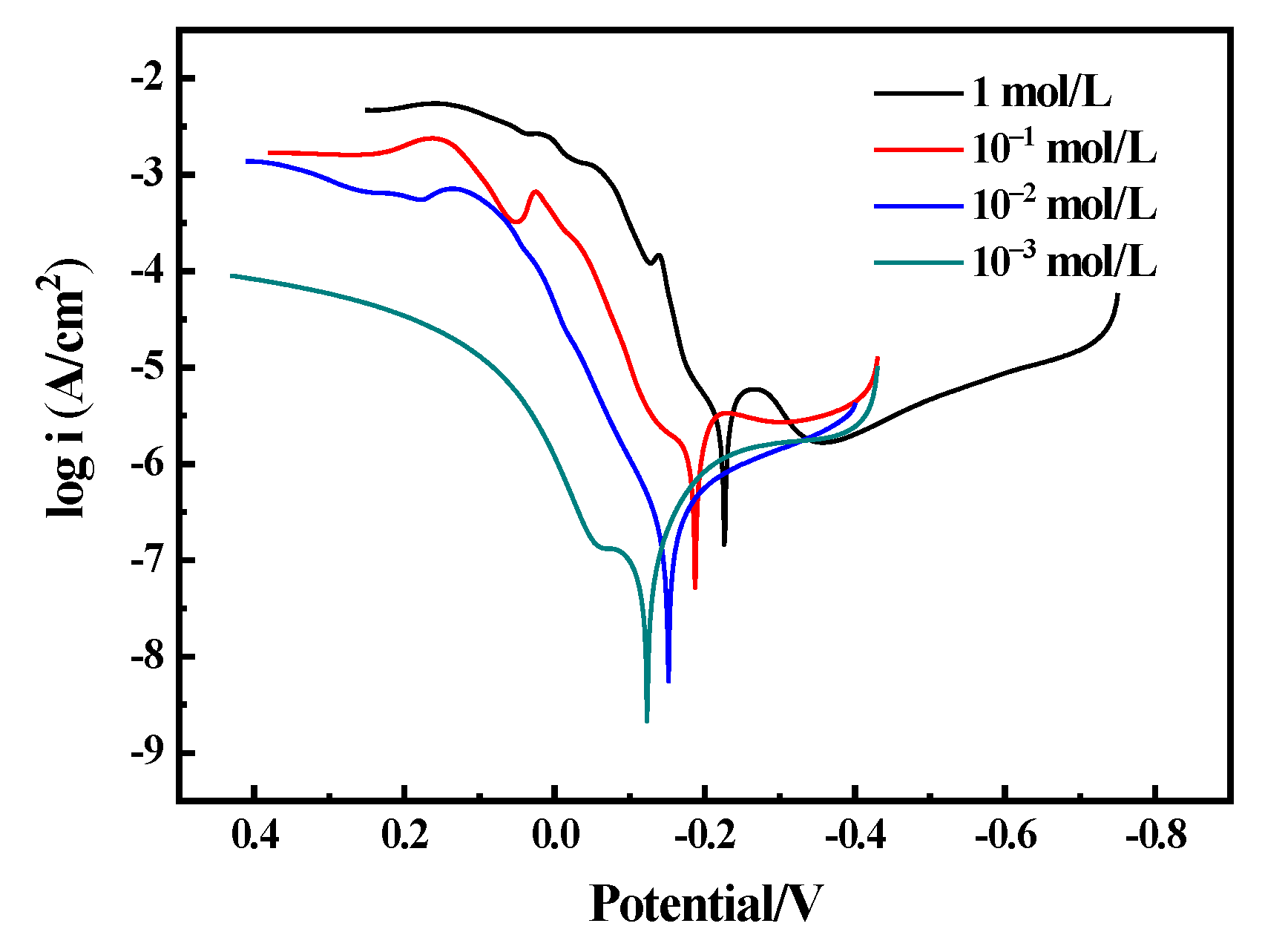
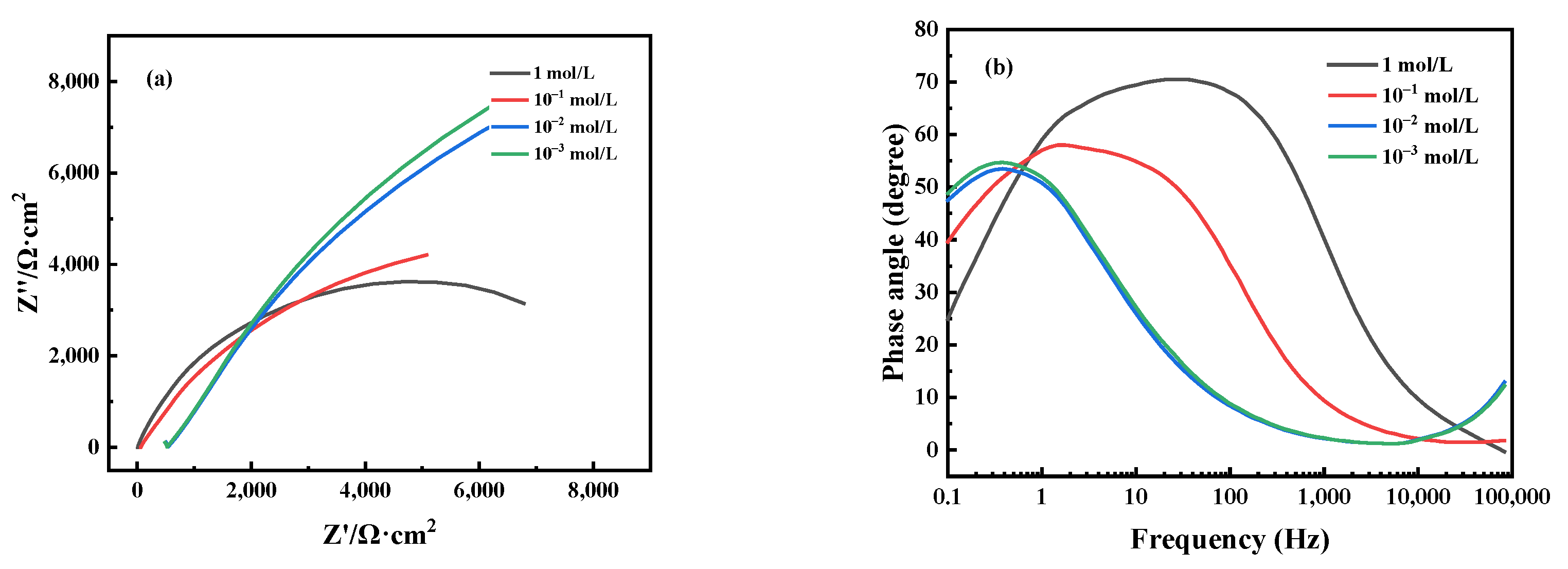
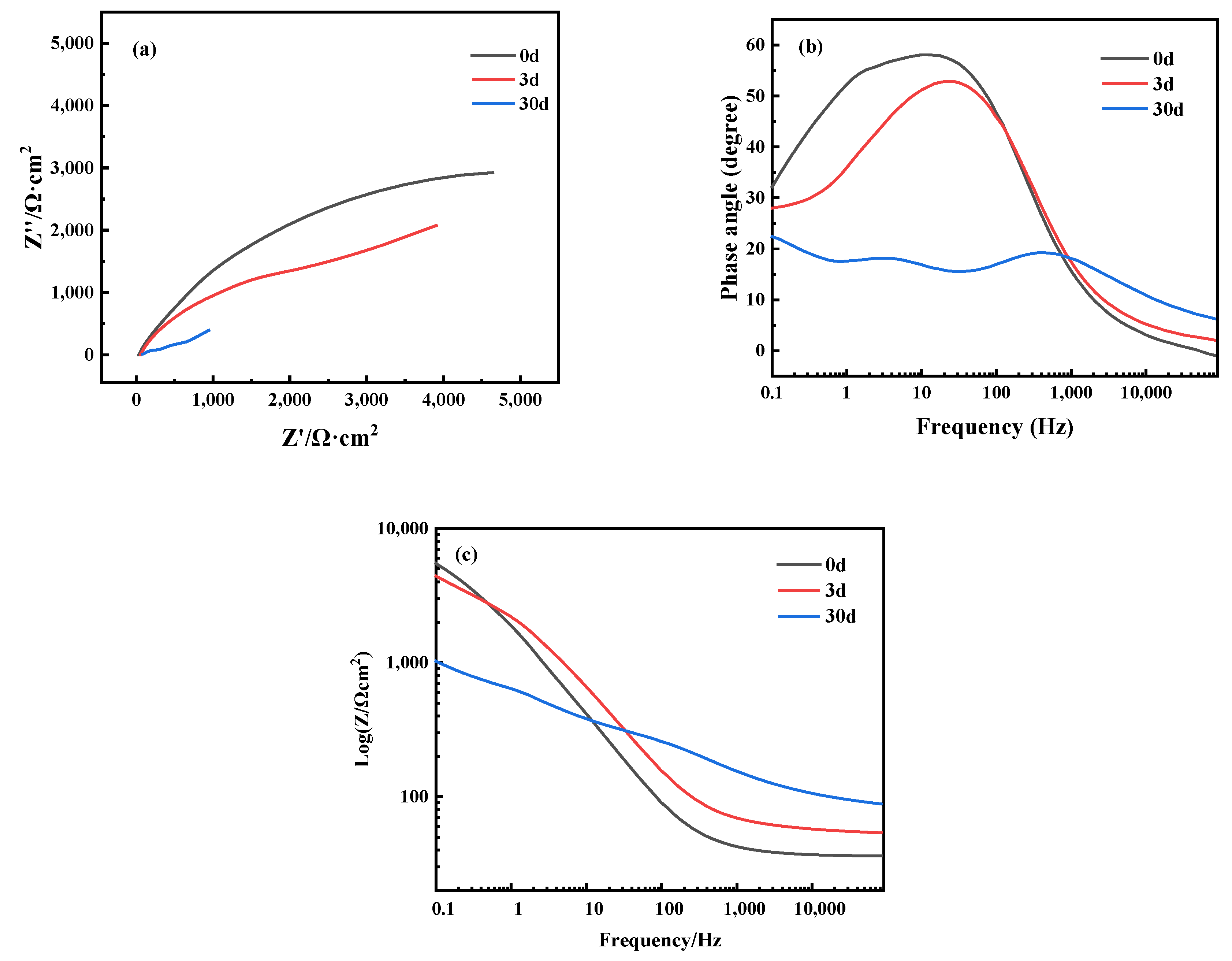
3.3. Electrochemical Corrosion Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohler, F.; Campanella, T.; Nakanishi, S.; Rappaz, M. Application of single pan thermal a nalysis to Cu–Sn peritectic alloys. Acta Mater. 2008, 56, 1519–1528. [Google Scholar] [CrossRef]
- Sürme, Y.; Gürten, A.A.; Bayol, E.; Ersoy, E. Systematic corrosion investigation of various Cu–Sn alloys electrodeposited on mild steel in acidic solution: Dependence of alloy composition. J. Alloys Compd. 2009, 485, 98–103. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Jiang, X.; Pan, C. Research progress on ancient bronze corrosion in different environments and using different conservation techniques: A review. MRS Adv. 2017, 2, 2033–2041. [Google Scholar] [CrossRef]
- Liu, L.; Zhong, Q.; Jiang, L.; Li, P.; Xiao, L.; Gong, Y.; Zhu, Z.; Yang, J. Metallurgical and corrosion characterization of warring states period bronzes excavated from Pujiang, Chengdu, China. Heritage Sci. 2022, 10, 36. [Google Scholar] [CrossRef]
- Privitera, A.; Corbascio, A.; Calcani, G.; Della Ventura, G.; Ricci, M.A.; Sodo, A. Raman approach to the forensic study of bronze patinas. J. Archaeol. Sci. Rep. 2021, 39, 103115. [Google Scholar] [CrossRef]
- Li, H.; Zuo, Z.; Cui, J.; Tian, J.; Yang, Y.; Yi, L.; Zhou, Z.; Fan, J. Copper alloy production in the Warring States period (475-221 BCE) of the Shu state: A metallurgical study on copper alloy objects of the Baishoulu cemetery in Chengdu, China. Heritage Sci. 2020, 8, 67. [Google Scholar] [CrossRef]
- Mu, D.; Luo, W.; Song, G.; Qiao, B.; Wang, F. The features as a county of Chu State: Chemical and metallurgical characteristics of the bronze artifacts from the Bayilu site. Archaeol. Anthropol. Sci. 2019, 11, 1123–1129. [Google Scholar] [CrossRef]
- Li, B.; Jiang, X.; Wu, R.; Wei, B.; Hu, T.; Pan, C. Formation of black patina on an ancient Chinese bronze sword of the Warring States Period. Appl. Surf. Sci. 2018, 455, 724–728. [Google Scholar] [CrossRef]
- Armetta, F.; Saladino, M.L.; Scherillo, A.; Caponetti, E. Microstructure and phase composition of bronze Montefortino helmets discovered Mediterranean seabed to explain an unusual corrosion. Sci. Rep. 2021, 11, 23022. [Google Scholar] [CrossRef]
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants, Conservation; Getty Publications: Los Angeles, CA, USA, 2002; pp. 125–128. Available online: https://www.getty.edu/publications/virtuallibrary/temp/9780892366385.pdf (accessed on 25 May 2023).
- Buccolieri, G.; Buccolieri, A.; Donati, P.; Marabelli, M.; Castellano, A. Portable EDXRF investigation of the patinas on the Riace Bronzes. Nucl. Instrum. Methods Phys. Res. Sect. B 2015, 343, 101–109. [Google Scholar] [CrossRef]
- Petitmangin, A.; Guillot, I.; Chabas, A.; Nowak, S.; Saheb, M.; Alfaro, S.C.; Blanc, C.; Fourdrin, C.; Ausset, P. The complex atmospheric corrosion of α/δ bronze bells in a marine environment. J. Cult. Herit. 2021, 52, 153–163. [Google Scholar] [CrossRef]
- Piccardo, P.; Mödlinger, M.; Ghiara, G.; Campodonico, S.; Bongiorno, V. Investigation on a “tentacle-like” corrosion feature on Bronze Age tin-bronze objects. Appl. Phys. A 2013, 113, 1039–1047. [Google Scholar] [CrossRef]
- Osório, W.R.; Freire, C.M.; Caram, R.; Garcia, A. The role of Cu-based intermetallics on the pitting corrosion behavior of Sn–Cu, Ti–Cu and Al–Cu alloys—ScienceDirect. Electrochim. Acta 2012, 77, 189–197. [Google Scholar] [CrossRef]
- Saud, S.; Hamzah, E.; Abubakar, T.; Bakhsheshi-Rad, H. Correlation of microstructural and corrosion characteristics of quaternary shape memory alloys Cu–Al–Ni–X (X = Mn or Ti). Trans. Nonferrous Met. Soc. China 2015, 25, 1158–1170. [Google Scholar] [CrossRef]
- Robbiola, L.; Blengino, J.-M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu–Sn alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Masi, G.; Esvan, J.; Josse, C.; Chiavari, C.; Bernardi, E.; Martini, C.; Bignozzi, M.C.; Gartner, N.; Kosec, T.; Robbiola, L. Characterization of typical patinas simulating bronze corrosion in outdoor conditions. Mater. Chem. Phys. 2017, 200, 308–321. [Google Scholar] [CrossRef]
- Luo, W.; Jin, R.; Qin, Y.; Huang, F.; Wang, C. Analysis of the corrosion products of the ancient bronzes excavated from Qiaojiayuan tombs. Appl. Phys. Res. 2010, 2, 156. [Google Scholar] [CrossRef]
- Oudbashi, O. A methodological approach to estimate soil corrosivity for archaeological copper alloy artefacts. Heritage Sci. 2018, 6, 2. [Google Scholar] [CrossRef]
- Arroyave, C.; Lopez, F.; Morcillo, M. The early atmospheric corrosion stages of carbon steel in acidic fogs. Corros. Sci. 1995, 37, 1751–1761. [Google Scholar] [CrossRef]
- Chang, T.; Herting, G.; Goidanich, S.; Amaya, J.S.; Arenas, M.; Le Bozec, N.; Jin, Y.; Leygraf, C.; Wallinder, I.O. The role of Sn on the long-term atmospheric corrosion of binary Cu-Sn bronze alloys in architecture. Corros. Sci. 2019, 149, 54–67. [Google Scholar] [CrossRef]
- De Oliveira, F.; Lago, D.; Senna, L.; De Miranda, L.; D’elia, E. Study of patina formation on bronze specimens. Mater. Chem. Phys. 2009, 115, 761–770. [Google Scholar] [CrossRef]
- Walker, R. Aqueous Corrosion of Tin-Bronze and Inhibition by Benzotriazole. Corrosion 2000, 56, 1211–1219. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, Y.; Li, L.; Zhang, J.; Chen, J. Same site, different corrosion phenomena caused by chloride: The effect of the archaeological context on bronzes from Sujialong Cemetery, China. J. Cult. Herit. 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Alfantazi, A.; Ahmed, T.; Tromans, D. Corrosion behavior of copper alloys in chloride media. Mater. Des. 2009, 30, 2425–2430. [Google Scholar] [CrossRef]
- Constantinides, I.; Adriaens, A.; Adams, F. Surface Characterization of Artificial Corrosion Layers on Copper Alloy Reference Materials. Appl. Surf. Sci. 2002, 189, 90–101. [Google Scholar] [CrossRef]
- Liang, Z.; Jiang, K.; Zhang, T. Corrosion behaviour of lead bronze from the Western Zhou Dynasty in an archaeological-soil medium. Corros. Sci. 2021, 191, 109721. [Google Scholar] [CrossRef]
- Wallinder, I.O.; Zhang, X.; Goidanich, S.; Le Bozec, N.; Herting, G.; Leygraf, C. Corrosion and runoff rates of Cu and three Cu-alloys in marine environments with increasing chloride deposition rate. Sci. Total Environ. 2014, 472, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qiu, R.; Chai, F.; Chen, L.; Li, T.; Zhu, H. A Study on Corrosion Behavior of Low-Alloy Steel for Ships in Simulated Seawater. J. Zhejiang Sci.-Tech. Univ. 2014, 31, 487–490. [Google Scholar] [CrossRef]
- González-Parra, R.; Covelo, A.; Barba, A.; Hernández, M. Electrochemical Polarization as a Sustainable Method for the Formation of Bronze Patina Layers on a Quaternary Copper Alloy: Insight into Patina Morphology and Corrosion Behaviour. Sustainability 2023, 15, 1899. [Google Scholar] [CrossRef]
- González-Parra, R.; Covelo, A.; Hernández, M. Determination of optimal electrochemical parameters to reproduce copper artistic patina on quaternary alloys. Mater. Lett. 2022, 309, 131414. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wu, Y. The inhibition effect and mechanism of L-cysteine on the corrosion of bronze covered with a CuCl patina. Corros. Sci. 2015, 97, 89–99. [Google Scholar] [CrossRef]
- Liao, X.N.; Cao, F.H.; Chen, A.N.; Liu, W.J.; Zhang, J.Q.; Cao, C.N. In-situ investigation of atmospheric corrosion behavior of bronze under thin electrolyte layers using electrochemical technique. Trans. Nonferrous Met. Soc. China 2012, 22, 1239–1249. [Google Scholar] [CrossRef]
- Yang, X.; Wu, W.; Chen, K. Investigation on the electrochemical evolution of the Cu-sn-Pb ternary alloy covered with CuCl in a simulated atmospheric environment. J. Electroanal. Chem. 2022, 921, 116636. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.; Walsh, F. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Chmielová, M.; Seidlerová, J.; Weiss, Z. X-ray diffraction phase analysis of crystalline copper corrosion products after treatment in different chloride solutions. Corros. Sci. 2003, 45, 883–889. [Google Scholar] [CrossRef]
- Grayburn, R.; Dowsett, M.; Hand, M.; Sabbe, P.J.; Thompson, P.; Adriaens, A. Tracking the progression of bronze disease—A synchrotron X-ray diffraction study of nantokite hydrolysis. Corros. Sci. 2015, 91, 220–223. [Google Scholar] [CrossRef]





| Position | Sn (wt%) | Cu (wt%) |
|---|---|---|
| average value | 20.12 | 79.88 |
| α-phase | 13.50 | 86.50 |
| (α + δ) phase | 23.82 | 76.18 |
| Position | Sn (wt%) | Cu (wt%) | O (wt%) | |
|---|---|---|---|---|
| Without corrosion | α-phase | 13.50 | 86.50 | <D.L. |
| δ-phase | 23.82 | 76.18 | <D.L. | |
| With corrosion | α-phase | 48.41 | 29.53 | 22.06 |
| δ-phase | 37.32 | 59.52 | 3.16 | |
| Solution | Ecorr/V | Icorr/A·cm2 | Ba/V−1 | Ba/V−1 | Rp/KΩ |
|---|---|---|---|---|---|
| 1 mol/L | −0.223 | 2.769 × 10−6 | 8.801 | 10.913 | 7.9648 |
| 10−1 mol/L | −0.187 | 4.578 × 10−7 | 2.745 | 11.160 | 68.301 |
| 10−2 mol/L | −0.152 | 3.032 × 10−7 | 3.921 | 13.843 | 80.723 |
| 10−3 mol/L | −0.123 | 1.101 × 10−7 | 31.944 | 9.348 | 95.635 |
| Solution | Rs/(Ω·cm2) | Qdl/(Ω−1·cm2·s n) | n | Rct/(Ω·cm2) |
|---|---|---|---|---|
| 1 mol/L | 6.44 | 6.148 × 10−5 | 0.8185 | 9406 |
| 10−1 mol/L | 54.92 | 7.777 × 10−5 | 0.7691 | 16,306 |
| 10−2 mol/L | 478.5 | 1.333 × 10−4 | 0.6672 | 37,271 |
| 10−3 mol/L | 58,556 | 1.168 × 10−4 | 0.6918 | 58,556 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Tegus, O. The Corrosion Properties of Bronze Alloys in NaCl Solutions. Materials 2023, 16, 5144. https://doi.org/10.3390/ma16145144
Song Z, Tegus O. The Corrosion Properties of Bronze Alloys in NaCl Solutions. Materials. 2023; 16(14):5144. https://doi.org/10.3390/ma16145144
Chicago/Turabian StyleSong, Zhiqiang, and Ojiyed Tegus. 2023. "The Corrosion Properties of Bronze Alloys in NaCl Solutions" Materials 16, no. 14: 5144. https://doi.org/10.3390/ma16145144
APA StyleSong, Z., & Tegus, O. (2023). The Corrosion Properties of Bronze Alloys in NaCl Solutions. Materials, 16(14), 5144. https://doi.org/10.3390/ma16145144





