Enhancing the Plasma-Resistance Properties of Li2O–Al2O3–SiO2 Glasses for the Semiconductor Etch Process via Alkaline Earth Oxide Incorporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Glass Sample Fabrication Method
2.2. Evaluation of Glass Properties
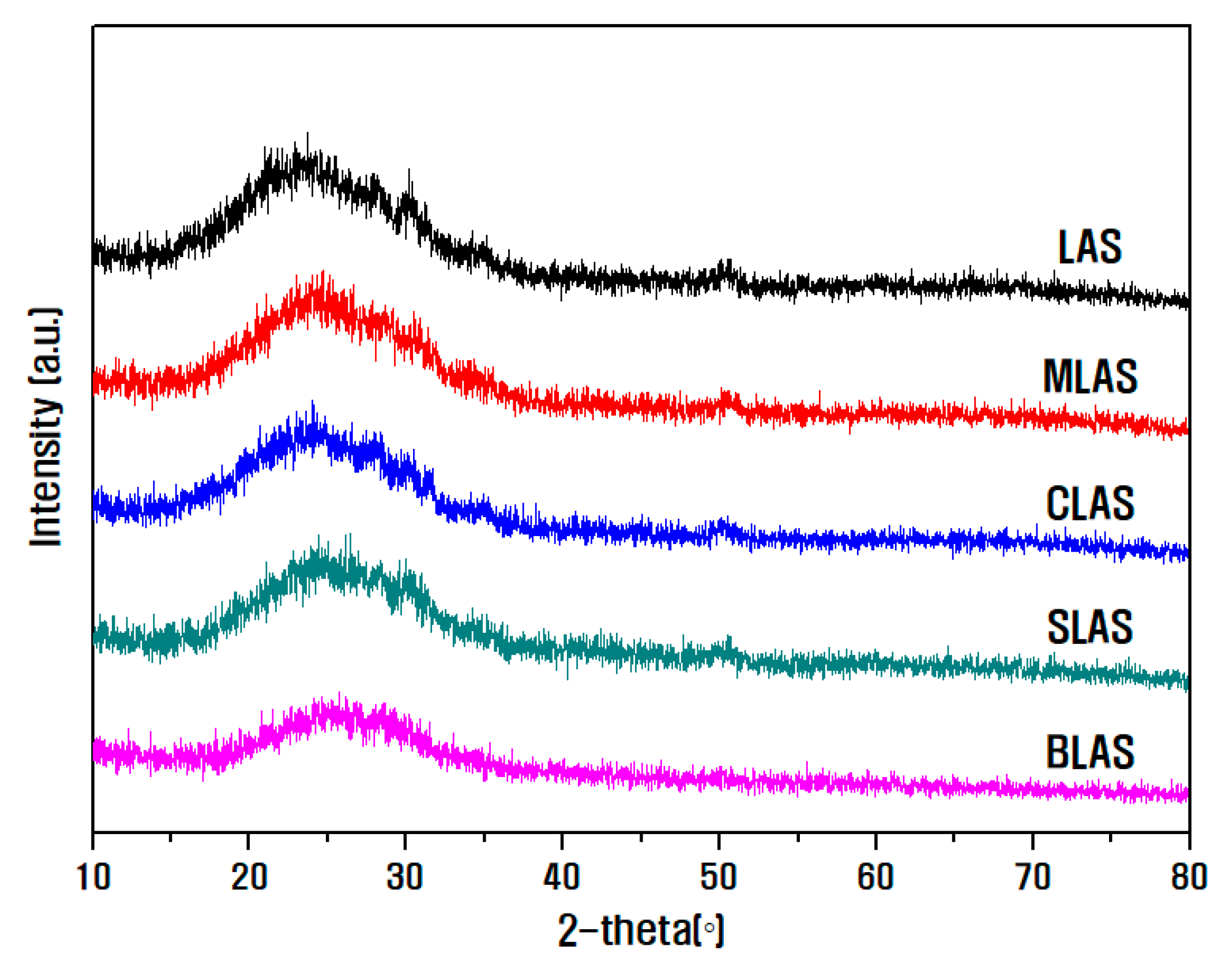
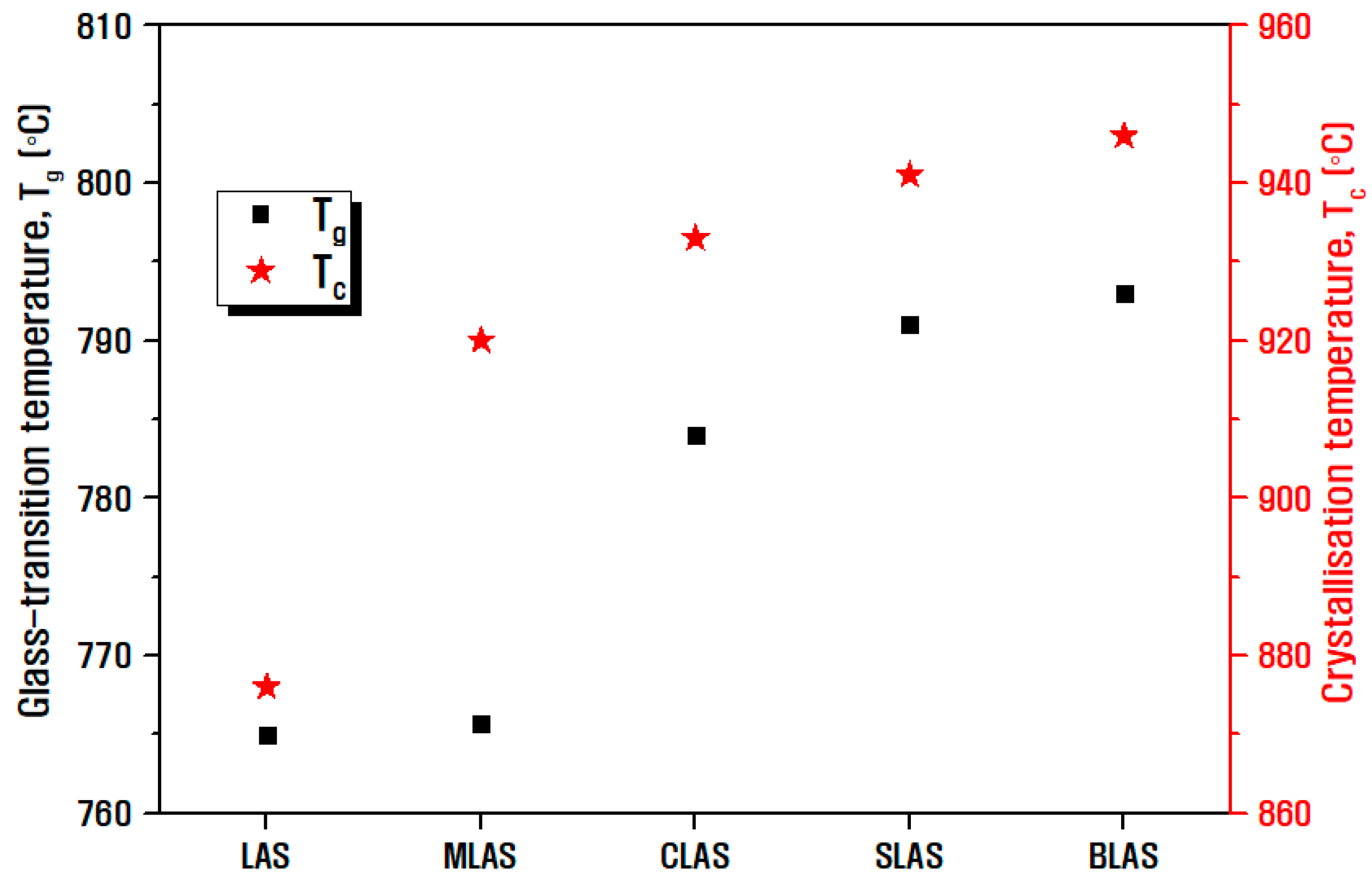
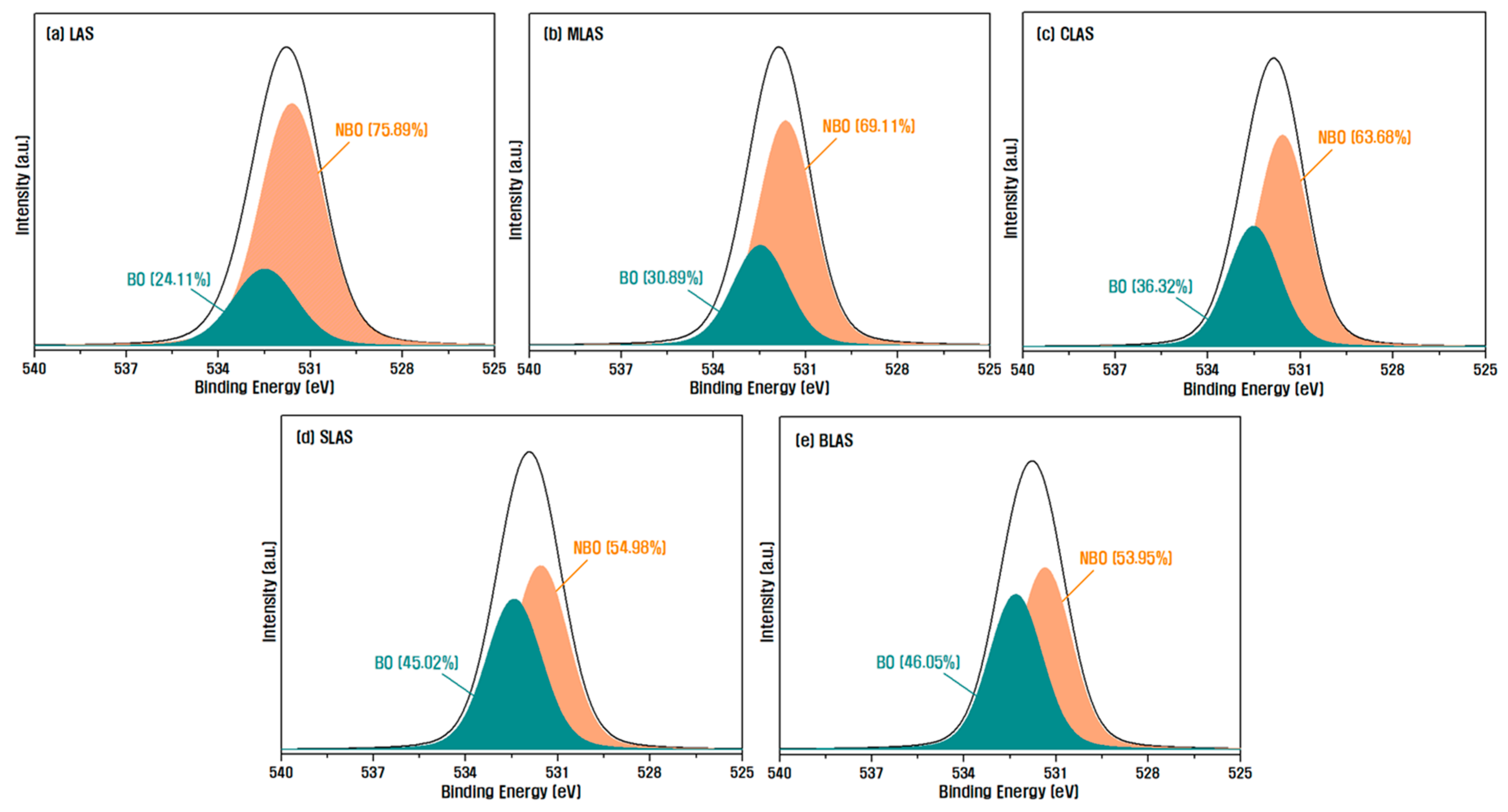
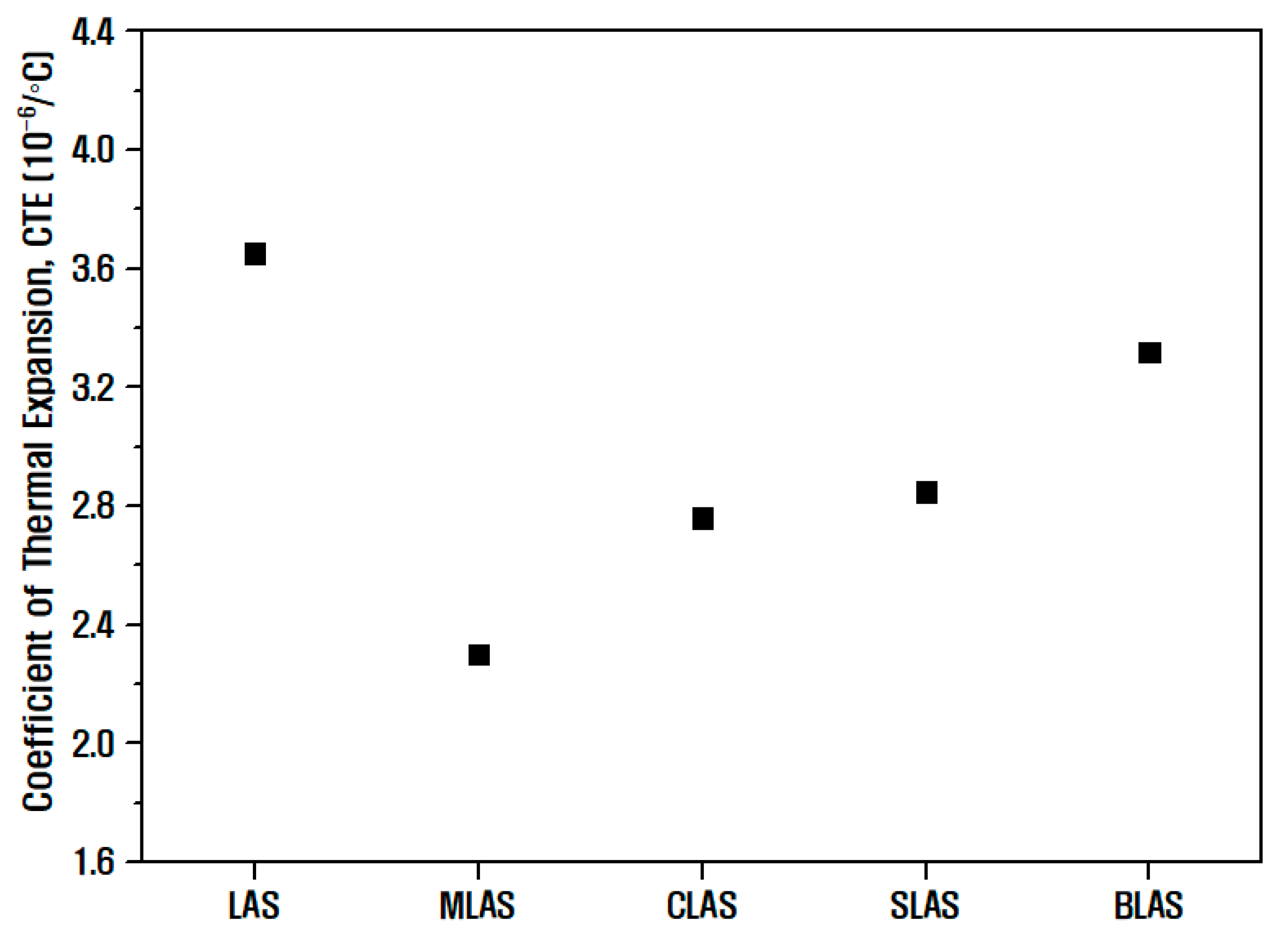
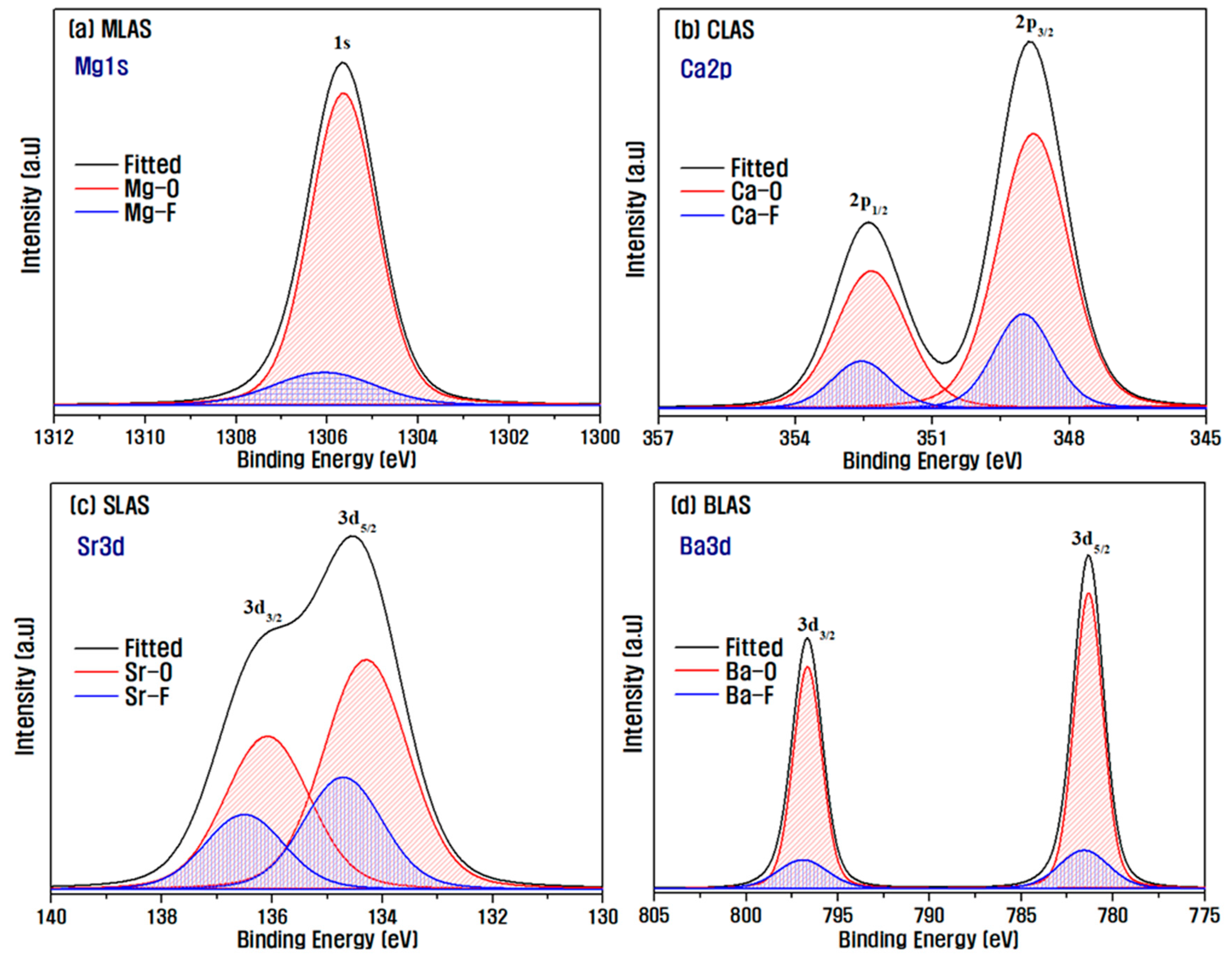
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abe, H.; Yoneda, M.; Fujiwara, N. Developments of plasma etching technology for fabricating semiconductor devices. Jpn. J. Appl. Phys. 2008, 47, 1435–1455. [Google Scholar] [CrossRef]
- Park, W.; Kwon, O.; Whang, K.-W.; Lee, J. Dry Etching Characteristics of TaN Absorber for Extreme Ultraviolet Mask with Ru Buffer Layer. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 041301. [Google Scholar] [CrossRef]
- Pranda, A.; Lin, K.-Y.; Engelmann, S.; Bruce, R.L.; Joseph, E.A.; Metzler, D.; Oehrlein, G.S. Significance of Plasma-Photoresist Interactions for Atomic Layer Etching Processes with Extreme Ultraviolet Photoresist. J. Vac. Sci. Technol. A 2020, 38, 052601. [Google Scholar] [CrossRef]
- Kim, G.W.; Chang, W.J.; Kang, J.E.; Kim, H.J.; Yeom, G.Y. Reduction of EUV Resist Damage by Neutral Beam Etching. Nanotechnology 2022, 33, 095301. [Google Scholar] [CrossRef]
- Yang, K.C.; Shin, Y.J.; Tak, H.W.; Lee, W.; Lee, S.B.; Yeom, G.Y. Effects of superimposed dual-frequency (13.56/2 MHz) inductively coupled plasma source on the uniformity of Ar/CF4 plasma. Vacuum 2019, 168, 108802. [Google Scholar] [CrossRef]
- Kim, D.M.; Jang, M.R.; Oh, Y.S.; Kim, S.; Lee, S.M.; Lee, S.H. Relative sputtering rates of oxides and fluorides of aluminum and yttrium. Surf. Coat. Technol. 2017, 309, 694–697. [Google Scholar] [CrossRef]
- Lee, J.-K.; Park, S.J.; Oh, Y.S.; Kim, S.; Kim, H.; Lee, S.M. Fragmentation behavior of Y2O3 suspension in axially fed suspension plasma spray. Surf. Coat. Technol. 2017, 309, 456–461. [Google Scholar] [CrossRef]
- Choi, J.H.; Na, H.; Park, J.; Kim, H.J. Plasma corrosion resistance of aluminosilicate glasses containing Ca, Y and B under fluorocarbon plasma with Ar+. J. Non. Cryst. Solids 2019, 521, 119498. [Google Scholar] [CrossRef]
- Kasashima, Y.; Motomura, T.; Nabeoka, N.; Uesugi, F. Numerous flaked particles instantaneously generated by micro-arc discharge in mass-production plasma etching equipment. Jpn. J. Appl. Phys. 2015, 54, 2–8. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, M.J.; Cho, H.; Park, T.E.; Yun, Y.H. Fabrication and plasma resistance of Y2O3 ceramics. Ceram. Int. 2015, 41, 12757–12762. [Google Scholar] [CrossRef]
- Lin, T.K.; Wang, W.K.; Huang, S.Y.; Tasi, C.T.; Wuu, D.S. Comparison of erosion behavior and particle contamination in mass-production CF4/O2 plasma chambers using Y2O3 and YF3 protective coatings. Nanomaterials 2017, 7, 183. [Google Scholar] [CrossRef]
- Kasashima, Y.; Tabaru, T.; Matsuda, O.; Motomura, T. Investigation of the relationship between plasma etching characteristics and microstructures of alumina ceramics for chamber parts. Jpn. J. Appl. Phys. 2019, 58, 041001. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Lee, S.; Kim, H. Effect of rare-earth elements on the plasma etching behavior of the RE-Si-Al-O glasses. J. Non. Cryst. Solids 2012, 358, 898–902. [Google Scholar] [CrossRef]
- Morin, E.I.; Wu, J.; Stebbins, J.F. Modifier cation (Ba, Ca, La, Y) field strength effects on aluminum and boron coordination in aluminoborosilicate glasses: The roles of fictive temperature and boron content. Appl. Phys. A Mater. Sci. Process. 2014, 116, 479–490. [Google Scholar] [CrossRef]
- Choi, J.H.; Han, Y.S.; Lee, S.M.; Park, H.B.; Choi, S.C.; Kim, H.J. Characteristics of carbon tetrafluoride plasma resistance of various glasses. J. Korean Ceram. Soc. 2016, 53, 700–706. [Google Scholar] [CrossRef]
- Choi, J.H.; Bin Park, H.; Na, H.; Kim, H.J. Plasma corrosion resistance of RO-Al2O3-SiO2 (R: Alkaline earth) under fluorocarbon plasma with Ar+: Ⅱ. Plasma resistant glass. Corros. Sci. 2019, 146, 247–253. [Google Scholar] [CrossRef]
- Na, H.; Park, J.; Choi, S.C.; Kim, H.J. The effect of composition of plasma resistance of CaO–Al2O3–SiO2 glasses under Fluorocarbon Plasma with Ar+. Appl. Surf. Sci. 2019, 476, 663–667. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.H.; Na, H.; Kim, H.J. Effect of CaF2 on fluorocarbon plasma resistance and thermal properties of CaO-Al2O3-SiO2 glasses. J. Asian Ceram. Soc. 2021, 9, 311–317. [Google Scholar] [CrossRef]
- Park, J.; Na, H.; Park, J.H.; Kim, D.G.; Choi, S.C.; Kim, H.J. Divalent ion mixed effect of calcium aluminosilicate glasses (CAS) on thermal properties and fluorocarbon plasma resistance. J. Non. Cryst. Solids 2020, 533, 119897. [Google Scholar] [CrossRef]
- Goswami, M.; Deshpande, S.K.; Kumar, R.; Kothiyal, G.P. Electrical behaviour of Li2O-ZnO-SiO2 glass and glass-ceramics system. J. Phys. Chem. Solids 2010, 71, 739–744. [Google Scholar] [CrossRef]
- Mahani, R.M.; Marzouk, S.Y. AC conductivity and dielectric properties of SiO2-Na2O-B2O3-Gd2O3 glasses. J. Alloys Compd. 2013, 579, 394–400. [Google Scholar] [CrossRef]
- Huang, S.; Shim, S.; Nam, S.K.; Kushner, M.J. Pattern Dependent Profile Distortion during Plasma Etching of High Aspect Ratio Features in SiO2. J. Vac. Sci. Technol. A 2020, 38, 023001. [Google Scholar] [CrossRef]
- Sreenivasan, H.; Kinnunen, P.; Adesanya, E.; Patanen, M.; Kantola, A.M.; Telkki, V.V.; Huttula, M.; Cao, W.; Provis, J.L.; Illikainen, M. Field Strength of Network-Modifying Cation Dictates the Structure of (Na-Mg) Aluminosilicate Glasses. Front. Mater. 2020, 7, 267. [Google Scholar] [CrossRef]
- Qin, Q.; Stone-Weiss, N.; Zhao, T.; Mukherjee, P.; Ren, J.; Mauro, J.C.; Goel, A. Insights into the mechanism and kinetics of dissolution of aluminoborosilicate glasses in acidic media: Impact of high ionic field strength cations. Acta Mater. 2023, 242, 118468. [Google Scholar] [CrossRef]
- Hao, X.; Luo, Z.; Hu, X.; Song, J.; Tang, Y.; Lu, A. Effect of replacement of B2O3 by ZnO on preparation and properties of transparent cordierite-based glass-ceramics. J. Non. Cryst. Solids 2016, 432, 265–270. [Google Scholar] [CrossRef]
- Gui, H.; Li, C.; Lin, C.; Zhang, Q.; Luo, Z.; Han, L.; Liu, J.; Liu, T.; Lu, A. Glass forming, crystallization, and physical properties of MgO-Al2O3-SiO2-B2O3 glass-ceramics modified by ZnO replacing MgO. J. Eur. Ceram. Soc. 2019, 39, 1397–1410. [Google Scholar] [CrossRef]
- He, Y.; Shen, X.; Jiang, Y.; Lu, A. Effects of Li2O replacing Na2O on glass forming, structure and properties of Na2O–MgO–Al2O3–SiO2 glass and glass-ceramics. Mater. Chem. Phys. 2021, 258, 123865. [Google Scholar] [CrossRef]
- Abdel Wahab, F.A.; Abdel-Baki, M. Electrical conduction and dielectric properties of lithium aluminum silicate glasses doped with Cr3+ ions. J. Non. Cryst. Solids 2009, 355, 2239–2249. [Google Scholar] [CrossRef]
- Lanagan, M.T.; Cai, L.; Lamberson, L.A.; Wu, J.; Streltsova, E.; Smith, N.J. Dielectric polarizability of alkali and alkaline-earth modified silicate glasses at microwave frequency. Appl. Phys. Lett. 2020, 116, 222902. [Google Scholar] [CrossRef]
- Shao, X.; Hu, Y.; Zhao, Y.; Chen, S.; Liu, L.; Chen, J.; Li, J. Crystallization and dielectric properties of oxyfluoride aluminosilicate glasses added with Na2O. J. Non. Cryst. Solids 2022, 576, 121261. [Google Scholar] [CrossRef]
- Amma, S.I.; Lanagan, M.T.; Kim, S.H.; Pantano, C.G. Ionic Conductivity in Sodium-Alkaline Earth-Aluminosilicate Glasses. J. Am. Ceram. Soc. 2016, 99, 1239–1247. [Google Scholar] [CrossRef]
- Wu, J.; Stebbins, J.F. Effects of cation field strength on the structure of aluminoborosilicate glasses: High-resolution 11B, 27Al and 23Na MAS NMR. J. Non. Cryst. Solids 2009, 355, 556–562. [Google Scholar] [CrossRef]
- Bouhadja, M.; Jakse, N.; Pasturel, A. Striking role of non-bridging oxygen on glass transition temperature of calcium aluminosilicate glass-formers. J. Chem. Phys. 2014, 140, 234507. [Google Scholar] [CrossRef]
- Grund Bäck, L.; Ali, S.; Karlsson, S.; Möncke, D.; Kamitsos, E.I.; Jonson, B. Mixed alkali/alkaline earth-silicate glasses: Physical properties and structure by vibrational spectroscopy. Int. J. Appl. Glas. Sci. 2019, 10, 349–362. [Google Scholar] [CrossRef]
- Liao, M.; Sun, H.; Wen, L.; Fang, Y.; Hu, L. Effect of alkali and alkaline earth fluoride introduction on thermal stability and structure of fluorophosphate glasses. Mater. Chem. Phys. 2006, 98, 154–158. [Google Scholar] [CrossRef]
- Aly, K.A.; Dahshan, A.; Abdel-Rahim, F.M. Thermal stability of Ge-As-Te-In glasses. J. Alloys Compd. 2009, 470, 574–579. [Google Scholar] [CrossRef]
- Mukherjee, D.P.; Das, S.K. Synthesis and characterization of machinable glass-ceramics added with B2O3. Ceram. Int. 2014, 40, 12459–12470. [Google Scholar] [CrossRef]
- Shan, Z.; Li, C.; Tao, H. Mixed alkaline-earth effect on the mechanical and rheological properties of Ca–Mg silicate glasses. J. Am. Ceram. Soc. 2017, 100, 4570–4580. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, J.; Kang, J.; Yuan, J.; Cui, J. Structure, glass stability and rheological properties of Na2O–CaO–Al2O3–SiO2 Glasses doped with Y2O3. Ceram. Silikaty 2018, 62, 173–180. [Google Scholar] [CrossRef]
- Lunkenheimer, P.; Loidl, A.; Riechers, B.; Zaccone, A.; Samwer, K. Thermal expansion and the glass transition. Nat. Phys. 2023, 19, 694–699. [Google Scholar] [CrossRef]
- Aliyah, L.H.; Katrina, A.T.; Hasmaliza, M. Preliminary study on the development of new composition lithium aluminosilicate glass ceramic. Mater. Today Proc. 2019, 17, 946–952. [Google Scholar] [CrossRef]
- Schmitt, J.; Meier, A.; Wallrabe, U.; Völklein, F. Reactive ion etching (CF4/Ar) and ion beam etching of various glasses for diffractive optical element fabrication. Int. J. Appl. Glas. Sci. 2018, 9, 499–509. [Google Scholar] [CrossRef]
- Honma, T.; Maeda, K.; Nakane, S.; Shinozaki, K. Unique properties and potential of glass-ceramics. J. Ceram. Soc. Jpn. 2022, 130, 545–551. [Google Scholar] [CrossRef]
- Major, G.H.; Fairley, N.; Sherwood, P.M.A.; Linford, M.R.; Terry, J.; Fernandez, V.; Artyushkova, K. Practical Guide for Curve Fitting in X-Ray Photoelectron Spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 061203. [Google Scholar] [CrossRef]



| Notation | Chemical Composition (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Li2O | MgO | CaO | SrO | BaO | As2O3 | SnO2 | |
| LAS | 64 | 20 | 15 | - | - | - | - | 0.5 | 0.5 |
| MLAS | 64 | 20 | 7.5 | 7.5 | - | - | - | 0.5 | 0.5 |
| CLAS | 64 | 20 | 7.5 | - | 7.5 | - | - | 0.5 | 0.5 |
| SLAS | 64 | 20 | 7.5 | - | - | 7.5 | - | 0.5 | 0.5 |
| BLAS | 64 | 20 | 7.5 | - | - | - | 7.5 | 0.5 | 0.5 |
| Parameter | Condition |
|---|---|
| Frequency (MHz) | 13.56 |
| Power (W) | 300 |
| Pressure (mTorr) | 10 |
| Etch time (min) | 60 |
| CF4 (sccm) | 30 |
| O2 (sccm) | 10 |
| Ar (sccm) | 5 |
| Chiller Temp. (℃) | 20 |
| Si | Al | Li | Mg | Ca | Sr | Ba | |
|---|---|---|---|---|---|---|---|
| Ionic Radius (nm) | 0.039 | 0.057 | 0.078 | 0.078 | 0.106 | 0.127 | 0.217 |
| Atomic Radius (nm) | 0.117 | 0.143 | 0.152 | 0.160 | 0.197 | 0.215 | 0.217 |
| Atomic Weight (amu) | 28 | 27 | 7 | 24 | 40 | 88 | 137 |
| Electronegativity | 1.90 | 1.61 | 0.98 | 1.31 | 1 | 0.95 | 0.89 |
| Ionic Field Strength (nm−2) | 2500 | 1068 | 173.1 | 385.8 | 200 | 124 | 97.8 |
| R–F/R–O (R: Alkaline Earth) | Si–F/Si–O | Al–F/Al–O | |
|---|---|---|---|
| MLAS | 0.16 | 0.06 | 0.09 |
| CLAS | 0.28 | 0.05 | 0.07 |
| SLAS | 0.45 | 0.06 | 0.09 |
| BLAS | 0.20 | 0.08 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-W.; Lee, H.-S.; Jun, D.-S.; Lee, S.-E.; Lee, J.-H.; Lee, H.-C. Enhancing the Plasma-Resistance Properties of Li2O–Al2O3–SiO2 Glasses for the Semiconductor Etch Process via Alkaline Earth Oxide Incorporation. Materials 2023, 16, 5112. https://doi.org/10.3390/ma16145112
Kim S-W, Lee H-S, Jun D-S, Lee S-E, Lee J-H, Lee H-C. Enhancing the Plasma-Resistance Properties of Li2O–Al2O3–SiO2 Glasses for the Semiconductor Etch Process via Alkaline Earth Oxide Incorporation. Materials. 2023; 16(14):5112. https://doi.org/10.3390/ma16145112
Chicago/Turabian StyleKim, So-Won, Hwan-Seok Lee, Deok-Sung Jun, Seong-Eui Lee, Joung-Ho Lee, and Hee-Chul Lee. 2023. "Enhancing the Plasma-Resistance Properties of Li2O–Al2O3–SiO2 Glasses for the Semiconductor Etch Process via Alkaline Earth Oxide Incorporation" Materials 16, no. 14: 5112. https://doi.org/10.3390/ma16145112
APA StyleKim, S.-W., Lee, H.-S., Jun, D.-S., Lee, S.-E., Lee, J.-H., & Lee, H.-C. (2023). Enhancing the Plasma-Resistance Properties of Li2O–Al2O3–SiO2 Glasses for the Semiconductor Etch Process via Alkaline Earth Oxide Incorporation. Materials, 16(14), 5112. https://doi.org/10.3390/ma16145112







