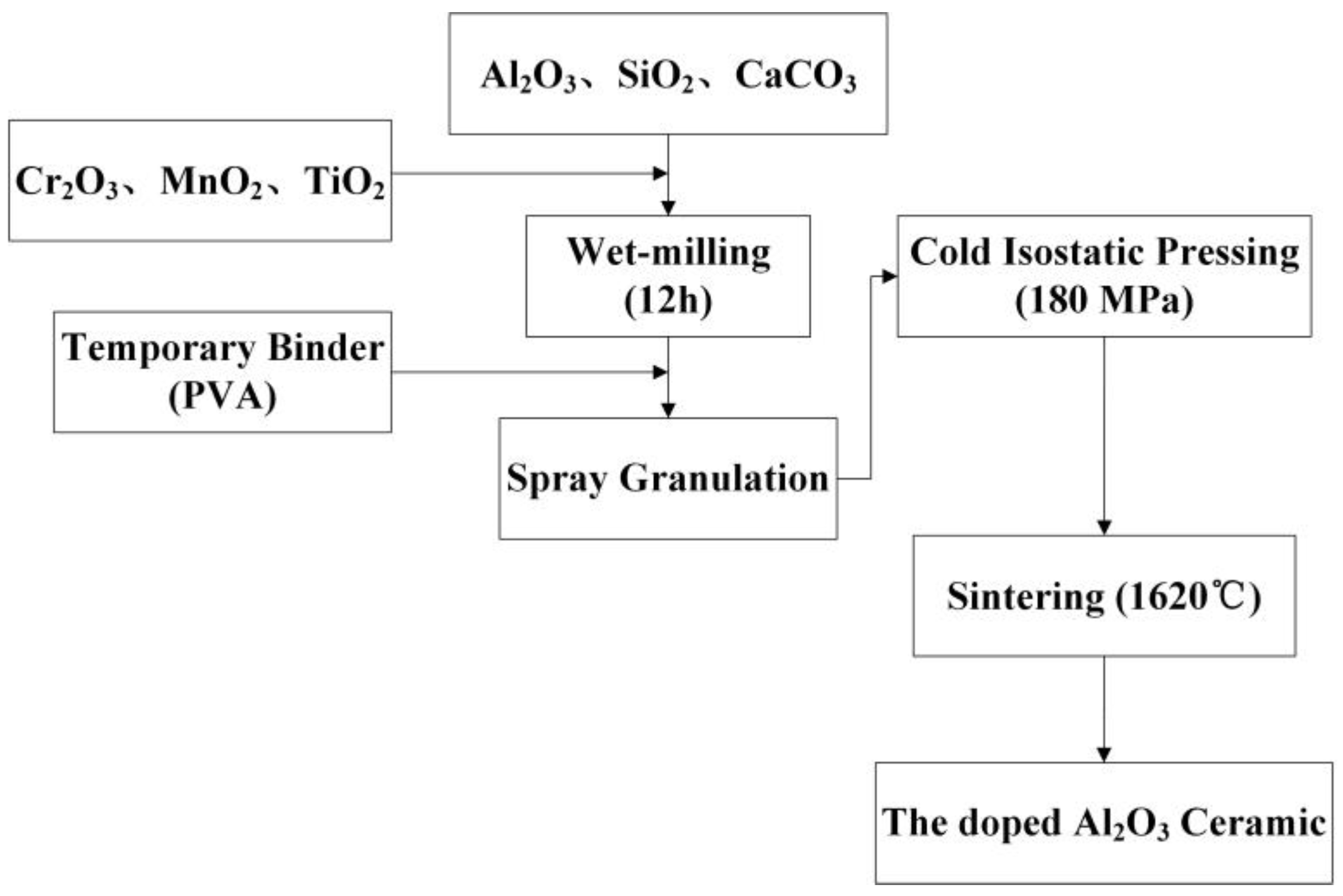
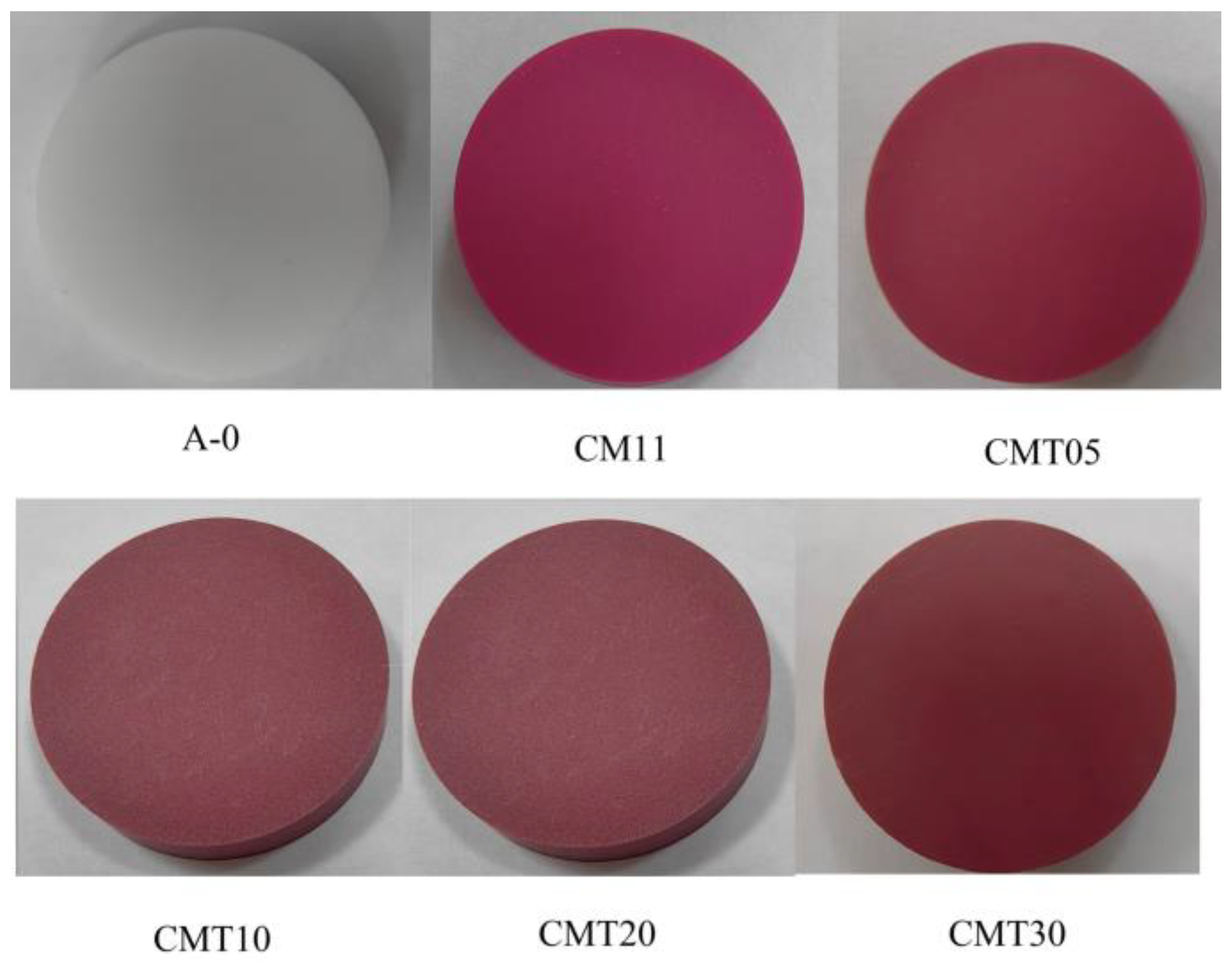
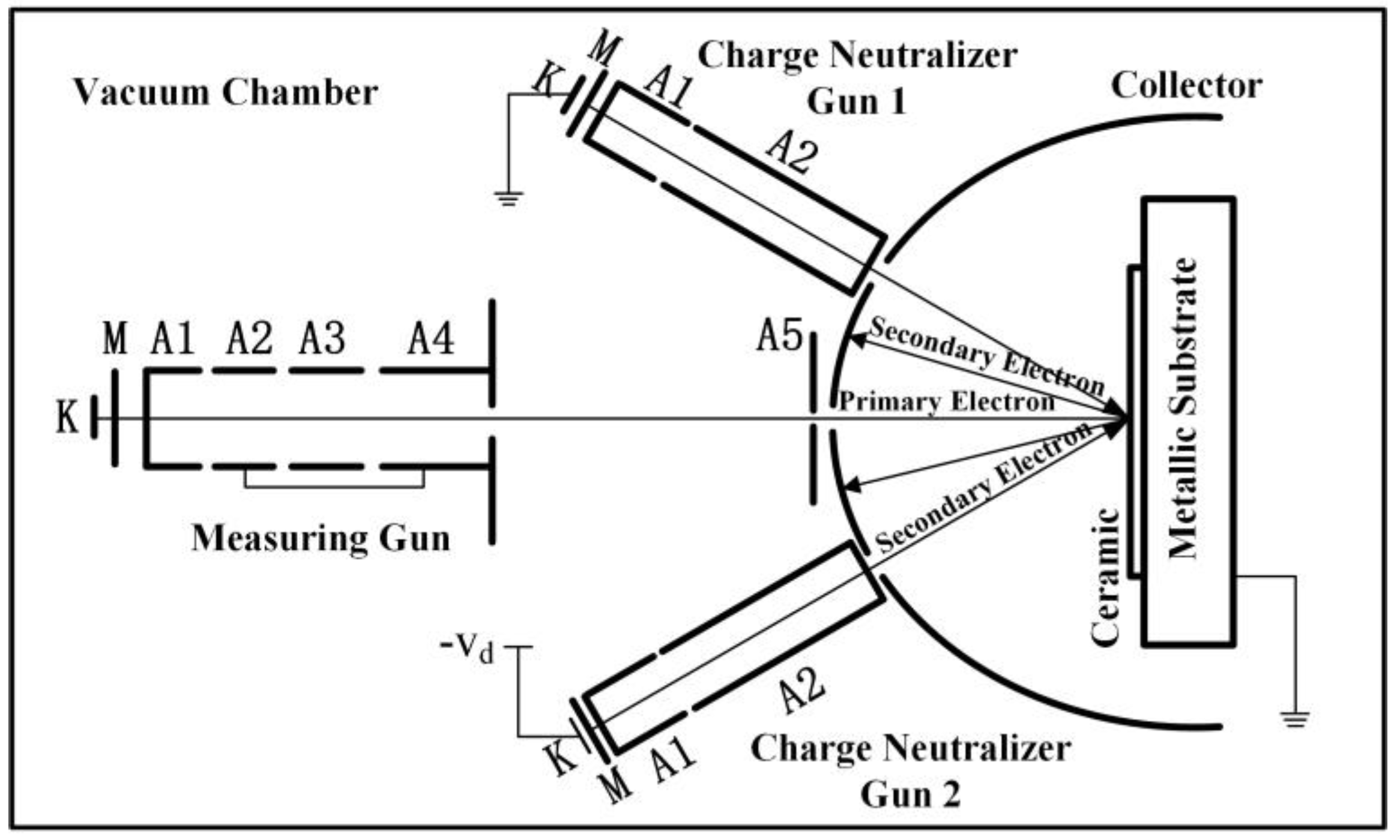
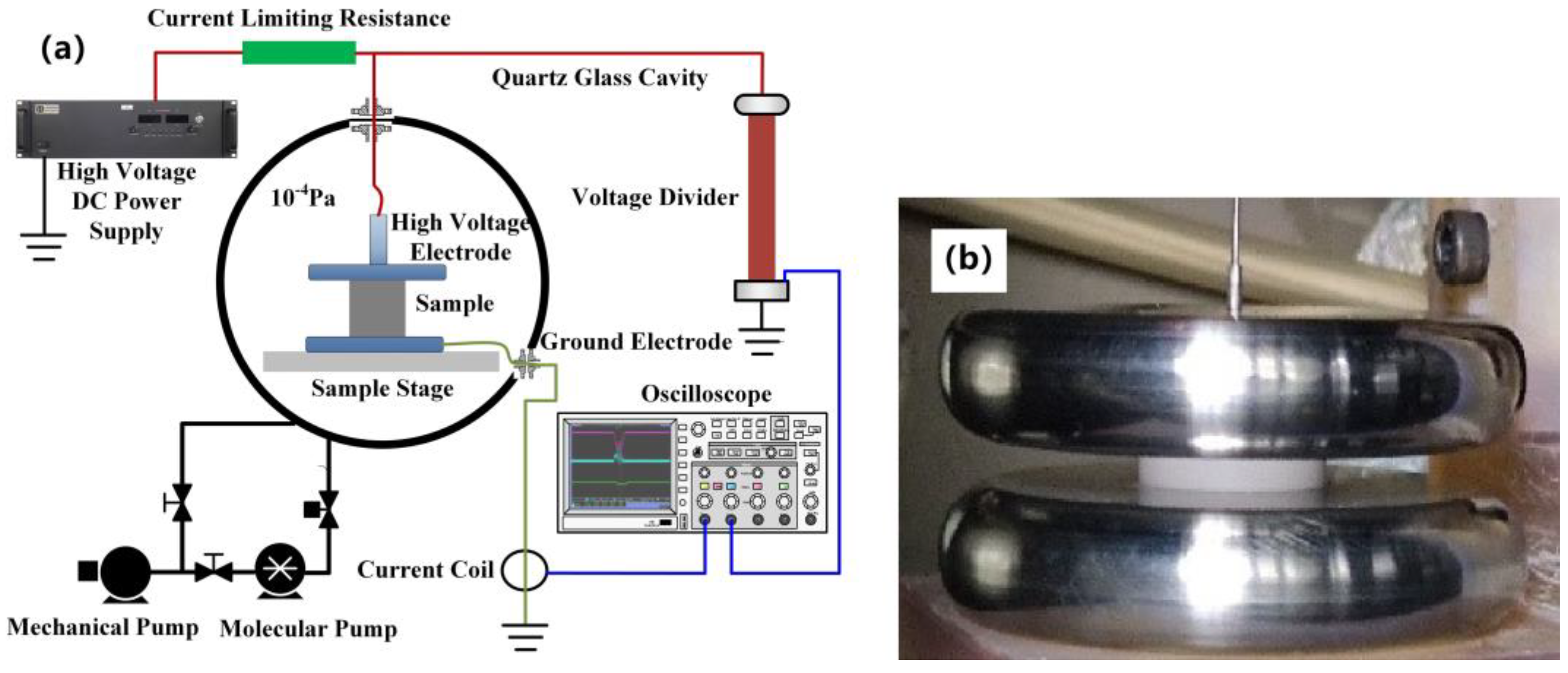
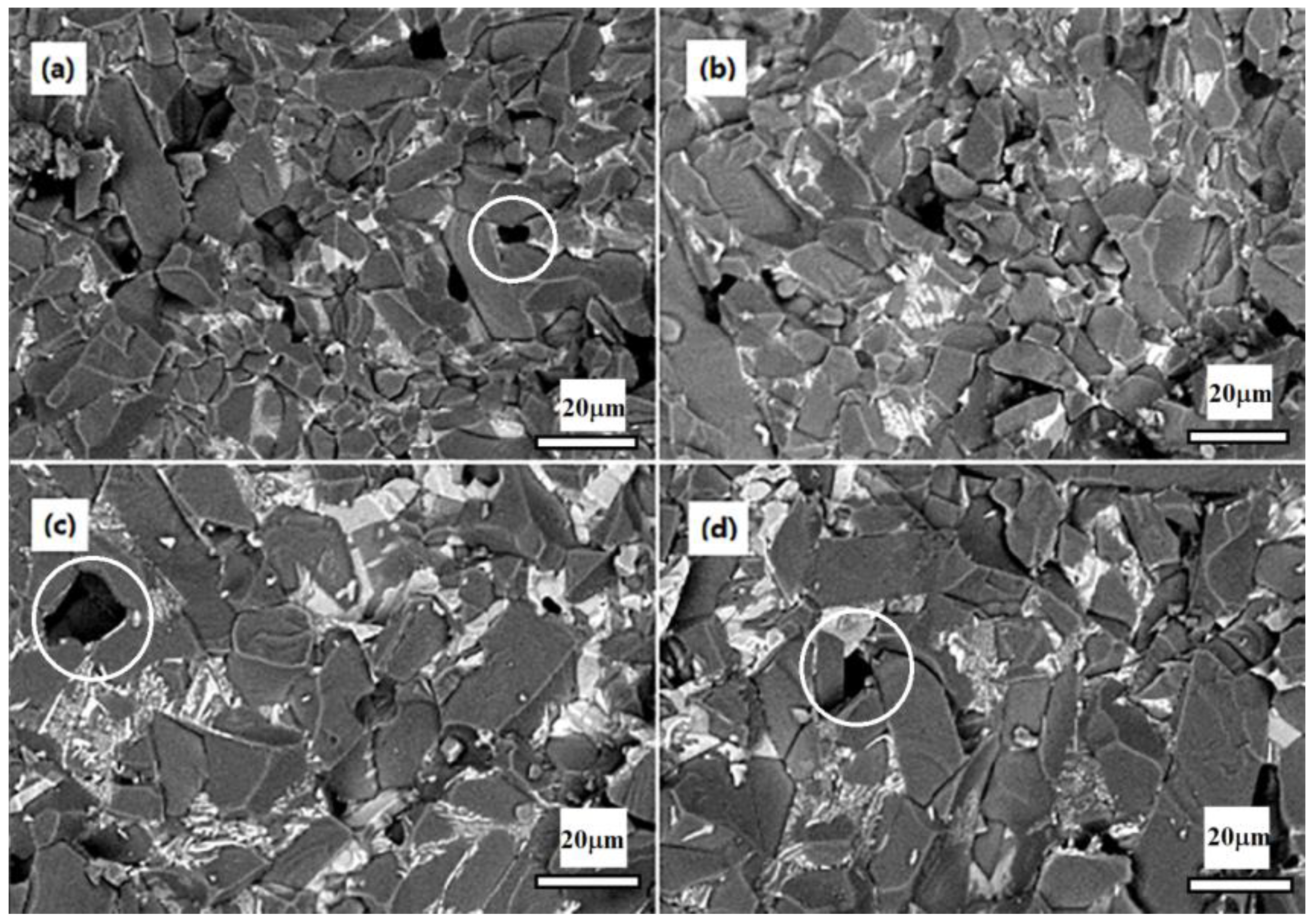
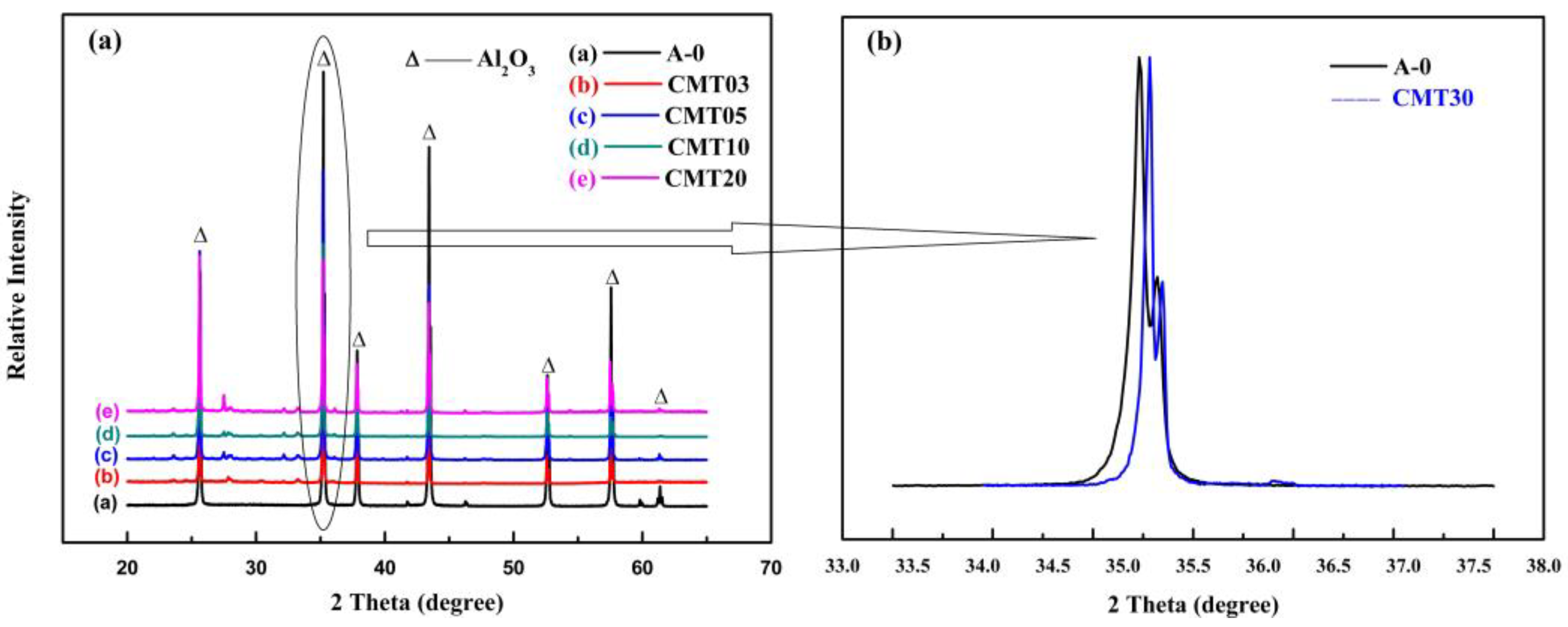
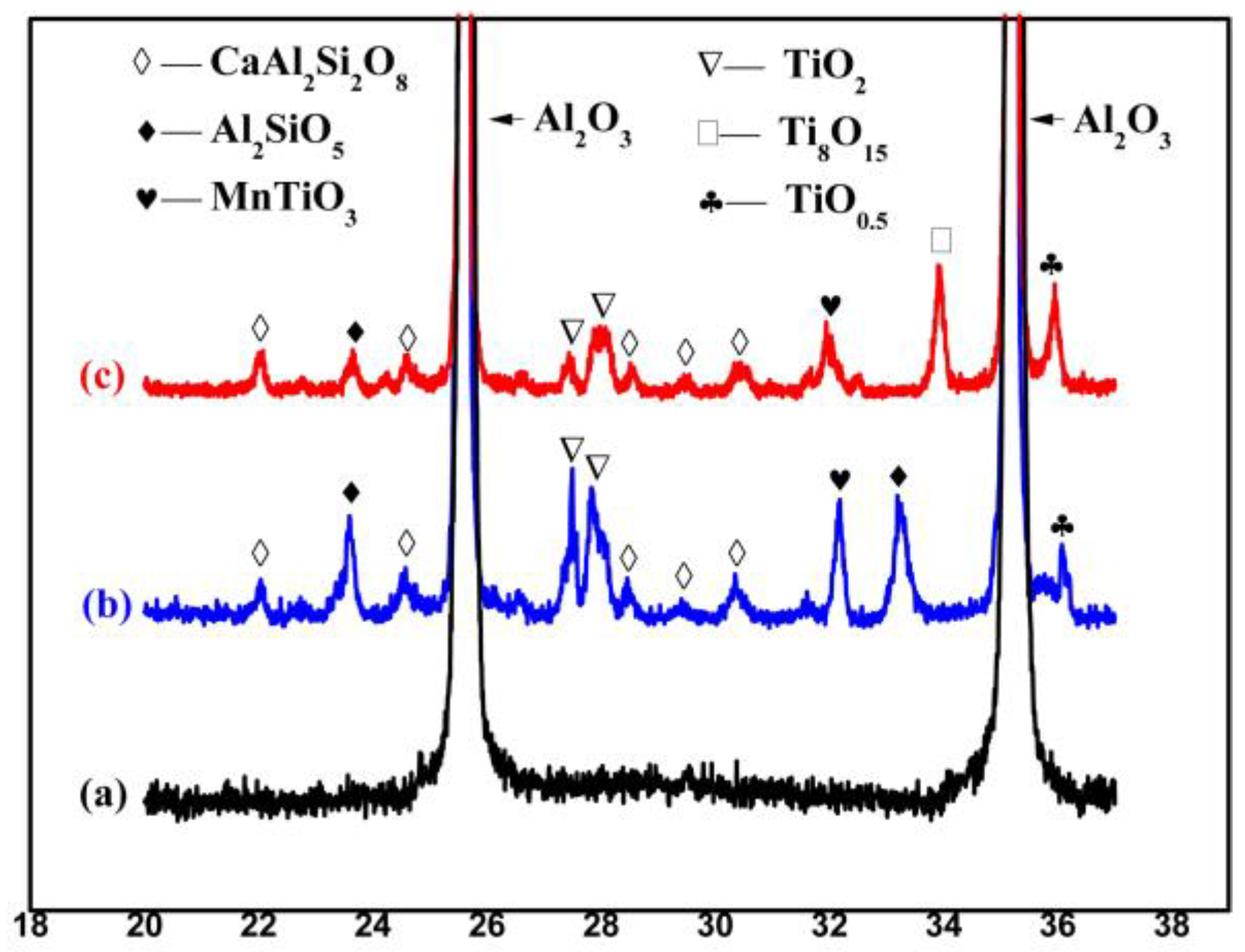
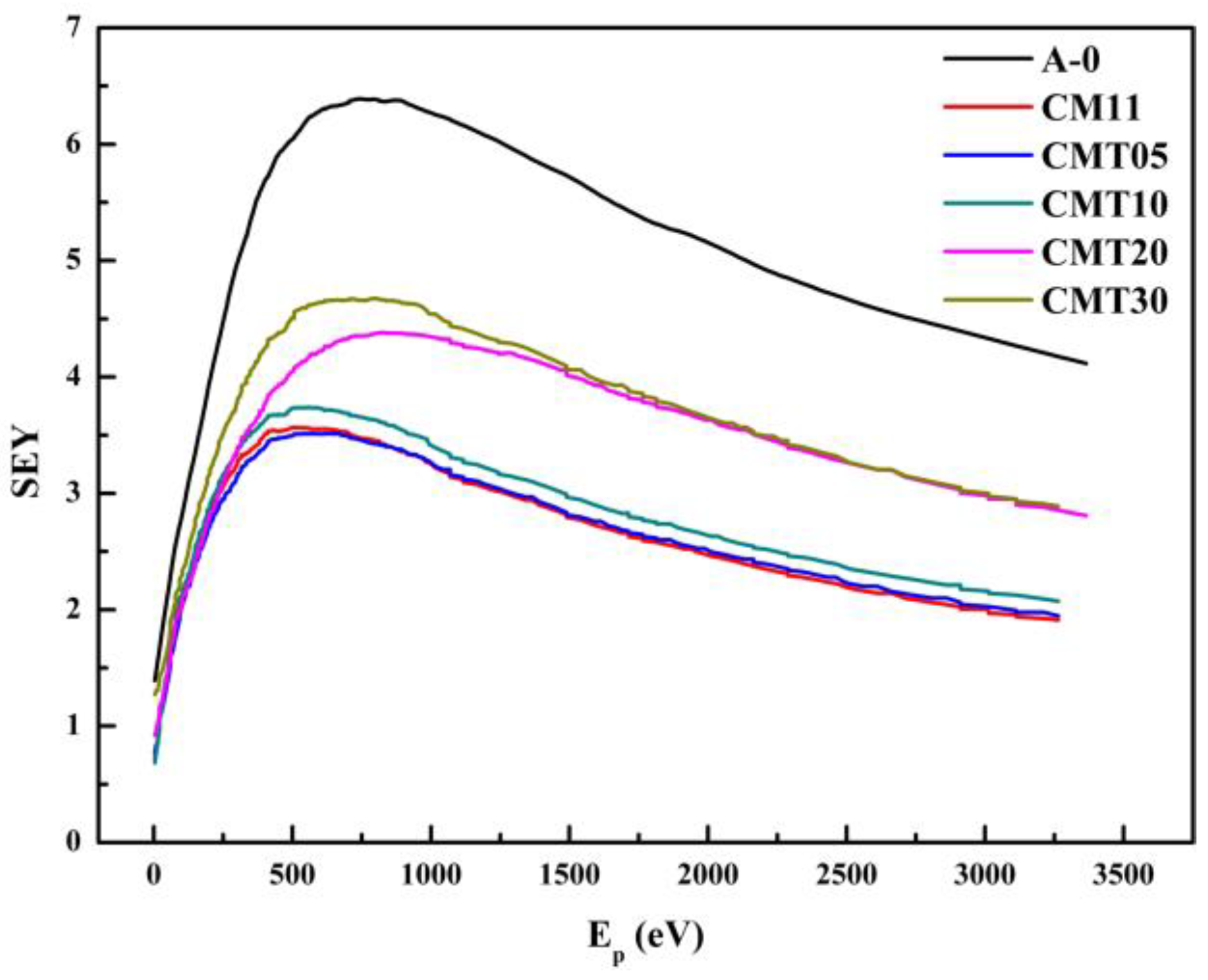
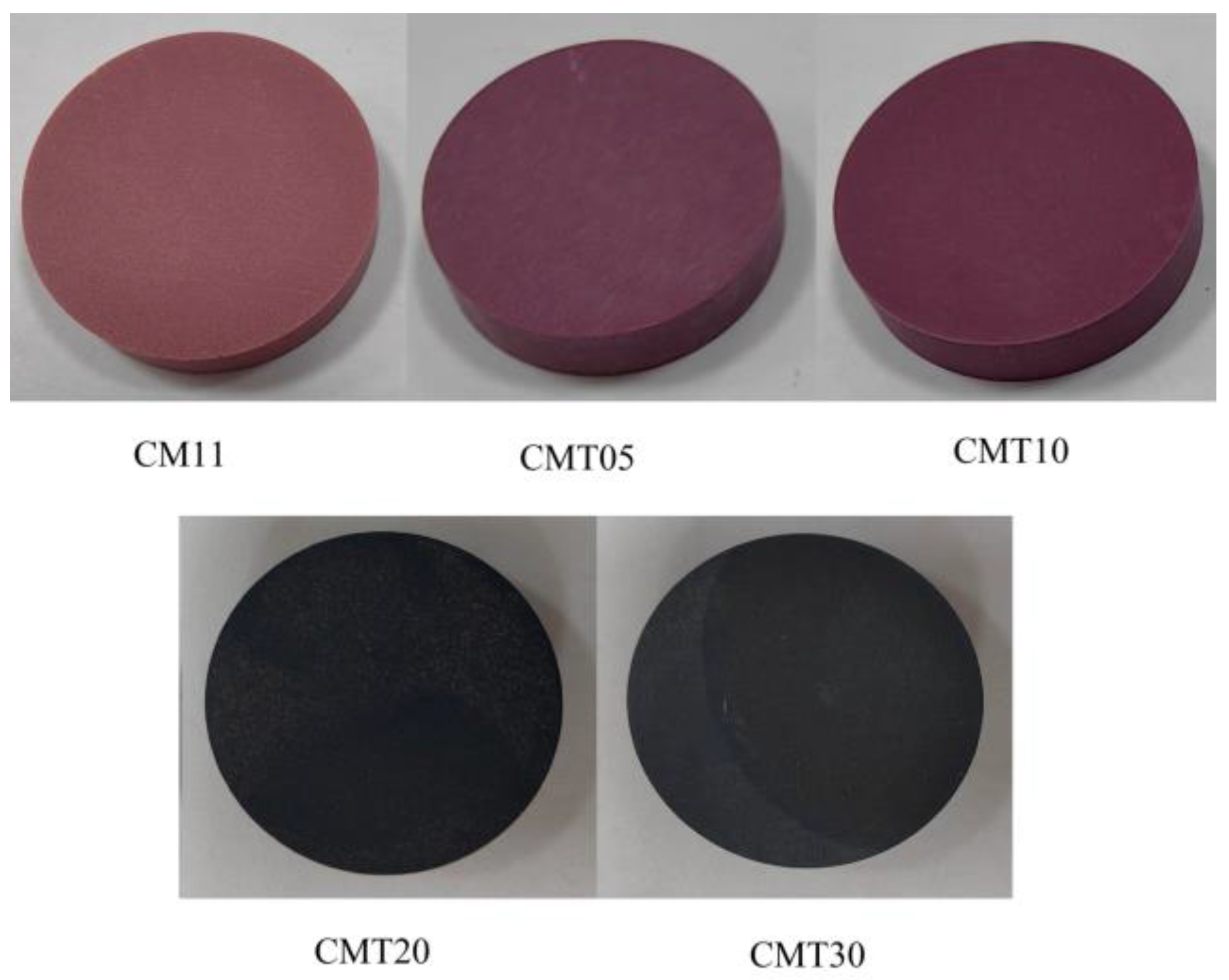
1. Introduction
Al
2O
3 ceramics play a significant role in vacuum electronic devices, such as high-voltage insulation, vacuum sealing, power transmission, and support fixation [
1,
2]. Studies have shown that as a solid insulator, the voltage holdoff capacity of Al
2O
3 ceramics in a vacuum is often lower than vacuum gaps of the same size. The surface of the insulator is the weakest point in vacuum-insulation systems because its bulk voltage holdoff capacity is generally greater than the vacuum gap of the same size [
3,
4]. Surface flashover occurs in many vacuum electronic devices when the applied voltage exceeds a certain value, resulting in the failure of or damage to the device [
5,
6,
7,
8,
9,
10]. With the development of vacuum electronic devices toward high power, high frequency, and miniaturization, such as high-power klystron and high-power pseudo spark switch, the operating voltage of the device increases exponentially, making surface flashover of Al
2O
3 ceramics become an important factor affecting their reliability and restricting the development of vacuum electronic devices [
11,
12,
13,
14,
15]. Therefore, improving the surface voltage holdoff capacity of Al
2O
3 ceramics has become one of the urgent problems to be solved in the field of vacuum electronics.
Surface flashover is related to many factors, such as the materials’ characteristics, shape structure, and surface roughness of the ceramic. According to secondary electron emission avalanche theory (SEEA) [
16,
17,
18], the initiation of a surface flashover is usually started by the emission of electrons (generally by field emission or thermal field emission) from the cathode triple junction (CTJ—the interface where the insulator, cathode, and vacuum are in close proximity). Some of the electrons impact the surface of the insulator, producing additional electrons by secondary emission. Some of these secondary electrons will again strike the surface, producing tertiary electrons. Continuation of this process results in the development of an SEEA. The final stages of surface flashover are predominantly thought to occur in desorbed surface gas or in vaporized insulator material. Hence, the secondary electron yield (SEY) of the ceramic is an important factor affecting its surface flashover voltage. Therefore, the SEY of the ceramic should be reduced as much as possible to improve its surface flashover voltage. In addition, the high surface resistivity of the ceramic is averse to charge leakage, which will increase the strength of the local electric field, leading to the occurrence of surface flashover.
Researchers have improved the surface voltage holdoff capacity of Al
2O
3 ceramics through surface modification and the bulk doping method. Tangal S. Sudarshan et al. [
19] reduced the SEY of Al
2O
3 ceramics and improved their surface flashover voltage in a vacuum by coating Cr
2O
3 on the surface. Yamamoto O. et al. [
20] studied the influence of surface roughness on the surface voltage holdoff capacity of dielectric materials and improved the surface flashover voltage of Al
2O
3 ceramics by roughening the surface. Zheng Jiagui et al. [
21] reduced the SEY of Al
2O
3 ceramics to 2~3 by coating Cr, Mn, and Ti on the surface. Zhang Hao et al. [
22] improved the surface flashover voltage of a glass ceramic by adding Cr
2O
3 to it. However, the mechanism of surface flashover of insulating materials in a vacuum is complex, and the research content involves multidisciplines, which are closely related to the composition and microstructure of materials. Therefore, further systematic and in-depth research on ceramic materials with a high-voltage holdoff capacity is needed to provide key basic technology support for the development of vacuum electronic devices toward high power, high frequency, and miniaturization.
Among the methods to improve the voltage holdoff capacity of insulating materials in a vacuum, surface modification can improve the surface properties without changing the intrinsic structure of the ceramic, thus increasing its surface flashover voltage. However, this method has large structural limitations, and the evaporation of coatings within the device is difficult to control. Bulk doping is easier to achieve for some special structures, and the material performance is relatively stable. The SEY of Cr
2O
3 [
16] is very low, and MnO
2 has low resistivity. They are usually used to improve the voltage holdoff capacity of Al
2O
3 ceramics as additives. The properties of Cr
2O
3 and MnO
2-doped Al
2O
3 ceramics were studied previously by our research group. The results show that the SEY of Al
2O
3 ceramics can be reduced effectively by adding Cr
2O
3 to the ceramics, while the surface resistivity of Al
2O
3 ceramics can be reduced by one order of magnitude with the addition of MnO
2. However, with the increase in the addition of MnO
2, the grain size of the Al
2O
3 ceramic increases, the bulk density of the Al
2O
3 ceramic decreases, and the uniformity of the microstructure becomes worse. In this paper, in order to further reduce the resistivity of the Al
2O
3 ceramic, on the basis of the previous research on Cr
2O
3 and MnO
2-doped Al
2O
3 ceramics, we chose TiO
2, with semiconductor properties, as an additive, and studied the effect of the TiO
2 addition on the performance of the Al
2O
3 ceramics. TiO
2 has low resistivity, but few studies have been reported on the effects of TiO
2 on the resistivity and voltage holdoff capacity of Al
2O
3 ceramics, since the valence of Ti changes in different compounds and its mechanism of action in materials is complicated [
23,
24]. On the basis of Cr/Mn-doped Al
2O
3 ceramics, we fixed Cr
2O
3:MnO
2 = 1:1 (mass ratio) in this paper and studied the effects of the additional amount of TiO
2 on the microstructure, phase composition, secondary electron emission characteristics, resistivity, and surface flash properties of the Al
2O
3 ceramic in a vacuum.