Abstract
Nickel antimonate (NiSb2O6) powders were synthesized using a wet chemistry process assisted by microwave radiation and calcination from 600 to 700 °C to evaluate their photocatalytic and gas-sensing properties. The crystalline phase obtained at 800 °C of trirutile-type nickel antimonate was confirmed with powder X-ray diffraction. The morphology and size of the nanostructures were analyzed employing electron microscopy (SEM and TEM), identifying irregular particles and microrods (~277 nm, made up of polyhedral shapes of size ~65 nm), nanorods with an average length of ~77 nm, and nanostructures of polyhedral type of different sizes. UV-vis analysis determined that the bandgap of the powders obtained at 800 °C was ~3.2 eV. The gas sensing tests obtained a maximum response of ~5 for CO (300 ppm) at 300 °C and ~10 for C3H8 (500 ppm) at 300 °C. According to these results, we consider that NiSb2O6 can be applied as a gas sensor. On the other hand, the photocatalytic properties of the antimonate were examined by monitoring the discoloration of malachite green (MG) at five ppm. MG concentration monitoring was carried out using UV-visible spectroscopy, and 85% discoloration was achieved after 200 min of photocatalytic reaction.
1. Introduction
The increase in toxic gas emissions, such as CO, NO2, SO2, and O3, seriously threatens public health because 90% of the world’s population is exposed to polluted air, resulting in an estimated 7 million deaths yearly due to air pollution []. On the other hand, these atmospheric pollutants cause what is known as acid rain. These pollutants are washed away by rain and seriously contaminate soils and water sources [].
Due to the harmful effects of polluted air, different gas detection materials have been applied to monitor and identify the toxic gases in the atmosphere. Among them, binary metal oxide semiconductors (MOx), such as TiO2, SnO, and ZnO, have been widely used. However, those binary sensors have several limitations, such as low stability under humidity, low selectivity, and high operating temperatures [,]. In the search for new materials with better gas detection characteristics, ternary semiconductor oxides, such as delafossites [], perovskites [,], ilmenites [], spinels [], and trirutiles [], have recently been studied. Among the latter, the antimonates MSb2O6 (where M = Mg, Co, Ca, Zn, Ni, or Mn) have gained much attention due to their promising characteristics as gas sensors [,,]. Particularly, NiSb2O6 has been shown to have interesting properties as a gas sensor. Singh et al. [] synthesized NiSb2O6 thin films to detect LPG and CO2 gases at very low operating temperatures, and Rodríguez-Betancourtt et al. [] obtained NiSb2O6 pellets highly sensitive to C3H8 and CO atmospheres. However, there is still a lack of studies on the application of this material as a gas sensor.
On the other hand, pollutants present in the atmosphere can end up in water sources, aggravating contamination problems. To mitigate the harmful effects of pollutants, nanotechnology has gained much interest in environmental remediation applications [,]. In this regard, trirutile-type antimonates have shown good photocatalytic characteristics. For example, Arunkumar and Naraginti [] studied for the first time the photocatalytic activity (under visible light conditions) of CoSb2O6, CuSb2O6, NiSb2O6, and FeSb2O6 for p-nitrophenol degradation. Liu et al. [] studied the photocatalytic effect of ZnSb2O6 nanoparticles using the discoloration of rhodamine B and methyl orange (MO); Zhang et al. [] synthesized nanoparticles of CaSb2O6 that showed high photocatalytic performance for the degradation of MO; Sunku et al. [] studied the degradation of MO by using MnSb2O6 under visible light conditions; and Papi et al. [] synthesized NiSb2O6 nanoparticles with high photocatalytic performance in the degradation of an aqueous solution of malachite green (MG). However, there have been few studies on trirutile-type antimonates applied in photocatalysis. Therefore, in this work, we evaluated the properties of NiSb2O6 as a gas sensor and photocatalyst, as this material has the potential to be applied for both air quality monitoring and environmental remediation.
The NiSb2O6 was synthesized using a microwave-assisted colloidal method. XRD microscopy was used to study its structural properties, and TEM and SEM microscopy were employed to analyze its morphological characteristics. The gas sensing response of NiSb2O6 nanoparticles was evaluated by measuring the variation in electrical resistance at different operating temperatures and concentrations of CO and C3H8. The photocatalytic performance of NiSb2O6 nanoparticles was evaluated by monitoring the discoloration of a 5 ppm aqueous solution of malachite green (MG).
2. Materials and Methods
2.1. Synthesis of NiSb2O6 Powders
The nickel antimonate synthesis was conducted using a wet chemistry method reported in [,]. A stoichiometric 2:1 molar ratio of nickel nitrate hexahydrate and antimony trichloride was used. A total of 1.45 g of Ni(NO3)2ꞏ6H2O, 2.80 g of SbCl3, and 2 mL of ethylenediamine (C2H8N2) were separately used; 5 mL of ethanol was added to the first two reactants, while 10 mL of ethanol was added to the C2H8N2. Each solution was kept under constant stirring at 300 rpm for 20 min at room temperature to homogenize the mixtures. After 20 min of stirring, the Ni(NO3)2ꞏ6H2O solution was added to the C2H8N2 solution. Finally, the SbCl3 solution was added to the mixture of Ni(NO3)2ꞏ6H2O and C2H8N2. The resulting solution was constantly stirred for 24 h at 25 °C. After completion of the stirring time, an evaporation process of the ethanol was carried out using microwave radiation, employing a General Electric ST0912C01352 domestic oven at a power of 70 W. The microwave radiation was applied in intervals of 60 s until a paste was formed, which was dried at 200 °C for 8 h and calcined at 600, 700, and 800 °C using a Novatech (Scottsdale, AZ, USA) muffle at a heating rate of 100 °C/h.
2.2. Physical Characterization
The crystallographic features of the NiSb2O6 calcined at different temperatures were studied using a Panalytical Empyrean diffractometer (Monterrey, Mexico) with Cuα radiation, a wavelength of 1.5406 Å, and a scan range of 10 to 90° at a rate of 0.02° per second. The band gap value of the material calcined at 800 °C was determined using UV-visible spectroscopy, applying a shift in the 200 to 600 nm range. A JEOL model JSM-6390LV scanning electron microscope (SEM, Mexico City, Mexico) in high vacuum mode was used for a detailed analysis of the compound’s microstructure, and a JEOL model JEM-2010 (Mexico City, Mexico) transmission electron microscope (TEM) with an acceleration voltage of 100 kV was employed to determine the average size of the material’s nanoparticles. For the TEM analysis, the powders were deposited in a container with 1 mL of ethyl alcohol to disperse them with ultrasound. Then, the dispersed material was poured dropwise onto a Formvar-coated copper microgrid.
2.3. Gas Sensing Test
To evaluate the nickel antimonate powders’ gas detection ability, pellets of 15 mm in diameter and 0.5 mm in thickness were made from the powders using a hydraulic press (Equip-25 Ton, Mexico City, Mexico) at a pressure of 10 Ton for 15 min; 0.4 g of NiSb2O6 calcined at 800 °C were used for this purpose. Subsequently, two ohmic contacts were attached to the pellets using colloidal silver paint (Alfa Aesar, Ward Hill, MA, USA 99%) to improve the contact between the pellets’ surface and the measurement system’s electrodes. The procedure to carry out the measurements is the following: firstly, the sample is placed in a 12 L chamber with ambient air, then a low vacuum is created in the chamber to approximate a 0 ppm concentration, then the concentration of the test gas to be analyzed is injected. The sample reacts after 50 s due to oxygen species adsorbed on the sample surface, the measurement is taken after 10 min in order to have a more stable measurement of the resistance change. It is important to mention that the equipment is designed for static measurements, that is, the response is affected exclusively by the test gas, for this reason there is no stabilizer gas flow, such as air.
The NiSb2O6 pellets were installed in the measurement chamber with a vacuum capacity of 10−3 Torr (Figure 1), and measurements were made in atmospheres of C3H8 and CO. For this, concentrations of 1, 5, 50, 100, 200, 300, 400, and 500 ppm of C3H8, and 1, 5, 50, 100, 200, and 300 ppm of CO were injected into the system. The operating temperatures were 100, 200, and 300 °C. The gas concentrations were controlled using a Leybold detector. The operating temperature was monitored using a highly sensitive type K thermocouple. The pellets’ varielectrical resistance was recorded using a Keithley 2001 digital multimeter (Beaverton, OR, USA). The pellets’ response was calculated using the equation reported in [,]:
where Gg and G0 are the pellets’ conductances (i.e., the reciprocal of the resistance) in the presence of gas (CO or C3H8) and air, respectively.
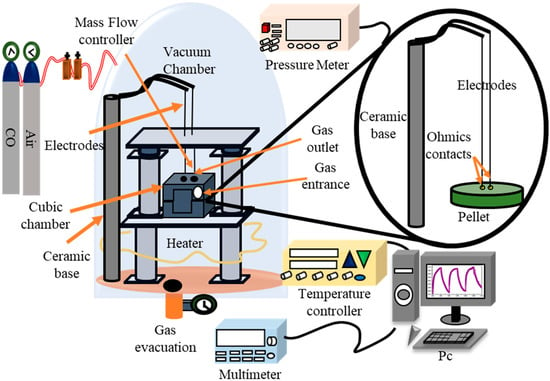
Figure 1.
Diagram of the detection system of controlled CO and C3H8 atmospheres at different operating temperatures.
2.4. Photocatalytic Activity Test
To analyze their photocatalytic activity, 0.25 g of NiSb2O6 powders were pressed into pellets (Ø 5 mm) and suspended in a quartz cell containing 3.5 mL of a 5 ppm malachite green aqueous solution. The suspension was kept in the dark for 30 min to reach the adsorption equilibrium. Then the suspension was put into an annular photoreactor equipped with a 15 W UV-254 nm lamp (Figure 2) and irradiated for 200 min. The discoloration was monitored at 30 min intervals using a JASCO V-670 UV-vis spectrophotometer.
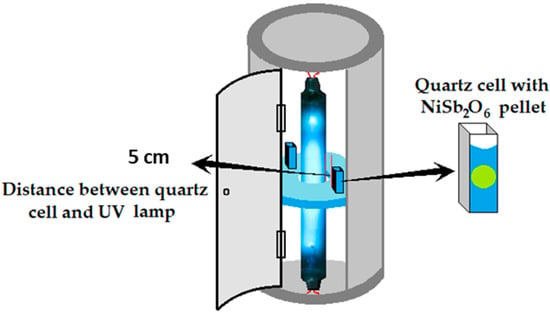
Figure 2.
Annular reactor for the photocatalytic tests of powders of NiSb2O6 calcined at 800 °C.
3. Results
3.1. XRD Analysis
Figure 3 shows diffractograms of the NiSb2O6 calcined at 600, 700, and 800 °C. According to PDF No. 38-1083, diffraction peaks corresponding to the NiSb2O6’s crystalline phase were identified at 2θ = 19.22°, 21.43°, 27.14°, 33.50°, 34.99°, 38.80°, 40.19°, 44.73°, 53.22°, 56.03°, 60.23°, 62.77°, 63.32°, 67.20°, 67.78°, 73.89°, 80.89°, and 86.83°. However, a small secondary phase associated with NiO was observed at 2θ = 36.82° (PDF No. 44-1159) and 62.76° (PDF No. 44-1159). Comparing the diffractograms with PDF No. 38-1083, the NiSb2O6 crystallized in a tetragonal trirutile-type crystal structure [] with cell parameters a = 4.641 Å and c = 9.223 Å, and space group P42/mnm [,]. Figure 3 shows pronounced broadening as well as the high intensity of NiSb2O6’s peaks. It has been reported in the literature that both features are indicative of a crystalline nanometric size []. To determine the crystallite size, Scherrer’s equation [] was used:
where λ is the radiation’s wavelength (Cu = 1.5406 Å), β is the peak width measured at half of the maximum intensity, and θ is Bragg’s angle. The most intense peak (110) corresponding to the last calcination (800 °C, see Figure 3) was considered for the calculation. A crystallite size of ~43.48 nm was obtained.
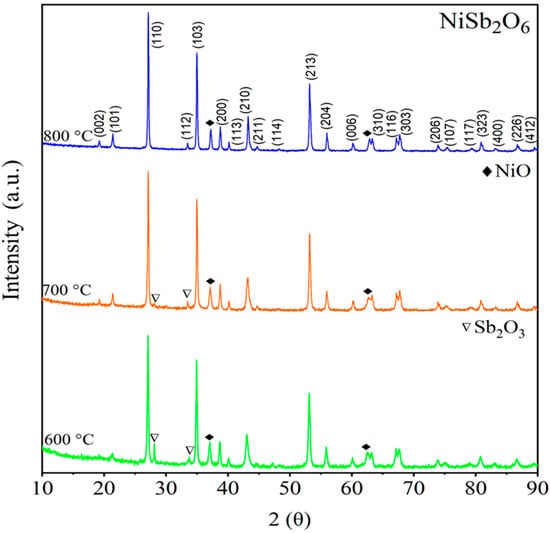
Figure 3.
X-ray diffraction of NiSb2O6 calcined in air at 600, 700, and 800 °C.
After extensive literature research, we compared our results with those of other groups that have prepared the same compound and found that, in our case, we obtained the NiSb2O6 crystalline phase at a lower calcination time (<5 h) from 600 to 800 °C. In the references [,], NiSb2O6’s crystalline phase formation was reported at a residence time of 3 days at 800 °C. Some authors used the solid-state reaction process (ceramic method), which required several days to obtain the crystalline phase of NiSb2O6, CoSb2O6, and CuSb2O6 []. In our case, we used a microwave-assisted wet chemistry method, which is economical, simple, and with easy control of chemical reactions and physical processes (such as low temperature to obtain the crystalline phase).
3.2. SEM Analysis
Figure 4 shows SEM micrographs of powders of the NiSb2O6 calcined at 800 °C. Magnifications used for the material’s microstructural analysis were 6.0, 10.0, 15.0, 60.0, and 70.0 kx. Based on the images, it was found that the material’s entire surface is composed of particles in the form of rods, polyhedra, and other irregular shapes. Figure 4a–c show a high agglomeration of polyhedral particles and other particles forming rods of different lengths. These particles agglomerated in such a way that they formed irregular structures of varying sizes (Figure 4d–f). In Figure 4d,f, the growth of smaller microrods can be observed, which had a common point of origin but grew in different directions. This formation of microrods is mainly attributed to the fact that, during the thermal treatment, the growth of very fine particles is favored, as well as their subsequent agglomeration, resulting in the formation of characteristic morphologies as shown. In addition, the residence time of the material (5 h) at 800 °C played a significant role in the morphologies’ growth.
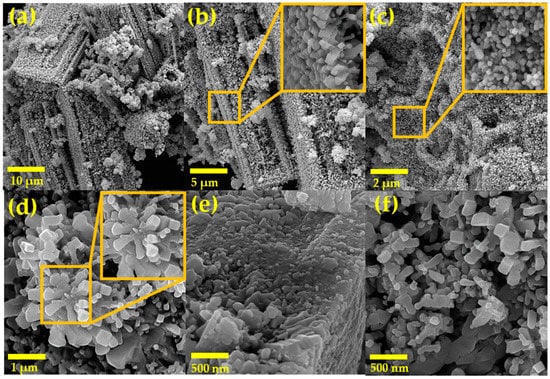
Figure 4.
SEM micrographs of powders of NiSb2O6 calcined at 800 °C at magnifications: (a) 6.0 kx, (b) 10.0 kx, (c) 15.0 kx, (d) 60.0 kx, (e) 70.0 kx, and (f) 70.0 kx.
The size of the microrods was estimated in the range of 100 to 500 nm, with an average of ~277 nm and a standard deviation of ~±83 nm (Figure 5a), while the size of the other particles was estimated in the range of 20 to 130 nm, with an average of ~65 nm and a standard deviation of ~±21 nm (Figure 5b). Multiple SEM micrographs, where the particles were clearly identifiable, were considered for the microstructures’ size calculation. Several authors have reported that the use of chelating agents such as ethylenediamine in the synthesis promotes the formation of such microstructures. This is because ethylenediamine reacts with temperature to form a mesh that captures metal particles, which agglomerate as the calcination temperature increases, resulting in the growth of rods and other morphologies []. Studies have also suggested that the use of ethylenediamine can lead to the formation of diverse nanostructures including nanoparticles, nanorods, nanowires, porous particles, and hexagonal nanostructures [,]. The microstructures in our study followed the crystallization principles established by LaMer and Dinegar []. According to these principles, nuclei formed due to chemical reactions until a saturation limit was reached, after which the particles grew until the system’s solubility reached equilibrium. This condition favored the production of morphologies such as those shown in Figure 4d–f.
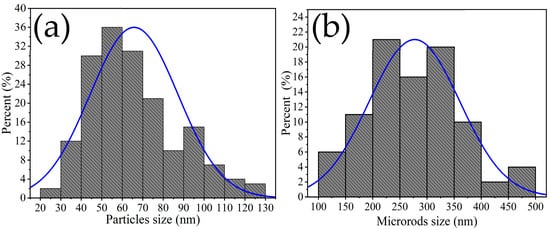
Figure 5.
Particle size distribution: (a) bars, (b) other types of particles.
3.3. TEM Analysis
To further examine the microstructure of the nickel antimonate calcined at 800 °C, transmission electron microscopy (TEM) in imaging mode was used. The resulting images, shown in Figure 6, reveal mainly nanorods and other particle types. This analysis confirmed the SEM results, which identified rods of different sizes and irregularly shaped nanoparticles measuring ~90 nm. Their thickness caused dark areas in the nanostructures, which impeded electron transmission. The nanorods and nanoparticles seen in Figure 6a–f agglomerated together due to the effect of the calcination temperature and ethylenediamine. These particles varied in shape and size and had small growth nuclei that caused the nanorods to grow in different directions []. The nanorod sizes ranged from 40 to 180 nm (Figure 6b–f), with a mean size of ~77 nm and a standard deviation of ±34 nm (see Figure 7).
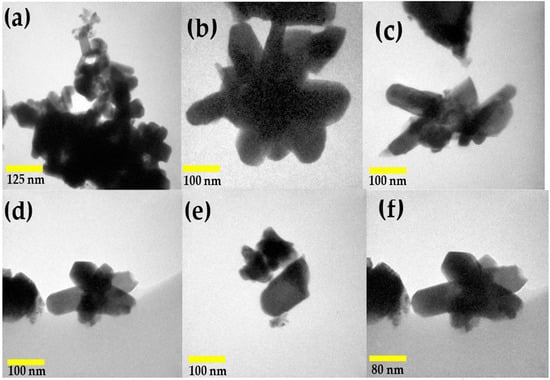
Figure 6.
TEM micrographs of (a) nanoparticles and (b–f) nanorods of NiSb2O6 calcined at 800 °C.
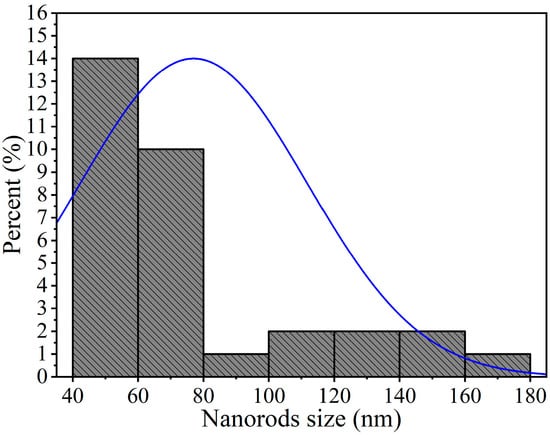
Figure 7.
Size distribution of nanorods of NiSb2O6 calcined at 800 °C.
Agreeing with other authors [], ethylenediamine helps to achieve specific sizes and morphologies, such as nanorods, microrods, nanowires, and polyhedra. In our case, we obtained nanorods and nanoparticles (Figure 6a–f) using ethylenediamine and an alternative synthesis process (wet chemistry), which allowed us to control the compound’s structure in short calcination times (5 h).
3.4. UV-Vis Analysis
To identify the characteristic absorbance bands and determine the value of the forbidden band of the oxide calcined at 800 °C, UV-vis spectroscopy was employed. In Figure 8, absorbance is shown as a function of the compound’s wavelength. In the 200–500 nm range, characteristic absorption bands of a material belonging to the family of semiconductor oxides with a trirutile-type structure were identified [,,]. On the other hand, in the 600–800 nm range, bands associated with the oxygen-metal bond (in our case, Ni-O) were identified [,].
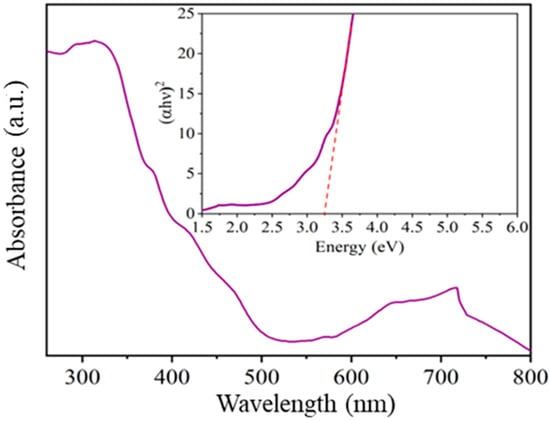
Figure 8.
Band gap value determined by the UV-Vis spectroscopy of NiSb2O6 calcined at 800 °C.
Tauc’s equation [] was employed to calculate the value of the band gap. Previous studies have indicated that materials like the NiSb2O6 exhibit direct transitions between their energy bands (n = ½) [,]. In Figure 8, (αℎυ)2 was plotted against energy, and the resulting spectrum was fitted to a line, yielding a forbidden band value of 3.2 eV. In reference [], the band gap was reported as 3.92 eV. Another study [] reported values in the range of 2.6–2.8 eV for a material calcined at 700 °C and 2.8–2.9 eV for a material calcined at 900 °C. It should be noted that the forbidden band value is strongly influenced by the synthesis method and the heat treatment employed, as mentioned by several authors [,,], which is consistent with the findings of this study.
3.5. Static Tests in CO and C3H8
To check nickel antimonate’s detection ability to different gas concentrations, its pellets were subjected to 1–300 ppm of CO and 1–500 ppm of C3H8 at temperatures 100, 200, and 300 °C. The results are shown in Figure 9 and Figure 10 as a function of the test gas concentration and the operating temperature. In the case of CO (Figure 9a,b), the material’s response increased as the concentration and operating temperature rose. This increase is mainly attributed to oxygen species (like , , or ) on the surface of the pellets, which rapidly reacted when the CO was injected into the measurement chamber. Furthermore, the excellent oxide response is associated with higher oxygen desorption due to the operating temperature [,]. When the pellet was at 100 and 200 °C, a slight increase in gas sensing response was recorded: ~0 and ~1 at 300 ppm of CO, respectively. This low response was because the thermal energy was insufficient for the oxygen species to react on the pellets’ surface [], which induced poor kinetic activity between them, the material’s particles, and the CO molecules [,]. However, when the temperature was raised to 300 °C, values of 0, 0, 1, 2, 4, and 5 were recorded, corresponding to 1, 5, 50, 100, 200, and 300 ppm of CO. The maximum response at 300 °C was ~5 at 300 ppm of CO. The excellent response is mainly attributed to the fact that the oxygen species and are highly reactive at temperatures greater than 150 °C compared to those species () that appear below that temperature [,]. It has been reported that an increase in the operating temperature favors a high interaction between the pellets’ surface with the CO molecules [], thereby provoking variation in electrical resistance and, therefore, an increase in response, as occurred in our case (Figure 9a,b).
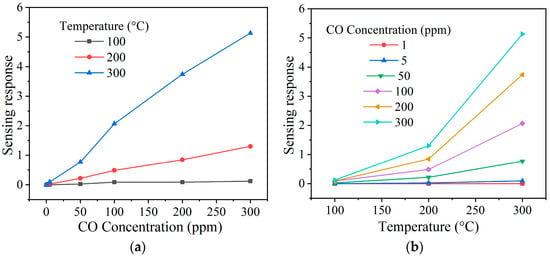
Figure 9.
Sensing response of the NiSb2O6 pellets as a function of (a) the concentration of CO and (b) the operating temperature.
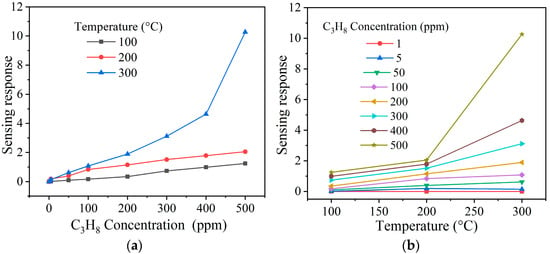
Figure 10.
Sensing response of the NiSb2O6 pellets as a function of (a) the concentration of C3H8 and (b) the operating temperature.
In Figure 10, the results of the tests in C3H8 atmospheres at 100, 200, and 300 °C are depicted. As in the previous case, when injecting the gas into the measurement chamber, the pellets showed varied electrical resistance due to temperature and C3H8 concentrations. However, when the gas was removed from the chamber, the pellets’ electrical resistance returned to their initial values (i.e., before starting the tests), thus corroborating the reproducibility of the experiments. According to Figure 10, the response magnitude rose significantly as the C3H8 concentration (from 1 to 500 ppm) and the operating temperature (from 100 to 300 °C) increased. Thus, at 100 and 200 °C and 500 ppm of C3H8, small response values were estimated: ~1 and ~2, respectively. The maximum response recorded was ~10 at 500 ppm of C3H8 and 300 °C. This trend in the increase in response is attributed to the fact that when the gas was injected into the chamber, the C3H8 molecules diffused on the pellets’ surface [], reacting with the oxygen available ( and ) due to the temperature effect [,]. According to the literature, the different oxygen species (, or ) that reacted at temperatures close to 300 °C [,] are responsible for the variation in the electrical resistance and, consequently, the increased NiSb2O6’s response in C3H8 atmospheres. The reaction between the test gas and the oxygen species caused a high gas–solid interaction [,,], causing the compound’s increased response. A possible chemical reaction between materials such as the one studied here and C3H8 has been proposed: C3H8 + 10O2− → 3CO2 + 4H2O + 10e− []. This means that when the pellets’ surface comes into contact with the C3H8, the latter dissociates before reacting with the ionosorbed oxygen species [,], causing a redox reaction that provokes changes in electrical resistance and, as a result, an increase in NiSb2O6’s response (Figure 10a,b).
3.6. Photocatalytic Tests
NiSb2O6’s photocatalytic activity was monitored with the discoloration of malachite green (MG). The evolution of the photo discoloration and the discoloration percentage are shown in Figure 11a,b, respectively. According to Figure 11b, after being in the dark for 30 min, the sample absorbed 30% of the dye. However, MG’s concentration decreased significantly during the UV light irradiation, which meant that when the NiSb2O6 particles acted as a photocatalyst, up to 85% of MG’s photo discoloration occurred after 200 min of irradiation. These results are competitive according to the literature. Table 1 shows the photocatalytic performance of different antimonates with a trirutile structure.
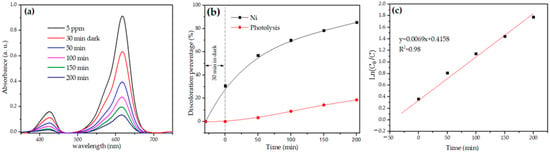
Figure 11.
(a) Evolution of MG’s photo discoloration, (b) MG’s discoloration percentage, (c) MG’s first-order kinetic photodegradation.

Table 1.
Photocatalitic performance of different trirutile antimonates.
The reaction kinetics of the photo discoloration was estimated using the first-order Langmuir–Hinshelwood equation []:
where C0 is MG’s concentration before the photo discoloration, C is the concentration at the beginning of the photocatalytic reaction, t is the reaction time, and k is the reaction rate constant []. The latter was estimated using the slope of Figure 11c (=0.0069).
The good photocatalytic performance of NiSb2O6 pellets can be attributed to the nanorods, since according to the literature, nanostructures such nanorods have shown to be efficient to enhance photocatalytic performance. C. J. Chang et al. [] studied the effect of nanorod-type nanostructures on the surface of ZnO films, concluding that the photocatalytic performance increases with the presence of these structures due to their higher surface area. S. Zhang et al. [] prepared TiO2, nanorods, TiO2 nanosheets, and TiO2 nanospheres; the best photocatalytic activity occurred in the nanorods due not only to the surface area, but also because this type of structures can provide direct conduction paths for photo-induced carriers, resulting in better photocatalytic activity. Y. C. Chang et al. [] synthesized sodium titanate nanorods that showed good photocatalytic activity. The better photocatalytic performance may be due to the higher amounts of oxygen vacancies that can occur in this type of structure, which act as electron traps, decreasing recombination and then improving the photocatalytic performance. Therefore, various phenomena can occur in this type of structures that may contribute to improving the photocatalytic activity.
Respecting the photocatalysis mechanism shown in Equations (4)–(7), it has been reported that the photon absorption by the photocatalyst leads to the excitation of electrons from the valence to the conduction bands, generating electron–hole (e−-h+) pairs (Equation (4)). The holes react with adsorbed water molecules on the photocatalyst’s surface (Equation (5)), producing hydroxyl radicals (OH●). Electrons react with dissolved oxygen molecules in the water (Equation (6)), producing superoxide radicals (O2−●) [,,]. Therefore, the MG may photodegrade by reacting with hydroxyl or superoxide radicals (Equation (7)).
The OH● generated is key in the photocatalytic process, since it reacts with the substances adsorbed on the surface and degrades them.
4. Conclusions
Applying a wet-chemistry method assisted with microwave radiation favored obtaining the crystalline phase of nickel antimonate (NiSb2O6) at a temperature of 800 °C in just five hours. SEM studies of the NiSb2O6’s microstructure allowed us to identify morphologies in the form of microrods, polyhedrons, and other particles with no apparent shape. With TEM, it was estimated that the average size of the nanorods was ~77 nm. UV-vis spectroscopy estimated a value of the NiSb2O6’s band gap of ~3.2 eV. This value is within the energy parameters for antimonates with a trirutile-type structure. NiSb2O6 pellets showed excellent responses of ~5 at 300 ppm of CO and ~10 at 500 ppm of C3H8, at 300 °C. These responses are attributed to the microstructure obtained during the synthesis. The particles’ morphological characteristics and size were closely related to the changes in electrical resistance and, therefore, to the increase in the compound’s response. The photocatalytic activity of NiSb2O6 nanostructures reached 85% degradation of malachite green. This suggests that NiSb2O6 is a potential photocatalytic material. However, it is a material little studied for applications as a gas sensor or photocatalyst. To encourage the study of this material, it is recommended to use our synthesis method, which is easy to carry out, relatively cheap, and does not require sophisticated equipment to control the physical and chemical parameters involved in the oxide’s preparation.
Author Contributions
H.G.-B., A.G.-B., J.T.G.-B. and V.-M.R.-B. were the research supervisors. Research: H.G.-B., J.M.-B., J.A.R.-O., V.-M.R.-B., J.T.G.-B. and A.G.-B. Formal analysis: A.G.-B., J.M.-B. and J.A.R.-O. Materials preparation: J.M.-B., J.A.R.-O., J.T.G.-B. and H.G.-B. Writing—review and editing: H.G.-B., J.M.-B., V.-M.R.-B., A.G.-B. and J.A.R.-O. All the authors participated in the conception and design of the work, meeting the authorship conditions. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.
Acknowledgments
The authors thank Mexico’s National Council of Science and Technology (CONACyT), and the University of Guadalajara for the support granted. Jacob Morales-Bautista thanks CONACyT for the scholarships received. Likewise, we thank M. de la Luz Olvera-Amador, Víctor-Manuel Soto-García, and Miguel-Ángel Luna-Arias for their technical assistance. This research was carried out following the line of research “Nanostructured Semiconductor Oxides” of the academic group UDG-CA-895 “Nanostructured Semiconductors” of CUCEI, University of Guadalajara.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petrica Bala, G.; Râjnoveanu, R.-M.; Tudorache, E.; Motișan, R.; Oancea, C. Air pollution exposure the (in)visible risk factor for respiratory diseases. Environ. Sci. Pollut. Res. 2021, 28, 19615–19628. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Agrawa, M. Acid rain and its ecological consequences. J. Environ. Biol. 2008, 29, 15–24. [Google Scholar] [PubMed]
- Masson, N.; Piedrahita, R.; Hannigan, M. Approach for quantification of metal oxide type semiconductor gas sensors used for ambient air quality monitoring. Sens. Actuators B 2015, 208, 339–345. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Deng, Z.; Meng, G.; Fang, X.; Dong, W.; Shao, J.; Wang, S.; Tong, B. A novel ammonia gas sensors based on p-type delafossite AgAlO2. J. Alloy. Compd. 2019, 17, 52–58. [Google Scholar] [CrossRef]
- Majura Bulemo, P.; Doo Kim, L. Recent advances in ABO3 perovskites: Their gas-sensing performance as resistive-type gas sensors. J. Korean Ceram. Soc. 2020, 57, 24–39. [Google Scholar] [CrossRef]
- Souri, M.; Amoli, H.S. Gas sensing mechanisms in ABO3 perovskite materials at room temperature: A review. Mater. Sci. Semicond. Process. 2023, 156, 107271. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, K.; Zheng, Z.; Debliquy, M. Defect engineering of nanostructured ZnSnO3 for conductometric room temperature CO2 sensors. Sens. Actuators B Chem. 2023, 384, 133628. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Meng, F. Spinel-Type Materials Used for Gas Sensing: A Review. Sensors 2020, 20, 5413. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Singh, A.; Rathore, S.; Yadav, B.-C.; Tandon, P. Nanostructured cobalt antimonate: A fast responsive and highly stable sensing material for liquefied petroleum gas detection at room temperature. RSC Adv. 2020, 10, 33770. [Google Scholar] [CrossRef]
- Guillén-Bonilla, H.; Flores-Martínez, M.; Rodríguez-Betancourtt, V.-M.; Guillen-Bonilla, A.; Reyes-Gómez, J.; Gildo-Ortiz, L.; de la Luz Olvera Amador, M.; Santoyo-Salazar, J. A Novel Gas Sensor Based on MgSb2O6 Nanorods to Indicate Variations in Carbon Monoxide and Propane Concentrations. Sensors 2016, 16, 177. [Google Scholar] [CrossRef]
- Michel, C.-R.; López-Contreras, N.-L.; López-Alvarez, M.-A.; Martínez-Preciado, A.-H. Gas selectivity of nanostructured ZnSb2O6 synthesized by a colloidal method. Sens. Actuators B Chem. 2012, 171–172, 686–690. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Singh, S.; Tandon, P. Nickel antimony oxide (NiSb2O6): A fascinating nanostructured material for gas sensing application. Chem. Phys. Lett. 2016, 646, 41–46. [Google Scholar] [CrossRef]
- Rodríguez-Betancourtt, V.-M.; Guillén-Bonilla, H.; Flores-Martínez, M.; Guillen-Bonilla, A.; Moran-Lazaro, J.-P.; Guillen-Bonilla, J.-T.; González, M.-A.; de la Luz Olvera Amador, M. Gas Sensing Properties of NiSb2O6 Micro and Nanoparticles in Propane and Carbon Monoxide Atmospheres. J. Nanomater. 2017, 2017, 8792567. [Google Scholar] [CrossRef]
- Guerra, F.-D.; Attia, M.-F.; Whitehead, D.-C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Ganie, A.-S.; Bano, S.; Nishat, K.; Sultana, S.; Rehman, Z.; Rahman-Mohammed, M.; Suhail, S.; Coulon, F.; Khan, M.-Z. Nanoremediation technologies for sustainable remediation of contaminated environments: Recent advances and challenges. Chemosphere 2021, 275, 130065. [Google Scholar] [CrossRef]
- Arunkumar, N.; Naraginti, S. Facile synthesis of nanostructured trirutile antimonates M(II)Sb2O6 (M = Co, Cu, Ni, Fe) and its visible photocatalytic studies. Inorg. Nano-Met. Chem. 2022, 52, 151–160. [Google Scholar] [CrossRef]
- Liu, W.; Lin, P.; Jin, H.; Xue, H.; Zhang, Y.; Li, Z. Nanocrystalline ZnSb2O6: Hydrothermal synthesis, electronic structure and photocatalytic activity. J. Mol. Catal. A Chem. 2011, 349, 80–85. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, L.; Yao, S.; Long, Y.; Li, W.; Wang, Z. Effect of calcination temperature on the photocatalytic activity of CaSb2O6 nanoparticles prepared by co-precipitation method. Catal. Commun. 2014, 48, 29–32. [Google Scholar] [CrossRef]
- Sunku, M.; Venkataswamy, P.; Bindu-Hima, G.; Srilekha, P.; Srinivas, M.; Vithal, M. A Novel Approach for Generation of Oxygen Vacancies in Trirutile MnSb2O6 and Their Impact on Photocatalytic Degradation of MO Dye. Eur. J. Inorg. Chem. 2022, 26, e202200550. [Google Scholar]
- Papi, N.; Hakimyfard, A.; Tahmasebi, N.; Samimifar, M. Photocatalytic degradation of water pollutant dye by solid state synthesized Ni1-xLnxSb2O6 (Ln=Eu, Gd, Ho and Yb) nanocomposites. Int. J. Nano Dimens. 2020, 11, 377–391. [Google Scholar]
- Ramírez-Ortega, J.A.; Guillén-Bonilla, J.T.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M.; Gildo-Ortiz, L.; Blanco-Alonso, O.; Soto-García, V.M.; Jiménez-Rodríguez, M.; Guillén-Bonilla, H. Preparation of powders containing Sb, Ni, and O for the design of a novel CO and C3H8 sensor. Appl. Sci. 2021, 11, 9536. [Google Scholar] [CrossRef]
- Guillén, H.; Rodríguez Betancourtt, V.M.; Guillen, J.T.; Gildo, L.; Guillen, A.; Casallas, Y.; Blanco, O.; Reyes, J. Sensitivity tests of pellets made from manganese antimonate nanoparticles in carbon monoxide and propane atmospheres. Sensors 2018, 18, 2299. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Sahoo, R.; Santra, S.; Ray, C.; Pal, A.; Negishi, Y.; Ray, S.K.; Pal, T. Hierarchical growth of ZnFe2O4 for sensing applications. New J. Chem. 2016, 40, 1861–1871. [Google Scholar] [CrossRef]
- Kato, M.; Kajimoto, K.; Yoshimura, K.; Kosuge, K.; Masakazu, N.; Kakurai, K. Magnetic Structure of CuSb2O6. J. Phys. Soc. Jpn. 2002, 71, 187–189. [Google Scholar] [CrossRef]
- Waseda, Y.; Matsubara, E.; Shinoda, K. X-Ray Diffraction Crystallography, 1st ed.; Springer: Aoba-Ku, Japan, 2011. [Google Scholar]
- Larcher, D.; Prakash, A.S.; Laffont, L.; Womes, M.; Jumas, J.C.; Olivier-Fourcade, J.; Hedge, M.S.; Tarascon, J.M. Reactivity of antimony oxides and MSb2O6 (M=Cu,Ni,Co), trirutile-type phases with metallic lithium. Electrochem. Soc. 2006, 153, 1778–1787. [Google Scholar]
- Hakimyfard, A. Effects of reaction temperature and raw material type on optical properties and crystal phase growth of Solid state synthesized NiSb2O6 nanomaterials. J. Adv. Mater. 2018, 5, 56–65. [Google Scholar]
- Wang, S.-F.; Sun, G.Z.; Fang, L.M.; Lei, L.; Xiang, X.; Zu, X.T. A comparative study of ZnAl2O4 nanoparticles synthesized from different aluminum salts for use as fluorescence materials. Sci. Rep. 2015, 5, 12849. [Google Scholar] [CrossRef]
- Barzinjy, A.A.; Salih, S.H.; Sadraden, Z.A.; Qadir, H.M. Nanostructured device in sensing applications: A review. Eurasian J. Sci. Eng. 2018, 4, 82–98. [Google Scholar]
- Esposito, S. “Traditional” sol-gel chemistry as a powerful tool for the preparation of supported metal and metal oxide catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef]
- Arshadi, S.; Moghaddam, J.; Eskandarian, M. LaMer diagram approach to study the nucleation and growth of Cu2O nanoparticles using supersaturation theory. Korean J. Chem. Eng. 2014, 31, 2020–2026. [Google Scholar] [CrossRef]
- Deng, Z.-X.; Wang, C.; Sun, X.-M.; Li, Y.D. Structure-directing coordination template effect of ethylenediamine in formations of ZnS and ZnSe nanocrystallites via solvothermal route. Inorg. Chem. 2002, 41, 869–873. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Peng, S.; Lu, G.; Li, S. Synthesis of CdS Nanorods by an Ethylenediamine Assisted Hydrothermal Method for Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2009, 113, 9352–9358. [Google Scholar] [CrossRef]
- Singh, J.; Bhardwaj, N.; Uma, S. Single step hydrothermal based synthesis of M(II)Sb2O6 (M = Cd and Zn) type antimonates and their photocatalytic properties. Bull. Mater. Sci. 2013, 36, 287–291. [Google Scholar] [CrossRef]
- Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.M.; Guillén-Bonilla, J.T.; Sánchez-Martínez, A.; Gildo-Ortiz, L.; Santoyo-Salazar, J.; Morán-Lázaro, J.P.; Guillén-Bonilla, H.; Blanco-Alonso, O. A novel CO and C3H8 sensor made of CuSb2O6 nanoparticles. Ceram. Int. 2017, 43, 13635–13644. [Google Scholar] [CrossRef]
- Balamurugan, C.; Maheswari, A.R.; Lee, D.W. Structural, optical, and selective ethanol sensing properties of p-type semiconducting CoNb2O6 nanopowder. Sens. Actuators B Chem. 2014, 205, 289–297. [Google Scholar] [CrossRef]
- Barakat, A.; Al Noaimi, M.; Suleiman, M.; Aldwayyan, A.; Hammouti, B.; Hadda, T.; Haddad, S.; Boshaala, A.; Warad, I. One step synthesis of NiO nanoparticles via solid-state thermal decomposition at low-temperature of novel aqua (2,9-dimethyl-1,10-phenanthroline) NiCl2 complex. Int. J. Mol. Sci. 2013, 14, 23941–23954. [Google Scholar] [CrossRef] [PubMed]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P., III. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi B 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Du, X.; Du, Y.; George, S.M. CO Gas Sensing by Ultrathin Tin Oxide Films Grown by Atomic Layer Deposition Using Transmission FTIR Spectroscopy. J. Phys. Chem. A 2008, 112, 9211–9219. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Vohs, J.M. Site requirements for the adsorption and reaction of oxygenates. Chem. Rev. 2013, 113, 4136–4163. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, A.; Puri, V.; Le, D.-N. A comprehensive review of semiconductor-type gas sensors for environmental monitoring. Rev. Comput. Eng. Res. 2016, 3, 55–64. [Google Scholar] [CrossRef]
- Hua, Z.; Tian, C.; Huang, D.; Yuan, W.; Zhang, C.; Tian, X.; Wang, M.; Li, E. Power-law response of metal oxide semiconductor gas sensors to oxygen in presence of reducing gases. Sens. Actuators B Chem. 2018, 267, 510–518. [Google Scholar] [CrossRef]
- Jayaraman, V.K.; Maldonado Álvarez, A.; de la Luz Olvera Amador, M. A simple and cost-effective zinc oxide thin film sensor for propane gas detection. Mater. Lett. 2015, 157, 169–171. [Google Scholar] [CrossRef]
- Naz, F.; Saeed, K. Synthesis of barium oxide nanoparticles and its novel application as a catalyst for the photodegradation of malachite green dye. Appl. Water Sci. 2022, 12, 121. [Google Scholar] [CrossRef]
- Chang, C.J.; Hsu, M.-H.; Weng, Y.-C.; Tsay, C.-Y.; Lin, C.-K. Hierarchical ZnO nanorod-array films with enhanced photocatalytic performance. Thin Solid Film. 2013, 528, 167–174. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Y.; Jiang, H.; Liu, Y.; Chen, R. Controlled synthesis of TiO2 nanorod arrays immobilized on ceramic membranes with enhanced photocatalytic performance. Ceram. Int. 2017, 43, 7261–7270. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lin, J.-C.; Wu, S.-H. One-step growth of Na2Ti3O7 nanorods for enhanced photocatalytic activities and recyclability. J. Alloy. Compd. 2018, 749, 955–960. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, G.; Fang, J.; Chen, J. Characterization, and Photocatalysis of Well-Dispersible Phase-Pure Anatase TiO2 Nanoparticles. Int. J. Photoenergy 2013, 2013, 726872. [Google Scholar] [CrossRef]
- Xu, A.-W.; Gao, Y.; Liu, H.Q. The Preparation, Characterization, and their Photocatalytic Activities of Rare-Earth-Doped TiO2 Nanoparticles. J. Catal. 2002, 207, 151–157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).