Precise Construction and Growth of Submillimeter Two-Dimensional WSe2 and MoSe2 Monolayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Growth Preparation and Processes
2.3. Characterization
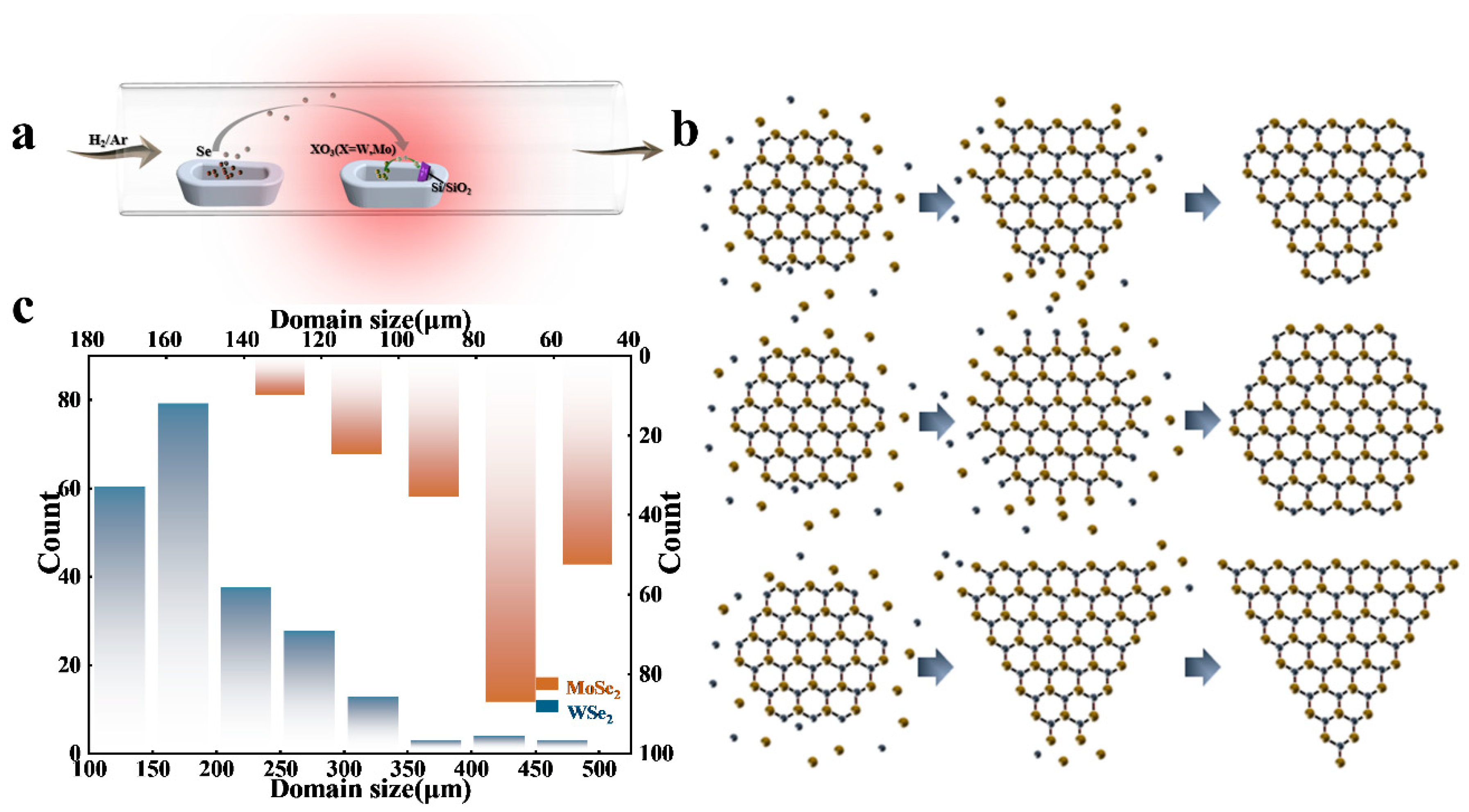
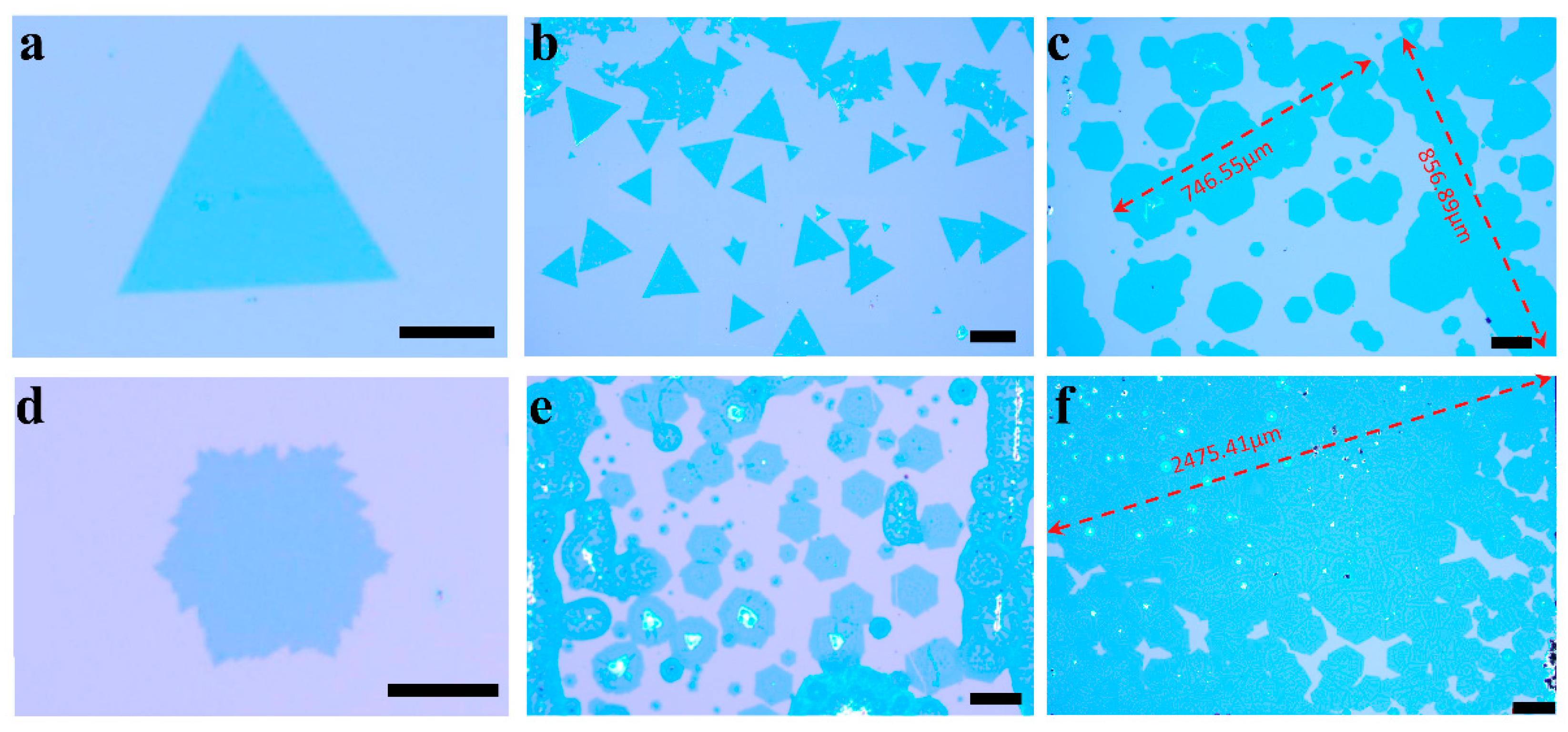
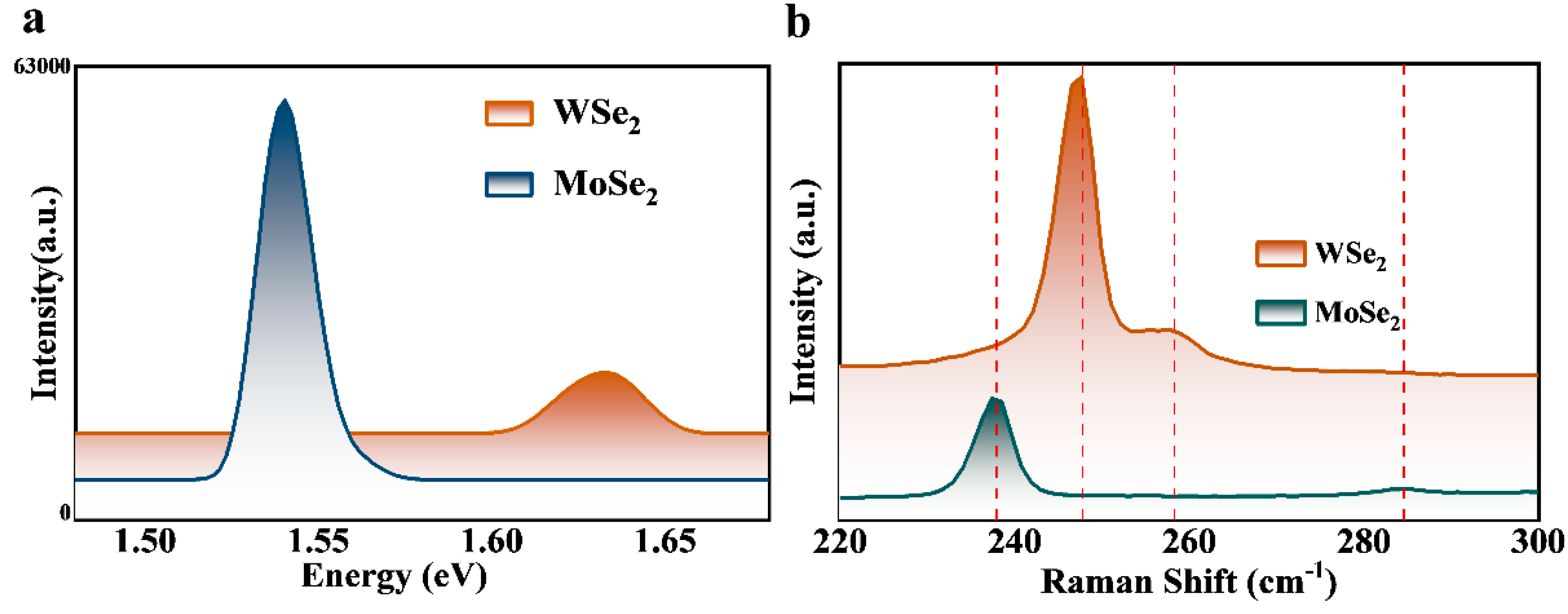
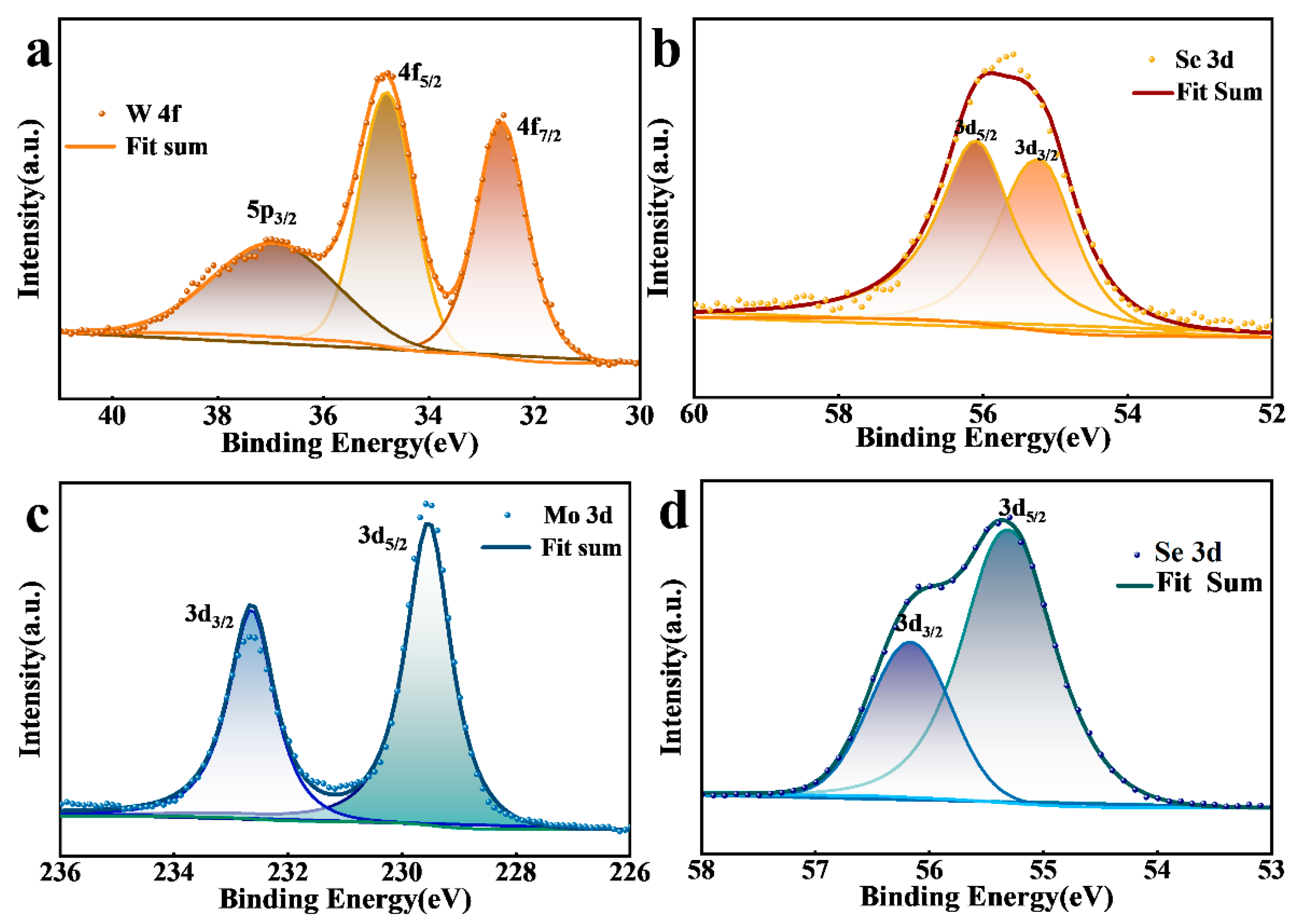
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A.B.; Vaghasiya, J.V.; Chauhan, P.; Sumesh, C.K.; Patel, V.; Soni, S.S.; Patel, K.D.; Garg, P.; Solanki, G.K.; Pathak, V.M. Synergistic 2D MoSe2@WSe2 nanohybrid heterostructure toward superior hydrogen evolution and flexible supercapacitor. Nanoscale 2022, 14, 6636–6647. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Chauhan, P.; Machhi, H.K.; Narayan, S.; Sumesh, C.K.; Patel, K.D.; Soni, S.S.; Jha, P.K.; Solanki, G.K.; Pathak, V.M. Transferrable thin film of ultrasonically exfoliated MoSe2 nanocrystals for efficient visible-light photodetector. Phys. E Low Dimens. Syst. Nanostruct. 2020, 119, 114019. [Google Scholar] [CrossRef]
- Kaasbjerg, K.; Thygesen, K.S.; Jacobsen, K.W. Phonon-limited mobility in n-type single-layer MoS2 from first principles. Phys. Rev. B 2012, 85, 115317. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Z.; Zhang, W.; Li, Y. Two-dimensional semiconductors with possible high room temperature mobility. Nano Res. 2014, 7, 1731–1737. [Google Scholar] [CrossRef]
- Huang, C.; Du, Y.; Wu, H.; Xiang, H.; Deng, K.; Kan, E. Prediction of Intrinsic Ferromagnetic Ferroelectricity in a Transition-Metal Halide Monolayer. Phys. Rev. Lett. 2018, 120, 147601. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Yang, F.; Huang, J.-K.; Li, L.-J.; Yao, W.; Ji, W.; Shih, C.-K. Engineering point-defect states in monolayer WSe2. ACS Nano 2019, 13, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Ezawa, M. Valley-polarized metals and quantum anomalous Hall effect in silicene. Phys. Rev. Lett. 2012, 109, 055502. [Google Scholar] [CrossRef]
- Jiang, S.; Li, L.; Wang, Z.; Mak, K.F.; Shan, J. Controlling magnetism in 2D CrI3 by electrostatic doping. Nat. Nanotechnol. 2018, 13, 549–553. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- McGuire, M.A.; Dixit, H.; Cooper, V.R.; Sales, B.C. Coupling of crystal structure and magnetism in the layered, ferromagnetic insulator CrI3. Chem. Mater. 2015, 27, 612–620. [Google Scholar] [CrossRef]
- Jiang, D.; Hu, T.; You, L.; Li, Q.; Li, A.; Wang, H.; Mu, G.; Chen, Z.; Zhang, H.; Yu, G. High-T c superconductivity in ultrathin Bi2Sr2CaCu2O8+x down to half-unit-cell thickness by protection with graphene. Nat. Commun. 2014, 5, 5708. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, C.; Wong, D.; Pedramrazi, Z.; Tsai, H.-Z.; Jia, C.; Moritz, B.; Claassen, M.; Ryu, H.; Kahn, S. Quantum spin Hall state in monolayer 1T’-WTe2. Nat. Phys. 2017, 13, 683–687. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, J.; Moore, R.; Li, W.; Yi, M.; Hashimoto, M.; Lu, D.; Devereaux, T.; Lee, D.-H.; Shen, Z.-X. Superconducting gap anisotropy in monolayer FeSe thin film. Phys. Rev. Lett. 2016, 117, 117001. [Google Scholar] [CrossRef]
- Huang, B.; Clark, G.; Klein, D.R.; MacNeill, D.; Navarro-Moratalla, E.; Seyler, K.L.; Wilson, N.; McGuire, M.A.; Cobden, D.H.; Xiao, D. Electrical control of 2D magnetism in bilayer CrI3. Nat. Nanotechnol. 2018, 13, 544–548. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Wu, X.; Shao, Y.; Qi, J.; Cao, Y.; Huang, L.; Liu, C.; Wang, J.-O.; Zheng, Q.; Zhu, Z.-L. Epitaxially grown monolayer VSe2: An air-stable magnetic two-dimensional material with low work function at edges. Sci. Bull. 2018, 63, 419–425. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Yang, J.; Li, W.; Huang, J.-K.; Li, S. Tungsten diselenides (WSe2) quantum dots: Fundamental, properties, synthesis and applications. Prog. Mater Sci. 2023, 132, 101042. [Google Scholar] [CrossRef]
- Kang, J.; Tongay, S.; Zhou, J.; Li, J.; Wu, J. Band offsets and heterostructures of two-dimensional semiconductors. Appl. Phys. Lett. 2013, 102, 012111. [Google Scholar] [CrossRef]
- Rehman, A.U.; Khan, M.F.; Shehzad, M.A.; Hussain, S.; Bhopal, M.F.; Lee, S.H.; Eom, J.; Seo, Y.; Jung, J.; Lee, S.H. n-MoS2/p-Si Solar Cells with Al2O3 Passivation for Enhanced Photogeneration. ACS Appl. Mater. Interfaces 2016, 8, 29383–29390. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-L.; Li, M.-Y.; Retamal, J.R.D.; Lam, K.-T.; Lin, Y.-C.; Suenaga, K.; Chen, L.-J.; Liang, G.; Li, L.-J.; He, J.-H. Single Atomically Sharp Lateral Monolayer p-n Heterojunction Solar Cells with Extraordinarily High Power Conversion Efficiency. Adv. Mater. 2017, 29, 1701168. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef]
- Liu, Y.; Weiss, N.O.; Duan, X.; Cheng, H.-C.; Huang, Y.; Duan, X. Van der Waals heterostructures and devices. Nat. Rev. Mater. 2016, 1, 16042. [Google Scholar] [CrossRef]
- Chen, D.-R.; Hofmann, M.; Yao, H.-M.; Chiu, S.-K.; Chen, S.-H.; Luo, Y.-R.; Hsu, C.-C.; Hsieh, Y.-P. Lateral two-dimensional material heterojunction photodetectors with ultrahigh speed and detectivity. ACS Appl. Mater. Interfaces 2019, 11, 6384–6388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, C.; Zou, X.; Liao, L. Recent advances in optoelectronic devices based on 2D materials and their heterostructures. Adv. Opt. Mater. 2019, 7, 1800441. [Google Scholar] [CrossRef]
- Xia, J.; Zeng, Q.; Zhou, J.; Zhou, W.; Zhang, Q.; Yan, J.; Liu, Z.; Shen, Z.X. Current rectification and asymmetric photoresponse in MoS2 stacking-induced homojunctions. 2D Mater. 2017, 4, 035011. [Google Scholar] [CrossRef]
- Liu, H.; Si, M.; Najmaei, S.; Neal, A.T.; Du, Y.; Ajayan, P.M.; Lou, J.; Ye, P.D. Statistical study of deep submicron dual-gated field-effect transistors on monolayer chemical vapor deposition molybdenum disulfide films. Nano Lett. 2013, 13, 2640–2646. [Google Scholar] [CrossRef]
- Gong, Y.; Ye, G.; Lei, S.; Shi, G.; He, Y.; Lin, J.; Zhang, X.; Vajtai, R.; Pantelides, S.T.; Zhou, W. Synthesis of millimeter-scale transition metal dichalcogenides single crystals. Adv. Funct. Mater. 2016, 26, 2009–2015. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Q.; Wang, H.; Zhao, S.; Xiong, Q.; Lv, R.; Zhang, X. Simultaneous electrical and thermal rectification in a monolayer lateral heterojunction. Science 2022, 378, 169–175. [Google Scholar] [CrossRef]
- Tong, T.; Gan, Y.; Li, W.; Zhang, W.; Song, H.; Zhang, H.; Liao, K.; Deng, J.; Li, S.; Xing, Z. Boosting the Sensitivity of WSe2 Phototransistor via Janus Interfaces with 2D Perovskite and Ferroelectric Layers. ACS Nano 2022, 17, 530–538. [Google Scholar] [CrossRef]
- Xue, H.; Wang, Y.; Dai, Y.; Kim, W.; Jussila, H.; Qi, M.; Susoma, J.; Ren, Z.; Dai, Q.; Zhao, J. A MoSe2/WSe2 heterojunction-based photodetector at telecommunication wavelengths. Adv. Funct. Mater. 2018, 28, 1804388. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Y.-H.; Yang, R.; Bao, L.-H.; Meng, L.; Luo, H.-L.; Cai, Y.-Q.; Liu, G.-D.; Zhao, W.-J.; Zhou, Z. Universal mechanical exfoliation of large-area 2D crystals. Nat. Commun. 2020, 11, 2453. [Google Scholar] [CrossRef]
- Huang, Y.; Sutter, E.; Shi, N.N.; Zheng, J.; Yang, T.; Englund, D.; Gao, H.-J.; Sutter, P. Reliable exfoliation of large-area high-quality flakes of graphene and other two-dimensional materials. ACS Nano 2015, 9, 10612–10620. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, C.; Zhou, Y.; Dong, J.; Li, P.; Zhang, Z.; Wang, Z.; Lin, Y.-C.; Shi, J.; Zhang, R. Composition and phase engineering of metal chalcogenides and phosphorous chalcogenides. Nat. Mater. 2022, 22, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical vapor deposition growth and applications of two-dimensional materials and their heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Tan, J.; Nong, H.; Liu, B.; Cheng, H.-M. Chemical vapor deposition growth of two-dimensional compound materials: Controllability, material quality, and growth mechanism. Acc. Mater. Res. 2020, 2, 36–47. [Google Scholar] [CrossRef]
- Zhu, W.; Low, T.; Wang, H.; Ye, P.; Duan, X. Nanoscale electronic devices based on transition metal dichalcogenides. 2d Mater. 2019, 6, 032004. [Google Scholar] [CrossRef]
- Chubarov, M.; Choudhury, T.H.; Hickey, D.R.; Bachu, S.; Zhang, T.; Sebastian, A.; Bansal, A.; Zhu, H.; Trainor, N.; Das, S.; et al. Wafer-Scale Epitaxial Growth of Unidirectional WS2 Monolayers on Sapphire. ACS Nano 2021, 15, 2532–2541. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Pan, S.; Tang, B.; Liang, Y.; Zhao, X.; Zhang, Z.; Shi, J.; Huan, Y.; Shi, Y.; et al. Epitaxial Growth of Centimeter-Scale Single-Crystal MoS2 Monolayer on Au(111). ACS Nano 2020, 14, 5036–5045. [Google Scholar] [CrossRef] [PubMed]
- Magda, G.Z.; Peto, J.; Dobrik, G.; Hwang, C.; Biro, L.P.; Tapaszto, L. Exfoliation of large-area transition metal chalcogenide single layers. Sci. Rep. 2015, 5, 14714. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Zhang, W.; Huang, J.-K.; Liu, K.-K.; Lee, Y.-H.; Liang, C.-T.; Chu, C.-W.; Li, L.-J. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale 2012, 4, 6637–6641. [Google Scholar] [CrossRef]
- Kang, K.; Xie, S.; Huang, L.; Han, Y.; Huang, P.Y.; Mak, K.F.; Kim, C.-J.; Muller, D.; Park, J. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature 2015, 520, 656–660. [Google Scholar] [CrossRef]
- Gao, Y.; Hong, Y.L.; Yin, L.C.; Wu, Z.; Yang, Z.; Chen, M.L.; Liu, Z.; Ma, T.; Sun, D.M.; Ni, Z. Ultrafast growth of high-quality monolayer WSe2 on Au. Adv. Mater. 2017, 29, 1700990. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, X.; Tan, S.J.; Xu, H.; Wu, B.; Liu, B.; Fu, D.; Fu, W.; Geng, D.; Liu, Y. Chemical vapor deposition of large-size monolayer MoSe2 crystals on molten glass. J. Am. Chem. Soc. 2017, 139, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, A.; Liu, Y.; Cui, D.; Li, Z.; Chung, Y.-H.; Mutyala, S.P.; Mecklenburg, M.; Nie, X.; Xu, C. Gold-vapor-assisted chemical vapor deposition of aligned monolayer WSe2 with large domain size and fast growth rate. Nano Res. 2020, 13, 2625–2631. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Liu, S.; Han, B.; Gu, P.; Li, S.; Xu, W.; Peng, Y.; Han, Z.; Chen, J. Seeded 2D epitaxy of large-area single-crystal films of the van der Waals semiconductor 2H MoTe2. Science 2021, 372, 195–200. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, B.; Wang, Z.; Xu, T.; Pan, C.; Zou, J.; Zhang, X.; Qi, Z.; Liu, H.; Feng, Y. Van der Waals epitaxial growth and optoelectronics of large-scale WSe2/SnS2 vertical bilayer p–n junctions. Nat. Commun. 2017, 8, 1906. [Google Scholar] [CrossRef]
- Du, J.; Liao, Q.; Hong, M.; Liu, B.; Zhang, X.; Yu, H.; Xiao, J.; Gao, L.; Gao, F.; Kang, Z. Piezotronic effect on interfacial charge modulation in mixed-dimensional van der Waals heterostructure for ultrasensitive flexible photodetectors. Nano Energy 2019, 58, 85–93. [Google Scholar] [CrossRef]
- Najafidehaghani, E.; Gan, Z.; George, A.; Lehnert, T.; Ngo, G.Q.; Neumann, C.; Bucher, T.; Staude, I.; Kaiser, D.; Vogl, T. 1D p–n junction electronic and optoelectronic devices from transition metal dichalcogenide lateral heterostructures grown by one-pot chemical vapor deposition synthesis. Adv. Funct. Mater. 2021, 31, 2101086. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, D.; Jiang, F.; Zhao, P.; Wang, H.; Zhang, Z.; Chen, X.; Jin, C. Revealing the microscopic CVD growth mechanism of MoSe2 and the role of hydrogen gas during the growth procedure. Nanotechnology 2018, 29, 314001. [Google Scholar] [CrossRef]
- Lauritsen, J.; Bollinger, M.; Lægsgaard, E.; Jacobsen, K.W.; Nørskov, J.K.; Clausen, B.; Topsøe, H.; Besenbacher, F. Atomic-scale insight into structure and morphology changes of MoS2 nanoclusters in hydrotreating catalysts. J. Catal. 2004, 221, 510–522. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Liu, Y.; Tang, W.; Nai, C.T.; Li, L.; Zheng, J.; Gao, L.; Zheng, Y.; Shin, H.S. Chemical vapor deposition of large-sized hexagonal WSe2 crystals on dielectric substrates. Adv. Mater. 2015, 27, 6722–6727. [Google Scholar] [CrossRef]
- Einax, M.; Dieterich, W.; Maass, P. Colloquium: Cluster growth on surfaces: Densities, size distributions, and morphologies. Rev. Mod. Phys. 2013, 85, 921. [Google Scholar] [CrossRef]
- Ji, H.G.; Lin, Y.-C.; Nagashio, K.; Maruyama, M.; Solís-Fernández, P.; Sukma Aji, A.; Panchal, V.; Okada, S.; Suenaga, K.; Ago, H. Hydrogen-assisted epitaxial growth of monolayer tungsten disulfide and seamless grain stitching. Chem. Mater. 2018, 30, 403–411. [Google Scholar] [CrossRef]
- Kim, K.K.; Hsu, A.; Jia, X.; Kim, S.M.; Shi, Y.; Hofmann, M.; Nezich, D.; Rodriguez-Nieva, J.F.; Dresselhaus, M.; Palacios, T. Synthesis of monolayer hexagonal boron nitride on Cu foil using chemical vapor deposition. Nano Lett. 2012, 12, 161–166. [Google Scholar] [CrossRef]
- Ismach, A.; Chou, H.; Ferrer, D.A.; Wu, Y.; McDonnell, S.; Floresca, H.C.; Covacevich, A.; Pope, C.; Piner, R.; Kim, M.J. Toward the controlled synthesis of hexagonal boron nitride films. ACS Nano 2012, 6, 6378–6385. [Google Scholar] [CrossRef]
- Guo, N.; Wei, J.; Fan, L.; Jia, Y.; Liang, D.; Zhu, H.; Wang, K.; Wu, D. Controllable growth of triangular hexagonal boron nitride domains on copper foils by an improved low-pressure chemical vapor deposition method. Nanotechnology 2012, 23, 415605. [Google Scholar] [CrossRef] [PubMed]
- Susarla, S.; Kutana, A.; Hachtel, J.A.; Kochat, V.; Apte, A.; Vajtai, R.; Idrobo, J.C.; Yakobson, B.I.; Tiwary, C.S.; Ajayan, P.M. Quaternary 2D transition metal dichalcogenides (TMDs) with tunable bandgap. Adv. Mater. 2017, 29, 1702457. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Duchamp, M.; Zeng, Q.S.; Wang, X.W.; Tsang, S.H.; Li, H.L.; Jing, L.; Yu, T.; Teo, E.H.T.; et al. Large-Area Atomic Layers of the Charge-Density-Wave Conductor TiSe2. Adv. Mater. 2018, 30, 1704382. [Google Scholar] [CrossRef]
- Meng, L.; Zhou, Z.; Xu, M.; Yang, S.; Si, K.; Liu, L.; Wang, X.; Jiang, H.; Li, B.; Qin, P.; et al. Anomalous thickness dependence of Curie temperature in air-stable two-dimensional ferromagnetic 1T-CrTe2 grown by chemical vapor deposition. Nat. Commun. 2021, 12, 809. [Google Scholar] [CrossRef]
- Li, X.; Dai, X.; Tang, D.; Wang, X.; Hong, J.; Chen, C.; Yang, Y.; Lu, J.; Zhu, J.; Lei, Z.; et al. Realizing the Intrinsic Anisotropic Growth of 1T‘ ReS2 on Selected Au(101) Substrate toward Large-Scale Single Crystal Fabrication. Adv. Funct. Mater. 2021, 31, 2102138. [Google Scholar] [CrossRef]
- Yu, P.; Lin, J.; Sun, L.; Le, Q.L.; Yu, X.; Gao, G.; Hsu, C.-H.; Wu, D.; Chang, T.-R.; Zeng, Q.; et al. Metal-Semiconductor Phase-Transition in WSe2(1-x)Te2x Monolayer. Adv. Mater. 2017, 29, 1603991. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, J.; Yu, W.; Chen, L.; Huang, Z.; Jiang, H.; He, J.; Liu, Q.; Lu, Y.; Zhu, D.; et al. Monolayer WxMo1-xS2 Grown by Atmospheric Pressure Chemical Vapor Deposition: Bandgap Engineering and Field Effect Transistors. Adv. Funct. Mater. 2017, 27, 1606469. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, H.; Meng, M.; Chen, C.; Sun, Y.; Chen, Z.; Dang, W.; Tan, C.; Liu, Y.; Yin, J.; et al. High electron mobility and quantum oscillations in non-encapsulated ultrathin semiconducting Bi2O2Se. Nat. Nanotechnol. 2017, 12, 530–534. [Google Scholar] [CrossRef]
- Hong, Y.-L.; Liu, Z.; Wang, L.; Zhou, T.; Ma, W.; Xu, C.; Feng, S.; Chen, L.; Chen, M.-L.; Sun, D.-M.; et al. Chemical vapor deposition of layered two-dimensional MoSi2N4 materials. Science 2020, 369, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, X.; Li, J.; Lin, L.; Sun, L.; Sun, X.; Zhao, S.; Tan, C.; Chen, C.; Dang, W.; et al. Surface Monocrystallization of Copper Foil for Fast Growth of Large Single-Crystal Graphene under Free Molecular Flow. Adv. Mater. 2016, 28, 8968–8974. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Qiu, L.; Zhuang, J.; Zhang, L.; Wang, H.; Liao, C.; Song, H.; Qiao, R.; Gao, P.; et al. Ultrafast growth of single-crystal graphene assisted by a continuous oxygen supply. Nat. Nanotechnol. 2016, 11, 930–935. [Google Scholar] [CrossRef]
- Zhou, J.D.; Lin, J.H.; Huang, X.W.; Zhou, Y.; Chen, Y.; Xia, J.; Wang, H.; Xie, Y.; Yu, H.M.; Lei, J.C.; et al. A library of atomically thin metal chalcogenides. Nature 2018, 556, 355–359. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, X.; Xu, J.; Tang, B.; Shang, Q.; Shi, J.; Huan, Y.; Liao, J.; Chen, Q.; Hou, Y.; et al. Controlled Growth and Thickness-Dependent Conduction-Type Transition of 2D Ferrimagnetic Cr2S3 Semiconductors. Adv. Mater. 2020, 32, e1905896. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, J.; Yin, L.; Shifa, T.A.; Cheng, Z.; Cheng, R.; Wang, F.; Wen, Y.; Zhan, X.; Wang, Z.; et al. Ultrathin Magnetic 2D Single-Crystal CrSe. Adv. Mater. 2019, 31, e1900056. [Google Scholar] [CrossRef]
- Yang, P.; Zou, X.; Zhang, Z.; Hong, M.; Shi, J.; Chen, S.; Shu, J.; Zhao, L.; Jiang, S.; Zhou, X.; et al. Batch production of 6-inch uniform monolayer molybdenum disulfide catalyzed by sodium in glass. Nat. Commun. 2018, 9, 979. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-H.; Wu, D.; Lin, S.-H.; Xie, C.; Yuan, H.-Y.; Lu, W.; Lau, S.P.; Chai, Y.; Luo, L.-B.; Li, Z.-J.; et al. Controlled Synthesis of 2D Palladium Diselenide for Sensitive Photodetector Applications. Adv. Funct. Mater. 2019, 29, 1806878. [Google Scholar] [CrossRef]
- Zhou, L.; Zubair, A.; Wang, Z.; Zhang, X.; Ouyang, F.; Xu, K.; Fang, W.; Ueno, K.; Li, J.; Palacios, T.; et al. Synthesis of High-Quality Large-Area Homogenous 1T‘ MoTe2 from Chemical Vapor Deposition. Adv. Mater. 2016, 28, 9526–9531. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, Q.; Gan, X.; Hu, M.; Zhang, T.; Li, C.; Kang, F.; Terrones, M.; Lv, R. Ultrasensitive Pressure Detection of Few-Layer MoS2. Adv. Mater. 2017, 29, 1603266. [Google Scholar] [CrossRef]

| Triangular | Hexagonal | Large-Area | |
|---|---|---|---|
| WSe2 | 31.8/10 | 32.4/8.5 | 32.2/10 |
| MoSe2 | 32.1/15.5 | 32.7/11 | 32.3/15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, Y.; Wang, X.; Liu, W.; He, J.; Luo, X.; Liu, J.; Liu, Y. Precise Construction and Growth of Submillimeter Two-Dimensional WSe2 and MoSe2 Monolayers. Materials 2023, 16, 4795. https://doi.org/10.3390/ma16134795
Li Y, Zhao Y, Wang X, Liu W, He J, Luo X, Liu J, Liu Y. Precise Construction and Growth of Submillimeter Two-Dimensional WSe2 and MoSe2 Monolayers. Materials. 2023; 16(13):4795. https://doi.org/10.3390/ma16134795
Chicago/Turabian StyleLi, Yuqing, Yuyan Zhao, Xiaoqian Wang, Wanli Liu, Jiazhen He, Xuemin Luo, Jinfeng Liu, and Yong Liu. 2023. "Precise Construction and Growth of Submillimeter Two-Dimensional WSe2 and MoSe2 Monolayers" Materials 16, no. 13: 4795. https://doi.org/10.3390/ma16134795
APA StyleLi, Y., Zhao, Y., Wang, X., Liu, W., He, J., Luo, X., Liu, J., & Liu, Y. (2023). Precise Construction and Growth of Submillimeter Two-Dimensional WSe2 and MoSe2 Monolayers. Materials, 16(13), 4795. https://doi.org/10.3390/ma16134795






