Author Contributions
Conceptualization, E.G. and D.K.; Methodology, K.B.-K., A.S. and D.K.; Formal analysis, K.B.-K.; Investigation, K.B.-K.; Resources, K.B.-K.; Data curation, K.B.-K.; Writing—original draft, K.B.-K.; Writing—review & editing, K.B.-K., A.S. and D.K.; Supervision, A.S. and D.K. All authors have read and agreed to the published version of the manuscript.
Figure 1.
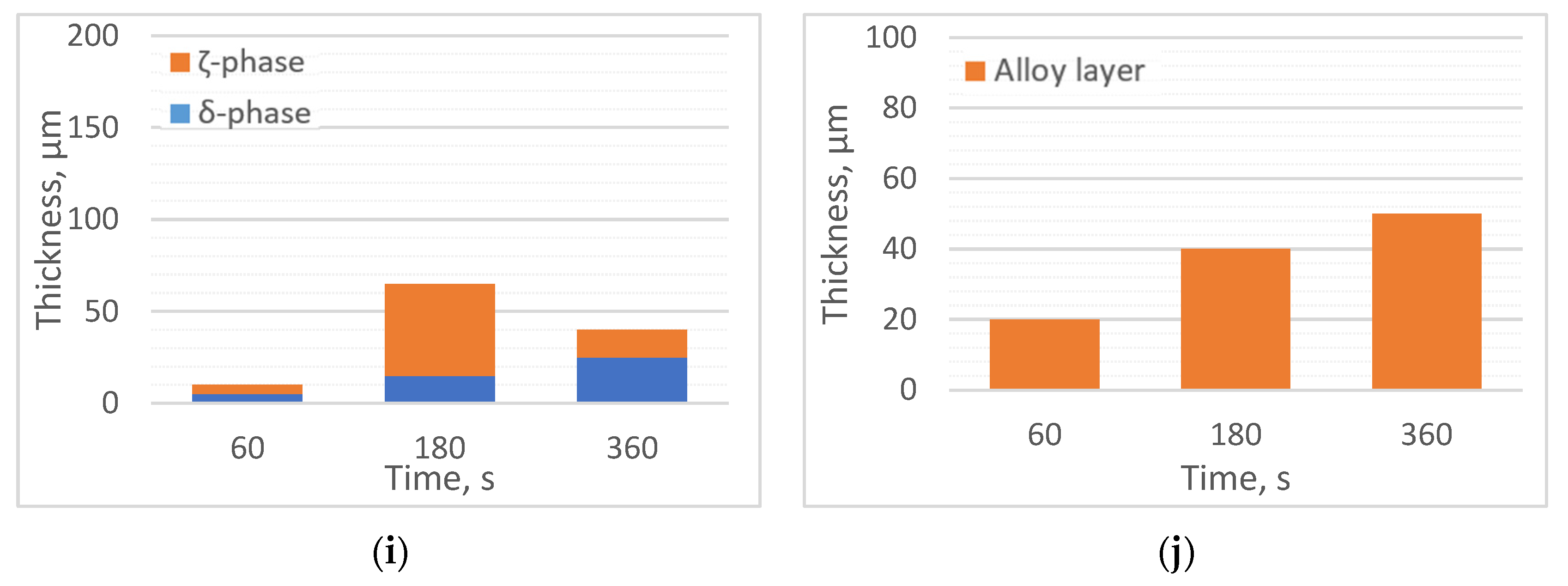
Thicknesses of alloy layers: (a) 450 °C, Bath 0; (b) 550 °C, Bath 0; (c) 450 °C, Bath A; (d) 550 °C, Bath A; (e) 450 °C, Bath B; (f) 550 °C, Bath B; (g) 450 °C, Bath C; (h) 550 °C, Bath C; (i) 450 °C, Bath D; (j) 550 °C, Bath D.
Figure 1.
Thicknesses of alloy layers: (a) 450 °C, Bath 0; (b) 550 °C, Bath 0; (c) 450 °C, Bath A; (d) 550 °C, Bath A; (e) 450 °C, Bath B; (f) 550 °C, Bath B; (g) 450 °C, Bath C; (h) 550 °C, Bath C; (i) 450 °C, Bath D; (j) 550 °C, Bath D.
Figure 2.
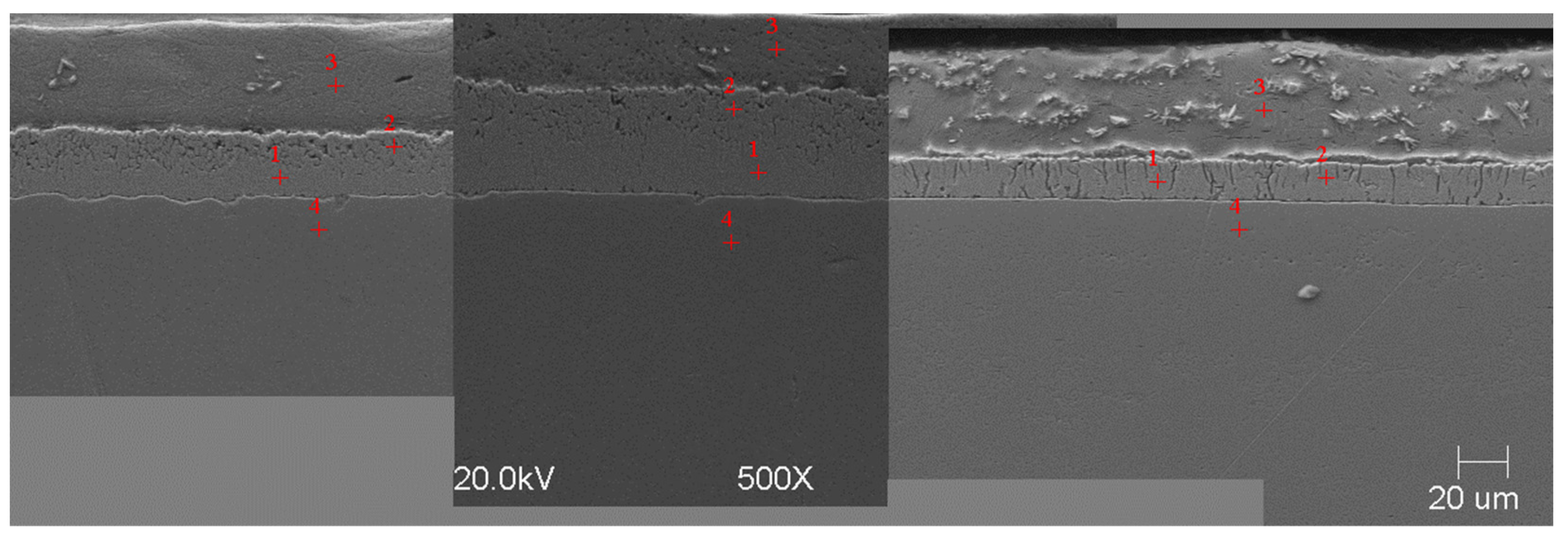
Microstructure of obtained coating in Bath 0 at 450 °C (from left—60, 180, and 360 s).
Figure 2.
Microstructure of obtained coating in Bath 0 at 450 °C (from left—60, 180, and 360 s).
Figure 3.
Microstructure of obtained coating in Bath A at 450 °C (from left—60, 180, and 360 s).
Figure 3.
Microstructure of obtained coating in Bath A at 450 °C (from left—60, 180, and 360 s).
Figure 4.
Microstructure of obtained coating in Bath B at 450 °C (from left—60, 180, and 360 s).
Figure 4.
Microstructure of obtained coating in Bath B at 450 °C (from left—60, 180, and 360 s).
Figure 5.
Microstructure of obtained coating in Bath C at 450 °C (from left—60, 180, and 360 s).
Figure 5.
Microstructure of obtained coating in Bath C at 450 °C (from left—60, 180, and 360 s).
Figure 6.
Microstructure of obtained coating in Bath 0 at 550 °C (from left—60, 180, and 360 s).
Figure 6.
Microstructure of obtained coating in Bath 0 at 550 °C (from left—60, 180, and 360 s).
Figure 7.
Microstructure of obtained coating in Bath A at 550 °C (from left—60, 180, and 360 s).
Figure 7.
Microstructure of obtained coating in Bath A at 550 °C (from left—60, 180, and 360 s).
Figure 8.
Microstructure of obtained coating in Bath B at 550 °C (from left—60, 180, and 360 s).
Figure 8.
Microstructure of obtained coating in Bath B at 550 °C (from left—60, 180, and 360 s).
Figure 9.
Microstructure of obtained coating in Bath C at 550 °C (from left—60, 180, and 360 s).
Figure 9.
Microstructure of obtained coating in Bath C at 550 °C (from left—60, 180, and 360 s).
Figure 10.
Microstructure of obtained coating in Bath D at 550 °C (from left—60, 180, and 360 s).
Figure 10.
Microstructure of obtained coating in Bath D at 550 °C (from left—60, 180, and 360 s).
Figure 11.
Results of analysis with LineScan tool—Bath B, the temperature of 450 °C, and immersion time of 180 s: (a) study area; (b) distribution of chemical composition in the study area.
Figure 11.
Results of analysis with LineScan tool—Bath B, the temperature of 450 °C, and immersion time of 180 s: (a) study area; (b) distribution of chemical composition in the study area.
Table 1.
Addition of titanium to zinc baths.
Table 1.
Addition of titanium to zinc baths.
| Bath | Addition of Titanium |
|---|
| 0 | 0 |
| A | 0.01% |
| B | 0.05% |
| C | 0.1% |
| D | 0.5% |
Table 2.
Chemical composition of steel.
Table 2.
Chemical composition of steel.
| Element | C | Mn | Si | P | S | Cr | Ni | Cu | Mo | Al | Ti | V |
|---|
| wt.% | 0.16 | 0.5 | 0.18 | 0.026 | 0.013 | 0.07 | 0.11 | 0.31 | 0.02 | 0.002 | 0.001 | 0.002 |
Table 3.
Results of chemical composition analysis in Bath 0 at 450 °C.
Table 3.
Results of chemical composition analysis in Bath 0 at 450 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Fe | Zn | Si | Fe | Zn | Si | Fe | Zn |
|---|
| 1 | 0.22 | 6.471 | 93.309 | 0.294 | 7.202 | 92.504 | 0.22 | 6.732 | 93.048 |
| 2 | 0.299 | 4.91 | 94.791 | 0.411 | 4.839 | 94.75 | 0.128 | 4.973 | 94.9 |
| 3 | 0.575 | 2.353 | 97.072 | 0.163 | 4.463 | 95.374 | 0.308 | 5.47 | 94.222 |
| 4 | 0.461 | 0.082 | 99.457 | 0.217 | 0.182 | 99.601 | 0.525 | 99.104 | 0.371 |
| 5 | | | | 0.446 | 99.228 | 0.326 | | | |
Table 4.
Results of chemical composition analysis in Bath A at 450 °C.
Table 4.
Results of chemical composition analysis in Bath A at 450 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn |
|---|
| 1 | 0.308 | 0.399 | 9.479 | 89.814 | 0.299 | 0.256 | 11.128 | 88.316 | 0.205 | 0.057 | 7.714 | 92.024 |
| 2 | 0.149 | 0.395 | 8.055 | 91.401 | 0.486 | 0.295 | 6.402 | 92.818 | 0.151 | 0.405 | 7.929 | 91.515 |
| 3 | 0.139 | 0.032 | 0.191 | 99.639 | 0.546 | 0.211 | 7.16 | 92.082 | 0.156 | 0.083 | 6.519 | 93.242 |
| 4 | 0.393 | 0.092 | 97.918 | 1.598 | 0.212 | 0.35 | 0.252 | 99.186 | 0.459 | 0.099 | 0.265 | 99.177 |
| 5 | 0.375 | 0.189 | 0.344 | 99.093 | 0.48 | 0 | 98.66 | 0.86 | 0.455 | 0.086 | 98.547 | 0.912 |
Table 5.
Results of chemical composition analysis in Bath B at 450 °C.
Table 5.
Results of chemical composition analysis in Bath B at 450 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn |
|---|
| 1 | 0.662 | 0.277 | 9.576 | 89.485 | 0.453 | 0 | 9.642 | 89.905 | 0.729 | 0.649 | 10.042 | 88.58 |
| 2 | 0.354 | 0.117 | 5.629 | 93.9 | 0.65 | 0.118 | 7.79 | 91.443 | 0.287 | 0.495 | 6.957 | 92.262 |
| 3 | 0.132 | 0.283 | 0.1 | 99.485 | 0.406 | 0.261 | 8.75 | 90.583 | 0 | 0.391 | 5.562 | 94.046 |
| 4 | 0.346 | 0.102 | 99.423 | 0.13 | 0.249 | 0.128 | 5.369 | 94.255 | 0.433 | 0.23 | 4.27 | 95.067 |
| 5 | | | | | 0.177 | 0.677 | 5.028 | 94.117 | 0.119 | 3.449 | 6.141 | 90.29 |
| 6 | | | | | 0.253 | 1.849 | 3.683 | 94.214 | 0.187 | 2.837 | 6.375 | 90.6 |
| 7 | | | | | 0.142 | 0.273 | 0.11 | 99.475 | 0.208 | 0.449 | 0.539 | 98.804 |
| 8 | | | | | 0.532 | 0.238 | 99.23 | 0 | 0.55 | 0.025 | 98.442 | 0.984 |
Table 6.
Results of chemical composition analysis in Bath C at 450 °C.
Table 6.
Results of chemical composition analysis in Bath C at 450 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn |
|---|
| 1 | 0.432 | 0.121 | 9.929 | 89.519 | 0.245 | 0.093 | 11.009 | 88.653 | 0.182 | 0.257 | 9.778 | 89.783 |
| 2 | 0.638 | 0.259 | 6.328 | 92.776 | 0.388 | 0.131 | 7.234 | 92.246 | 0.672 | 0.251 | 6.215 | 92.863 |
| 3 | 0.128 | 0.123 | 0.339 | 99.411 | 0.461 | 0.182 | 6.178 | 93.179 | 0 | 4.016 | 5.542 | 90.443 |
| 4 | 0.779 | 0.148 | 98.513 | 0.56 | 0.167 | 3.128 | 6.031 | 90.674 | 0.198 | 3.374 | 5.972 | 90.456 |
| 5 | | | | | 0.516 | 0.217 | 0.148 | 99.065 | 0.038 | 0.379 | 0.289 | 99.295 |
| 6 | | | | | 0.287 | 0.108 | 99.29 | 0.315 | 0.459 | 0.026 | 98.601 | 0.915 |
Table 7.
Results of chemical composition analysis in Bath 0 at 550 °C.
Table 7.
Results of chemical composition analysis in Bath 0 at 550 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Fe | Zn | Si | Fe | Zn | Si | Fe | Zn |
|---|
| 1 | 0.194 | 7.805 | 92 | 0.354 | 7.823 | 91.823 | 0.357 | 8.156 | 91.486 |
| 2 | 0.389 | 6.491 | 93.119 | 0.401 | 6.962 | 92.636 | 0.098 | 7.161 | 92.741 |
| 3 | 0.322 | 0.31 | 99.365 | 0.391 | 0.424 | 99.184 | 0.295 | 0.053 | 99.653 |
| 4 | 0.484 | 98.935 | 0.582 | 0.24 | 98.706 | 1.054 | 0.573 | 98.849 | 0.578 |
Table 8.
Results of chemical composition analysis in Bath A at 550 °C.
Table 8.
Results of chemical composition analysis in Bath A at 550 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn |
|---|
| 1 | 0.204 | 0.144 | 10.351 | 89.301 | 0.272 | 0.15 | 9.614 | 89.965 | 0.121 | 0.035 | 7.677 | 92.168 |
| 2 | 0.179 | 0.296 | 7.006 | 92.519 | 0.087 | 0.034 | 6.899 | 92.98 | 0.092 | 0.091 | 5.164 | 94.653 |
| 3 | 0.144 | 0.424 | 6.244 | 93.188 | 0.153 | 0.373 | 5.598 | 93.871 | 0.172 | 0.084 | 5.4 | 94.344 |
| 4 | 0.1 | 0.058 | 0.161 | 99.681 | 0.089 | 0.1 | 0.111 | 99.699 | 0.179 | 0.03 | 5.153 | 94.637 |
| 5 | 1.088 | | 98.912 | | 1.25 | | 98.75 | | 0.135 | 0.022 | 0.228 | 99.615 |
| 6 | | | | | | | | | 0.037 | 0.213 | 4.421 | 95.329 |
| 7 | | | | | | | | | 1.212 | 0.105 | 98.192 | 0.491 |
| 8 | | | | | | | | | 0.222 | 0.049 | 7.723 | 92.006 |
Table 9.
Results of chemical composition analysis in Bath B at 550 °C.
Table 9.
Results of chemical composition analysis in Bath B at 550 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn |
|---|
| 1 | 0.668 | 0.084 | 11.577 | 87.671 | 0.443 | 0.486 | 9.166 | 89.904 | 0.281 | 0.43 | 8.966 | 90.324 |
| 2 | 0.47 | 0.536 | 9.031 | 89.963 | 0.34 | 0.135 | 10.14 | 89.385 | 0.189 | 0.264 | 6.872 | 92.676 |
| 3 | 0.75 | 0.072 | 98.987 | 0.19 | 0.345 | 0.061 | 0.6 | 98.994 | 0 | 0.634 | 0.391 | 98.974 |
| 4 | 0.238 | 0.246 | 6.699 | 92.817 | 0.393 | 0.102 | 98.812 | 0.693 | 0.392 | 0.361 | 98.437 | 0.81 |
| 5 | 0.618 | 0.245 | 0.479 | 98.658 | | | | | | | | |
| 6 | 0.111 | 0.537 | 0.371 | 98.981 | | | | | | | | |
| 7 | 0.224 | 0.394 | 0.974 | 98.408 | | | | | | | | |
| 8 | 0.337 | 0.139 | 0.399 | 99.124 | | | | | | | | |
Table 10.
Results of chemical composition analysis in Bath C at 550 °C.
Table 10.
Results of chemical composition analysis in Bath C at 550 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn |
|---|
| 1 | 0.264 | 0.453 | 11.361 | 87.922 | 0.149 | 0.022 | 8.022 | 91.807 | 0.284 | 0.143 | 9.705 | 89.867 |
| 2 | 0.314 | 0.229 | 0.881 | 98.577 | 0.391 | 3.352 | 6.582 | 89.676 | 0.367 | 0.127 | 98.739 | 0.766 |
| 3 | 0.149 | 0.135 | 99.379 | 0.337 | 0.247 | 0.119 | 98.878 | 0.756 | 0.432 | 0.138 | 7.107 | 92.323 |
| 4 | | | | | 0.121 | 0.143 | 0.348 | 99.387 | 0.373 | 0.184 | 0.801 | 98.642 |
| 5 | | | | | | | | | 0.302 | 0.277 | 11.755 | 87.666 |
Table 11.
Results of chemical composition analysis in Bath D at 550 °C.
Table 11.
Results of chemical composition analysis in Bath D at 550 °C.
| | 60 s | 180 s | 360 s |
|---|
| | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn | Si | Ti | Fe | Zn |
|---|
| 1 | 0.346 | 0.229 | 11.409 | 88.016 | 0.399 | 0.253 | 8.915 | 90.433 | 0.646 | 0.19 | 8.632 | 90.532 |
| 2 | 0.169 | 3.823 | 6.191 | 89.818 | 0.47 | 3.9 | 6.635 | 88.996 | 0.73 | 3.516 | 6.014 | 89.74 |
| 3 | 0.171 | 0.909 | 0.664 | 98.256 | 0.32 | 0 | 98.989 | 0.691 | 0.743 | 0.04 | 98.357 | 0.86 |
| 4 | 0.534 | 0.114 | 99.02 | 0.331 | 0.058 | 0.335 | 0.456 | 99.152 | 0.614 | 0.352 | 0.453 | 98.581 |
Table 12.
Spot analyses of chemical compositions of observed Zn-Fe-Ti phase.
Table 12.
Spot analyses of chemical compositions of observed Zn-Fe-Ti phase.
| Measuring Point | Si | Ti | Fe | Zn |
|---|
| Figure 4, time 180 s, Point 6 | 0.253 | 1.849 | 3.683 | 94.214 |
| Figure 4, time 360 s, Point 6 | 0.187 | 2.837 | 6.375 | 90.6 |
| Figure 5, time 180 s, Point 4 | 0.167 | 3.128 | 6.031 | 90.674 |
| Figure 5, time 360 s, Point 3 | 0 | 4.016 | 5.542 | 90.443 |
| Figure 9, time 180 s, Point 2 | 0.391 | 3.352 | 6.582 | 89.676 |
| Figure 10, time 60 s, Point 2 | 0.169 | 3.823 | 6.191 | 89.818 |
| Figure 10, time 180 s, Point 2 | 0.47 | 3.9 | 6.635 | 88.996 |
| Figure 10, time 360 s, Point 2 | 0.73 | 3.516 | 6.014 | 89.74 |