Study on Electrochemical Performance of MnO@rGO/Carbon Fabric-Based Wearable Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
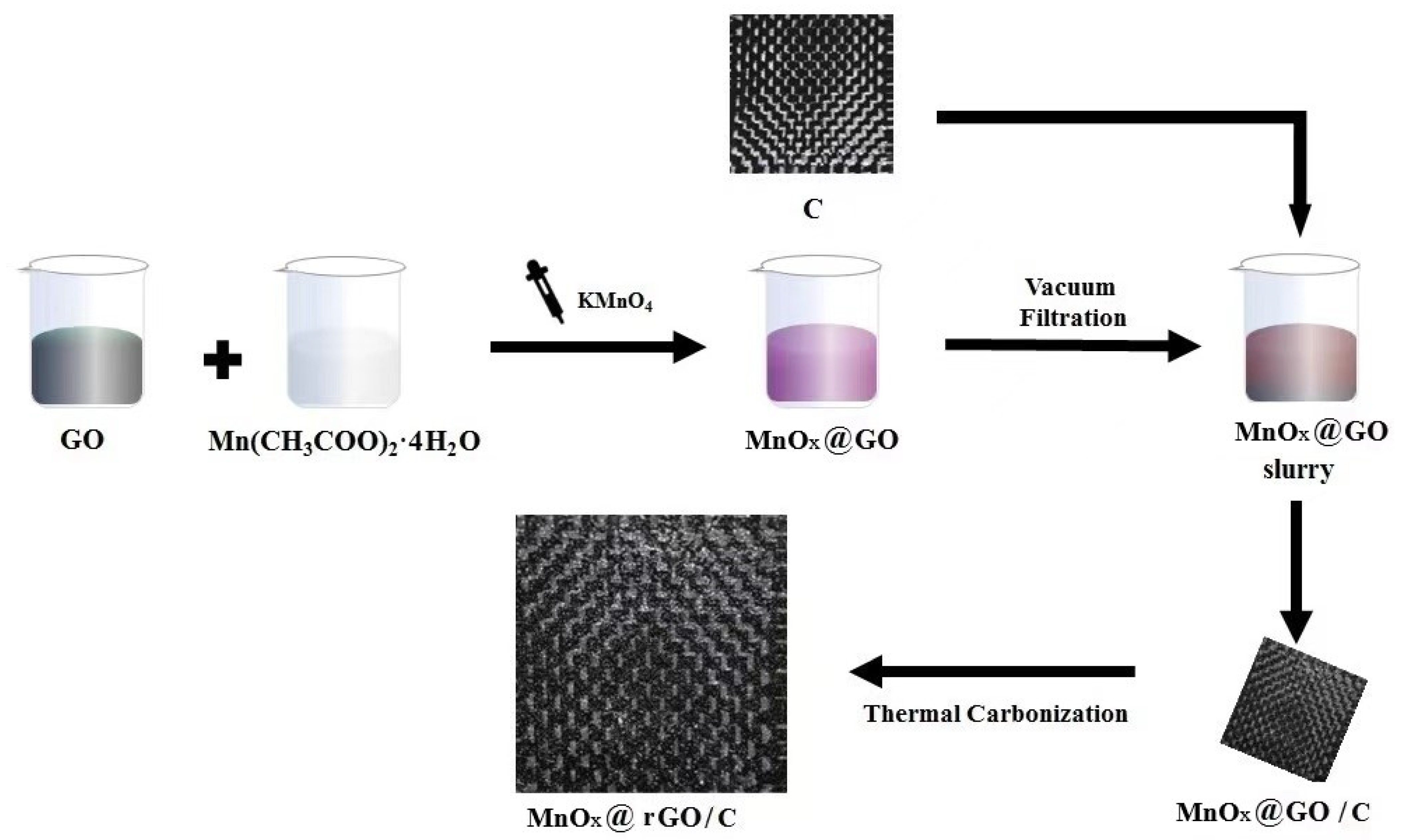
2.2. Preparation of MnOx@GO Slurry
2.3. Preparation of MnOx@rGO/C
2.4. Preparation of MnOx@rGO/C Electrodes and Supercapacitors
2.5. Characterizations
2.6. Characterizations of Electrochemical Performances
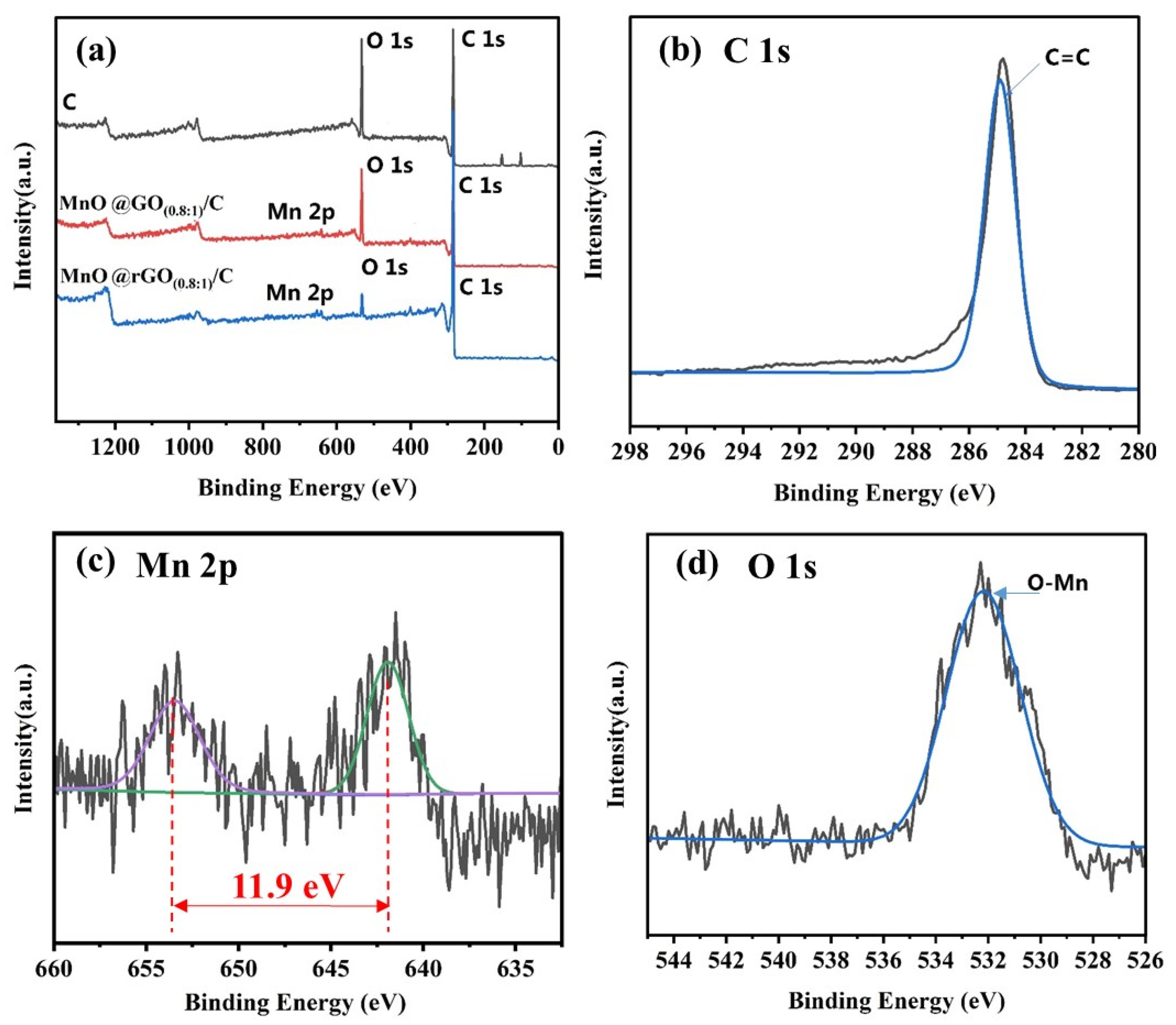
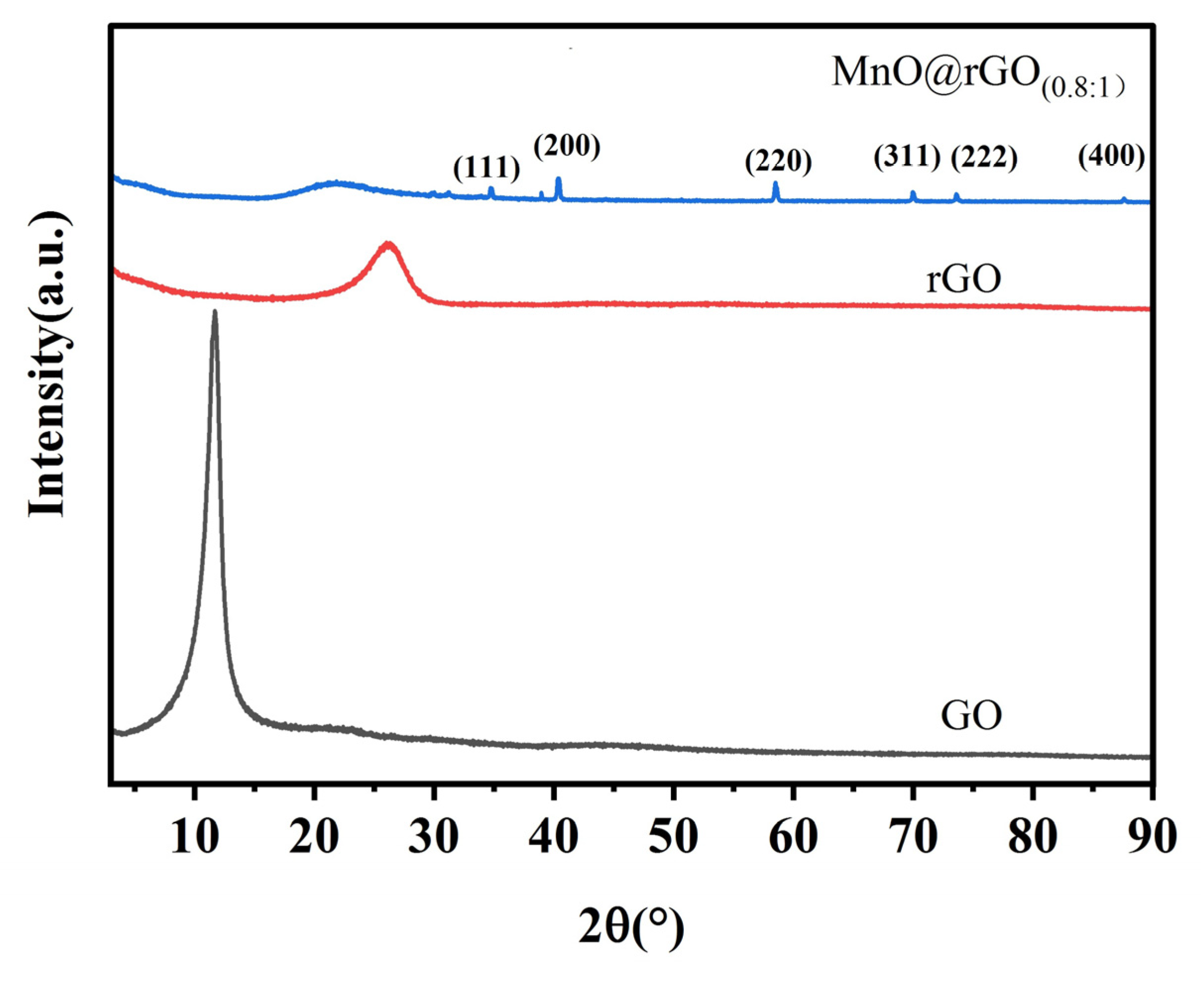
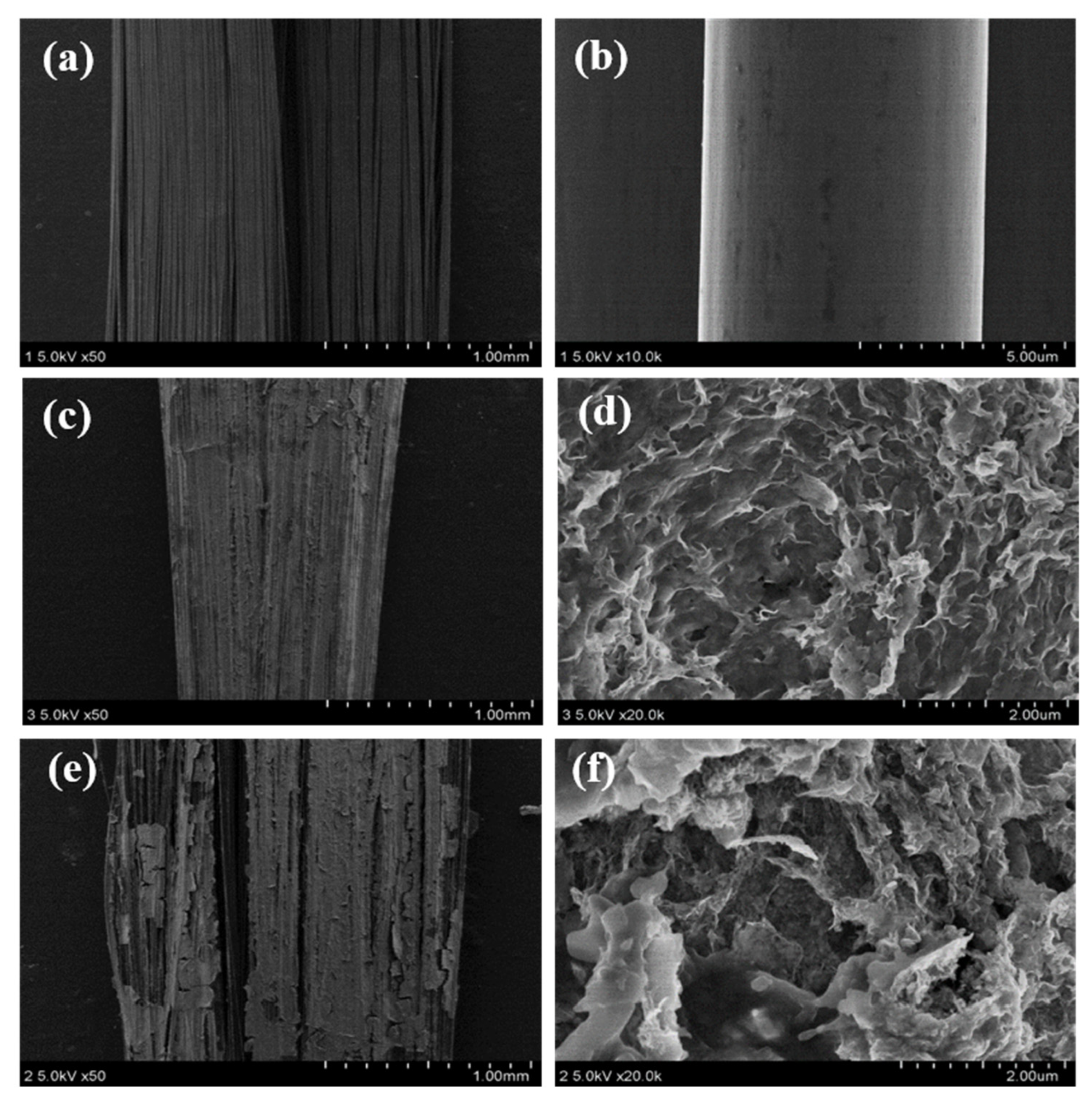
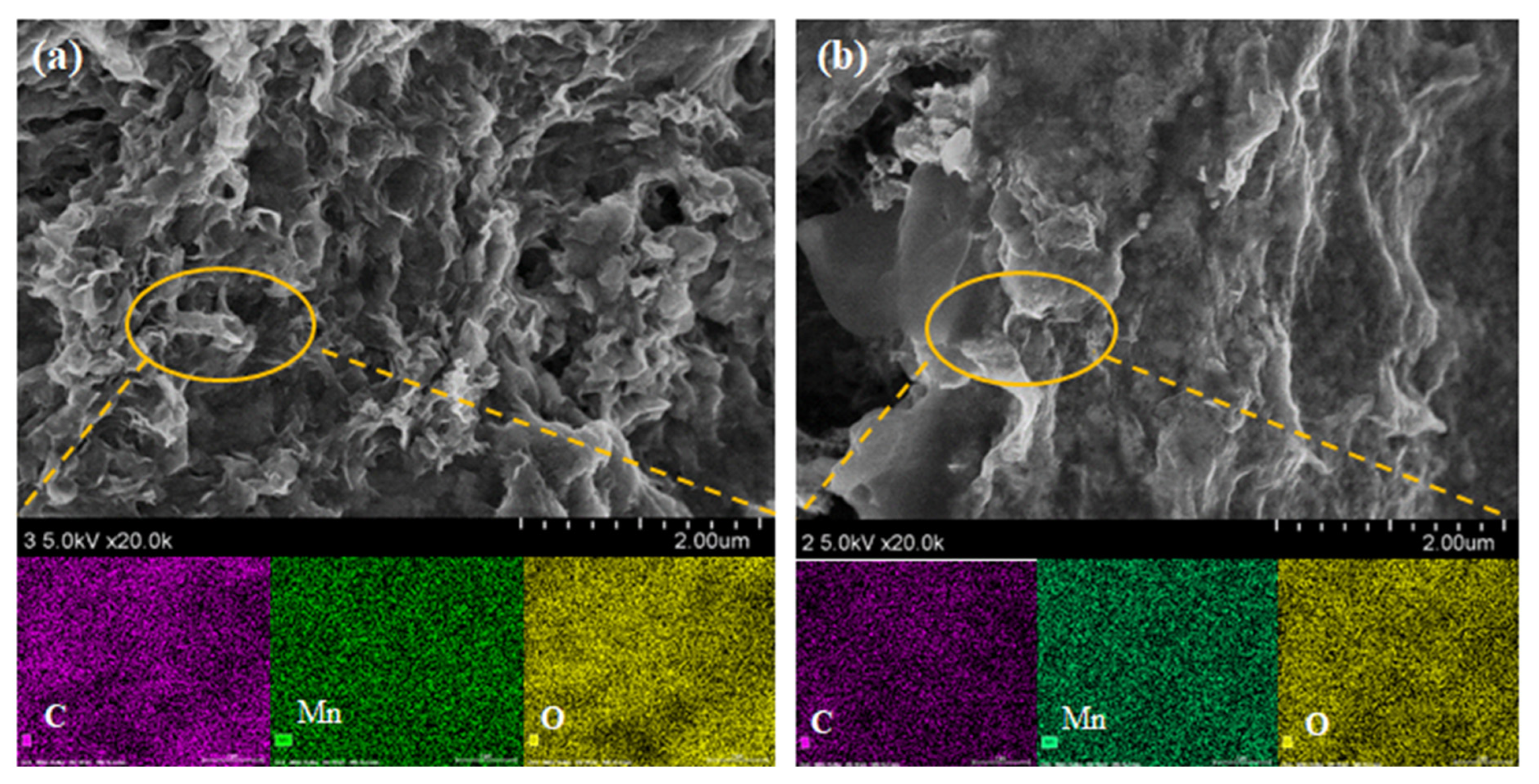
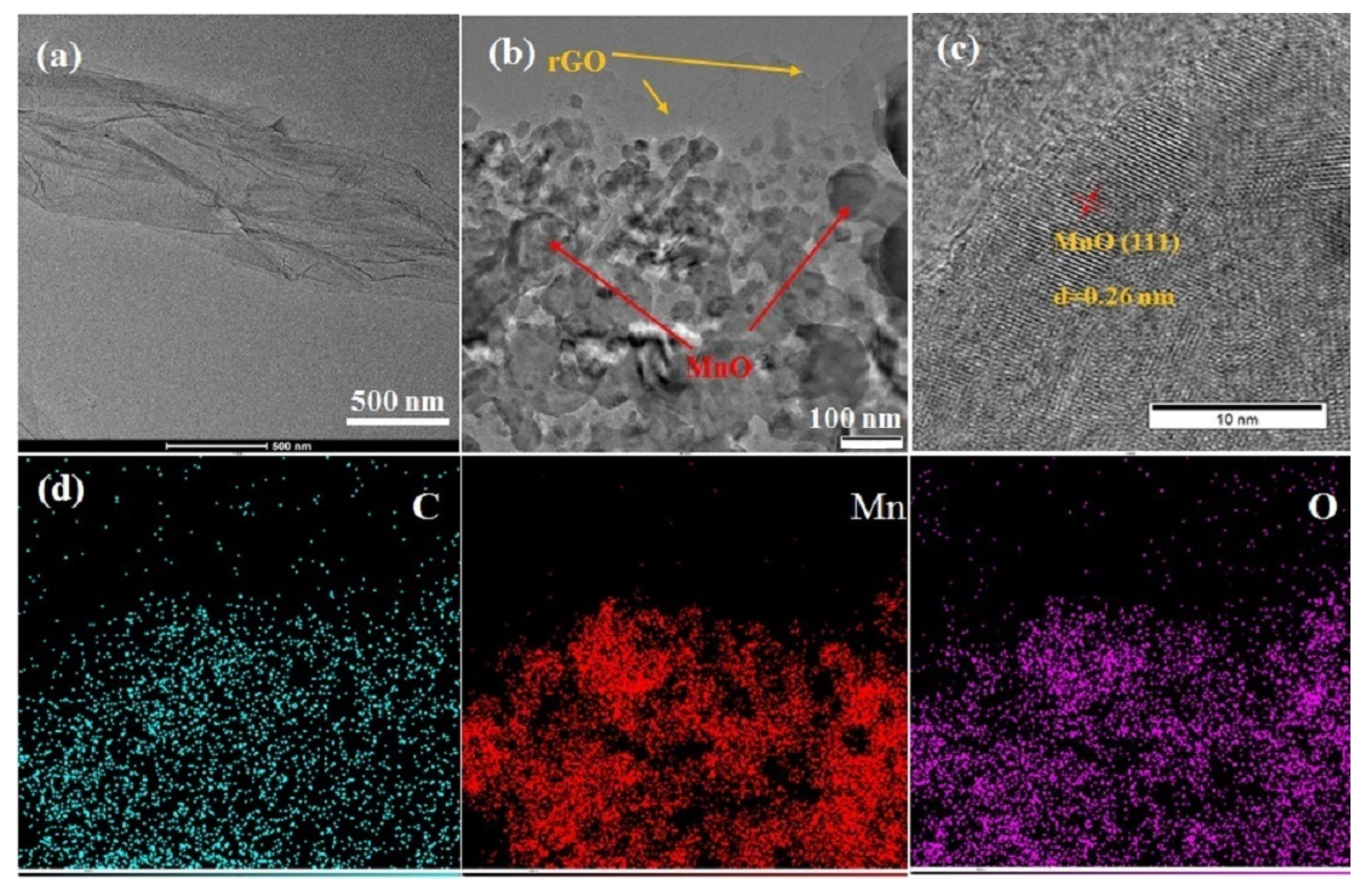
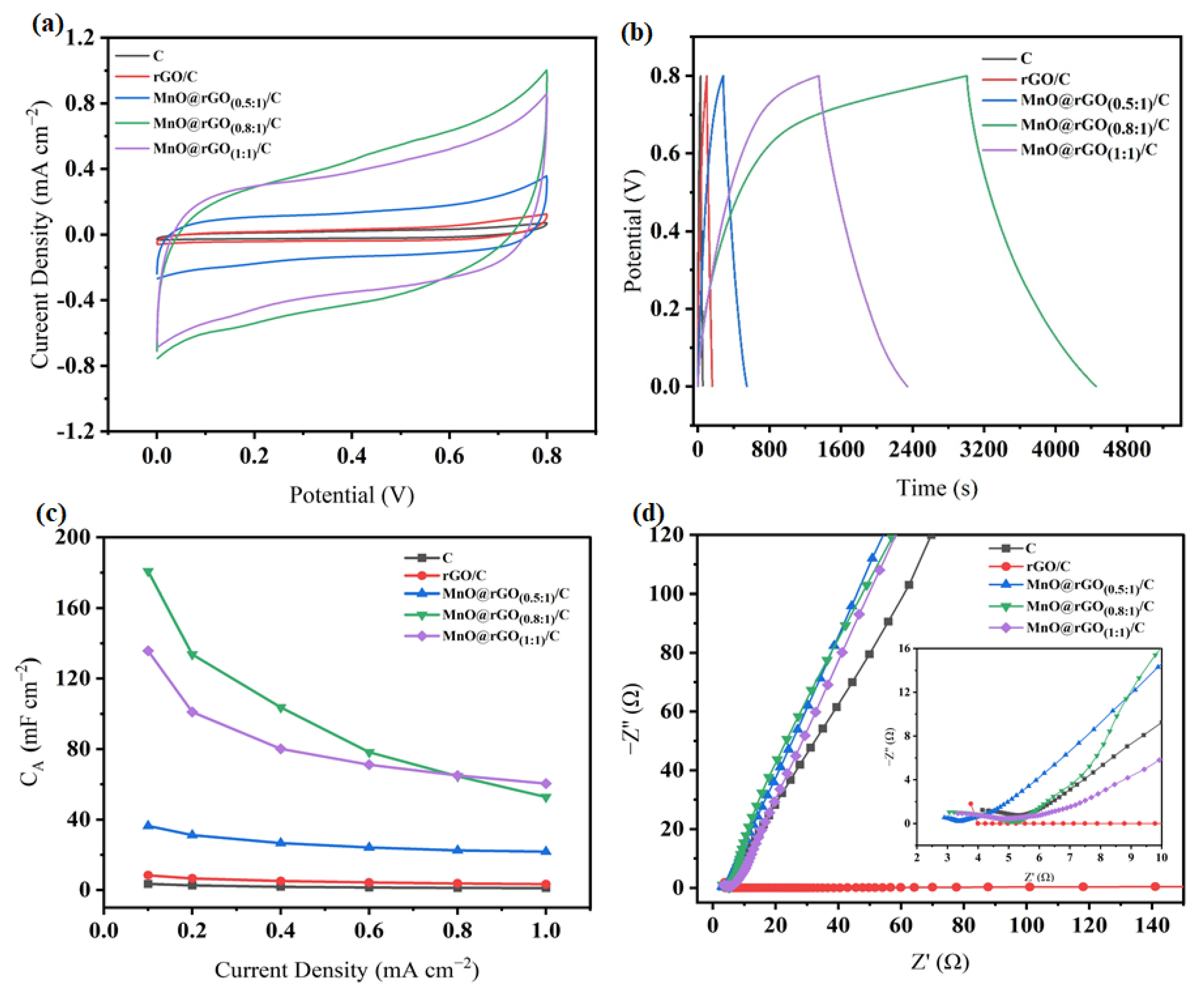
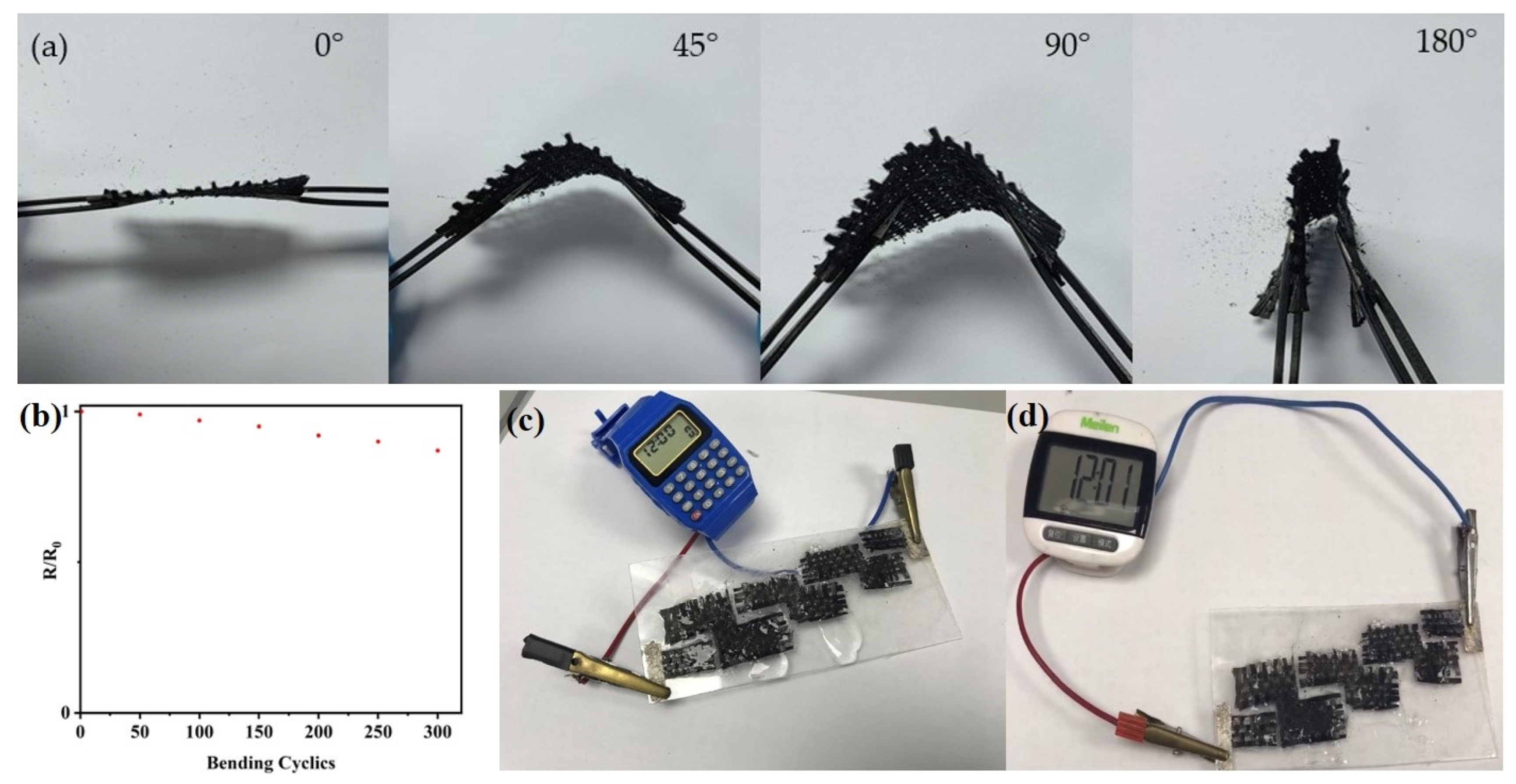
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.; Yang, J.; Lin, J.; Wang, X.; Chen, D.; Jiao, L.; Zhang, Q.; Hou, Y.; Ye, Z.; Lu, J. Mesoporous Co0.85Se nanowire arrays for flexible asymmetric supercapacitors with high energy and power densities. J. Energy Storage 2023, 65, 107360. [Google Scholar] [CrossRef]
- Asl, M.S.; Hadi, R.; Salehghadimi, L.; Tabrizi, A.G.; Farhoudian, S.; Babapoor, A.; Pahlevani, M. Flexible all-solid-state supercapacitors with high capacitance, long cycle life, and wide operational potential window: Recent progress and future perspectives. J. Energy Storage 2022, 50, 104223. [Google Scholar] [CrossRef]
- Gao, D.; Luo, Z.; Liu, C.; Fan, S. A survey of hybrid energy devices based on supercapacitors. Green Energy Environ. 2022. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Ruan, Y.; Wang, J.; Han, X.; Sun, X. Electrostatic self-assembly of citrus based carbon nanosheets and MXene: Flexible film electrodes and patterned interdigital electrodes for all-solid supercapacitors. J. Energy Storage 2023, 58, 106392. [Google Scholar] [CrossRef]
- Sun, C.; Guo, Z.; Zhou, M.; Li, X.; Cai, Z.; Ge, F. Heteroatoms-doped porous carbon electrodes with three-dimensional self-supporting structure derived from cotton fabric for high-performance wearable supercapacitors. J. Power Sources 2020, 482, 228934. [Google Scholar] [CrossRef]
- Shabeeba, A.; Rajan, L.; Sidheekha, M.P.; Thayyil, M.S.; Ismail, Y.A. Polypyrrole/hydrogel hybrid films as multi sensing supercapacitor electrodes. J. Energy Storage 2022, 55, 105724. [Google Scholar] [CrossRef]
- Ghasemi, M.; Fahimi, Z.; Moradlou, O.; Sovizi, M.R. Porous gel polymer electrolyte for the solid state metal oxide supercapacitor with a wide potential window. J. Taiwan Inst. Chem. Eng. 2021, 118, 223–231. [Google Scholar] [CrossRef]
- Eom, S.; Jung, J.; Kim, D.H. One-Pot Synthesis of Nanostructured Ni@Ni(OH)2 and Co-Doped Ni@Ni(OH)2 via Chemical Reduction Method for Supercapacitor Applications. Materials 2022, 16, 380. [Google Scholar] [CrossRef]
- Ma, C.; Ruan, S.; Wang, J.; Long, D.; Qiao, W.; Ling, L. Free-standing carbon nanofiber fabrics for high performance flexible supercapacitor. J. Colloid Interface Sci. 2018, 531, 513–522. [Google Scholar] [CrossRef]
- Dai, P.; Zhang, S.; Liu, H.; Yan, L.; Gu, X.; Li, L.; Liu, D.; Zhao, X. Cotton fabrics-derived flexible nitrogen-doped activated carbon cloth for high-performance supercapacitors in organic electrolyte. Electrochim. Acta 2020, 354, 136717. [Google Scholar] [CrossRef]
- Jin, X.; Wang, H.; Liu, Y.; Wang, H.; Wang, W.; Lin, T. Hydrogen-bonding power interfacial load transfer of carbon fabric/polypyrrole composite pseudosupercapacitor electrode with improved electrochemical stability. Appl. Surf. Sci. 2018, 470, 783–791. [Google Scholar] [CrossRef]
- Li, B.; Sasikala, S.P.; Kim, D.H.; Bak, J.; Kim, I.-D.; Cho, E.; Kim, S.O. Fe-N4 complex embedded free-standing carbon fabric catalysts for higher performance ORR both in alkaline & acidic media. Nano Energy 2019, 56, 524–530. [Google Scholar] [CrossRef]
- Wang, M.; Huang, J.; Li, S.; Ni, Y.; Dong, X.; Wang, X.; Chen, Z.; Li, X.; Cai, W.; Lai, Y. A sandwich-like structured superhydrophobic fabric for versatile and highly efficient emulsion separation. Sep. Purif. Technol. 2021, 275, 119253. [Google Scholar] [CrossRef]
- Shi, T.; Chen, M.; Zhang, C.; Mao, Z.; Liang, J.; Liu, Z.; Zhang, J.; Zhang, Q.; Pan, L.; Wang, Y.; et al. Modifying carbon fiber fabric for flexible thermoelectric energy conversion. Appl. Surf. Sci. 2023, 610, 155479. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; Cai, Z.; Ge, F. Free-standing carbon electrode materials with three-dimensional hierarchically porous structure derived from waste dyed silk fabrics. Mater. Res. Bull. 2018, 107, 355–360. [Google Scholar] [CrossRef]
- Lee, M.E.; Jang, D.; Lee, S.; Yoo, J.; Choi, J.; Jin, H.-J.; Lee, S.; Cho, S.Y. Silk Protein-Derived carbon fabric as an electrode with high Electro-Catalytic activity for All-Vanadium redox flow batteries. Appl. Surf. Sci. 2021, 567, 150810. [Google Scholar] [CrossRef]
- Hu, L.; Zang, L.; Yang, J.; Liu, Q.; Qiao, X.; Qiu, J.; Yang, C.; Li, H. A scalable strategy for carbon derived from complex six-membered ring-like tannin on glass fiber for 1D/2D flexible all solid state supercapacitors. J. Electroanal. Chem. 2019, 856, 113693. [Google Scholar] [CrossRef]
- Artigas-Arnaudas, J.; Sánchez-Romate, X.F.; Sánchez, M.; Ureña, A. Effect of electrode surface treatment on carbon fiber based structural supercapacitors: Electrochemical analysis, mechanical performance and proof-of-concept. J. Energy Storage 2023, 59, 106599. [Google Scholar] [CrossRef]
- Yao, Z.; Quan, B.; Yang, T.; Li, J.; Gu, C. Flexible supercapacitors based on vertical graphene/carbon fabric with high rate performance. Appl. Surf. Sci. 2023, 610, 155535. [Google Scholar] [CrossRef]
- Liang, J.; Rawal, A.; Yu, M.; Xiao, K.; Liu, H.; Jiang, Y.; Lennon, A.; Wang, D.-W. Low-potential solid-solid interfacial charging on layered polyaniline anode for high voltage pseudocapacitive intercalation Li-ion supercapacitors. Nano Energy 2023, 105, 108010. [Google Scholar] [CrossRef]
- Kolathodi, M.S.; Rao, S.N.H.; Natarajan, T.S.; Singh, G. Beaded manganese oxide (Mn2O3) nanofibers: Preparation and application for capacitive energy storage. J. Mater. Chem. A 2016, 4, 7883–7891. [Google Scholar] [CrossRef]
- Zdolšek, N.; Perović, I.; Brković, S.; Tasić, G.; Milović, M.; Vujković, M. Deep Eutectic Solvent for Facile Synthesis of Mn3O4@N-Doped Carbon for Aqueous Multivalent-Based Supercapacitors: New Concept for Increasing Capacitance and Operating Voltage. Materials 2022, 15, 8540. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-W.; Xu, Y.-T.; Yang, B.; Lang, H.-Z.; Shen, Z.-X.; Wang, N.; Wang, X.-F.; Wang, S.-H.; Sun, C.-L. In-situ Co-precipitated α-MnO2@2-methylimidazole cathode material for high performance zinc ion batteries. J. Alloy. Compd. 2021, 896, 162785. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.; Wang, A.; Xiao, L.; Hou, L. Facile synthesis of N, S co-doped hierarchical porous carbon/MnO2 composites for supercapacitor electrodes via sodium alginate crosslinking. J. Alloy. Compd. 2022, 923, 166333. [Google Scholar] [CrossRef]
- Ullah, A.; Rahman, L.; Hussain, S.Z.; Abbas, W.; Tawab, A.; Jilani, A.; Bajwa, S.Z.; Khan, W.S.; Riaz, R.; Hussain, I.; et al. Mechanistic insight of dye degradation using TiO2 anchored α-MnO2 nanorods as promising sunlight driven photocatalyst. Mater. Sci. Eng. B 2021, 271, 115257. [Google Scholar] [CrossRef]
- Radhakanth, S.; Singhal, R. In–situ synthesis of MnO dispersed carbon nanofibers as binder-free electrodes for high-performance supercapacitors. Chem. Eng. Sci. 2023, 265, 118224. [Google Scholar] [CrossRef]
- Zhao, N.; Deng, L.; Luo, D.; Zhang, P. One-step fabrication of biomass-derived hierarchically porous carbon/MnO nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci. 2020, 526, 146696. [Google Scholar] [CrossRef]
- Xiao, L.; Jia, L.; Zhao, S.; Tang, X.; Zhu, C.; Huang, H.; Jiang, J.; Li, M. Solvent-free synthesis of sheet-like carbon coated MnO with three-dimensional porous structure for simultaneous detection of dopamine and uric acid. J. Electroanal. Chem. 2020, 858, 113823. [Google Scholar] [CrossRef]
- Nie, J.; Fu, H.; Li, Z.; Yao, S. Using Prussian blue as a self-sacrificial template to construct MnO/MnFe2O4 microcubes as anodes for lithium-ion batteries. J. Alloy. Compd. 2021, 882, 160693. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Liu, Y.; Li, J. Selective electrochemical detection of dopamine using nitrogen-doped graphene/manganese monoxide composites. RSC Adv. 2015, 5, 85065–85072. [Google Scholar] [CrossRef]
- Guo, W.; Guo, X.; Yang, L.; Wang, T.; Zhang, M.; Duan, G.; Liu, X.; Li, Y. Synthetic melanin facilitates MnO supercapacitors with high specific capacitance and wide operation potential window. Polymer 2021, 235, 124276. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.; Weng, W. High-performance supercapacitor based on MnO/carbon nanofiber composite in extended potential windows. Electrochim. Acta 2021, 370, 137713. [Google Scholar] [CrossRef]
- Luo, Y.; Ji, P.; Li, J.; Wu, P.; Wang, Y.; Meng, G.; Wu, J.; Yang, S.; Chen, L.; Shuang, Z.; et al. Self-supporting electrodes with in situ built aniline on carbon fibers and reduced graphene oxide covalently for stable flexible supercapacitors. J. Energy Storage 2023, 64, 106898. [Google Scholar] [CrossRef]
- Dywili, N.; Ntziouni, A.; Ndipingwi, M.M.; Ikpo, C.; Nwanya, A.C.; Kordatos, K.; Iwuoha, E. High power asymmetric supercapacitor based on activated carbon/reduced graphene oxide electrode system. Mater. Today Commun. 2023, 35, 105653. [Google Scholar] [CrossRef]
- Shruti, M.S.; Khilari, S.; Samuel, E.J.J.; Han, H.; Nayak, A.K. Recent trends in graphene assisted vanadium based nanocomposites for supercapacitor applications. J. Energy Storage 2023, 63, 107006. [Google Scholar] [CrossRef]
- BinSabt, M.H.; Galal, A.; Nazeer, A.A. Enhancement of Supercapacitor Performance of Electrochemically Grown Nickel Oxide by Graphene Oxide. Materials 2023, 16, 3068. [Google Scholar] [CrossRef]
- Maseed, H.; Srikanth, V.V.; Narayana, A.L.; Hussain, O.M.; Shaikshavali, P. MnO/few-layered-graphene composite as a high performance electrode material in aqueous supercapacitors. Mater. Lett. 2020, 277, 128370. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Z.; Huang, X.; Li, Y.; Li, H.; Zhou, B.; Liu, J.; Xu, K.; Wang, M.; Ogata, H.; et al. One-step hydrothermal synthesis of manganese oxide nanosheets with graphene quantum dots for high-performance supercapacitors. J. Energy Storage 2023, 62, 106948. [Google Scholar] [CrossRef]
- Gangwar, A.; Das, T.; Shaw, S.; Prasad, N. Nanocomposite of (α-Mn3O4/MnO)@rGO as a high performance electrode material for supercapacitors. Electrochim. Acta 2021, 390, 138823. [Google Scholar] [CrossRef]
- Li, Z.; Mi, Y.; Liu, X.; Liu, S.; Yang, S.; Wang, J. Flexible graphene/MnO2 composite papers for supercapacitor electrodes. J. Mater. Chem. 2011, 21, 14706–14711. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Hou, X.; Ye, H.; Guo, Z.; Chen, Z.; Jin, Y.; Du, Y.; Ren, P. MnO decorated biomass derived carbon based on hyperaccumulative characteristics as advanced electrode materials for high-performance supercapacitors. Diam. Relat. Mater. 2023, 136, 109888. [Google Scholar] [CrossRef]
- Martínez-Galera, A.J.; Guo, H.; Jiménez-Sánchez, M.D.; Michel, E.G.; Gómez-Rodríguez, J.M. Dirac cones in graphene grown on a half-filled 4d-band transition metal. Carbon 2023, 205, 294–301. [Google Scholar] [CrossRef]
- Gong, X.; Li, R.; Chen, H.; He, C.; Gao, Z.; Xie, H. (1 1 1)-Oriented crystalline plane MnO loaded by biomass carbon separator to facilitate sulfur redox kinetics in lithium–sulfur batteries. Arab. J. Chem. 2023, 16, 104752. [Google Scholar] [CrossRef]
- Pinilla-Sánchez, A.; Chávez-Angel, E.; Murcia-López, S.; Carretero, N.M.; Palardonio, S.M.; Xiao, P.; Rueda-García, D.; Torres, C.M.S.; Gómez-Romero, P.; Martorell, J.; et al. Controlling the electrochemical hydrogen generation and storage in graphene oxide by in-situ Raman spectroscopy. Carbon 2022, 200, 227–235. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Tan, C.; Zhang, T.; Xin, X.; Li, W.; Li, J.; Lu, R. Study of the interaction mechanism between GO/rGO and trypsin. J. Hazard. Mater. Adv. 2021, 3, 100011. [Google Scholar] [CrossRef]
- Nakayasu, Y.; Goto, Y.; Katsuyama, Y.; Itoh, T.; Watanabe, M. Highly crystalline graphite-like carbon from wood via low-temperature catalytic graphitization. Carbon Trends 2022, 8, 100190. [Google Scholar] [CrossRef]
- Bousiakou, L.G.; Qindeel, R.; Al-Dossary, O.M.; Kalkani, H. Synthesis and characterization of graphene oxide (GO) sheets for pathogen inhibition: Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. J. King Saud Univ. Sci. 2022, 34, 102002. [Google Scholar] [CrossRef]
- Munonde, T.S.; Nqombolo, A.; Hobongwana, S.; Mpupa, A.; Nomngongo, P.N. Removal of methylene blue using MnO2@rGO nanocomposite from textile wastewater: Isotherms, kinetics and thermodynamics studies. Heliyon 2023, 9, e15502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Q.; Qian, X.; Wang, X.; Zhang, K. A scalable strategy toward compliant tandem yarn-shaped supercapacitors with high voltage output. J. Mater. Chem. A 2021, 9, 13916–13925. [Google Scholar] [CrossRef]
- Abdalrahman, A.A.; Aziz, S.B.; Karim, W.O. EIS and FTIR approaches to study the ion transport parameters and relaxation dynamics of Na+1 ion in SPE based on MC polymer inserted with sodium salt. Results Phys. 2022, 36, 105439. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, Q.; Zhang, Y.; Fu, Y.; Yang, C.; Wu, F.; Li, Z.; Wei, Y.; Zhang, K. Study on Electrochemical Performance of MnO@rGO/Carbon Fabric-Based Wearable Supercapacitors. Materials 2023, 16, 4687. https://doi.org/10.3390/ma16134687
Ke Q, Zhang Y, Fu Y, Yang C, Wu F, Li Z, Wei Y, Zhang K. Study on Electrochemical Performance of MnO@rGO/Carbon Fabric-Based Wearable Supercapacitors. Materials. 2023; 16(13):4687. https://doi.org/10.3390/ma16134687
Chicago/Turabian StyleKe, Qianlan, Yuhui Zhang, Yuanheng Fu, Chenxi Yang, Fan Wu, Zhongxiu Li, Yi Wei, and Kun Zhang. 2023. "Study on Electrochemical Performance of MnO@rGO/Carbon Fabric-Based Wearable Supercapacitors" Materials 16, no. 13: 4687. https://doi.org/10.3390/ma16134687
APA StyleKe, Q., Zhang, Y., Fu, Y., Yang, C., Wu, F., Li, Z., Wei, Y., & Zhang, K. (2023). Study on Electrochemical Performance of MnO@rGO/Carbon Fabric-Based Wearable Supercapacitors. Materials, 16(13), 4687. https://doi.org/10.3390/ma16134687





