3.1. Raw Bauxite Characterization
Raw bauxite from the Timan Deposit was pre-crushed using a rod mill and subsequently classified on vibrating sieves (NKP Mekhanobr-Tekhnika, Saint-Petersburg, Russia) to achieve a particle size of 80% less than 71 μm. The crushed bauxite before the experiments was subjected to the sieve procedure to obtain three fractions: −50 μm, +50–71 μm, and +71 μm. The average particle size of each fraction was: 48 μm, 62 μm, and 87 μm. The chemical composition of these three fractions and the raw bauxite is shown in
Table 1.
According to the data presented in
Table 1, the raw bauxite is high-iron and highly siliceous. The silica modulus (the ratio of Al
2O
3 to SiO
2) of bauxite is 8.36 units, which is at the lower limit of profitability for Bayer’s method.
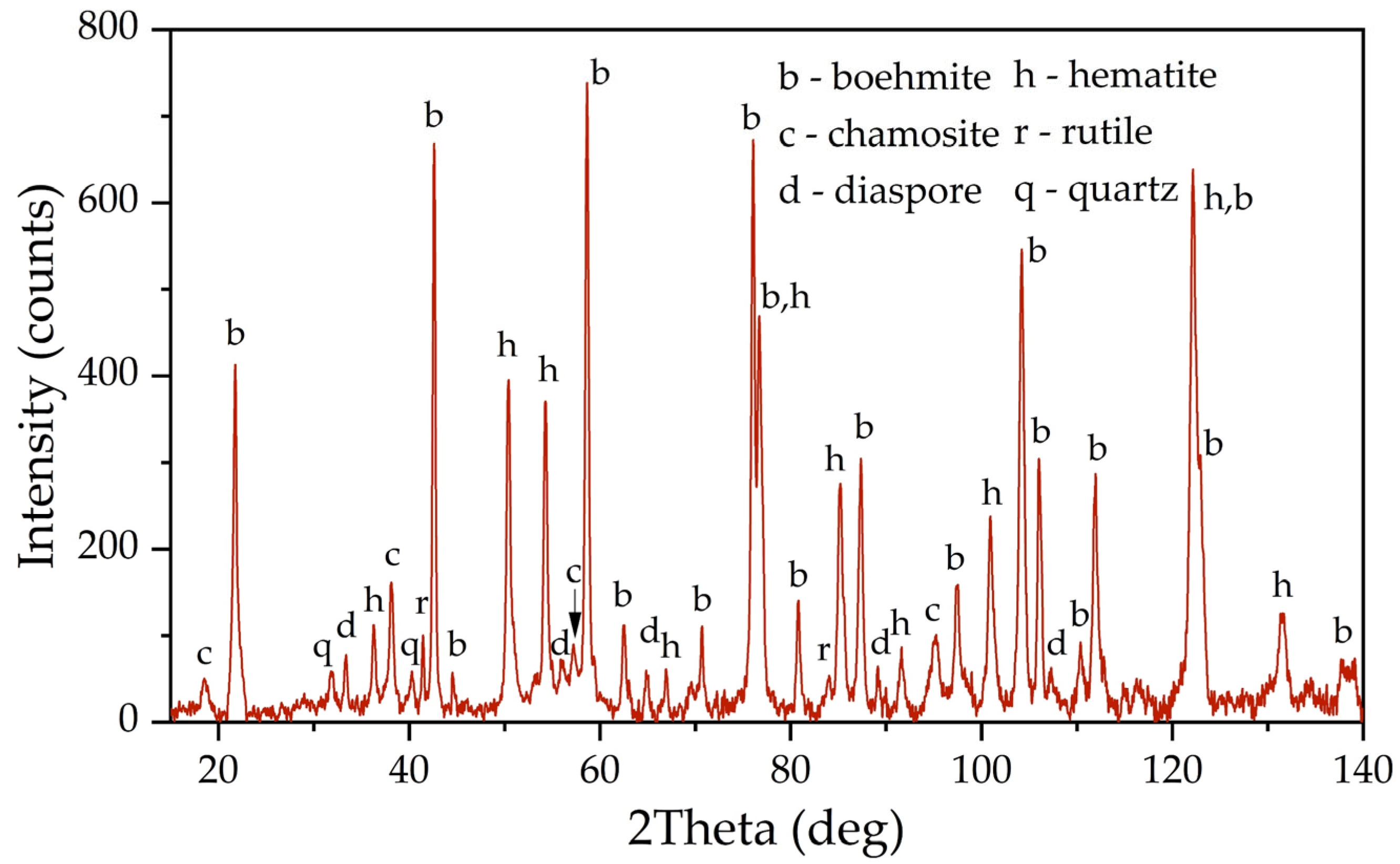
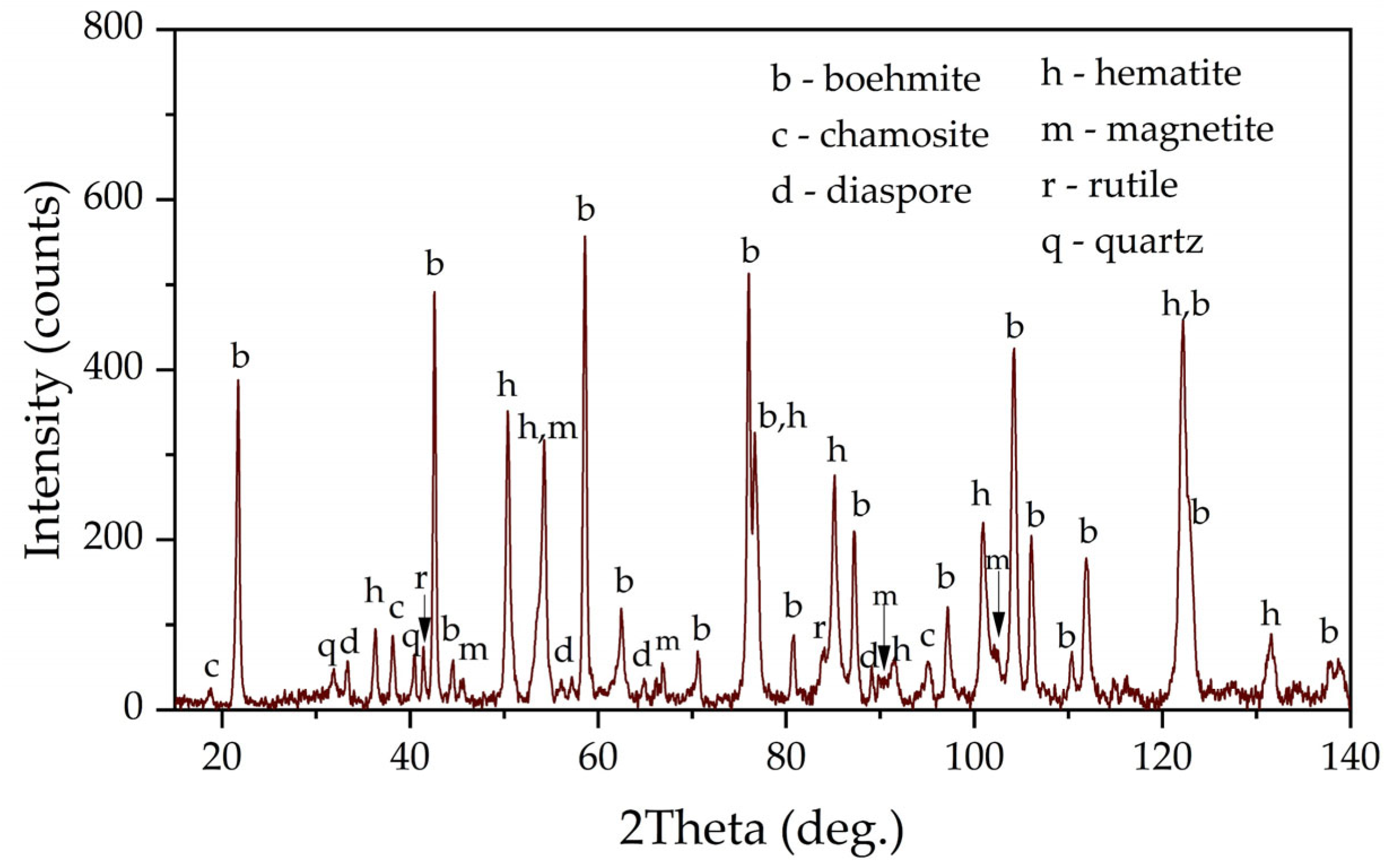
Figure 1 shows an X-ray diagram of the raw bauxite. Raw bauxite consists mainly of boehmite (AlOOH) and hematite (Fe
2O
3). Small amounts of rutile (TiO
2), quartz (SiO
2), diaspore (AlOOH), chamosite ((Fe
2⁺,Mg,Al,Fe
3⁺)
6(Si,Al)
4O
10(OH,O)
8) are also present. A semi-quantitative analysis of the crystalline phases of the bauxite sample is shown in
Table 2. According to
Table 2, more than 62% of the original bauxite is represented by boehmite, more than 25% by hematite, the rest is quartz, rutile, and chamosite. However, it should be noted that chamosite also has in its composition both alumina and silica, which may lead to subsequent problems during leaching (secondary aluminum losses due to the formation of DSP). According to the literature [
6], kaolinite is often found in high-silica bauxite, but its content in this sample of Timan bauxite was insignificant.
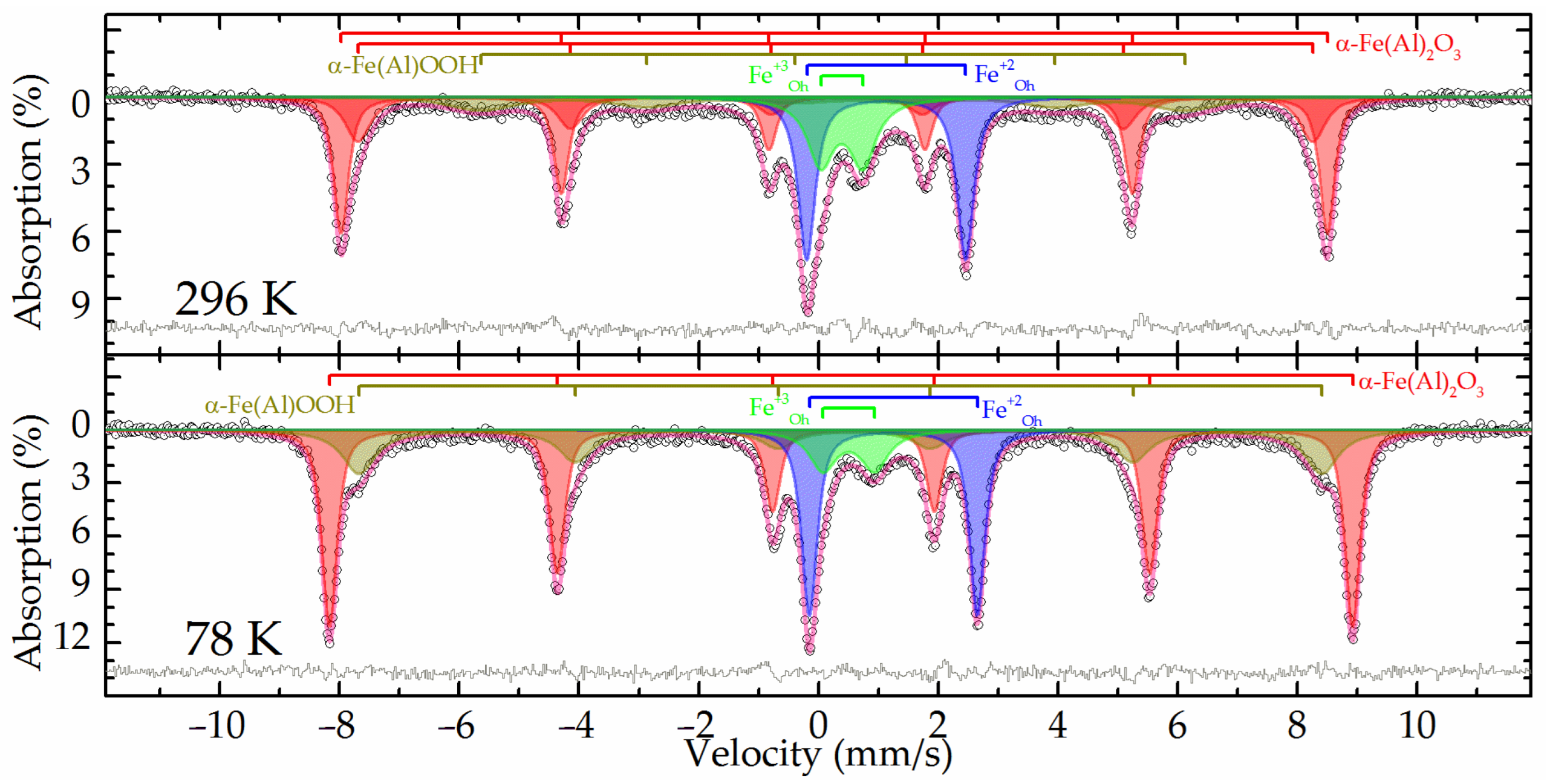
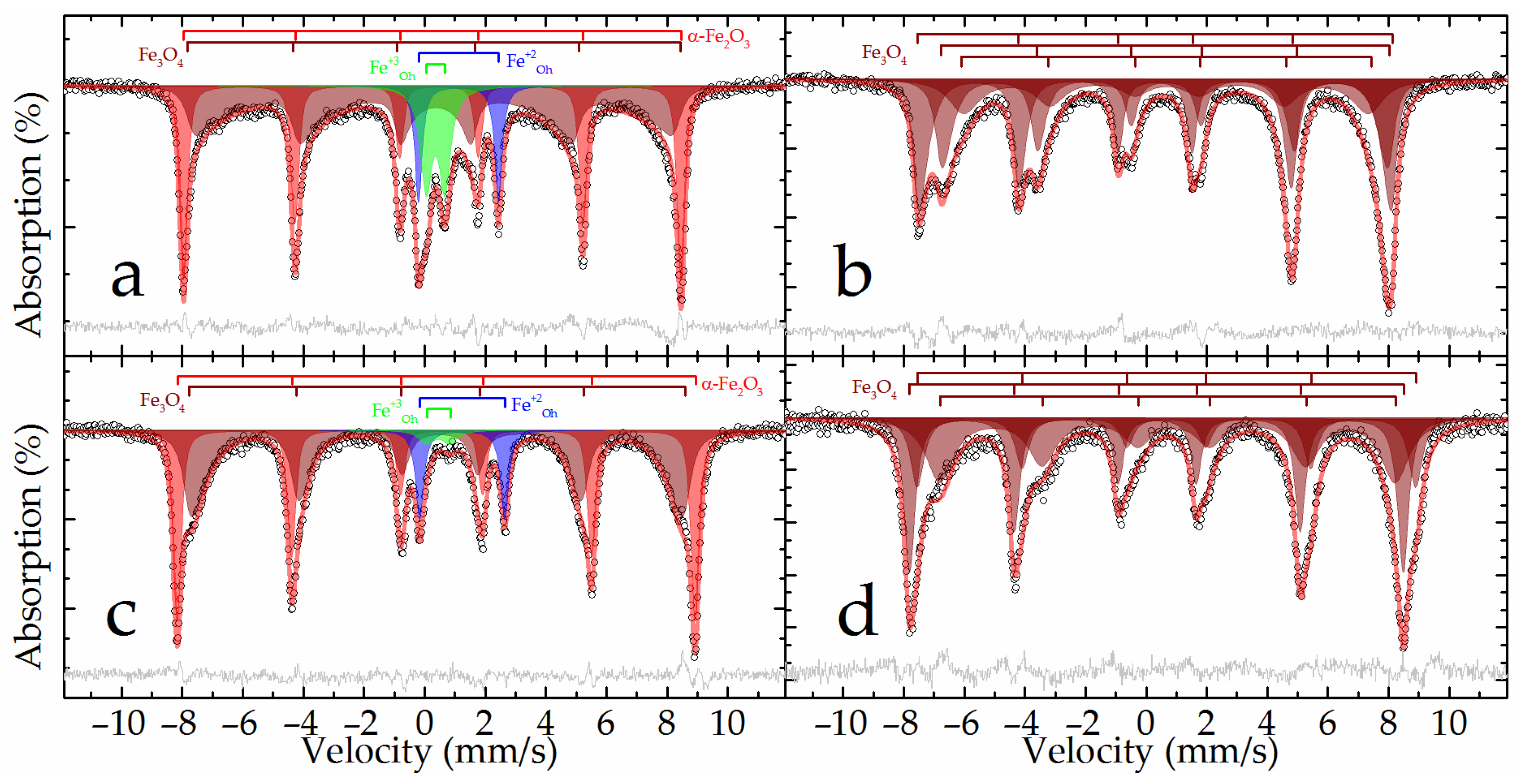
The Mössbauer spectra at both temperatures of the raw bauxite sample showed a set of rather narrow resonance lines in which the presence of a sextet and a doublet with a large quadrupole splitting was clearly distinguished (
Figure 2). The experimental spectra could be satisfactorily described by a superposition of four or five subspectra including two symmetrical doublets and two or three symmetrical sextets (
Table 3).
In the spectrum obtained at room temperature, two sextets with the maximum values of hyperfine magnetic splitting (
Table 3, subspectra 1 and 2) corresponded to hematite—α-Fe
2O
3 as well as aluminum-substituted hematite [
25]. When the sample was cooled to the boiling point of nitrogen, these two sextets combined into one sextet (
Figure 2). In this case, the value of the quadrupole shift did not change sign, which indicates the absence of the Morrin transition characteristic of pure hematite, and confirms the hypothesis of aluminohematite formation [
27]. The remaining sextet demonstrated a strong temperature dependence of both its profile and the hyperfine magnetic splitting (
Table 3, subspectrum 3). The hyperfine parameters of this subspectrum and the features of its temperature changes allow it to be attributed to aluminogoethite, which we considered in detail in [
5]. The rest of the spectrum can be described by a pair of doublets corresponding to iron atoms with charges of +3 and +2 (
Table 3, subspectra 4 and 5) in the high-spin state and octahedral oxygen environment [
28]. Considering that the intensity of subspectrum 4 (
Table 3) decreases almost twofold with decreasing temperature, it can be assumed that superparamagnetic aluminogoethite is partly responsible for the formation of this subspectrum. The rest of this subspectrum as well as subspectrum 5 obviously belonged to a layered aluminosilicate mineral, in particular, the hyperfine parameters made it possible to reliably assign them to chamosite [
29,
30,
31].
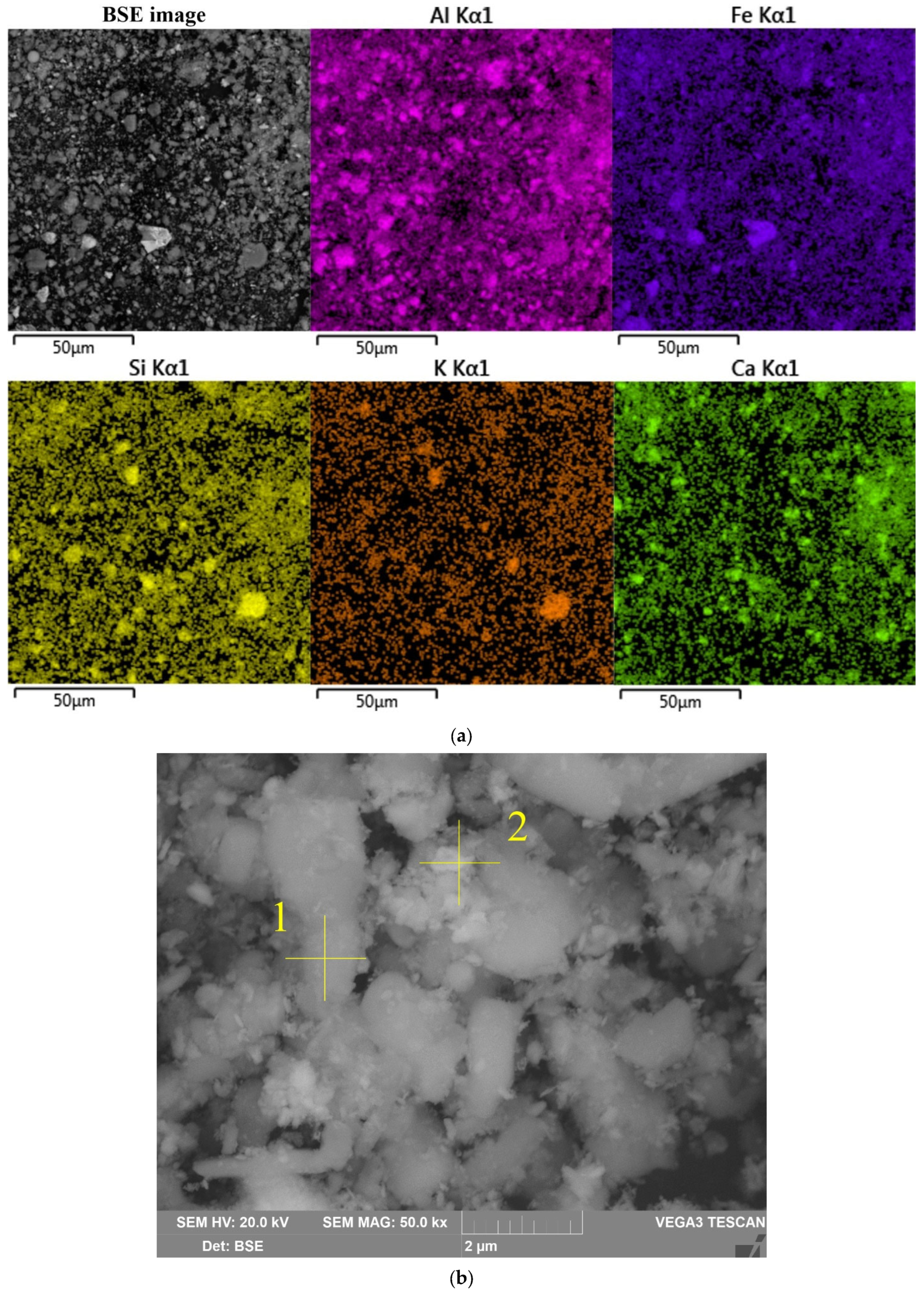
The morphology and chemical composition of the raw bauxite particles were evaluated by SEM-EDS analysis (
Figure 3,
Table 4). The SEM-EDS images in
Figure 3 show that aluminum, iron, silicon, and calcium were uniformly distributed over the surface of the bauxite particles, but single particles with a high content of these elements could be identified. Potassium has a close association with silica, indicating its content in the aluminosilicates.
Figure 3b shows that the particles of raw bauxite had an irregular shape. After grinding, it was possible to observe particles with sizes from 100 nm to 10 μm. SEM-EDS image of the boehmite and hematite particles are shown in
Table 4. There was a close relationship between them (i.e., the boehmite particles were covered with hematite particles and vice versa).
3.2. Effect of Leaching Conditions on Bauxite Desilication and Transformation of Iron Minerals on the First Stage
Modeling of the process of bauxite pretreatment with highly concentrated alkaline solutions was carried out using the SANN model.
As revealed in a previous study [
25], the use of high alkali concentrations and the L:S ratio eliminates the formation of DSP due to silicon retention in the solution. This allows for a complete extraction of alumina, even from such highly silica raw materials such as fly ash, regardless of how much silica was contained in the feedstock. Moreover, the boiling temperature of the highly concentrated NaOH solution (more than 330 g L
−1 Na
2O) exceeded 120 °C. This makes leaching at atmospheric pressure possible at temperatures above 100 °C. The matrix of experiments created with the Statistica 13 software package and the results of Al and Si extraction from the different bauxite fractions and the Fe and Na
2O content in the solid residue are shown in
Table 5.
As was shown [
32,
33], the machine learning produces more accurate models than the use of convenient methods. The closest to the experimental data SANN model (R
2 = 0.96) obtained for alumina extraction was multilayer perceptron (MLP) 6.10.4, where 6 was the number of input parameters, 10 was the number of hidden layers, and 4 the number of output layers.
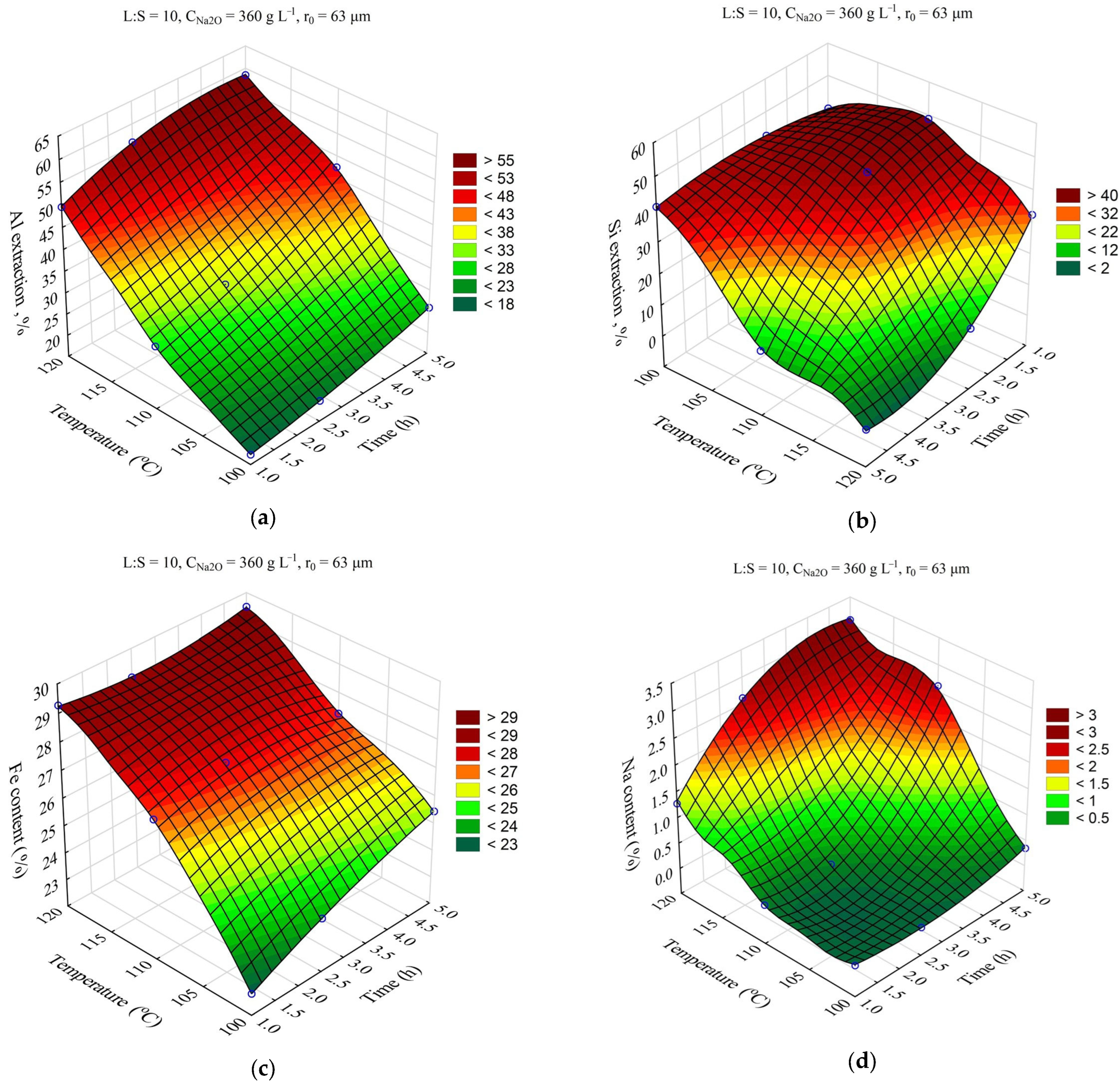
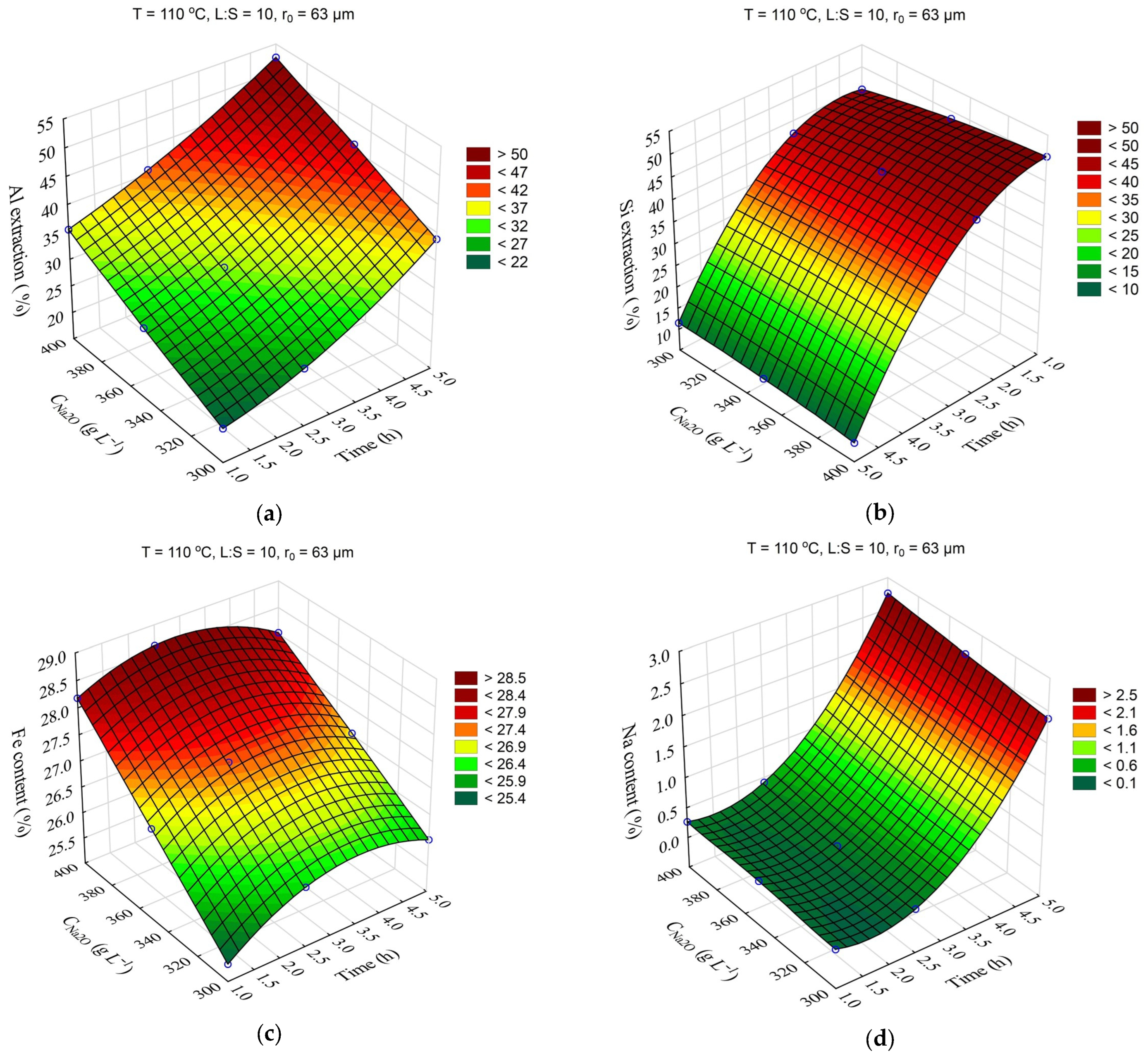
The response surfaces predicted by the SANN model for the effect of time and temperature on the degree of Al and Si extraction and the Fe and Na content in the solid residue are shown in
Figure 4. The leaching time (τ, min) was varied from 1 to 5 h, and the temperature (T, °C) from 100 to 120 °C. The L:S ratio, Na
2O concentration (C
Na2O, g L
–1), initial Al
2O
3 concentration (C
Al2O3, g L
–1), and initial mean particle size (r
0, μm) were fixed at L:S = 10, r
0 = 63 μm, C
Na2O = 360 g L
–1, and C
Al2O3 = 0 g L
–1.
Obviously, increasing the temperature and time allowed for an increase in the Al and Si extraction up to 60 and 40–50%, respectively. However, the effect of time on the Fe content in the solid residue was low, especially at high temperature. This may have been due to the fact that the desilication was completed in the first hour. Then, according to
Figure 4d, the DSP began to precipitate, which led to an increase in the Na
2O content in the precipitate up to 3.2%, and accordingly, it led to a decrease in the Fe content.
The response surfaces predicted by the SANN model for the effect of the time and L:S ratio on the Al and Si extraction as well as the Fe and Na content in the solid residue are shown in
Figure 5. The leaching duration (τ, h) was varied from 1 to 5 h, and the ratio L:S from 5 to 20. Other parameters were fixed at T = 110 °C, r
0 = 63 µm, C
Na2O = 360 g L
–1, and C
Al2O3 = 0 g L
–1.
The increase in L:S from 5 to 20 allowed to increase the solutional extraction from 28 to 41% after 1 h of leaching (
Figure 5a). After 5 h of leaching, the increase in L:S from 5 to 20 resulted in an increase in the Al extraction by only 6%. At the same time, the increase in L:S allowed for a significant increase in the Si extraction (
Figure 5b), which was associated with its retention in the solution, as evidenced by the Na content in the solid residue, which increased to 3% after 5 h at L:S = 5. The high Al and Si extraction at L:S above 10 also led to an increased Fe content in the solid residue, since Fe is not leached out during the alkaline treatment and concentrates in the residue.
The response surfaces predicted by the SANN model for the effects of time and Na
2O concentration on the Al and Si extraction and the Fe and Na content in the solid residue are shown in
Figure 6. The leaching time (τ, min) was varied from 1 to 5 h, and the Na
2O concentration from 330 to 400 g L
–1. The other parameters were fixed at T = 110 °C, r
0 = 63 µm, L:S = 10, and C
Al2O3 = 0 g L
–1.
The data in
Figure 6a show that the solution composition had a significant impact on the Al extraction, which seems to be associated with an increase in the caustic modulus and as a consequence, with the increased equilibrium concentration of Al in solution. Thus, an increase in Na
2O concentration from 330 to 400 g L
–1 after 5 h of leaching led to an increase in Al extraction from 40 to 54%. The effect of the solution concentration on the Si extraction and Na
2O content in the solid residue was insignificant (
Figure 6b,d). Increased Al extraction at a high concentration reduced the yield of the solid residue and, accordingly, increased the iron content. As the leaching duration increased from 3 h to 5 h, DSP began to form, resulting in an increased yield and higher Na
2O content in the solid residue (
Figure 6c,d).
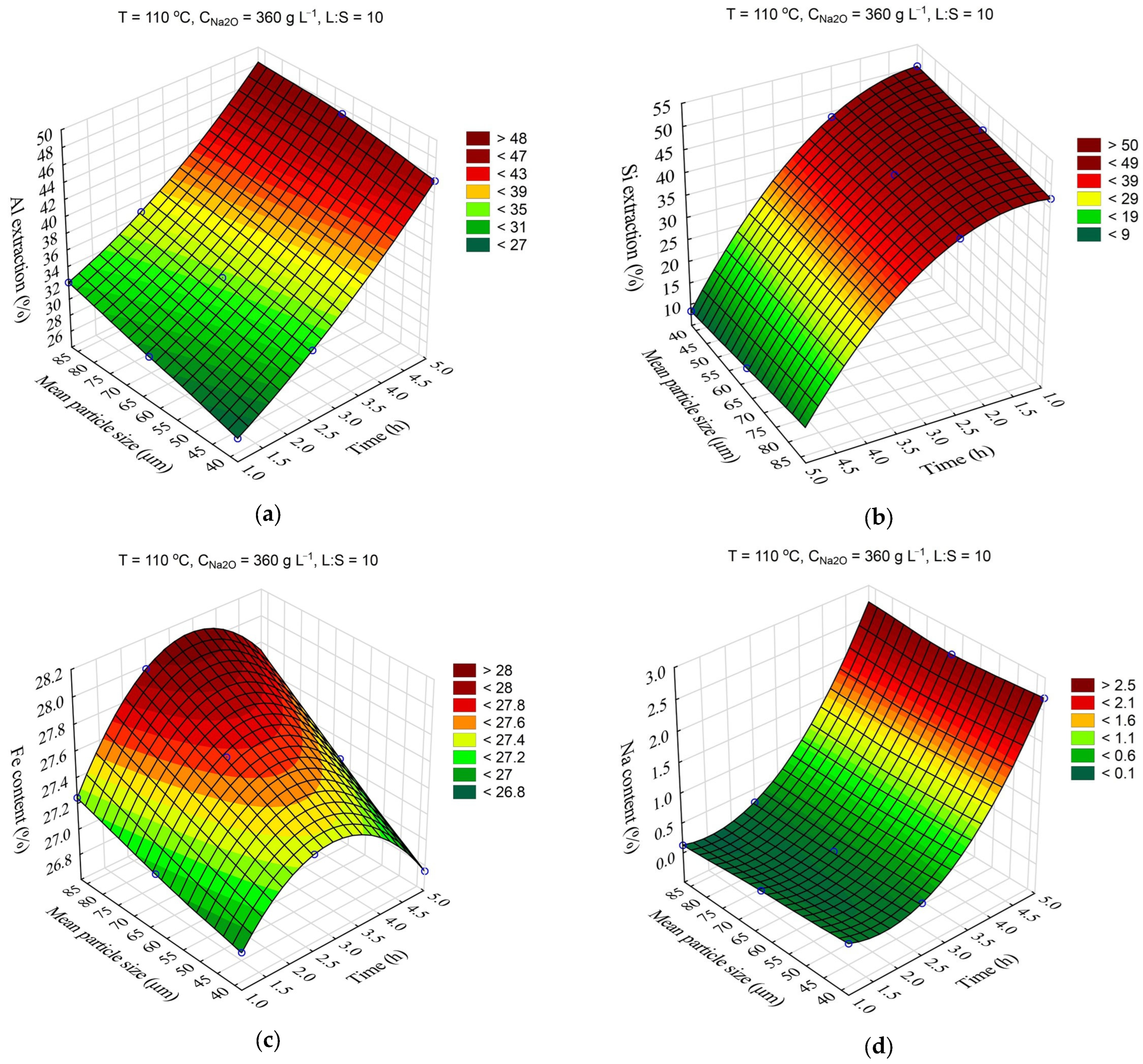
The response surfaces predicted by the SANN model for the effects of time and the initial mean particle size (r
0) on the Al and Si extraction and the Fe and Na content in the solid residue are shown in
Figure 7. The leaching time was varied from 1 to 5 h, and the mean particle size from 38 to 78 μm. Other parameters were fixed at T = 110 °C, C
Na2O = 360 g L
–1, L:S = 10, and C
Al2O3 = 0 g L
–1.
A decrease in the average particle size from 78 to 38 μm resulted in only a slight (2–4%) increase in Al and Si extraction (
Figure 7a,b). The Fe content also slightly increased with the decrease in the r
0 (
Figure 8c), which was associated with a higher Al and Si extraction. After 2.5 h of leaching, the Fe content began to decrease, which is connected to the beginning of DSP formation (
Figure 7d).
The data presented in
Table 4 were further processed in Statistica using the ANOVA (analysis of variance) method to study the statistical significance of certain process parameters. Pareto diagrams for each variable were constructed based on the results of the ANOVA (
Figure 8).
According to the results shown in
Figure 8 with a 0.95 confidence level (or significance level of 0.05), the temperature, time, concentration, and L:S ratio were statistically significant for Al and Si extraction: L:S ratio, time (negative effect), temperature (negative effect), average particle size (Q—quadratic dependence); for Na in solid residue, temperature and duration were significant, while the L:S ratio was significant for Na reduction in solid residue. Thus, if the task of the first stage is a selective Si extraction with minimization of Al extraction, it is necessary to take the minimum values of temperature and time and the maximum value L:S. The other parameters were of little importance for Al and Si extraction. Accordingly, the recommended parameters can be seen as follows: T = 100 °C, τ = 1 h, and L:S = 20. Using these parameters, it is possible to extract up to 60% of Si, and the Al extraction can be as low as 20–24%.
Experiments on desilication with the use of aluminate solutions of different Al concentrations were carried out to investigate the possibility of reducing Al co-extraction. It is known that the solubility of boehmite at atmospheric pressure is very low [
34], but when using highly concentrated NaOH solutions, it is sufficient to extract more than 50% of aluminum at a L:S above 10. The effect of Al concentration (in terms of Al
2O
3, g L
–1) on the Al extraction from bauxite during the first stage of leaching are shown in
Figure 9.
It is obvious that the use of an aluminate solution can suppress the process of Al co-extraction from bauxite during its desilication, even under the most severe conditions: T = 120 °C, Na
2O = 330 g L
–1, and the L:S ratio = 20. When the aluminate solution used for desilication at 100 °C, τ = 1 h, and L:S ratio = 20, the Al and Si extraction were 5.1% and 60.5%, respectively. The chemical composition of the concentrate (desilicated bauxite—DB) obtained under these conditions is shown in
Table 6. As can be seen, the silica modulus of bauxite after desilication increased to 21.34 units compared with 8.36 units for the original bauxite. The maximum theoretical Al extraction (maximal Al extraction minus Al that will be precipitated as DSP) from this bauxite by the Bayer method is 95.3%.
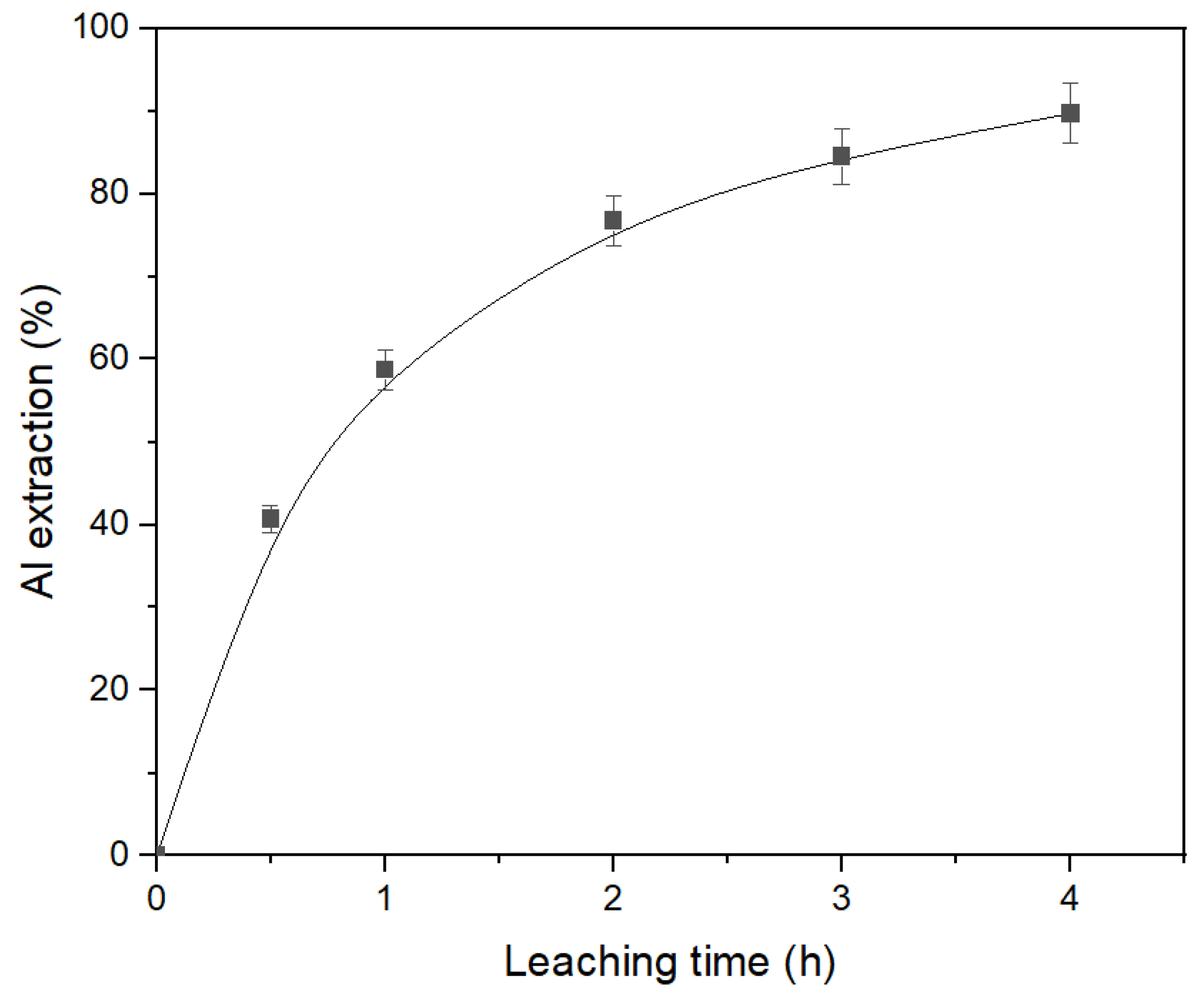
3.3. The Effect of Leaching Conditions on Al Extraction from Desilicated Bauxite (DB)
The desilicated bauxite obtained in
Section 3.1,
Table 5 was subjected to second stage leaching under atmospheric pressure. The parameters of the leaching were: T = 120 °C, C
Na2O = 360 g L
–1, C
Al2O3 = 0 g L
−1, and the L:S ratio = 20. The result of the effect of time on Al extraction under these condition are shown in
Figure 10. As can be seen, DB can be efficiently treated using the atmospheric leaching process. After leaching, the hematite transformation to magnetite was completed of 75.6%. However, the leaching time should be more than 4 h for the extraction of 90% of Al. The resulting aluminate solution contained only 32.6 g L
−1 Al
2O
3 and could not be processed using the Bayer process. Therefore, the high-pressure leaching of DB was studied.
The results of high-pressure Al leaching from DB were also processed using neural network modeling in the Statistica application package. The matrix of experiments and the results of Al extraction from the desilicated bauxite are shown in
Table 7.
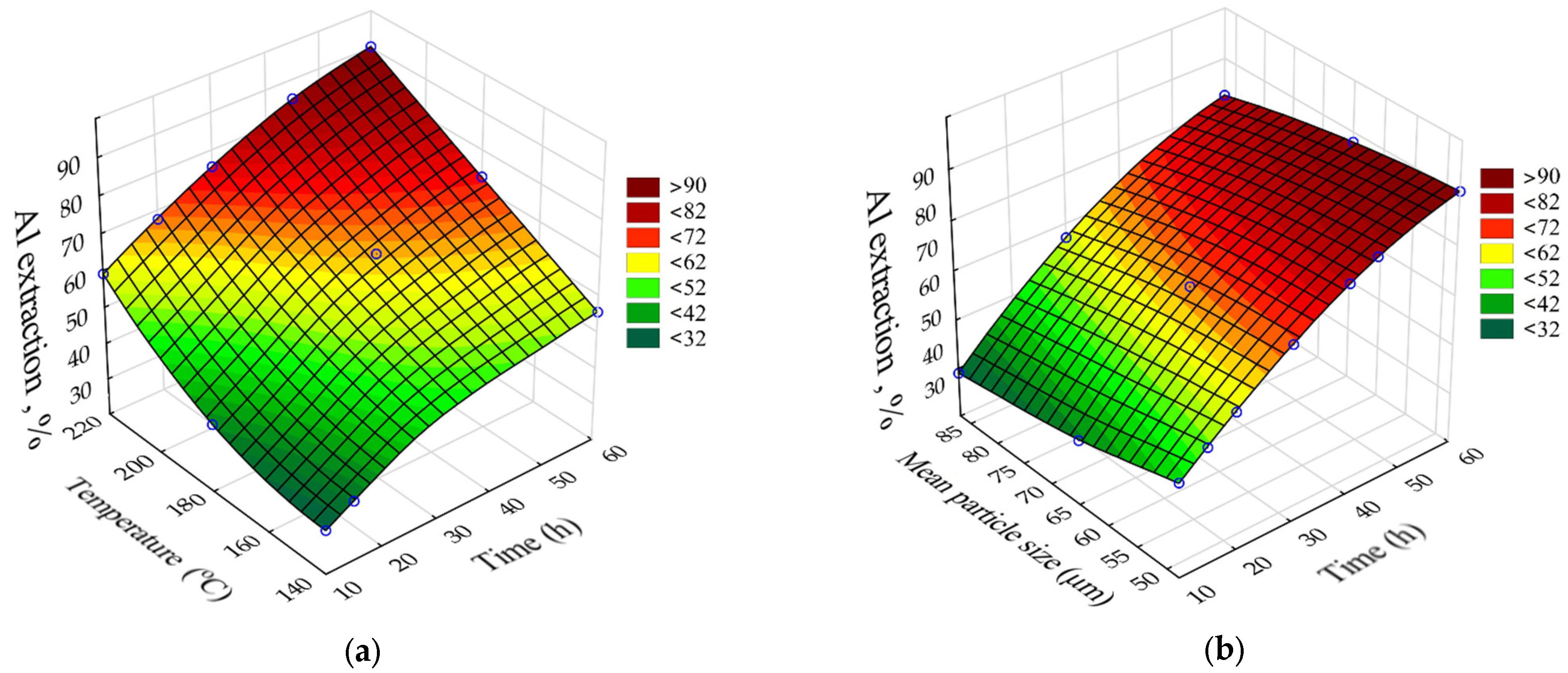
The SANN model that was better fitted to the experimental data of Al extraction was multilayer perceptron (MLP) 5.9.1 (R
2 = 0.98). The response surfaces predicted by the SANN model for Al recovery as a function of leaching time (τ, h), temperature (T, °C), and initial average bauxite particle size (r
0, μm) are shown in
Figure 11. The fixed values were C
Na2O = 330 g L
–1, C
Al2O3 = 150 g L
–1, and the L:S for the obtaining caustic modulus of the aluminate solution α
k (molar ratio of Na
2O to Al
2O
3) = 1.65.
The greatest influence (
Figure 11) on Al extraction was caused by the leaching time and temperature. Increasing the temperature from 140 °C to 220 °C increased the Al extraction after 60 min of leaching from 56 to 92% (
Figure 11a). This may indicate that the surface chemical reaction is the limiting stage of the process. Increasing the initial particle size from 48 μm to 78 μm resulted in a decrease in Al extraction from 90 to 85% (
Figure 11b), which may indicate that diffusion has no influence on the kinetics of the leaching process.
3.4. Solid Residue Characterization
Figure 12 shows the elemental surface distribution maps for the desilicated bauxite after the first stage of leaching at T = 100 °C, C
Na2O = 330 g L
–1 C
Al2O3 150 g L
–1, and L:S ratio = 20. According to the elemental distribution, Fe, Si, Ca, and Ti were evenly distributed over the particle surfaces. Particles of boehmite (particles with high Al content) were clearly visible. Fragmented iron particles were also found, but in general, the iron particles after magnetization appeared to be sufficiently finely dispersed.
Figure 13 shows an XRD pattern of bauxite desilicated under the optimum conditions. After desilication in the presence of divalent iron, a new phase, magnetite, appeared, although the intensity of the peaks was low. Furthermore, after desilication and magnetization, the chamosite peaks disappeared, but quartz, which is insoluble at atmospheric pressure, was visible in the solid residue. It should be noted that under the parameters of the Bayer process, chamosite was almost inert up until 200 °C [
35].
The chemical composition of the solid residue obtained after leaching of the DB under conditions similar to the industrial one (T = 220 °C, τ = 120 min, C
Na2O = 330 g L
–1, C
Al2O3 = 150 g L
–1, and L:S ratio needed to obtain the caustic modulus of 1.65 in the aluminate solution) is presented in
Table 8. The yield of the solid residue was 41.5% from the initial mass of the bauxite sample before desilication. It can be seen that the Fe and Ti content in the residue increased significantly compared to the feedstock, and according to the XRD analysis, almost all iron was represented by magnetite. The alumina content was decreased by a factor of 20, and the silica content by a factor of two. This indicates that practically all of the alumina from the chamosite and all of the boehmite were leached after 2 h of leaching—the total Al extraction after two stages was 97%. At the same time, the Na
2O content remained very low, even after two stages; this means that the DSP was practically not formed in the leaching of DB in the simultaneous presence of ferrous iron, which was also confirmed by XRD analysis (
Figure 14).
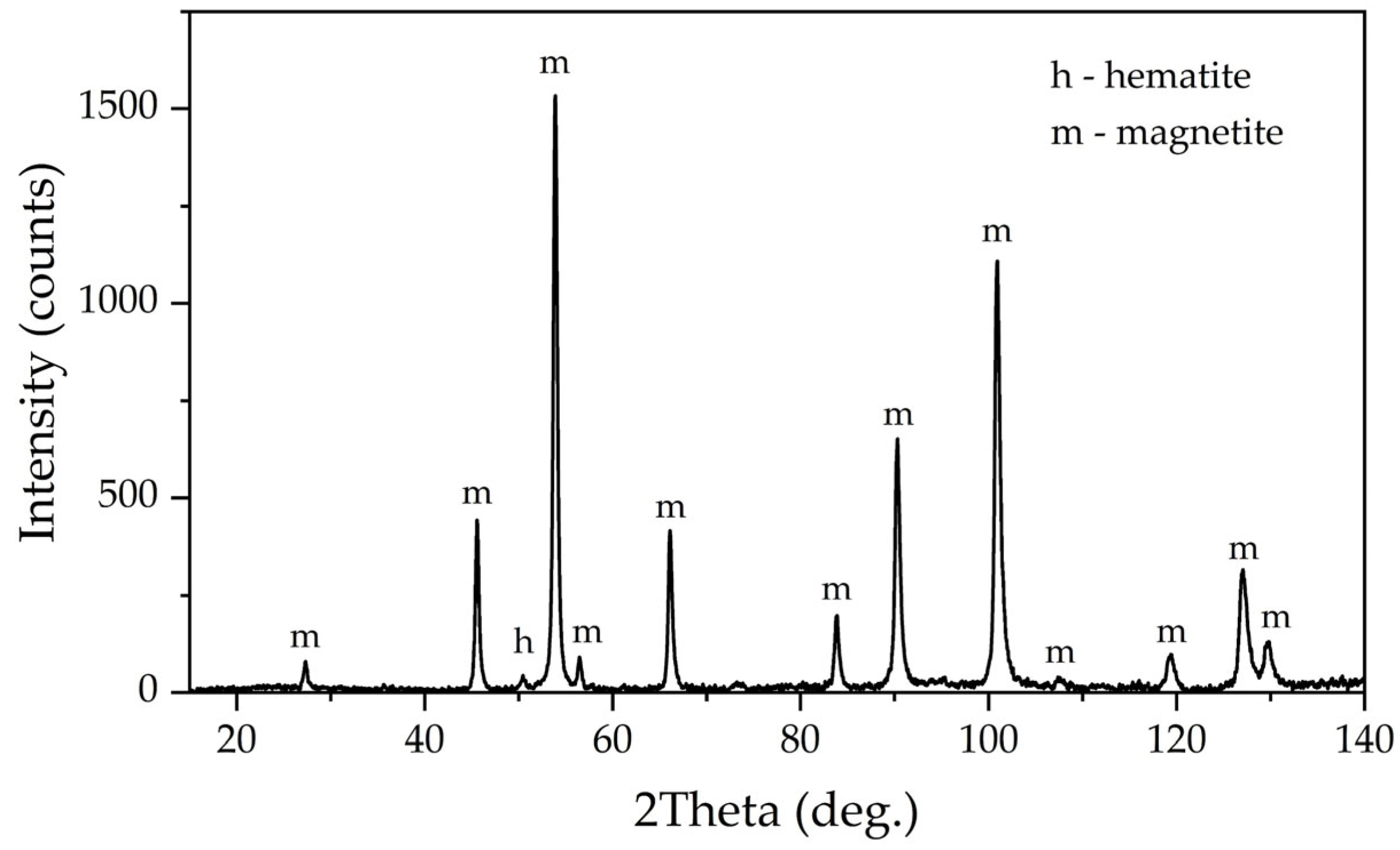
XRD pattern in
Figure 14 shows that the peaks of boehmite and hematite disappeared, while the peaks of magnetite increased significantly—magnetite remained almost the only phase in this BR. This fact suggests that the leaching of boehmite was complete. However, the absence of DSP and rutile on the XRD means that magnetization also helps to transform both silica and titania in a new phase [
36]. The possible chemical reactions of the raw bauxite mineral phases with the leaching agents are listed in
Table 9. Fe
2+ transforms in the alkali media into HFeO
2−, which reacts with the iron and titanium minerals with the formation of magnetite and titanomagnetite. Other minerals dissolve in the solution with the formation of sodium silicate and aluminate without DSP precipitation. Similar XRD patterns of magnetite and titanomagnetite help to explain the absence of the individual mineral of titanium in
Figure 14.
The results of the XRD patterns in
Figure 13 and
Figure 14 were confirmed by Mössbauer spectroscopy. The Mössbauer spectra of the DB sample obtained at both temperatures (
Figure 15a,c) can be satisfactorily described by the superposition of two sextets and two doublets (
Table 3).
The outer sextet with the maximum hyperfine magnetic splitting and narrow resonance lines corresponded to hematite partially substituted by aluminum and was close in parameters to the analogous subspectrum of the raw bauxite sample (
Table 3). The intensity of this sextet noticeably increased when going from 296 to 78 K, with a simultaneous decrease in the intensity of the doublet described by subspectrum 3 (
Table 3), which suggests that this doublet mainly corresponded to the superparamagnetic nanosized hematite. The hyperfine parameters of subspectrum 4, corresponding to iron(+2) atoms, were similar to the corresponding parameters for the initial bauxite, and obviously corresponded to the incompletely reacted chamosite (
Table 3). There were no subspectra that corresponded to goethite in this sample. The rest of the spectrum could only be described using the many-state superparamagnetic relaxation model [
37]. The models for the spectra obtained at different temperatures were consistent with each other through the ratio of the energy of the magnetic anisotropy of particles to the thermal energy:
where K—magnetic anisotropy constant; V—volume of the magnetic domain; k
B—Boltzmann constant; T—temperature [
38]. Obviously, this subspectrum refers to the forming particles of nanomagnetite, possibly partially oxidized [
39]. From the parameters obtained using Equation (5) and making the assumption that the particles are spherical and that the magnetic anisotropy constant does not depend on temperature and is equal to 2 × 10
4 J m
−3 [
40,
41], one can estimate the size of the magnetic domain for nanomagnetite as 10.32 ± 0.06 nm.
The Mössbauer spectra of the BR sample had a form characteristic of magnetite [
42]. There was a sextet with a characteristic splitting of 1–3 resonance lines, an increased intensity of 4–6 lines in the spectra at room temperature, and a noticeable asymmetric distortion of the sextet resonance lines in the spectra at the boiling point of nitrogen (
Figure 15b,d). The general broadening of resonance lines to the inner region of the spectrum indicates the manifestation of superparamagnetism by the material [
38]. Both spectra were satisfactorily described by the superposition of three sextets. The profile of each was specified within the many-state superparamagnetic relaxation model [
37] (
Table 3). In this case, within the same spectrum, the sextets were interconnected by relaxation parameters, and the spectra at different temperatures were also consistent with each other through the ratio of the energy of the magnetic anisotropy of particles to the thermal energy (Equation (4)). Similar to the method described above for the example of the DB sample, the sizes of the magnetic domains of nanomagnetite were estimated, which amounted to 19.2 ± 0.2 nm. No other components corresponding to those observed in the raw bauxite or DB samples or not observed in them were recorded in the described spectra, which indicates the complete magnetization of iron minerals after the high-pressure leaching of the DB.
The morphology and elemental composition of the bauxite residue particles were also investigated using SEM-EDS analysis (
Figure 16).
The data obtained above were confirmed by SEM-EDS analysis (
Figure 16).
Figure 16a,b shows that the particle size of BR (mostly magnetite) was less than 200 nm. At the same time, Al, Si, Ti, and Ca were evenly distributed on the particle surface, which may indicate their inclusion in the iron containing phases. It should be noted that, because of the complete Al extraction and no DSP formation, the obtained BR was enriched in rare-earth elements (REE). For example, the scandium content in BR reached 130 mg kg
−1. Therefore, the high iron content and concentration of REE make this BR a valuable by-product for the extraction of metals.