Low-Density Unsaturated Polyester Resin with the Presence of Dual-Initiator
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LDUPR Samples
2.3. Methods
2.3.1. Gel Time Determination
2.3.2. Apparent Density Testing
2.3.3. Detection of Compressive Strength
2.3.4. Thermal Analysis
2.3.5. Microscopic Analysis
3. Results and Discussion
3.1. Gel Time of Resin Glue with the Presence of One Single Initiator
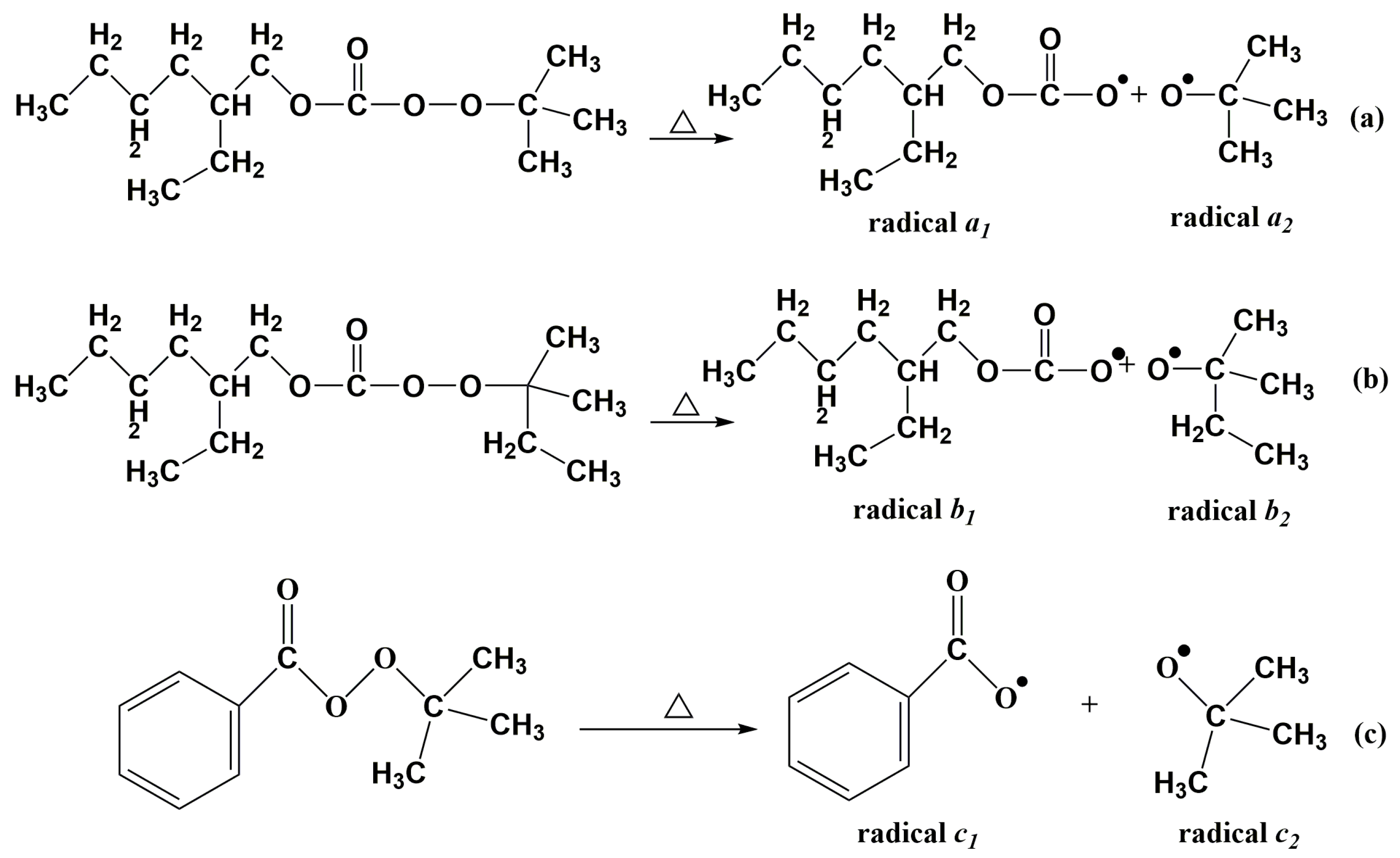
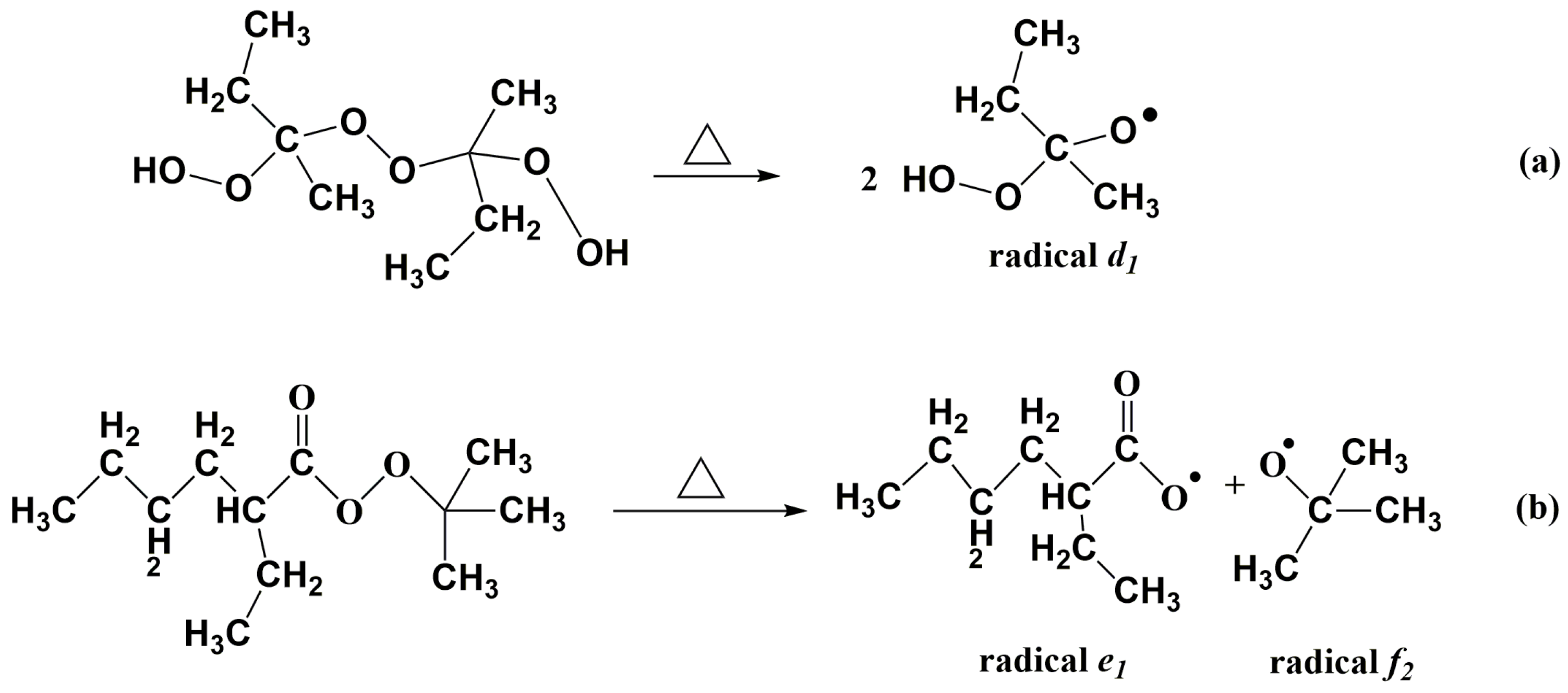
3.2. Gel Time of Resin Glue with the Presence of Dual-Initiator
3.3. Preparation of LDUPR Samples
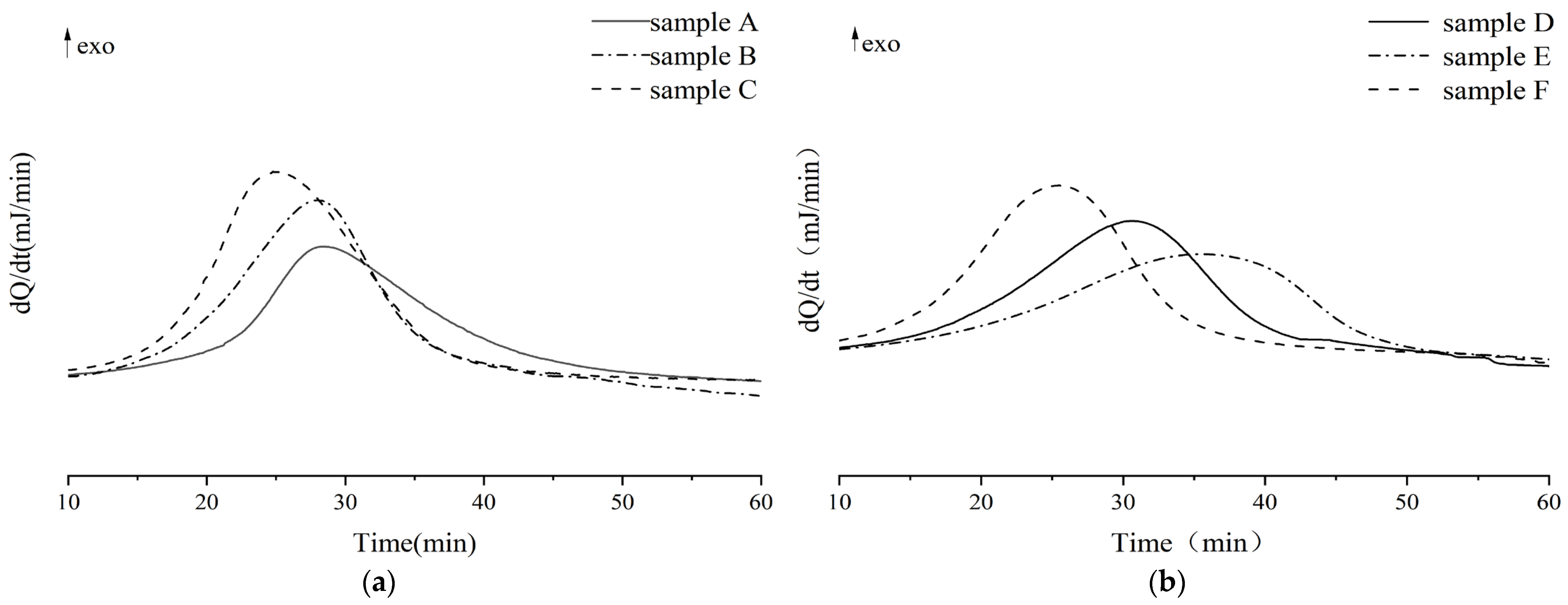
3.4. Influences of Dual-Initiator on Curing Degree of LDUPR Samples
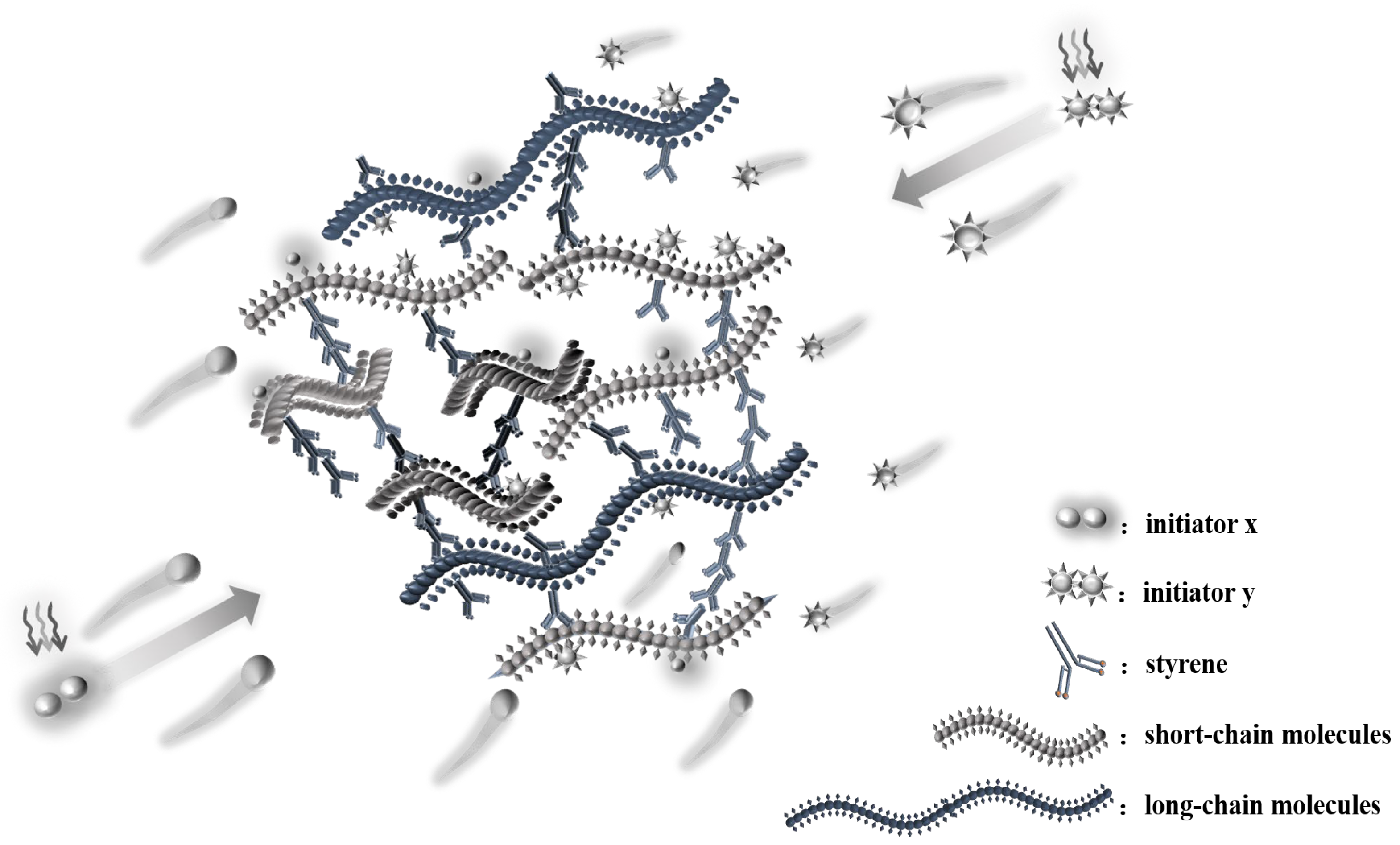
3.5. Analysis of the Microstructure of LDUPR Prepared by Dual-Initiation System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, J.B.; Musa, A.B.; Danladi, A.; Bukhari, M.M.; Nyakuma, B.B. Physico-mechanical Properties of Unsaturated Polyester Resin Reinforced Maize Cob and Jute Fiber Composites. J. Nat. Fibers 2020, 19, 3195–3207. [Google Scholar] [CrossRef]
- Hu, S.-L.; Li, Y.-M.; Hu, W.-J.; Hobson, J.; Wang, D.-Y. Strategic design unsaturated polyester resins composites with excellent flame retardancy and high tensile strength. Polym. Degrad. Stab. 2022, 206, 110190. [Google Scholar] [CrossRef]
- Tasnim, S.; Shaikh, F.U.A.; Sarker, P.K. Mechanical properties and microstructure of lightweight polymer composites containing mono and hybrid fillers sourced from recycled solid wastes. Constr. Build. Mater. 2021, 277, 122369. [Google Scholar] [CrossRef]
- Ji, G.-F.; Wang, X.-J.; Zhang, Y.-F.; Kong, P.; Li, C. Triethanolamine-azodiisobutyronitrile mixture as a foaming agent for low-density unsaturated polyester resin manufacturing at a low temperature. J. Appl. Polym. Sci. 2017, 134, 44797. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X. Mechanical enhancement of ripples and dimples in CaCO3/low-density unsaturated polyester resin composites. Mater. Res. Express 2020, 7, 065302. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Pan, Z.; Huang, H. Sodium bicarbonate/azodiisobutyronitrile synergistic effect on low-density unsaturated polyester resin fabrication. Iran. Polym. J. 2018, 27, 207–216. [Google Scholar] [CrossRef]
- Liu, Y.; Ganewatta, M. Thermoset Resins and Performance Novel Environmentally Friendly Bio-Based Thermoset Resins and Performance. Sampe. J. 2023, 59, 30–35. [Google Scholar]
- Melilli, G.; Guigo, N.; Robert, T.; Sbirrazzuoli, N. Radical Oxidation of Itaconic Acid-Derived Unsaturated Polyesters under Thermal Curing Conditions. Macromolecules 2022, 55, 9011–9021. [Google Scholar] [CrossRef]
- Chen, T.; Su, C.; Zeng, Y.; Chen, Y.; Qiu, R.; Liu, W. Effects of hydrogen bonds on soybean oil-based thermosets and their bamboo fibers composites. Compos. Commun. 2022, 33, 101231. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Zhu, J.; Chen, M. Long Chopped Glass Fiber Reinforced Low-Density Unsaturated Polyester Resin under Different Initiation. Materials 2021, 14, 7307. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Fu, X. High Mechanic Enhancement of Chopped Carbon Fiber Reinforced-Low-Density Unsaturated Polyester Resin Composite at Low Preparation Temperature with Facile Polymerization. Materials 2021, 14, 4273. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Jahanfar, M.; Ogino, K. Increased storage stability of marble adhesive based on unsaturated polyester resin. Int. J. Adhes. Adhes. 2023, 122, 103321. [Google Scholar] [CrossRef]
- Moujdin, I.A.; Totah, H.S.; Abulkhair, H.A.; Alsaiari, A.O.; Shaiban, A.A.; Organji, H.A. Development of Low Shrinkage Curing Techniques for Unsaturated Polyester and Vinyl Ester Reinforced Composites. Materials 2022, 15, 2972. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Zhang, J.; Zhu, J. Synergistic action of initiator and accelerator on curing thermodynamics and kinetics of chopped carbon fiber-reinforced low-density unsaturated polyester resin with rapid polymerization. J. Therm. Anal. Calorim. 2022, 147, 14195–14209. [Google Scholar] [CrossRef]
- Marín, D.; Gañán, P.; Tercjak, A.; Castro, C.; Builes, D.H. Phase distribution changes of neat unsaturated polyester resin and their effects on both thermal stability and dynamic—mechanical properties. J. Appl. Polym. Sci. 2021, 138, 51308. [Google Scholar] [CrossRef]
- Mustapha, S.N.H.; Rahmat, A.R.; Mustapha, R. Interactions and performance analysis of epoxidized palm oil/unsaturated polyester resin: Mechanical, thermal, and thermo-mechanical properties. Polym. Polym. Compos. 2022, 30, 09673911221095704. [Google Scholar] [CrossRef]
- Jothibasu, S.; Chandramohan, A.; Kumar, A.A.; Alagar, M. Polyhedral oligomeric silsesquioxane (POSS) reinforced-unsaturated polyester hybrid nanocomposites: Thermal, thermomechanical and morphological properties. J. Macromol. Sci. Part A 2018, 55, 433–439. [Google Scholar] [CrossRef]
- Abdul Amir, H.F.; Li, H.; Zhu, F.; Li, C.; Khiew, P.S. Preparation of a self-curable unsaturated polyester with vinyl double bonds and Investigation of its self-curing properties. MATEC Web Conf. 2017, 108, 01003. [Google Scholar]
- Zhang, Y.-F.; Wang, X.-J.; Pan, Z.-G.; Hong, C.-M.; Ji, G.-F. Urea-activated 4,4′-oxibis-(benzenesulfonyl hydrazide) as foaming agent for low-density unsaturated polyester resin manufacturing. J. Appl. Polym. Sci. 2015, 132, 42824. [Google Scholar] [CrossRef]
- Delaite, C.; Bistac, S.; Dreyer, E.; Schuller, A.S. Influence of glass transition temperature of crosslinked unsaturated polyester resin/styrene formulations on the final conversion after an isothermal curing at 100 °C. Polym. Adv. Technol. 2020, 31, 2031–2037. [Google Scholar] [CrossRef]
- Berman, A.; DiLoreto, E.; Moon, R.J.; Kalaitzidou, K. Hollow glass spheres in sheet molding compound composites: Limitations and potential. Polym. Compos. 2020, 42, 1279–1291. [Google Scholar] [CrossRef]
- Rajaee, P.; Ashenai Ghasemi, F.; Fasihi, M.; Saberian, M. Effect of styrene-butadiene rubber and fumed silica nano-filler on the microstructure and mechanical properties of glass fiber reinforced unsaturated polyester resin. Compos. Part B Eng. 2019, 173, 106803. [Google Scholar] [CrossRef]
- Delaite, C.; Bistac, S.; Dreyer, E.; Schuller, A.S. Influence of initiator on the curing of unsaturated polyester resin at 100 °C. Polym. Int. 2019, 69, 1089–1096. [Google Scholar] [CrossRef]
- Poyraz, B. Kompozit malzeme üretiminde kullanılan polyesterlerin mekanik, termal ve kimyasal özelliklerine başlatıcı etkisinin incelenmesi. Gazi Üniversitesi Mühendislik Mimar. Fakültesi Derg. 2018, 33, 1383–1396. [Google Scholar] [CrossRef]
- Derosa, R.; Telfeyan, E.; Gaustad, G.; Mayes, S. Strength and Microscopic Investigation of Unsaturated Polyester BMC Reinforced with SMC-Recyclate. J. Thermoplast. Compos. Mater. 2016, 18, 333–349. [Google Scholar] [CrossRef]
- Cousinet, S.; Ghadban, A.; Fleury, E.; Lortie, F.; Pascault, J.-P.; Portinha, D. Toward replacement of styrene by bio-based methacrylates in unsaturated polyester resins. Eur. Polym. J. 2015, 67, 539–550. [Google Scholar] [CrossRef]
- Naderi, N.; Mazinani, S.; Hosain Beheshty, M.; Mahdi Rajab, M. Cure kinetics of hot cured unsaturated polyester (UP)/nanoclay nanocomposite including dual initiators. Plast. Rubber Compos. 2014, 44, 19–25. [Google Scholar] [CrossRef]
- Kunwen, D.U.; Kunwu, D.U.; Qiu, G.; Mao, P.; Wang, L. Initiator composition, unsaturated polyester resin composition comprising same, and method for curing resin. US20150011713A1, 5 October 2016. [Google Scholar]
- Guo, B.; Wang, X.; Wang, R.; Ma, Y. Mild-thermal fabrication and phase conformation of chopped glass fiber-reinforced low-density unsaturated polyester resin with NH4HCO3. Iran. Polym. J. 2018, 27, 997–1010. [Google Scholar] [CrossRef]
- GB/T 24148.2-2009 (ISO 3672-2:2000); National Standard of the People’s Republic of China. Plastics—Unsaturated-polyester resins (UP-R)—Part 2: Preparation of test specimens and determination of properties. Standards Press of China: Beijing, China, 2009.
- GB/T 24148.7-2014 (ISO 2535:2001); National Standard of the People’s Republic of China. Plastics—Unsaturated polyester resins—Part 7: Measurement of gel time at ambient temperature. Standards Press of China: Beijing, China, 2014.
- GB/T 6343-2009 (ISO 845:2006); National Standard of the People’s Republic of China. Cellular plastics and rubbers—Determination of apparent density. Standards Press of China: Beijing, China, 2009.
- GB/T 8813-2020 (ISO 844:2014); National Standard of the People’s Republic of China. Rigid cellular plastics—Determination of compression properties. Standards Press of China: Beijing, China, 2020.
- Hong, C.; Wang, X.; Pan, Z.; Zhang, Y. Curing thermodynamics and kinetics of unsaturated polyester resin with different chain length of saturated aliphatic binary carboxylic acid. J. Therm. Anal. Calorim. 2015, 122, 427–436. [Google Scholar] [CrossRef]
- Farsane, M.; Lhasnaoui, S.; Anouar, A.; Dagdag, S.; Bouzziri, M. A Review of Measuring the Gelation Time in Unsaturated Polyester Resins. Mater. Tehnol. 2022, 56, 323–329. [Google Scholar] [CrossRef]
- Echeverri, D.A.; Jaramillo, F.; Rios, L.A. Curing copolymerization kinetics of styrene with maleated castor oil glycerides obtained from biodiesel-derived crude glycerol. J. Appl. Polym. Sci. 2015, 132, 41344. [Google Scholar] [CrossRef]
- Ujah, C.O.; Popoola, A.P.I.; Ezema, I.C. Gel time prediction of polyester resin for lamination of polymer composites. Bull. Chem. Soc. Ethiop. 2020, 34, 163–174. [Google Scholar] [CrossRef]
- Hadzich, A.; Flores, S.; Masucci, A.E.; Gomez, E.D.; Groß, G.A. NMR and GPC Analysis of Alkyd Resins: Influence of Synthesis Method, Vegetable Oil and Polyol Content. Polymers 2023, 15, 1993. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Mahmoud, Z.; Hubert-Roux, M.; Azaroual, N.; Afonso, C.; Schuller, A.S.; Rolando, C. Deciphering the structure of itaconate-based unsaturated polyester resins by high resolution mass spectrometry. Polym. Int. 2020, 69, 1140–1151. [Google Scholar] [CrossRef]

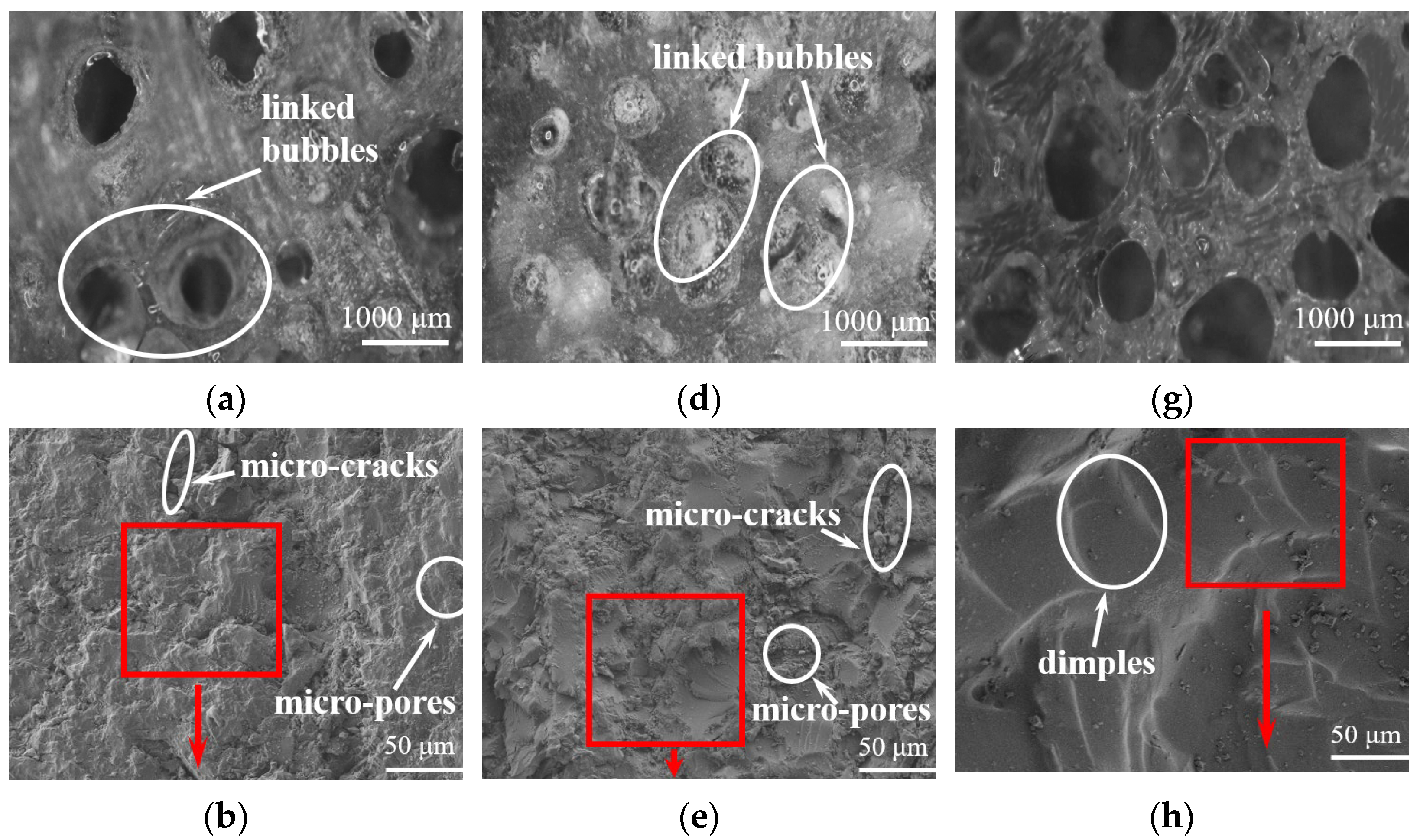
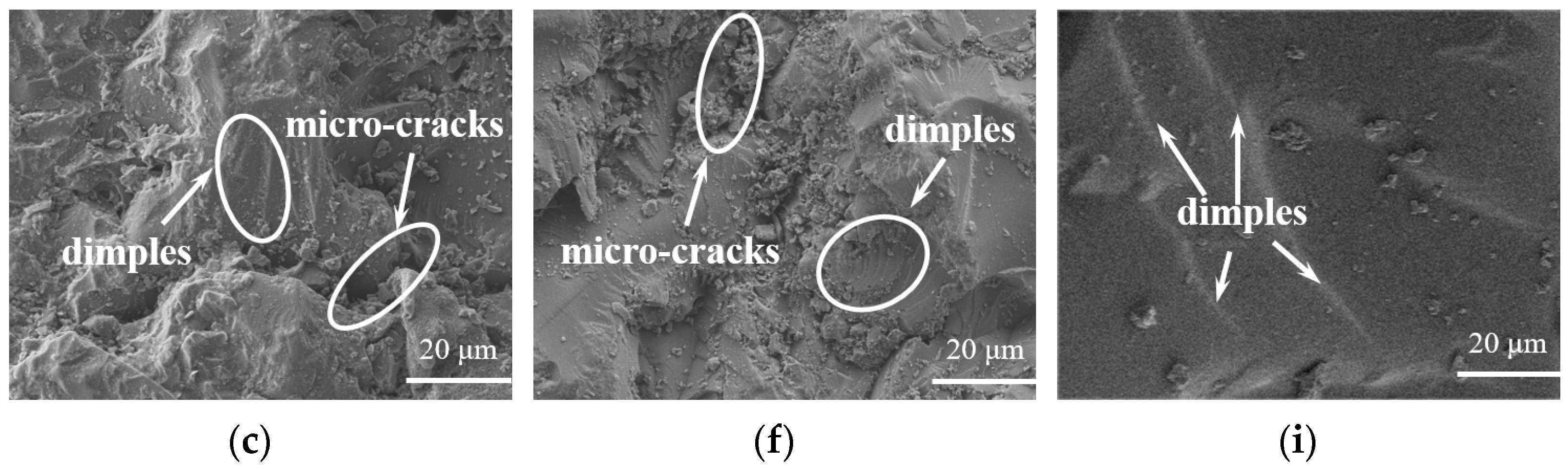
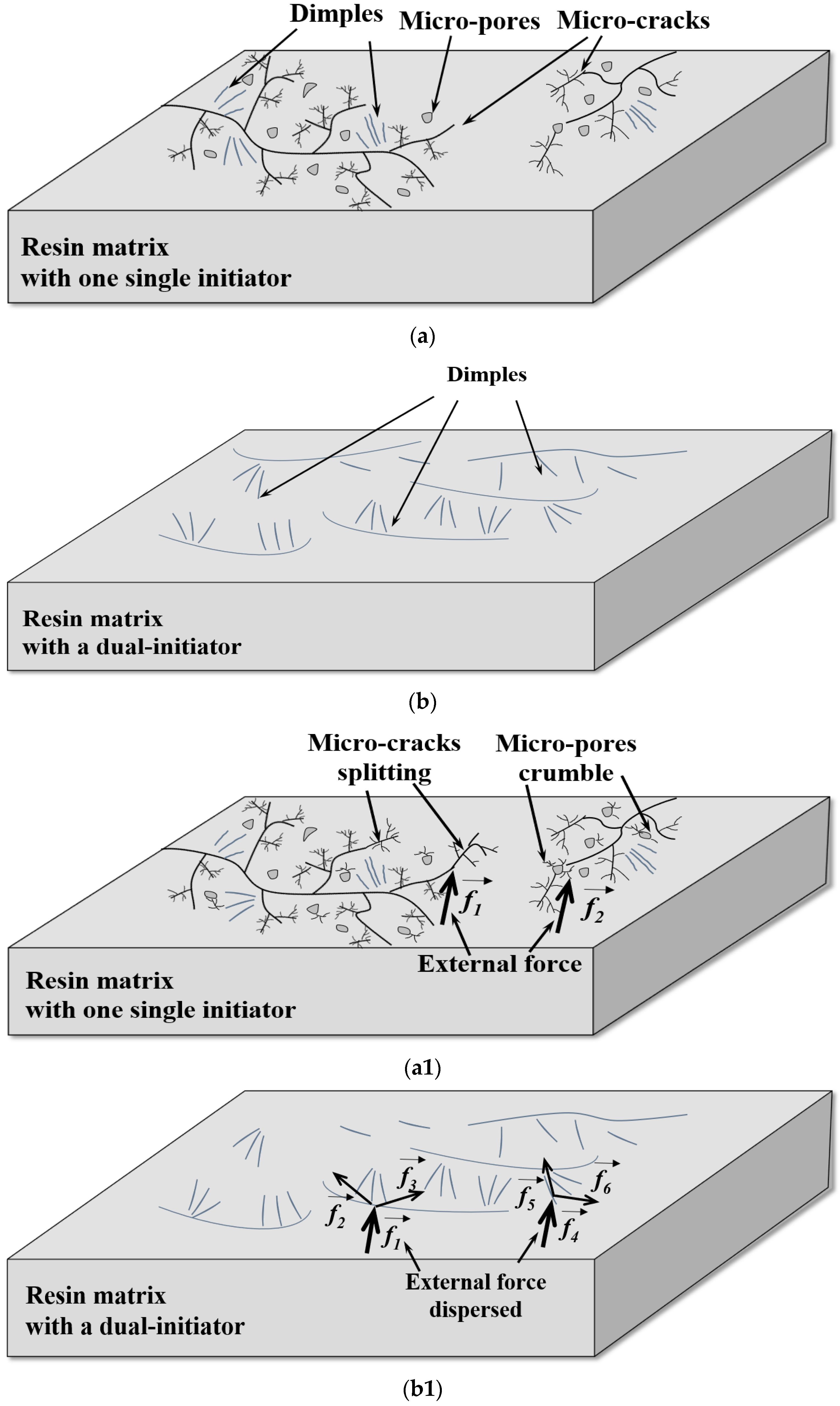
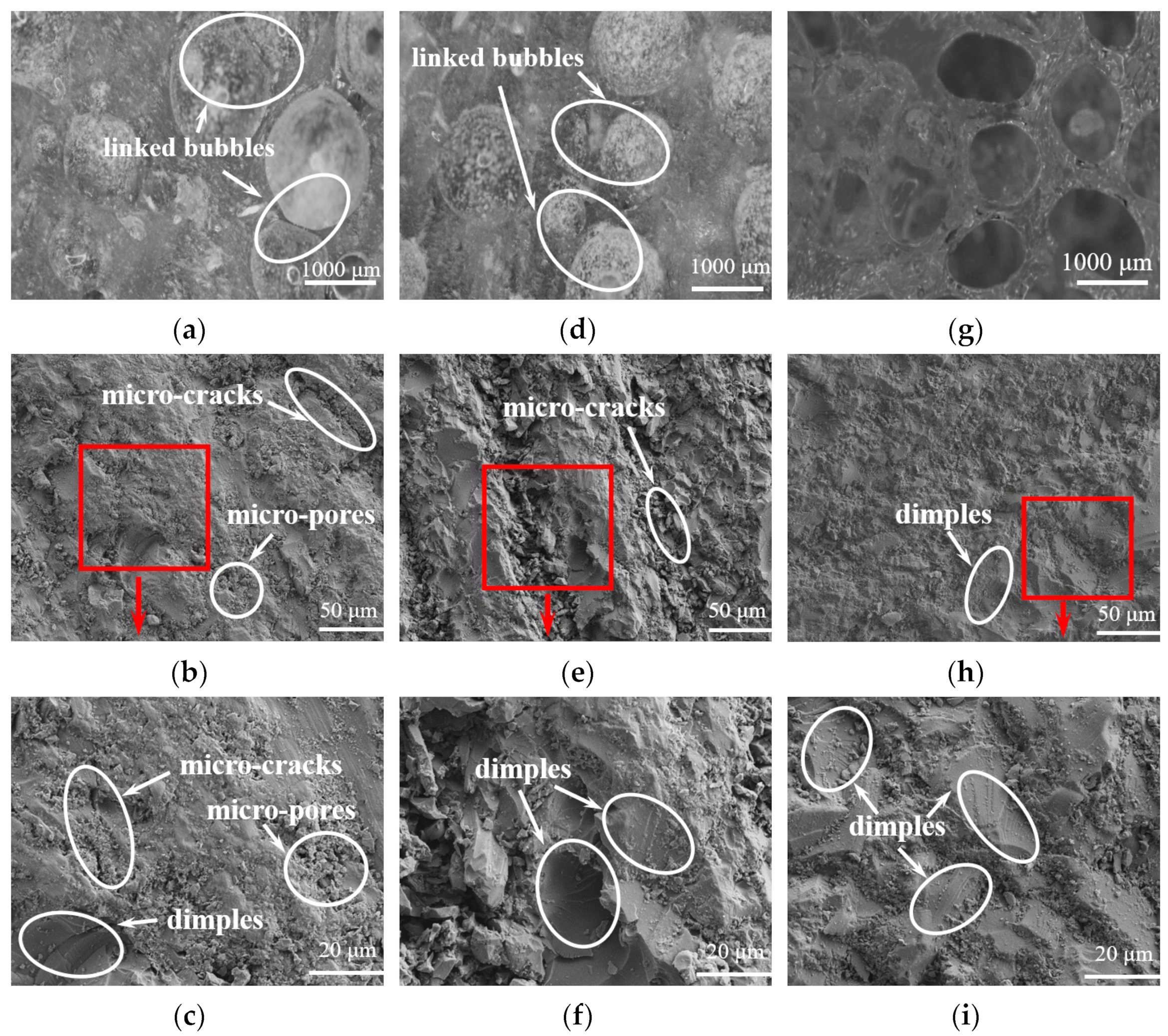
| Type of Initiator | Dosage of Initiator (phr) | Temperature Range (°C) | Gel Time (min) |
|---|---|---|---|
| CYHP | 1.00 | 65.0–73.0 | 34.6–23.4 |
| 2.00 | 62.0–71.0 | 35.4–23.5 | |
| 3.00 | 60.0–70.0 | 36.0–22.4 | |
| MEKP | 1.00 | 65.0–75.0 | 34.9–23.9 |
| 2.00 | 63.0–73.0 | 34.8–23.5 | |
| 3.00 | 62.0–72.0 | 35.3–23.0 | |
| TBPO | 1.00 | 71.0–78.0 | 34.8–22.5 |
| 2.00 | 67.0–75.0 | 35.6–22.4 | |
| 3.00 | 65.0–73.0 | 36.3–23.1 | |
| TAEC | 1.00 | 80.0–90.0 | 35.0–22.9 |
| 2.00 | 77.0–87.0 | 35.4–23.1 | |
| 3.00 | 75.0–85.0 | 35.1–22.6 | |
| TBEC | 1.00 | 73.0–90.0 | 35.3–23.1 |
| 2.00 | 72.0–87.0 | 35.4–23.1 | |
| 3.00 | 71.0–85.0 | 35.2–22.8 | |
| TBPB | 1.00 | 85.0–95.0 | 34.9–23.6 |
| 2.00 | 82.0–93.0 | 34.3–23.1 | |
| 3.00 | 77.0–90.0 | 35.3–22.8 |
| Initiator Combination | Temperature Range (°C) | Component Initiator | Dosages of Component Initiators (phr) |
|---|---|---|---|
| CYHP/MEKP | 60.0–68.0 | CYHP | 1.00, 2.00 |
| MEKP | 1.00, 2.00 | ||
| CYHP/TBPO | 62.0–70.0 | CYHP | 1.00, 2.00 |
| TBPO | 1.00, 2.00 | ||
| MEKP/TBPO | 62.0–70.0 | MEKP | 1.00, 2.00 |
| TBPO | 1.00, 2.00 |
| Initiator Combination | Temperature Range (°C) | Component Initiator | Dosages of Component Initiators (phr) |
|---|---|---|---|
| TAEC/TBPB | 72.0–80.0 | TAEC | 1.00, 2.00 |
| TBPB | 1.00, 2.00 | ||
| TAEC/TBEC | 72.0–80.0 | TAEC | 1.00, 2.00 |
| TBEC | 1.00, 2.00, 3.00 | ||
| TBEC/TBPB | 72.0–80.0 | TBEC | 1.00, 2.00 |
| TBPB | 1.00, 2.00 |
| Sample Serial Number | Curing Temperature (°C) (A) | Content of CYHP (phr) (B) | Content of MEKP (phr) (C) | Content of NH4HCO3 (phr) (D) | ρ (g·cm−3) | P (MPa) | Ps (MPa·g−1·cm3) |
|---|---|---|---|---|---|---|---|
| 1 | 60.0 | 1.00 | 1.00 | 1.00 | 0.69 ± 0.02 | 17.66 ± 0.26 | 25.41 ± 0.48 |
| 2 | 60.0 | 1.25 | 1.25 | 1.50 | 0.58 ± 0.01 | 15.38 ± 0.21 | 26.45 ± 0.47 |
| 3 | 60.0 | 1.50 | 1.50 | 2.00 | 0.44 ± 0.01 | 13.73 ± 0.23 | 31.21 ± 0.45 |
| 4 | 60.0 | 1.75 | 1.75 | 2.50 | 0.42 ± 0.01 | 12.25 ± 0.14 | 29.17 ± 0.34 |
| 5 | 60.0 | 2.00 | 2.00 | 3.00 | 0.40 ± 0.02 | 10.87 ± 0.11 | 27.35 ± 0.29 |
| 6 | 62.0 | 1.00 | 1.25 | 2.00 | 0.41 ± 0.01 | 14.33 ± 0.21 | 35.38 ± 0.62 |
| 7 | 62.0 | 1.25 | 1.50 | 2.50 | 0.39 ± 0.01 | 12.58 ± 0.11 | 31.85 ± 0.45 |
| 8 | 62.0 | 1.50 | 1.75 | 3.00 | 0.37 ± 0.02 | 9.97 ± 0.13 | 26.95 ± 0.47 |
| 9 | 62.0 | 1.75 | 2.00 | 1.00 | 0.73 ± 0.03 | 16.32 ± 0.21 | 22.21 ± 0.31 |
| 10 | 62.0 | 2.00 | 1.00 | 1.50 | 0.63 ± 0.02 | 16.40 ± 0.19 | 25.84 ± 0.48 |
| 11 | 64.0 | 1.00 | 1.50 | 3.00 | 0.39 ± 0.01 | 8.99 ± 0.15 | 23.34 ± 0.26 |
| 12 | 64.0 | 1.25 | 1.75 | 1.00 | 0.73 ± 0.02 | 17.67 ± 0.31 | 24.37 ± 0.26 |
| 13 | 64.0 | 1.50 | 2.00 | 1.50 | 0.57 ± 0.01 | 16.68 ± 0.19 | 29.24 ± 0.53 |
| 14 | 64.0 | 1.75 | 1.00 | 2.00 | 0.41 ± 0.02 | 16.53 ± 0.22 | 40.06 ± 0.45 |
| 15 | 64.0 | 2.00 | 1.25 | 2.50 | 0.40 ± 0.01 | 10.47 ± 0.12 | 26.17 ± 0.47 |
| 16 | 66.0 | 1.00 | 1.75 | 1.50 | 0.57 ± 0.03 | 15.96 ± 0.29 | 27.79 ± 0.36 |
| 17 | 66.0 | 1.25 | 2.00 | 2.00 | 0.43 ± 0.01 | 13.85 ± 0.22 | 32.25 ± 0.38 |
| 18 | 66.0 | 1.50 | 1.00 | 2.50 | 0.38 ± 0.01 | 9.45 ± 0.18 | 24.55 ± 0.34 |
| 19 | 66.0 | 1.75 | 1.25 | 3.00 | 0.39 ± 0.01 | 9.71 ± 0.17 | 24.92 ± 0.34 |
| 20 | 66.0 | 2.00 | 1.50 | 1.00 | 0.72 ± 0.03 | 16.85 ± 0.31 | 23.24 ± 0.36 |
| 21 | 68.0 | 1.00 | 2.00 | 2.50 | 0.39 ± 0.01 | 9.36 ± 0.11 | 23.74 ± 0.41 |
| 22 | 68.0 | 1.25 | 1.00 | 3.00 | 0.34 ± 0.01 | 8.45 ± 0.13 | 24.93 ± 0.29 |
| 23 | 68.0 | 1.50 | 1.25 | 1.00 | 0.70 ± 0.02 | 18.39 ± 0.29 | 26.09 ± 0.32 |
| 24 | 68.0 | 1.75 | 1.50 | 1.50 | 0.54 ± 0.02 | 16.57 ± 0.18 | 30.73 ± 0.43 |
| 25 | 68.0 | 2.00 | 1.75 | 2.00 | 0.44 ± 0.02 | 11.39 ± 0.13 | 25.86 ± 0.33 |
| Factor | Mean | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | R1, R2 or R3 (kmax–kmin) |
|---|---|---|---|---|---|---|---|
| Curing temperature (A) | (g·cm−3) | 0.51 | 0.51 | 0.50 | 0.50 | 0.48 | 0.02 |
| (MPa) | 13.98 | 13.92 | 14.07 | 13.16 | 12.83 | 1.24 | |
| (MPa·g−1·cm3) | 27.92 | 28.45 | 28.69 | 26.55 | 26.27 | 2.42 | |
| Content of CYHP (B) | (g·cm−3) | 0.49 | 0.49 | 0.49 | 0.50 | 0.52 | 0.03 |
| (MPa) | 13.26 | 13.59 | 13.65 | 14.28 | 13.20 | 1.08 | |
| (MPa·g−1·cm3) | 27.13 | 27.97 | 27.61 | 29.47 | 25.69 | 3.77 | |
| Content of MEKP (C) | (g·cm−3) | 0.49 | 0.50 | 0.50 | 0.51 | 0.51 | 0.01 |
| (MPa) | 13.70 | 13.66 | 13.74 | 14.28 | 13.42 | 0.86 | |
| (MPa·g−1·cm3) | 28.20 | 27.80 | 28.08 | 26.83 | 26.97 | 1.38 | |
| Content of NH4HCO3 (D) | (g·cm−3) | 0.72 | 0.58 | 0.42 | 0.40 | 0.38 | 0.34 |
| (MPa) | 17.38 | 16.20 | 13.97 | 10.82 | 9.60 | 7.78 | |
| (MPa·g−1·cm3) | 24.26 | 28.02 | 33.00 | 27.10 | 25.50 | 8.74 |
| Initiation System | CYHP/MEKP | CYHP/TBPO | MEKP/TBPO | TAEC/TBPB | TAEC/TBEC | TBEC/TBPB |
|---|---|---|---|---|---|---|
| Temperature (°C) | 64.0 | 66.0 | 66.0 | 78.0 | 76.0 | 76.0 |
| Initiator x/initiator y (phr) | 1.75/1.00 | 2.00/1.25 | 2.00/1.25 | 1.50/1.00 | 1.75/1.00 | 1.50/1.50 |
| Content of NH4HCO3 (phr) | 2.00 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| ρ (g·cm−3) | 0.41 ± 0.02 | 0.41 ± 0.01 | 0.40 ± 0.01 | 0.36 ± 0.01 | 0.37 ± 0.02 | 0.37 ± 0.01 |
| P (MPa) | 16.53 ± 0.22 | 17.10 ± 0.34 | 16.90 ± 0.26 | 15.60 ± 0.27 | 15.34 ± 0.25 | 15.13 ± 0.22 |
| Ps (MPa·g−1·cm3) | 40.06 ± 0.45 | 41.28 ± 0.26 | 42.08 ± 0.26 | 43.32 ± 0.45 | 41.46 ± 0.32 | 40.89 ± 0.29 |
| Sample Number | Curing Temperature (°C) | The Composition of the Sample |
|---|---|---|
| A | 66.0 | UPR/MEKP(2.00 phr)/NH4HCO3(2.50 phr) |
| B | 66.0 | UPR/TBPO(1.25 phr)/NH4HCO3(2.50 phr) |
| C | 66.0 | UPR/MEKP(2.00 phr)/TBPO(1.25 phr)/NH4HCO3(2.50 phr) |
| D | 78.0 | UPR/TAEC(1.50 phr)/NH4HCO3(2.50 phr) |
| E | 78.0 | UPR/TBPB(1.00 phr)/NH4HCO3(2.50 phr) |
| F | 78.0 | UPR/TAEC(1.50 phr)/TBPB(1.00 phr)/NH4HCO3(2.50 phr) |
| Sample Number | Temperature (°C) | Onset (min) | Peak (min) | End (min) | Curing Time (min) | QP (J/g) | QR (J/g) | QT (J/g) | α |
|---|---|---|---|---|---|---|---|---|---|
| A | 66.0 | 18.9 | 28.7 | 45.1 | 26.2 | 117.6 | 57.6 | 175.2 | 0.67 |
| B | 66.0 | 16.1 | 27.4 | 38.0 | 21.9 | 132.2 | 52.8 | 185.0 | 0.71 |
| C | 66.0 | 13.5 | 24.6 | 39.1 | 25.6 | 165.8 | 45.9 | 206.7 | 0.78 |
| D | 78.0 | 18.9 | 30.6 | 41.5 | 22.6 | 150.1 | 63.1 | 213.2 | 0.70 |
| E | 78.0 | 22.5 | 36.2 | 44.2 | 21.7 | 139.8 | 63.9 | 203.7 | 0.69 |
| F | 78.0 | 12.1 | 25.4 | 36.8 | 24.7 | 169.5 | 54.1 | 223.6 | 0.76 |
| Sample Number | ρ (g·cm−3) | P (MPa) | Ps (MPa·g−1·cm3) |
|---|---|---|---|
| A | 0.57 ± 0.02 | 14.31 ± 0.22 | 25.10 ± 0.49 |
| B | 0.47 ± 0.01 | 14.56 ± 0.20 | 30.99 ± 0.51 |
| C | 0.40 ± 0.01 | 16.90 ± 0.26 | 42.08 ± 0.26 |
| Sample Number | ρ (g·cm−3) | P (MPa) | Ps (MPa·g−1·cm3) |
|---|---|---|---|
| D | 0.47 ± 0.02 | 13.49 ± 0.18 | 28.82 ± 0.53 |
| E | 0.43 ± 0.02 | 12.68 ± 0.14 | 29.36 ± 0.57 |
| F | 0.36 ± 0.01 | 15.60 ± 0.27 | 43.32 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wang, X.; Chen, M. Low-Density Unsaturated Polyester Resin with the Presence of Dual-Initiator. Materials 2023, 16, 4677. https://doi.org/10.3390/ma16134677
Zhu J, Wang X, Chen M. Low-Density Unsaturated Polyester Resin with the Presence of Dual-Initiator. Materials. 2023; 16(13):4677. https://doi.org/10.3390/ma16134677
Chicago/Turabian StyleZhu, Jinjian, Xiaojun Wang, and Minzhuang Chen. 2023. "Low-Density Unsaturated Polyester Resin with the Presence of Dual-Initiator" Materials 16, no. 13: 4677. https://doi.org/10.3390/ma16134677
APA StyleZhu, J., Wang, X., & Chen, M. (2023). Low-Density Unsaturated Polyester Resin with the Presence of Dual-Initiator. Materials, 16(13), 4677. https://doi.org/10.3390/ma16134677






