Iron, Cobalt, and Nickel Phthalocyanine Tri-Doped Electrospun Carbon Nanofibre-Based Catalyst for Rechargeable Zinc–Air Battery Air Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Physical Characterization
2.3. Electrochemical Characterization
2.4. Zn–Air Battery Testing
3. Results and Discussion
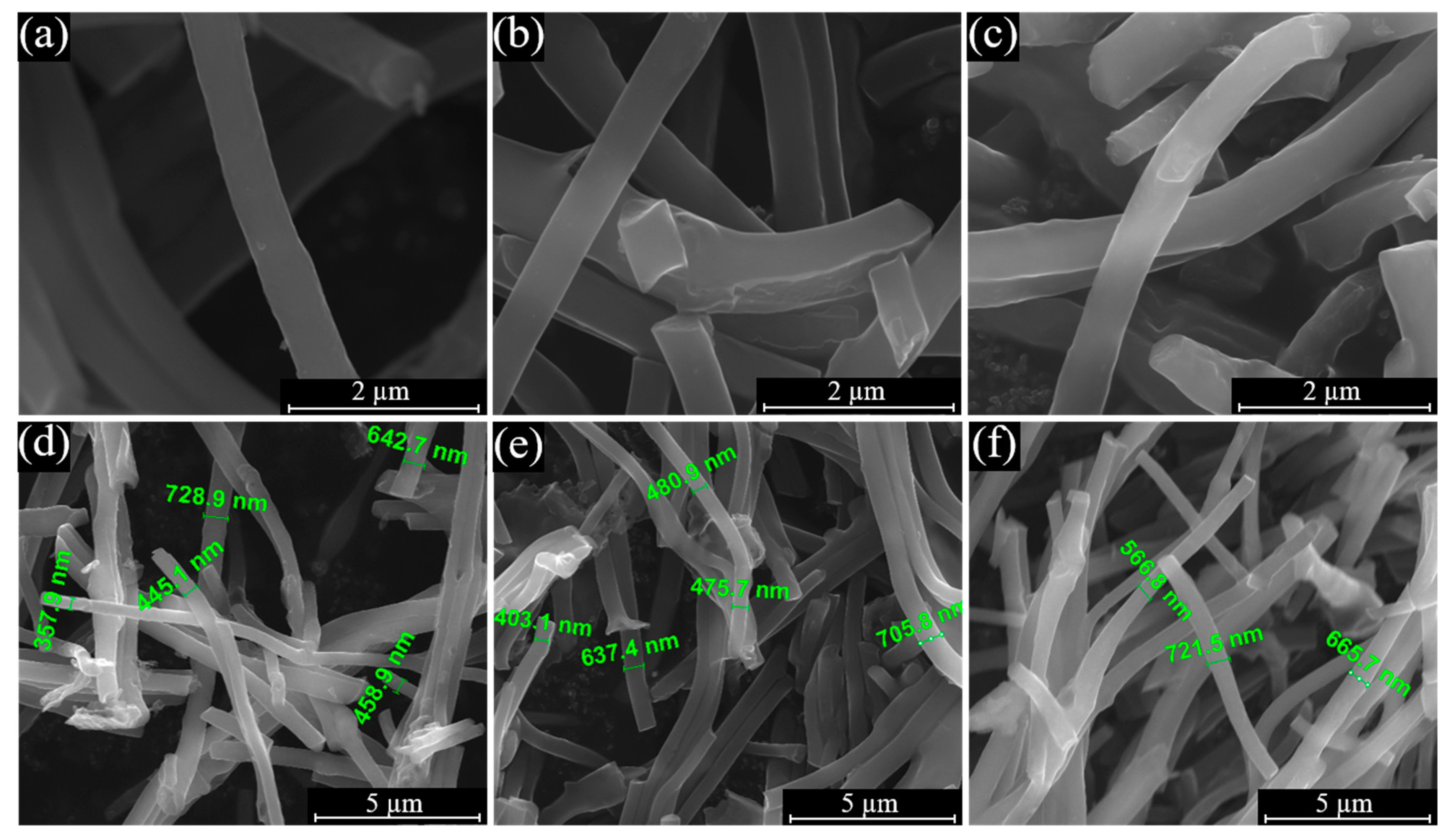
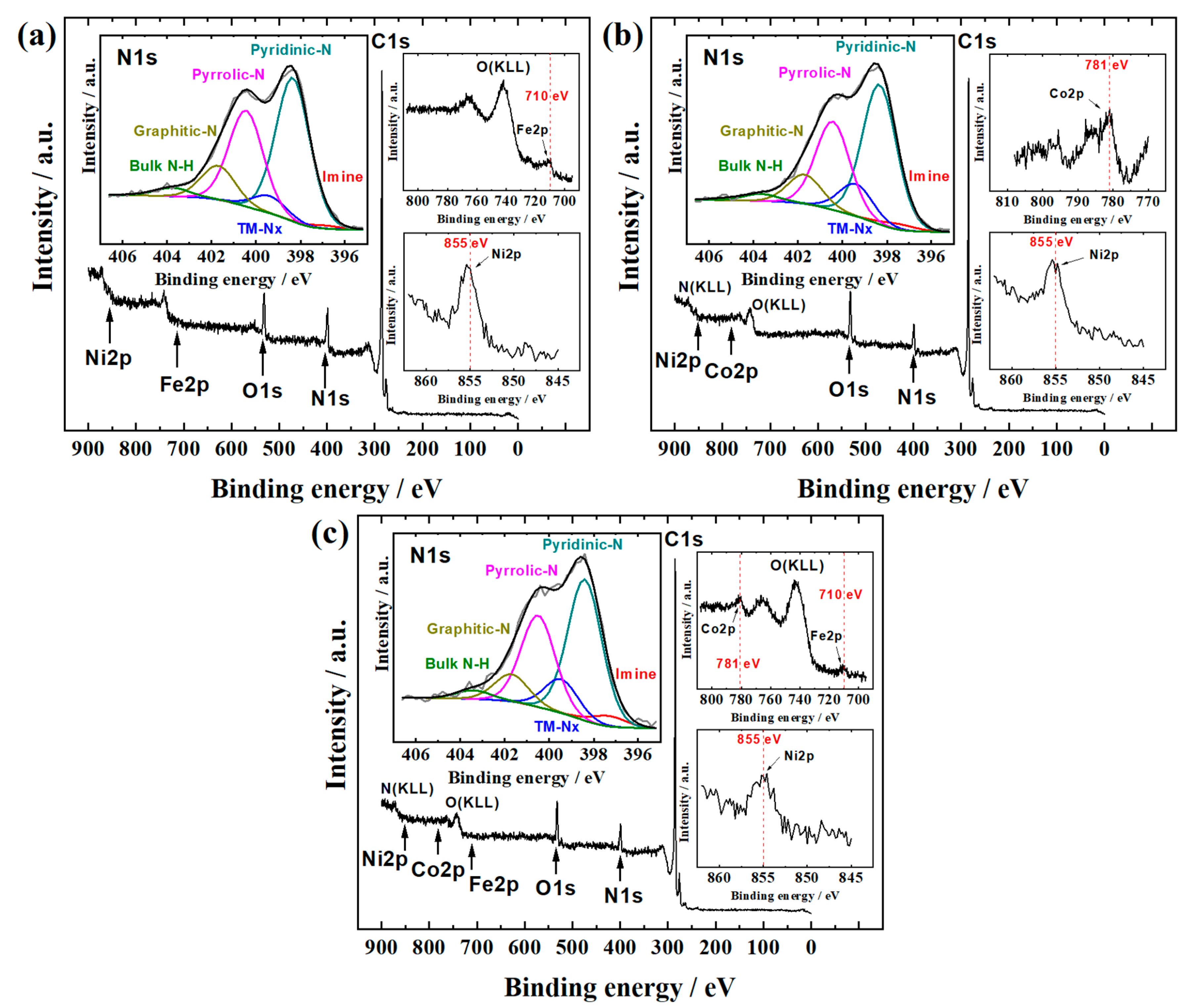
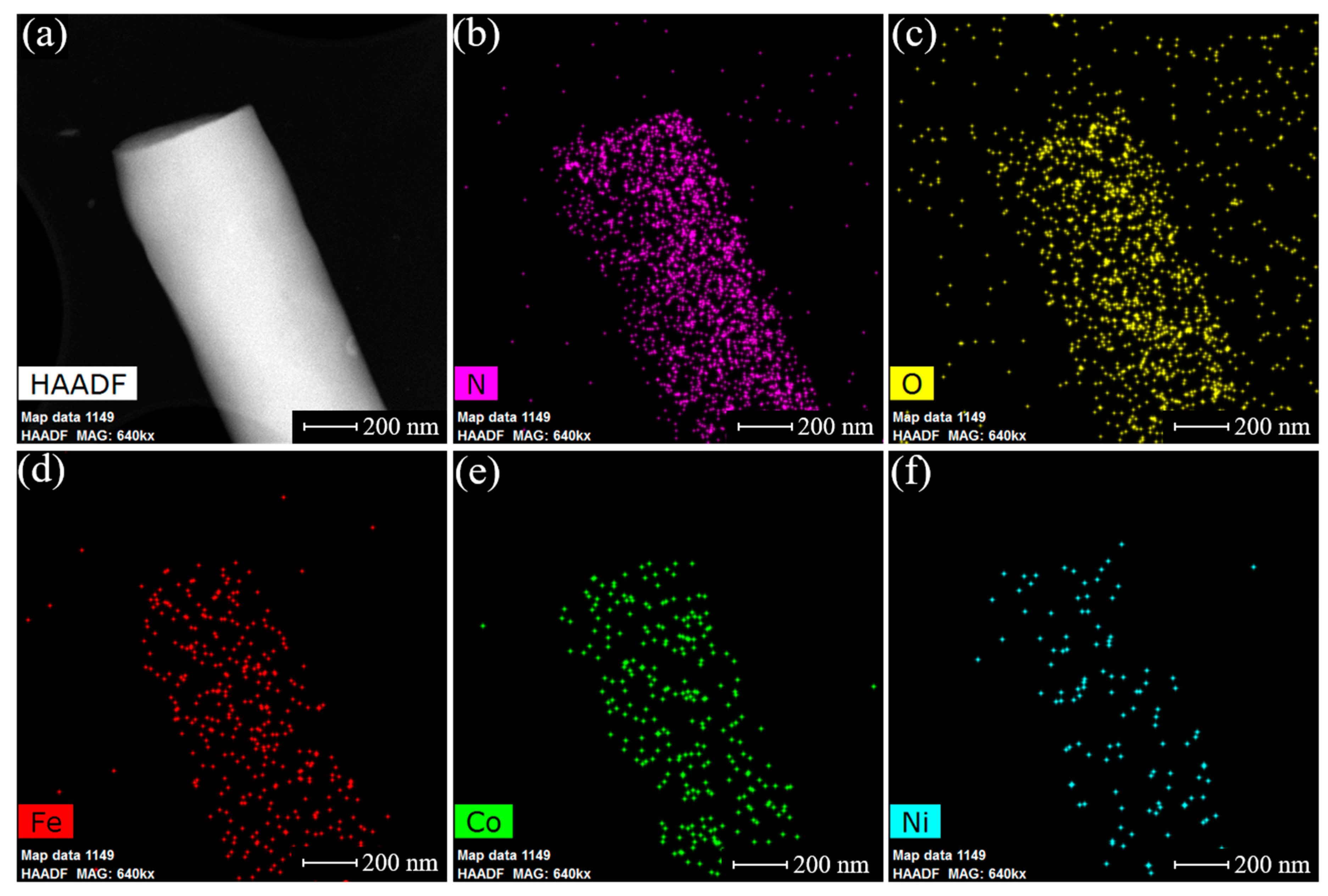
3.1. Physical Characterization
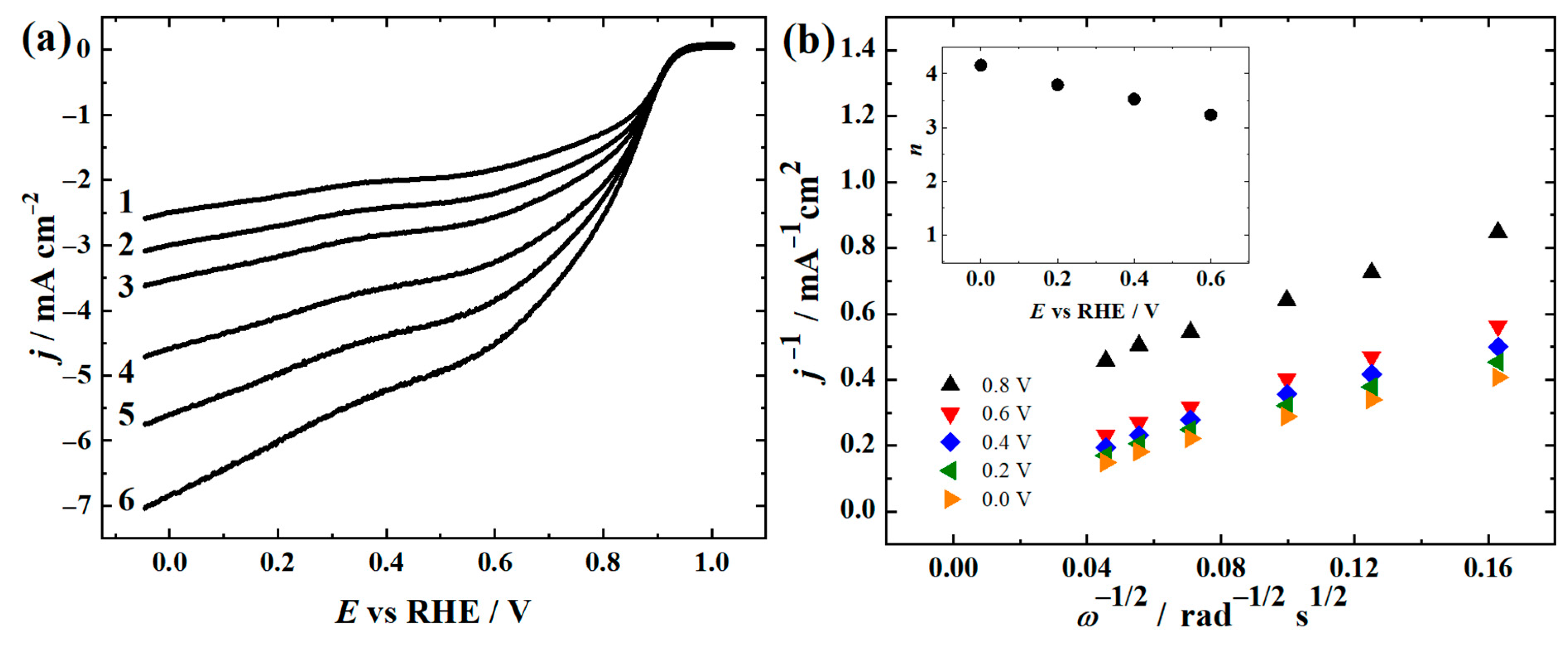
3.2. ORR and OER Studies in 0.1 M KOH
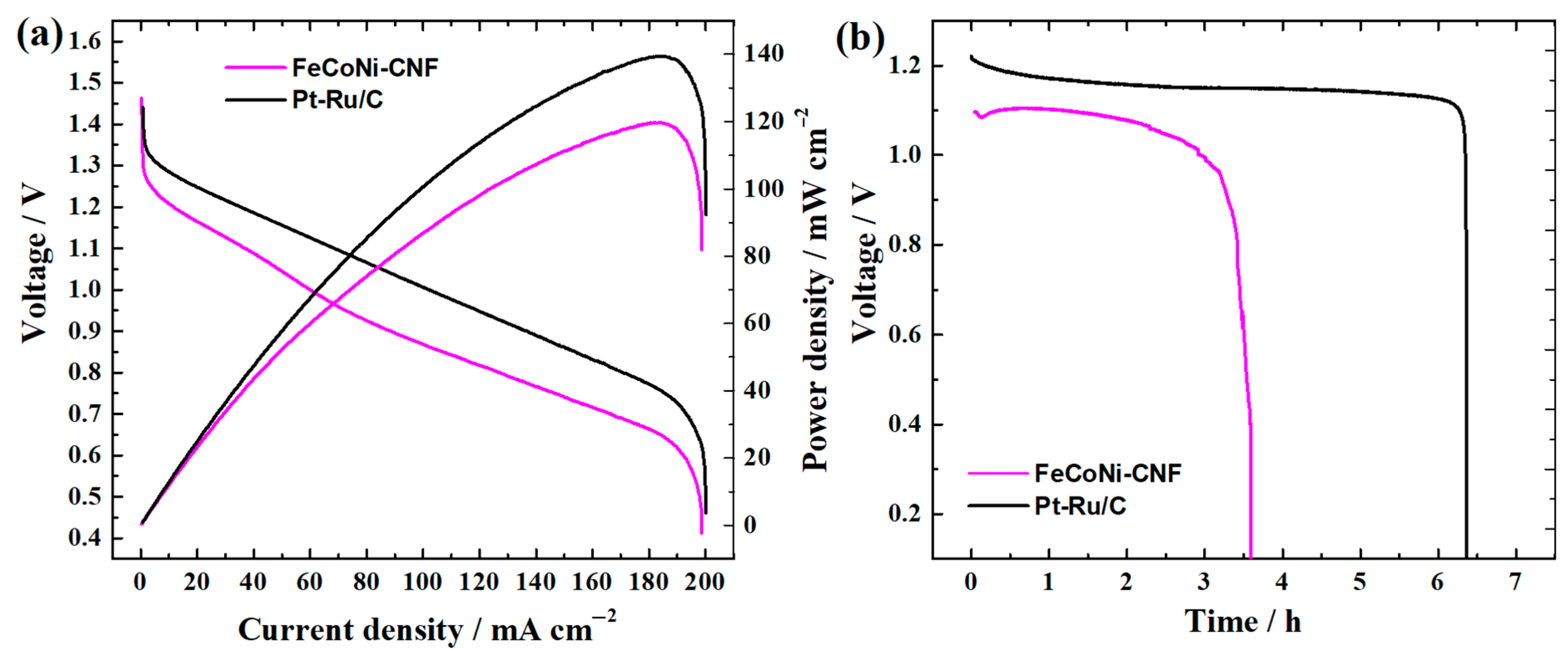
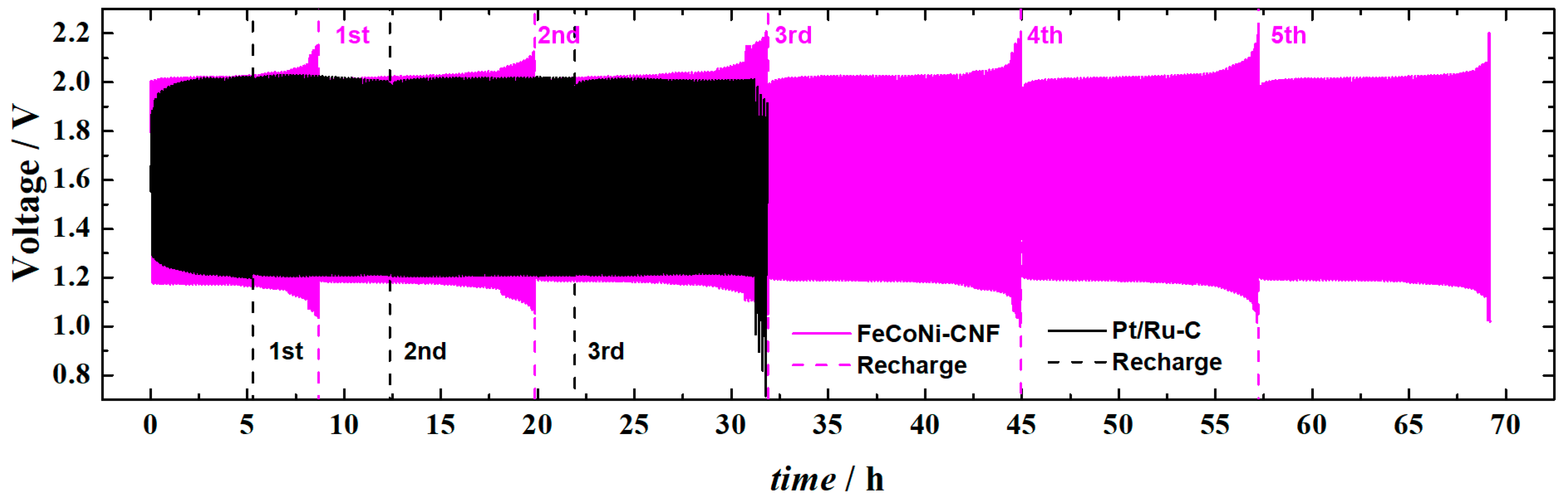
3.3. Zn–Air Battery Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Axsen, J.; Bhardwaj, C.; Crawford, C. Comparing policy pathways to achieve 100% zero-emissions vehicle sales by 2035. Transp. Res. D Transp. Environ. 2022, 112, 103488. [Google Scholar] [CrossRef]
- Ipadeola, A.K.; Haruna, A.B.; Gaolatlhe, L.; Lebechi, A.K.; Meng, J.S.; Pang, Q.Q.; Eid, K.; Abdullah, A.M.; Ozoemena, K.I. Efforts at Enhancing Bifunctional Electrocatalysis and Related Events for Rechargeable Zinc-Air Batteries. ChemElectroChem 2021, 8, 3998–4018. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Y.; Mao, L.C.; Yang, X.X.; Peng, W.; Jin, J.H.; Yang, S.L.; Li, G. Rational assembly of hybrid carbon nanotubes grafted on the carbon nanofibers as reliable and robust bifunctional catalyst for rechargeable zinc-air battery. J. Power Sources 2019, 421, 68–75. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.P.; Fowler, M.; Chen, Z.W. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef]
- Batool, N.; Ahmad, N.; Liu, J.; Han, X.F.; Zhang, T.H.; Wang, W.T.; Yang, R.Z.; Tian, J.H. Electrospun nanofibers and their applications in rechargeable zinc-air batteries. Mater. Chem. Front. 2021, 5, 2950–2966. [Google Scholar] [CrossRef]
- Li, H.F.; Ma, L.T.; Han, C.P.; Wang, Z.F.; Liu, Z.X.; Tang, Z.J.; Zhi, C.Y. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Ma, J.M.; Li, Y.T.; Grundish, N.S.; Goodenough, J.B.; Chen, Y.H.; Guo, L.M.; Peng, Z.Q.; Qi, X.Q.; Yang, F.Y.; Qie, L.; et al. The 2021 battery technology roadmap. J. Phys. D 2021, 54, 183001. [Google Scholar] [CrossRef]
- Sun, J.; Wang, N.; Qiu, Z.Z.; Xing, L.X.; Du, L. Recent Progress of Non-Noble Metal Catalysts for Oxygen Electrode in Zn-Air Batteries: A Mini Review. Catalysts 2022, 12, 843. [Google Scholar] [CrossRef]
- Hao, Y.A.; Hu, F.; Chen, Y.; Wang, Y.H.; Xue, J.J.; Yang, S.Y.; Peng, S.J. Recent Progress of Electrospun Nanofibers for Zinc-Air Batteries. Adv. Fiber Mater. 2022, 4, 185–202. [Google Scholar] [CrossRef]
- Nie, G.D.; Zhang, Z.Y.; Wang, T.T.; Wang, C.; Kou, Z.K. Electrospun One-Dimensional Electrocatalysts for Oxygen Reduction Reaction: Insights into Structure-Activity Relationship. ACS Appl. Mater. Interfaces 2021, 13, 37961–37978. [Google Scholar] [CrossRef]
- Bhoyate, S.D.; Kim, J.; de Souza, F.M.; Lin, J.R.Y.; Lee, E.H.; Kumar, A.; Gupta, R.K. Science and engineering for non-noble-metal-based electrocatalysts to boost their ORR performance: A critical review. Coord. Chem. Rev. 2023, 474, 214854. [Google Scholar] [CrossRef]
- Ullah, N.; Ullah, R.; Khan, S.; Xu, Y.G. Boron nitride-based electrocatalysts for HER, OER, and ORR: A mini-review. Front. Mater. Sci. 2021, 15, 543–552. [Google Scholar] [CrossRef]
- Zagal, J.H.; Koper, M.T.M. Reactivity Descriptors for the Activity of Molecular MN4 Catalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2016, 55, 14510–14521. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, X.M.; Rageloa, J.; Liu, Z.G.; Wang, W. Coordination strategy to prepare high-performance Fe-Nx catalysts for Al-air batteries. J. Power Sources 2023, 567, 232988. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Chao, Y.G.; Zhang, W.S.; Zhou, J.H.; Lv, F.; Wang, K.; Lin, F.X.; Luo, H.; Li, J.; Tong, M.P.; et al. Emerging Dual-Atomic-Site Catalysts for Efficient Energy Catalysis. Adv. Mater. 2021, 33, 2102576. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Q.A.; Wang, J.; Zhang, J.J.; Zhao, Y.F. Supported dual-atom catalysts: Preparation, characterization, and potential applications. Chin. J. Catal. 2020, 41, 783–798. [Google Scholar] [CrossRef]
- Gollasch, M.; Müller-Hülstede, J.; Schmies, H.; Schonvogel, D.; Wagner, P.; Dyck, A.; Wark, M. Elucidating Synergistic Effects of Different Metal Ratios in Bimetallic Fe/Co-N-C Catalysts for Oxygen Reduction Reaction. Catalysts 2021, 11, 841. [Google Scholar] [CrossRef]
- Gao, T.T.; Jin, Z.Y.; Zhang, Y.J.; Tan, G.Q.; Yuan, H.Y.; Xiao, D. Coupling cobalt-iron bimetallic nitrides and N-doped multi-walled carbon nanotubes as high-performance bifunctional catalysts for oxygen evolution and reduction reaction. Electrochim. Acta 2017, 258, 51–60. [Google Scholar] [CrossRef]
- Yusibova, G.; Assafrei, J.M.; Ping, K.F.; Aruväli, J.; Paiste, P.; Käärik, M.; Leis, J.; Piirsoo, H.M.; Tamm, A.; Kikas, A.; et al. Bimetallic metal-organic-framework-derived porous cobalt manganese oxide bifunctional oxygen electrocatalyst. J. Electroanal. Chem. 2023, 930, 117161. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, X.R.; Liu, X.Z.; Huang, S.; Liu, Y.; Yao, Z.C.; Zhang, Y.; Zhang, Q.H.; Gu, L.; Zheng, L.R.; et al. Interfacial assembly of binary atomic metal-Nx sites for high-performance energy devices. Nat. Commun. 2023, 14, 1822. [Google Scholar] [CrossRef]
- Pei, Y.B.; Zhu, W.K.; Yue, R.F.; Yao, J.; Liu, X.; Wang, L.Q.; Zhang, J.F.; Yin, Y.; Guiver, M.D. Fine adjustment of catalyst agglomerate for the controllable construction of Co/Fe-N-C catalyst layers. J. Power Sources 2023, 566, 232904. [Google Scholar] [CrossRef]
- Hao, R.; Gu, S.; Hu, J.; Chen, J.J.; Gan, Q.M.; Li, Y.Z.; Wang, Z.Q.; Liu, G.Y.; Yan, C.L.; Yuan, H.M.; et al. Introducing dual metal centers in high purity pyrrolic-N for superior oxygen reduction reaction. Carbon 2023, 209, 118031. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.M.; Liu, J.; Ma, X.Y.D.; Kong, J.H.; Zhang, Y.F.; Yan, T.; Lu, X.H. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2020, 263, 118344. [Google Scholar] [CrossRef]
- Ji, D.X.; Sun, J.G.; Tian, L.D.; Chinnappan, A.; Zhang, T.R.; Jayathilaka, W.; Gosh, R.; Baskar, C.; Zhang, Q.Y.; Ramakrishna, S. Engineering of the Heterointerface of Porous Carbon Nanofiber-Supported Nickel and Manganese Oxide Nanoparticle for Highly Efficient Bifunctional Oxygen Catalysis. Adv. Funct. Mater. 2020, 30, 1910568. [Google Scholar] [CrossRef]
- Niu, Y.L.; Teng, X.; Gong, S.Q.; Chen, Z.F. A bimetallic alloy anchored on biomass-derived porous N-doped carbon fibers as a self-supporting bifunctional oxygen electrocatalyst for flexible Zn-air batteries. J. Mater. Chem. A 2020, 8, 13725–13734. [Google Scholar] [CrossRef]
- Wang, X.T.; Ouyang, T.; Wang, L.; Zhong, J.H.; Liu, Z.Q. Surface Reorganization on Electrochemically-Induced Zn-Ni-Co Spinel Oxides for Enhanced Oxygen Electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 6492–6499. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Balamurugan, J.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Yoo, D.J. Nitrogen-doped graphene encapsulated FeCoMoS nanoparticles as advanced trifunctional catalyst for water splitting devices and zinc-air batteries. Appl. Catal. B Environ. 2020, 279, 119381. [Google Scholar] [CrossRef]
- Tang, X.N.; Cao, R.; Li, L.B.; Huang, B.Y.; Zhai, W.J.; Yuan, K.; Chen, Y.W. Engineering efficient bifunctional electrocatalysts for rechargeable zinc-air batteries by confining Fe-Co-Ni nanoalloys in nitrogen-doped carbon nanotube@nanosheet frameworks. J. Mater. Chem. A 2020, 8, 25919–25930. [Google Scholar] [CrossRef]
- Jiang, R.Z.; Baker, D.R.; Tran, D.T.; Li, J.T.; Leff, A.C.; Zhang, S.S. Multimetallic FeCoNiOx Nanoparticles Covered with Nitrogen-Doped Graphene Layers as Trifunctional Catalysts for Hydrogen Evolution and Oxygen Reduction and Evolution. ACS Appl. Nano Mater. 2020, 3, 7119–7129. [Google Scholar] [CrossRef]
- Bejar, J.; Alvarez-Contreras, L.; Espinosa-Magana, F.; Ledesma-Garcia, J.; Arjona, N.; Arriaga, L.G. Zn-air battery operated with a 3DOM trimetallic spinel (Mn0.5Ni0.5Co2O4) as the oxygen electrode. Electrochim. Acta 2021, 391, 138900. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhou, X.Y.; Fang, G.; Xie, G.Q.; Liu, X.J.; Lin, X.; Qiu, H.J. Multicomponent Spinel Metal Oxide Nanocomposites as High-Performance Bifunctional Catalysts in Zn-Air Batteries. ACS Appl. Energy Mater. 2020, 3, 7710–7718. [Google Scholar] [CrossRef]
- Guo, Z.W.; Ma, Y.A.; Zhao, Y.J.; Song, Y.; Tang, S.R.; Wang, Q.; Li, W. Trimetallic ZIFs-derived porous carbon as bifunctional electrocatalyst for rechargeable Zn-air battery. J. Power Sources 2022, 542, 231723. [Google Scholar] [CrossRef]
- Kumar, Y.; Mooste, M.; Tammeveski, K. Recent progress of transition metal-based bifunctional electrocatalysts for rechargeable zinc–air battery application. Curr. Opin. Electrochem. 2023, 38, 101229. [Google Scholar] [CrossRef]
- Xie, L.S.; Zhang, X.P.; Zhao, B.; Li, P.; Qi, J.; Guo, X.N.; Wang, B.; Lei, H.T.; Zhang, W.; Apfel, U.P.; et al. Enzyme-Inspired Iron Porphyrins for Improved Electrocatalytic Oxygen Reduction and Evolution Reactions. Angew. Chem. Int. Ed. 2021, 60, 7576–7581. [Google Scholar] [CrossRef]
- Yang, S.X.; Yu, Y.H.; Dou, M.L.; Zhang, Z.P.; Wang, F. Edge-Functionalized Polyphthalocyanine Networks with High Oxygen Reduction Reaction Activity. J. Am. Chem. Soc. 2020, 142, 17524–17530. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wang, Q.T.; Bian, Z.N.; Wang, G.M.; Xu, Y.X. Supramolecular Modulation of Molecular Conformation of Metal Porphyrins toward Remarkably Enhanced Multipurpose Electrocatalysis and Ultrahigh-Performance Zinc-Air Batteries. Adv. Energy Mater. 2021, 11, 2102062. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Põldsepp, E.; Mooste, M.; Kozlova, J.; Kikas, A.; Aruväli, J.; Käärik, M.; Kisand, V.; Leis, J.; Tamm, A.; et al. Iron and Nickel Phthalocyanine-Modified Nanocarbon Materials as Cathode Catalysts for Anion-Exchange Membrane Fuel Cells and Zinc-Air Batteries. ChemElectroChem 2022, 9, e202200717. [Google Scholar] [CrossRef]
- Feng, Y.X.; Tian, G.; Peng, Q.L.; Wu, Y.B.; Li, Y.L.; Luo, X.Q.; Han, Y.J.; Li, Q.B. Fe-N Doped Peanut Shell Activated Carbon as a Superior Electrocatalyst for Oxygen Reduction and Cathode Catalyst for Zinc-Air Battery. ChemElectroChem 2021, 8, 4797–4803. [Google Scholar] [CrossRef]
- Loyola, C.Z.; Abarca, G.; Ureta-Zanartu, S.; Aliaga, C.; Zagal, J.H.; Sougrati, M.T.; Jaouen, F.; Orellana, W.; Tasca, F. Insights into the electronic structure of Fe penta-coordinated complexes. Spectroscopic examination and electrochemical analysis for the oxygen reduction and oxygen evolution reactions. J. Mater. Chem. A 2021, 9, 23802–23816. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Põldsepp, E.; Kozlova, J.; Kikas, A.; Käärik, M.; Aruväli, J.; Kisand, V.; Leis, J.; Tamm, A.; Tammeveski, K. Bimetal Phthalocyanine-Modified Carbon Nanotube-Based Bifunctional Catalysts for Zinc-Air Batteries. ChemElectroChem 2021, 8, 2662–2670. [Google Scholar] [CrossRef]
- Morozan, A.; Campidelli, S.; Filoramo, A.; Jousselme, B.; Palacin, S. Catalytic activity of cobalt and iron phthalocyanines or porphyrins supported on different carbon nanotubes towards oxygen reduction reaction. Carbon 2011, 49, 4839–4847. [Google Scholar] [CrossRef]
- Guo, X.S.; Huang, Z.Y.; Qi, X.W.; Si, L.P.; Zhang, H.; Liu, H.Y. The optimization of iron porphyrin@MOF-5 derived Fe-N-C electrocatalysts for oxygen reduction reaction in zinc-air batteries. J. Electroanal. Chem. 2023, 936, 117381. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Su, Z.; Zhao, Z.; Cai, Q.; Zhao, J. Phthalocyanine-supported single-atom catalysts as a promising bifunctional electrocatalyst for ORR/OER: A computational study. ChemPhysMater 2022, 1, 237–245. [Google Scholar] [CrossRef]
- Muuli, K.; Sokka, A.; Mooste, M.; Lilloja, J.; Gudkova, V.; Käärik, M.; Otsus, M.; Kikas, A.; Kisand, V.; Tamm, A.; et al. Iron and cobalt phthalocyanine embedded electrospun carbon nanofiber-based catalysts for anion exchange membrane fuel cell cathode. J. Catal. 2023, 422, 117–130. [Google Scholar] [CrossRef]
- Mooste, M.; Kibena-Põldsepp, E.; Vassiljeva, V.; Merisalu, M.; Kook, M.; Treshchalov, A.; Kisand, V.; Uibu, M.; Krumme, A.; Sammelselg, V.; et al. Electrocatalysts for oxygen reduction reaction based on electrospun polyacrylonitrile, styrene-acrylonitrile copolymer and carbon nanotube composite fibres. J. Mater. Sci. 2019, 54, 11618–11634. [Google Scholar] [CrossRef]
- Moni, P.; Mooste, M.; Tammeveski, K.; Rezwan, K.; Wilhelm, M. One-dimensional polymer-derived ceramic nanowires with electrocatalytically active metallic silicide tips as cathode catalysts for Zn-air batteries. RSC Adv. 2021, 11, 39707–39717. [Google Scholar] [CrossRef]
- Muuli, K.; Lyu, X.; Mooste, M.; Käärik, M.; Zulevi, B.; Leis, J.; Yu, H.; Cullen, D.A.; Serov, A.; Tammeveski, K. Outstanding platinum group metal-free bifunctional catalysts for rechargeable zinc-air batteries. Electrochim. Acta 2023, 446, 142126. [Google Scholar] [CrossRef]
- Sokka, A.; Mooste, M.; Käärik, M.; Gudkova, V.; Kozlova, J.; Kikas, A.; Kisand, V.; Treshchalov, A.; Tamm, A.; Paiste, P.; et al. Iron and cobalt containing electrospun carbon nanofibre-based cathode catalysts for anion exchange membrane fuel cell. Int. J. Hydrogen Energy 2021, 46, 31275–31287. [Google Scholar] [CrossRef]
- Wen, Y.; Kok, M.D.R.; Tafoya, J.P.V.; Sobrido, A.B.J.; Bell, E.; Gostick, J.T.; Herou, S.; Schlee, P.; Titirici, M.M.; Brett, D.J.L.; et al. Electrospinning as a route to advanced carbon fibre materials for selected low-temperature electrochemical devices: A review. J. Energy Chem. 2021, 59, 492–529. [Google Scholar] [CrossRef]
- Cavaliere, S.; Subianto, S.; Savych, I.; Jones, D.J.; Roziere, J. Electrospinning: Designed architectures for energy conversion and storage devices. Energy Environ. Sci. 2011, 4, 4761–4785. [Google Scholar] [CrossRef]
- Rauf, M.; Wang, J.W.; Zhang, P.X.; Iqbal, W.; Qu, J.L.; Li, Y.L. Non-precious nanostructured materials by electrospinning and their applications for oxygen reduction in polymer electrolyte membrane fuel cells. J. Power Sources 2018, 408, 17–27. [Google Scholar] [CrossRef]
- Sonkar, P.K.; Ganesan, V.; Gupta, R.; Yadav, D.K.; Yadav, M. Nickel phthalocyanine integrated graphene architecture as bifunctional electrocatalyst for CO2 and O2 reductions. J. Electroanal. Chem. 2018, 826, 1–9. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, J.; Liu, G.Y.; Luo, X.G.; Wu, F.S. Cobalt phthalocyanine-based nanodots as efficient catalysts for chemical conversion of CO2 under ambient conditions. J. Mater. Sci. 2021, 56, 10990–10999. [Google Scholar] [CrossRef]
- Liu, K.X.; Kattel, S.; Mao, V.; Wang, G.F. Electrochemical and Computational Study of Oxygen Reduction Reaction on Nonprecious Transition Metal/Nitrogen Doped Carbon Nanofibers in Acid Medium. J. Phys. Chem. C 2016, 120, 1586–1596. [Google Scholar] [CrossRef]
- Praats, R.; Käärik, M.; Kikas, A.; Kisand, V.; Aruväli, J.; Paiste, P.; Merisalu, M.; Sarapuu, A.; Leis, J.; Sammelselg, V.; et al. Electroreduction of oxygen on cobalt phthalocyanine-modified carbide-derived carbon/carbon nanotube composite catalysts. J. Solid State Electrochem. 2021, 25, 57–71. [Google Scholar] [CrossRef]
- Li, L.B.; Tang, X.N.; Huang, S.H.; Lu, C.B.; Lutzenkirchen-Hecht, D.; Yuan, K.; Zhuang, X.D.; Chen, Y.W. Longitudinally Grafting of Graphene with Iron Phthalocyanine-based Porous Organic Polymer to Boost Oxygen Electroreduction. Angew. Chem. Int. Ed. 2023, 62, e202301642. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Chuang, Y.Y.; Chen, J.S. Effect of surface bonding of FePC with electrospun carbon nanofiber on electrocatalytic performance for aprotic Li-O2 batteries. J. Colloid Interface Sci. 2020, 562, 213–223. [Google Scholar] [CrossRef]
- Frost, D.C.; McDowell, C.A.; Woolsey, I.S. X-ray photoelectron spectra of cobalt compounds. Mol. Phys. 1974, 27, 1473–1489. [Google Scholar] [CrossRef]
- Govan, J.; Orellana, W.; Zagal, J.H.; Tasca, F. Penta-coordinated transition metal macrocycles as electrocatalysts for the oxygen reduction reaction. J. Solid State Electrochem. 2021, 25, 15–31. [Google Scholar] [CrossRef]
- Orellana, W.; Zuñiga, C.; Gatica, A.; Ureta-Zanartu, M.-S.; Zagal, J.H.; Tasca, F. Effect of Electrolyte Media on the Catalysis of Fe Phthalocyanine toward the Oxygen Reduction Reaction: Ab Initio Molecular Dynamics Simulations and Experimental Analyses. ACS Catal. 2022, 12, 12786–12799. [Google Scholar] [CrossRef]
- Loyola, C.Z.; Ureta-Zanartu, S.; Zagal, J.H.; Tasca, F. Activity volcano plots for the oxygen reduction reaction using FeN4 complexes: From reported experimental data to the electrochemical meaning. Curr. Opin. Electrochem. 2022, 32, 100923. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Zhao, H.T.; Liang, J.; Wang, Y.; Li, T.S.; Luo, Y.S.; Shi, X.F.; Lu, S.Y.; Feng, Z.S.; Wu, Q.; et al. Noble-metal-free electrospun nanomaterials as electrocatalysts for oxygen reduction reaction. Mater. Today Phys. 2020, 15, 100280. [Google Scholar] [CrossRef]
- Meng, H.J.; Liu, Y.M.; Liu, H.R.; Pei, S.P.; Yuan, X.X.; Li, H.; Zhang, Y.M. ZIF67@MFC-Derived Co/N-C@CNFs Interconnected Frameworks with Graphitic Carbon-Encapsulated Co Nanoparticles as Highly Stable and Efficient Electrocatalysts for Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2020, 12, 41580–41589. [Google Scholar] [CrossRef]
- Lu, L.; Cao, X.J.; Shen, Z.Y.; Huo, J.J.; Chen, W.H.; Liu, C.T.; Liu, H. Electrospun nitrogen-doped carbon nano fibers for electrocatalysis. SM&T 2020, 26, e00221. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Role of Nitrogen Moieties in N-Doped 3D-Graphene Nanosheets for Oxygen Electroreduction in Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 11623–11632. [Google Scholar] [CrossRef]
- Bezerra, L.S.; Mooste, M.; Fortunato, G.V.; Cardoso, E.S.F.; Lanza, M.R.V.; Tammeveski, K.; Maia, G. Tuning NiCo2O4 bifunctionality with nitrogen-doped graphene nanoribbons in oxygen electrocatalysis for zinc-air battery application. J. Electroanal. Chem. 2023, 928, 117000. [Google Scholar] [CrossRef]
- Zhan, Z.M.; Liang, X.L.; Li, J.F.; Qian, J.M.; Liu, Y.G.; Yang, S.L.; Wang, Y.; Gao, D.Q.; Xue, D.S. Interfacial Engineering of NiO/NiCo2O4 Porous Nanofibers as Efficient Bifunctional Catalysts for Rechargeable Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 21661–21669. [Google Scholar] [CrossRef]
- Zeng, K.; Zheng, X.J.; Li, C.; Yan, J.; Tian, J.H.; Jin, C.; Strasser, P.; Yang, R.Z. Recent Advances in Non-Noble Bifunctional Oxygen Electrocatalysts toward Large-Scale Production. Adv. Funct. Mater. 2020, 30, 2000503. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhao, X.X.; Xi, S.B.; Zhang, L.L.; Chen, Z.X.; Zeng, Z.P.; Huang, M.; Yang, H.B.; Liu, B.; Pennycook, S.J.; et al. Atomically Dispersed Cobalt Trifunctional Electrocatalysts with Tailored Coordination Environment for Flexible Rechargeable Zn-Air Battery and Self-Driven Water Splitting. Adv. Energy Mater. 2020, 10, 2002896. [Google Scholar] [CrossRef]
- Sun, L.X.; Qin, Y.N.; Fu, L.S.; Di, Y.T.; Hu, K.D.; Li, H.L.; Li, L.; Zhang, W.M. A self-supported bifunctional air cathode composed of Co3O4/Fe2O3 nanoparticles embedded in nanosheet arrays grafted onto carbon nanofibers for secondary zinc-air batteries. J. Alloys Compd. 2022, 921, 166128. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Põldsepp, E.; Kozlova, J.; Rähn, M.; Treshchalov, A.; Kikas, A.; Kisand, V.; Aruväli, J.; Tamm, A.; Douglin, J.C.; et al. Bifunctional Oxygen Electrocatalysis on Mixed Metal Phthalocyanine-Modified Carbon Nanotubes Prepared via Pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 41507–41516. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Poschl, U. Raman micro spectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Buan, M.E.M.; Cognigni, A.; Walmsley, J.C.; Muthuswamy, N.; Ronning, M. Active sites for the oxygen reduction reaction in nitrogen-doped carbon nanofibers. Catal. Today 2020, 357, 248–258. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, Y.L.; Dong, L.L.; Yang, W.X.; Liu, X.J.; Long, L.; Liu, C.Y.; Dong, S.J.; Jia, J.B. Synergistic effect between atomically dispersed Fe and Co metal sites for enhanced oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 4369–4375. [Google Scholar] [CrossRef]
- Teppor, P.; Jäger, R.; Härmas, M.; Aruväli, J.; Volobujeva, O.; Koppel, M.; Lust, E. Bifunctional Platinum-Free Mixed Metal Oxygen Electrocatalysts Based on Naturally Abundant Peat. ECS Trans. 2022, 108, 29. [Google Scholar] [CrossRef]
- Ullah, N.; Zhao, W.T.; Lu, X.Q.; Oluigbo, C.J.; Shah, S.A.; Zhang, M.M.; Xie, J.M.; Xu, Y.G. In situ growth of M-MO (M = Ni, Co) in 3D graphene as a competent bifunctional electrocatalyst for OER and HER. Electrochim. Acta 2019, 298, 163–171. [Google Scholar] [CrossRef]
- Lu, X.Y.; Yim, W.L.; Suryanto, B.H.R.; Zhao, C. Electrocatalytic Oxygen Evolution at Surface-Oxidized Multiwall Carbon Nanotubes. J. Am. Chem. Soc. 2015, 137, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Pei, Z.X.; Li, H.F.; Huang, Y.; Xue, Q.; Zhu, M.S.; Wang, Z.F.; Zhi, C.Y. Texturing in situ: N, S-enriched hierarchically porous carbon as a highly active reversible oxygen electrocatalyst. Energy Environ. Sci. 2017, 10, 742–749. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wu, X.H.; Tu, T.X.; Zhang, P.F.; Li, J.T.; Zhou, Y.; Huang, L.; Sun, S.G. Controlled synthesis of FeNx-CoNx dual active sites interfaced with metallic Co nanoparticles as bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. Appl. Catal. B Environ. 2020, 278, 119259. [Google Scholar] [CrossRef]
- Zhang, T.R.; Bian, J.J.; Zhu, Y.Q.; Sun, C.W. FeCo Nanoparticles Encapsulated in N-Doped Carbon Nanotubes Coupled with Layered Double (Co, Fe) Hydroxide as an Efficient Bifunctional Catalyst for Rechargeable Zinc-Air Batteries. Small 2021, 17, 2103737. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Chen, S.; Yue, Q.; Kang, Y.J. Optimizing the Spin States of Mesoporous Co3O4 Nanorods through Vanadium Doping for Long-Lasting and Flexible Rechargeable Zn-Air Batteries. ACS Catal. 2021, 11, 8097–8103. [Google Scholar] [CrossRef]
- Dong, M.Y.; Liu, X.; Jiang, L.X.; Zhu, Z.J.; Shu, Y.J.; Chen, S.; Dou, Y.H.; Liu, P.R.; Yin, H.J.; Zhao, H.J. Cobalt-doped Mn3O4 nanocrystals embedded in graphene nanosheets as a high-performance bifunctional oxygen electrocatalyst for rechargeable Zn-Air batteries. Green Energy Environ. 2020, 5, 499–505. [Google Scholar] [CrossRef]
- Wang, S.J.; Wang, H.Y.; Huang, C.A.Q.; Ye, P.C.; Luo, X.T.; Ning, J.Q.; Zhong, Y.J.; Hu, Y. Trifunctional electrocatalyst of N-doped graphitic carbon nanosheets encapsulated with CoFe alloy nanocrystals: The key roles of bimetal components and high-content graphitic-N. Appl. Catal. B Environ. 2021, 298, 120512. [Google Scholar] [CrossRef]
- Thakur, N.; Kumar, M.; Mandal, D.; Nagaiah, T.C. Nickel Iron Phosphide/Phosphate as an Oxygen Bifunctional Electrocatalyst for High-Power-Density Rechargeable Zn-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 52487–52497. [Google Scholar] [CrossRef]
- Go, Y.; Min, K.; An, H.; Kim, K.; Shim, S.E.; Baeck, S.H. Oxygen-vacancy-rich CoFe/CoFe2O4 embedded in N-doped hollow carbon spheres as a highly efficient bifunctional electrocatalyst for Zn-air batteries. Chem. Eng. J. 2022, 448, 13. [Google Scholar] [CrossRef]



| Catalyst Designation | Electrospinning Solution Components |
|---|---|
| FeNi-CNF | 10% [BMIm]Ac; 58% PAN; 16% FePc; 16% NiPc |
| CoNi-CNF | 10% [BMIm]Ac; 58% PAN; 16% CoPc; 16% NiPc |
| FeCoNi-CNF | 10% [BMIm]Ac; 58% PAN; 10.6% FePc; 10.6% CoPc; 10.6% NiPc |
| Catalyst Material | C | N | O | Fe | Co | Ni |
|---|---|---|---|---|---|---|
| FeNi-CNF | 83.1 | 9.4 | 6.7 | 0.3 | 0 | 0.5 |
| CoNi-CNF | 82.4 | 7.7 | 9.2 | 0 | 0.4 | 0.3 |
| FeCoNi-CNF | 83.6 | 7.5 | 8.1 | 0.1 | 0.4 | 0.3 |
| Catalyst material | imine | pyridinic-N | TM-Nx | pyrrolic-N | graphitic-N | bulk N-H |
| FeNi-CNF | 1 | 47 | 6 | 32 | 11 | 3 |
| CoNi-CNF | 2 | 46 | 11 | 29 | 10 | 2 |
| FeCoNi-CNF | 3 | 45 | 11 | 29 | 9 | 3 |
| Electrode | E1/2 (V) | E10 (V) | ∆E (V) |
|---|---|---|---|
| FeNi-CNF | 0.68 | - | - |
| CoNi-CNF | 0.76 | - | - |
| FeCoNi-CNF | 0.77 | 1.66 | 0.89 |
| FeCoNi-CNF-750 | 0.76 | 1.72 | 0.96 |
| FeCoNi-CNF-850 | 0.77 | 1.71 | 0.94 |
| FeCoNi-CNF-1M | 0.78 | - | - |
| FeCoNi-CNF-3M | 0.71 | - | - |
| Catalyst | E1/2, V | E10, V | ΔE, V | OCV, V | Pmax, mW cm−2 | Specific Capacity, mAh gZn−1 | Stability, Time @j (mA cm−2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| FeCoNi-CNF | 0.77 | 1.66 | 0.89 | 1.51 | 120 | 407 | 68 h @5 | This work |
| Zn0.4Ni0.6Co2O4/NCNTs | 0.78 | 1.64 | 0.86 | 1.48 | 109 | 690 | 100 cycles @25 | [26] |
| Mn0.5Ni0.5Co2O4 3DOM | 0.76 | 1.63 | 0.87 | 1.38 | 117 | - | 21 h @10 | [30] |
| 5 wt%-Ni/PDC | 0.62 | 1.61 | 0.99 | 1.24 | 59 | 608 | 200 h @5 | [46] |
| FeNi/N-CPCF-950 | 0.87 | 1.59 | 0.72 | 1.48 | 161 | 751 | 640 h @10 | [23] |
| FeNiN-MWCNT | 0.87 | 1.59 | 0.72 | 1.45 | 84.5 | - | 48 h @1 | [37] |
| Co3O4/Fe2O3NAs@CNFs | 0.80 | 1.60 | 0.80 | 1.53 | 256 | - | 40 h @10 | [70] |
| Ni|MnO/CNF | 0.83 | 1.59 | 0.76 | 1.56 | 139 | - | 120 h @10 | [24] |
| CoFe-LDH@FeCo NPs-N-CNTs | 0.89 | 1.57 | 0.68 | 1.51 | 116 | 799 | 100 h @10 | [82] |
| H-Co@FeCo/N/C | 0.91 | 1.61 | 0.70 | 1.45 | 125.2 | - | 200 h @10 | [81] |
| FeCoNi-NC | 0.89 | 1.54 | 0.65 | 1.52 | 315 | 804 | 100 h @50 | [28] |
| Fe/Co/Zn-CNZIF | 0.85 | 1.58 | 0.73 | 1.46 | 157 | 769 | 137 h @5 | [32] |
| Vo-CoFe/CoFe2O4@NC | 0.86 | 1.59 | 0.73 | 1.53 | 140 | 775 | 45 h @10 | [87] |
| NiCo2O4(90):GNRN(10) | 0.76 | 1.62 | 0.86 | 1.43 | 69 | 702 | 96 h @2 | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muuli, K.; Kumar, R.; Mooste, M.; Gudkova, V.; Treshchalov, A.; Piirsoo, H.-M.; Kikas, A.; Aruväli, J.; Kisand, V.; Tamm, A.; et al. Iron, Cobalt, and Nickel Phthalocyanine Tri-Doped Electrospun Carbon Nanofibre-Based Catalyst for Rechargeable Zinc–Air Battery Air Electrode. Materials 2023, 16, 4626. https://doi.org/10.3390/ma16134626
Muuli K, Kumar R, Mooste M, Gudkova V, Treshchalov A, Piirsoo H-M, Kikas A, Aruväli J, Kisand V, Tamm A, et al. Iron, Cobalt, and Nickel Phthalocyanine Tri-Doped Electrospun Carbon Nanofibre-Based Catalyst for Rechargeable Zinc–Air Battery Air Electrode. Materials. 2023; 16(13):4626. https://doi.org/10.3390/ma16134626
Chicago/Turabian StyleMuuli, Kaur, Rohit Kumar, Marek Mooste, Viktoria Gudkova, Alexey Treshchalov, Helle-Mai Piirsoo, Arvo Kikas, Jaan Aruväli, Vambola Kisand, Aile Tamm, and et al. 2023. "Iron, Cobalt, and Nickel Phthalocyanine Tri-Doped Electrospun Carbon Nanofibre-Based Catalyst for Rechargeable Zinc–Air Battery Air Electrode" Materials 16, no. 13: 4626. https://doi.org/10.3390/ma16134626
APA StyleMuuli, K., Kumar, R., Mooste, M., Gudkova, V., Treshchalov, A., Piirsoo, H.-M., Kikas, A., Aruväli, J., Kisand, V., Tamm, A., Krumme, A., Moni, P., Wilhelm, M., & Tammeveski, K. (2023). Iron, Cobalt, and Nickel Phthalocyanine Tri-Doped Electrospun Carbon Nanofibre-Based Catalyst for Rechargeable Zinc–Air Battery Air Electrode. Materials, 16(13), 4626. https://doi.org/10.3390/ma16134626







