In Situ Growth of Nano-MoS2 on Graphite Substrates as Catalysts for Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Sample Preparation Method
2.3. Structural Characterizations
2.4. Electrochemical Tests
3. Results and Discussion
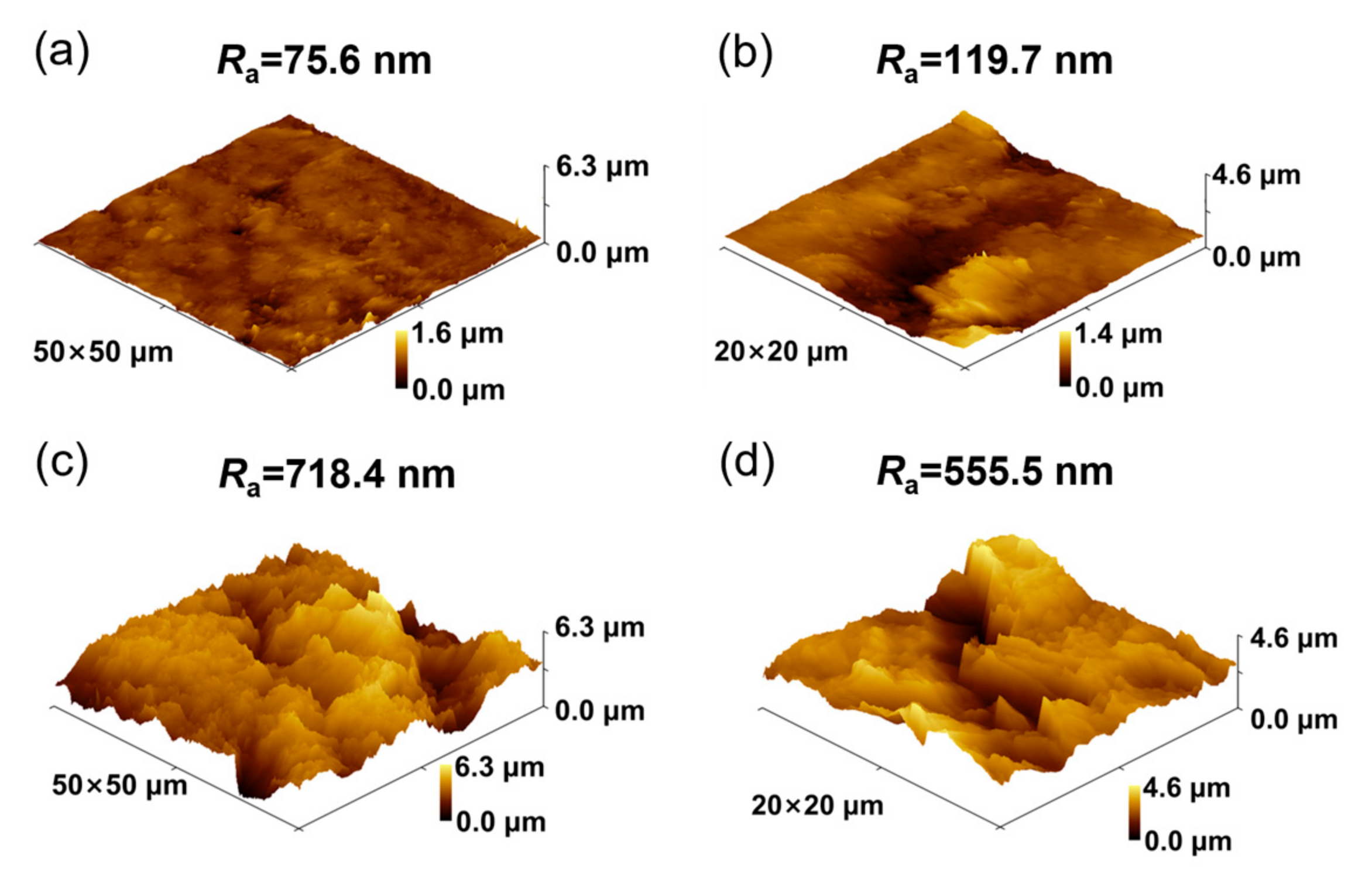
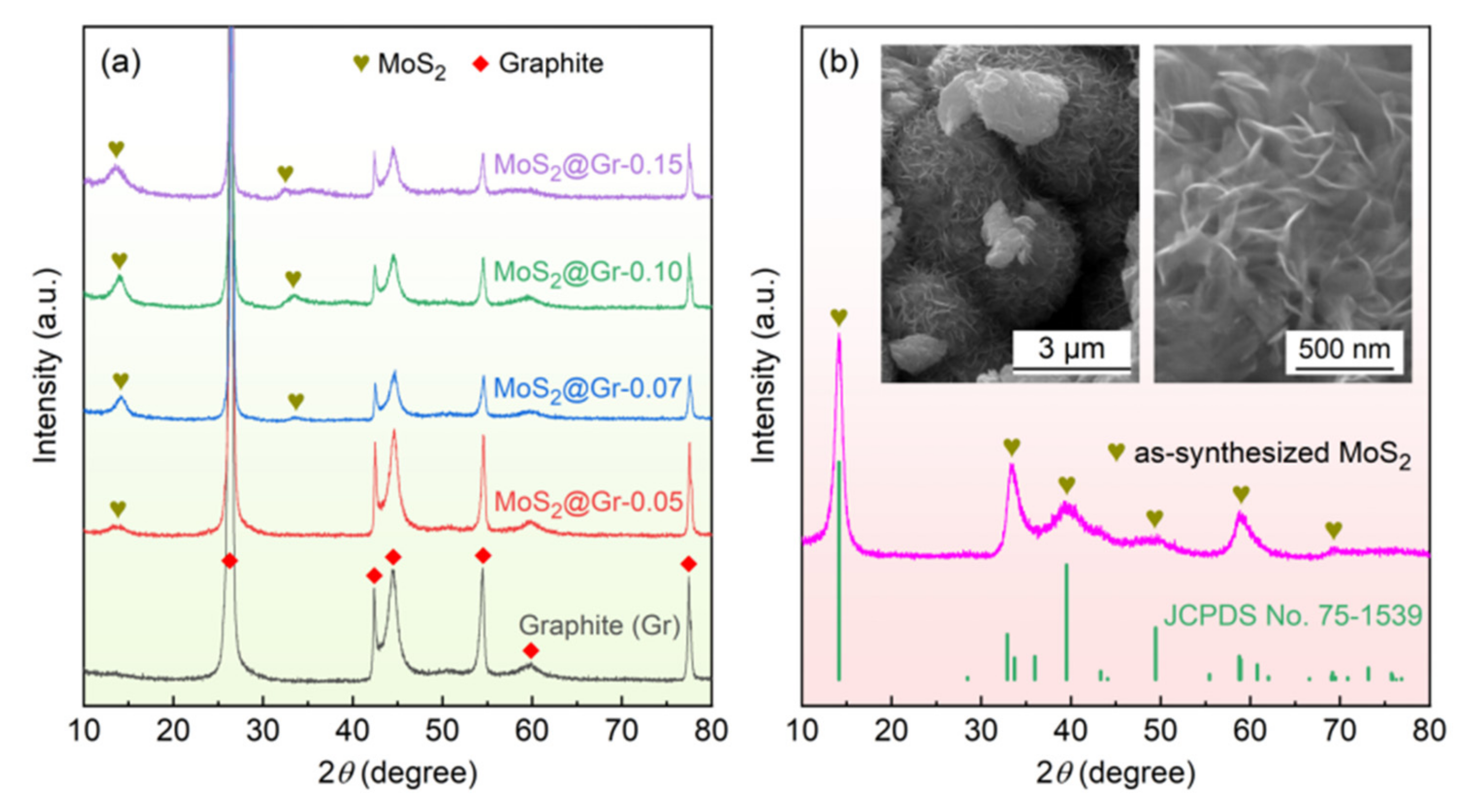
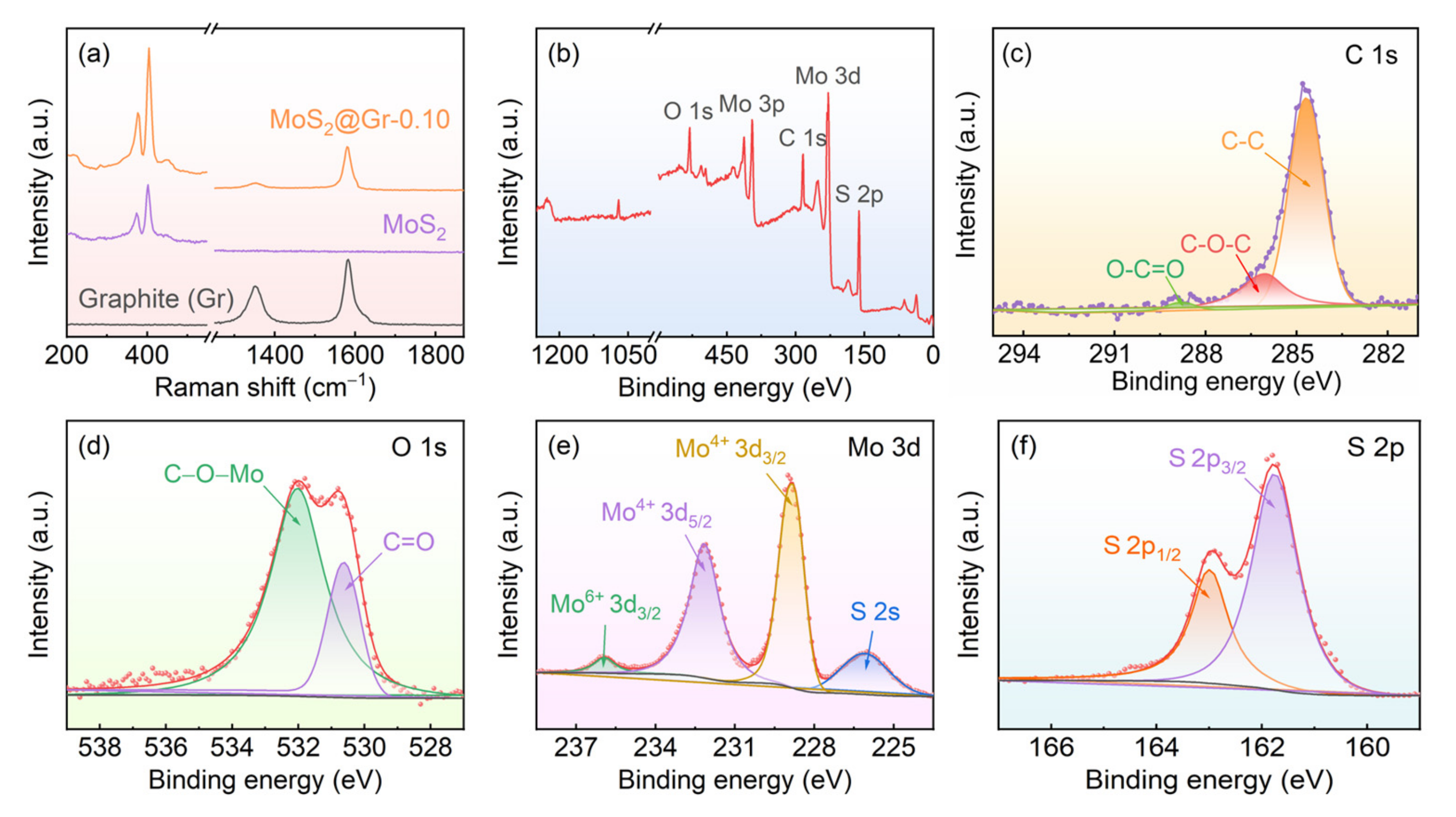
3.1. Morphology and Structure of MoS2@Gr Samples
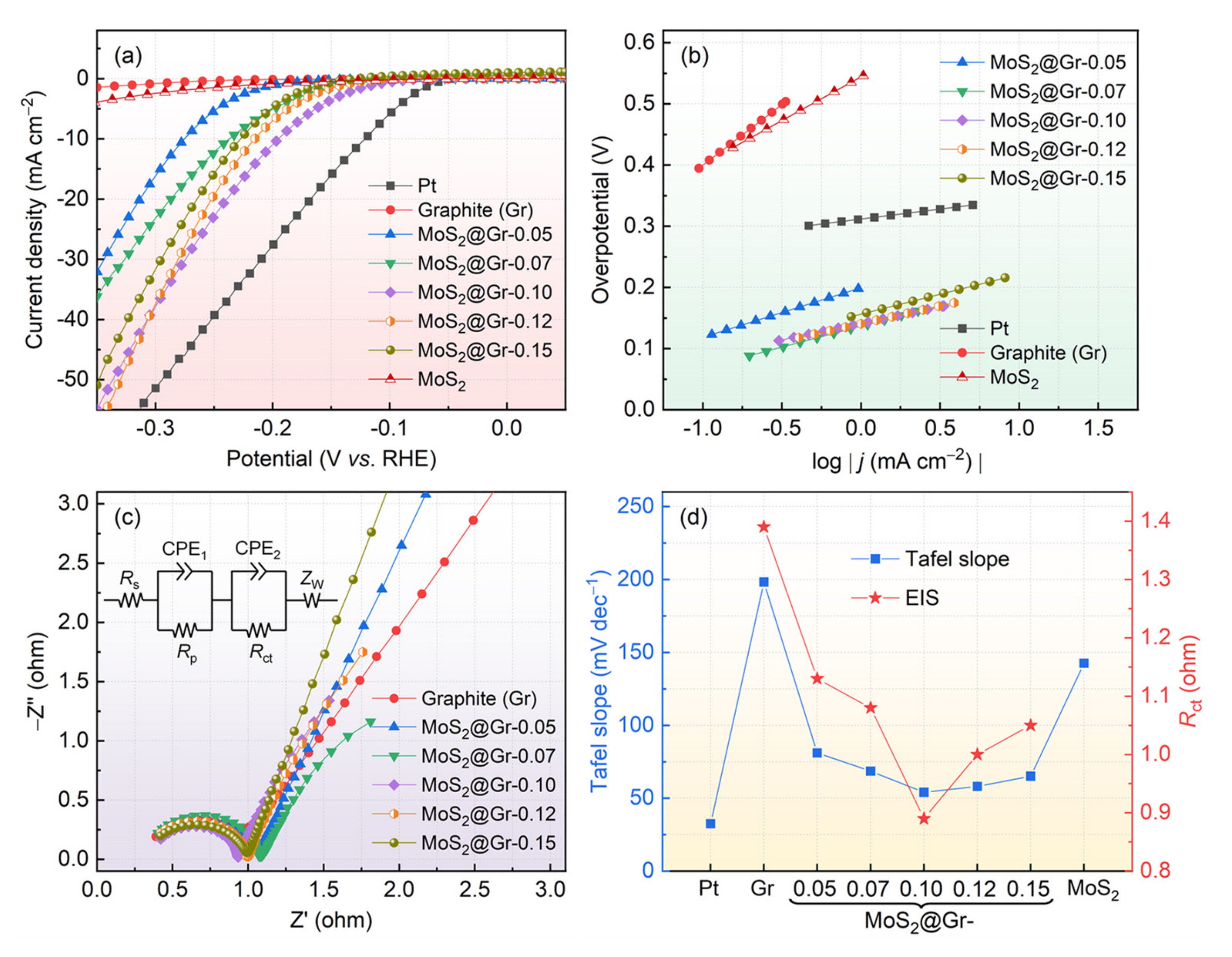
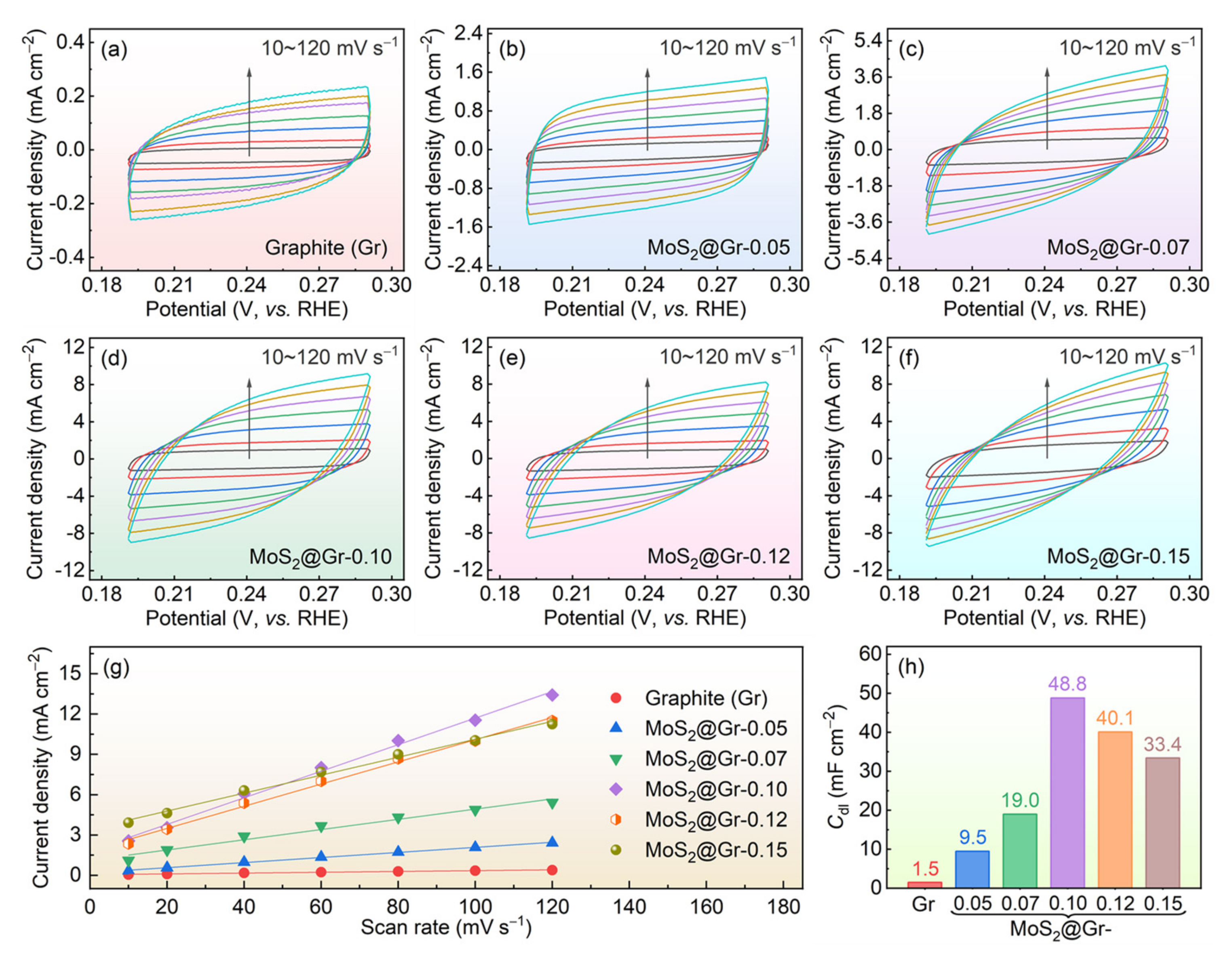
3.2. Electrochemical HER Performance
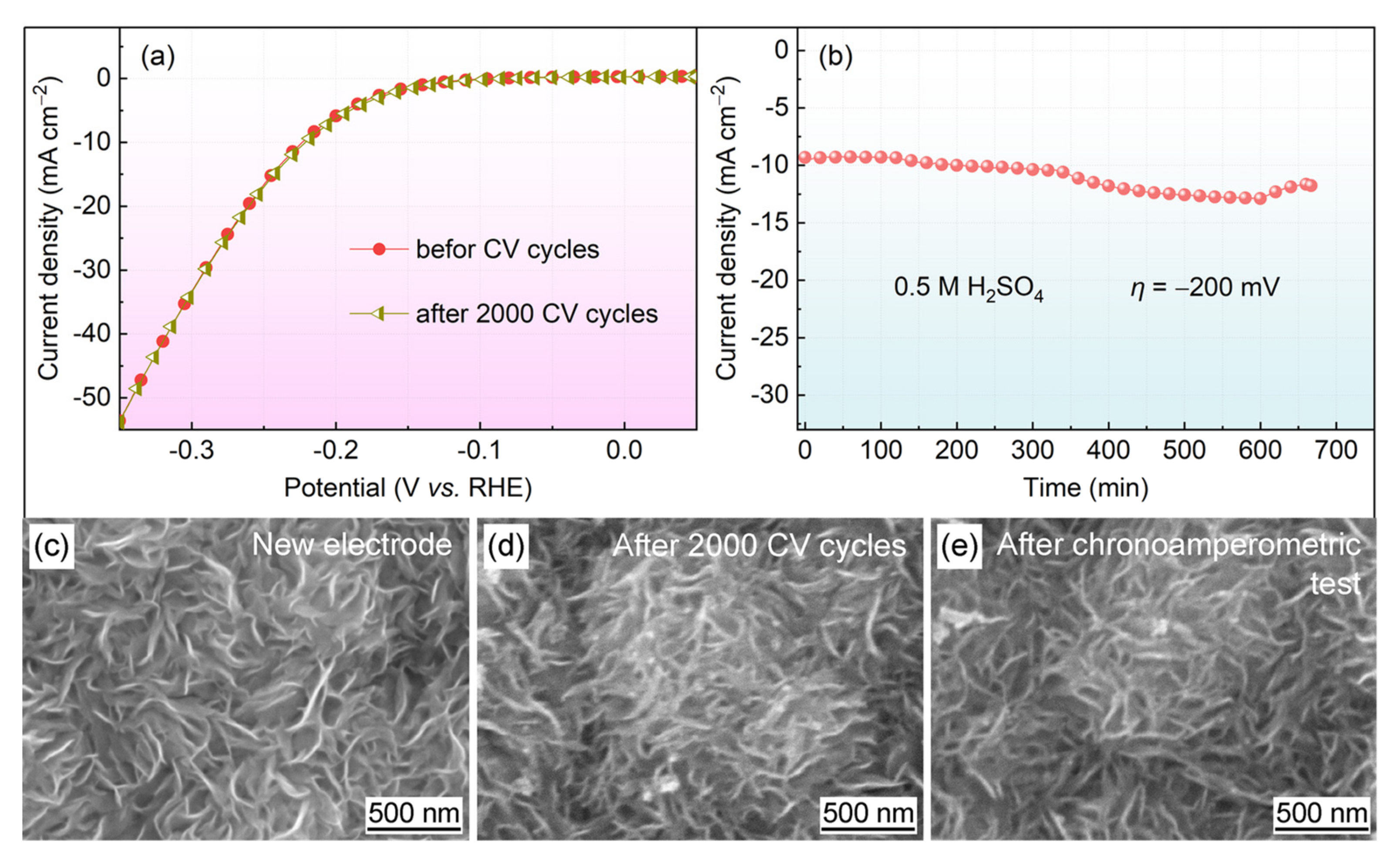
3.3. Cycling Stability
4. Conclusions
- (1)
- Nano-MoS2 was successfully deposited on the surface of a graphite substrate via a one-step hydrothermal method, and the microstructure of the MoS2 layers could be controlled by changing concentration of reactant.
- (2)
- A dense and uniform MoS2 layer was the key factor to improve the HER catalytic activity of the MoS2@Gr electrodes. However, a higher reactant concentration led to an increase in the deposited MoS2 layer thickness, which resulted in edge coverage of active sites and a decrease in the conductivity of the catalyst.
- (3)
- The MoS2@Gr-0.10 electrode showed the best electrochemical performance with an overpotential of 196 mV at 10 mA·cm−2 and a Tafel slope of 54.1 mV·dec−1.
- (4)
- There was no catalytic activity loss of the MoS2@Gr-0.10 electrode after 2000 CV cycles, and the electrode exhibited good stability performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Abbasi, K.R.; Shahbaz, M.; Zhang, J.; Irfan, M.; Alvarado, R. Analyze the environmental sustainability factors of China: The role of fossil fuel energy and renewable energy. Renew. Energy 2022, 187, 390–402. [Google Scholar] [CrossRef]
- Moustakas, K.; Loizidou, M.; Rehan, M.; Nizami, A.S. A review of recent developments in renewable and sustainable energy systems: Key challenges and future perspective. Renew. Sustain. Energy Rev. 2020, 119, 109418. [Google Scholar] [CrossRef]
- Gapp, E.; Pfeifer, P. Membrane reactors for hydrogen production from renewable energy sources. Curr. Opin. Green Sustain. Chem. 2023, 41, 100800. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Underground coal gasification: From fundamentals to applications. Prog. Energy Combust. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Yi, L.; Fan, Y.; Yang, R.; Zhu, R.; Zhu, Z.; Hu, J. Fabrication and optimization of CdS photocatalyst using nature leaf as biological template for enhanced visible-light photocatalytic hydrogen evolution. Catal. Today 2022, 402, 241–247. [Google Scholar] [CrossRef]
- Daulbayev, C.; Sultanov, F.; Korobeinyk, A.V.; Yeleuov, M.; Azat, S.; Bakbolat, B.; Umirzakov, A.; Mansurov, Z. Bio-waste-derived few-layered graphene/SrTiO3/PAN as efficient photocatalytic system for water splitting. Appl. Surf. Sci. 2021, 549, 149176. [Google Scholar] [CrossRef]
- Daulbayev, C.; Sultanov, F.; Bakbolat, B.; Daulbayev, O. 0D, 1D and 2D nanomaterials for visible photoelectrochemical water splitting. A Review. Int. J. Hydrogen Energy 2020, 45, 33325–33342. [Google Scholar] [CrossRef]
- Sultanov, F.; Daulbayev, C.; Azat, S.; Kuterbekov, K.; Bekmyrza, K.; Bakbolat, B.; Bigaj, M.; Mansurov, Z. Influence of Metal Oxide Particles on Bandgap of 1D Photocatalysts Based on SrTiO3/PAN Fibers. Nanomaterials 2020, 10, 1734. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Thomas, J.M.; Edwards, P.P.; Dobson, P.J.; Owen, G.P. Decarbonizing energy: The developing international activity in hydrogen technologies and fuel cells. J. Energy Chem. 2020, 51, 405–415. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, S.; Liang, Y.; Wu, S.; Li, Z.; Luo, S.; Cui, Z. Self-supported Ni3Se2@NiFe layered double hydroxide bifunctional electrocatalyst for overall water splitting. J. Colloid Interface Sci. 2021, 587, 79–89. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Guan, J. Strategies to improve electrocatalytic and photocatalytic performance of two-dimensional materials for hydrogen evolution reaction. Chin. J. Catal. 2021, 42, 511–556. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, C.; Liu, X.; Bai, J.; Xu, M.; Xu, Q.; Li, Y.; Long, L.; Zhang, G.; Li, S.; et al. Electrocatalytic hydrogen evolution of highly dispersed Pt/NC nanoparticles derived from porphyrin MOFs under acidic and alkaline medium. Int. J. Hydrogen Energy 2022, 47, 6631–6637. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.X.; Gou, W.; Zhang, M.; Xia, Z.; Zhang, S.; Chang, C.R.; Ma, Y.; Qu, Y. Ethylene-glycol ligand environment facilitates highly efficient hydrogen evolution of Pt/CoP through proton concentration and hydrogen spillover. Energy Environ. Sci. 2019, 12, 2298–2304. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthik, P.E.; Subramanian, B.; Kundu, S. Pt nanoparticle anchored molecular self-assemblies of DNA: An extremely stable and efficient HER electrocatalyst with ultralow Pt content. ACS Catal. 2016, 6, 4660–4672. [Google Scholar] [CrossRef]
- Laszczyńska, A.; Tylus, W.; Szczygieł, I. Electrocatalytic properties for the hydrogen evolution of the electrodeposited Ni–Mo/WC composites. Int. J. Hydrogen Energy 2021, 46, 22813–22831. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.; Yu, Z.; Islam, S.M.; He, H.; Yuan, M.; Yue, Y.; Xu, K.; Hao, W.; Sun, G.; et al. Hierarchical nanoassembly of MoS2/Co9S8/Ni3S2/Ni as a highly efficient electrocatalyst for overall water splitting in a wide pH range. J. Am. Chem. Soc. 2019, 141, 10417–10430. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; He, D.; Bai, X.; Yu, W.W. Synthesis of tungsten disulfide quantum dots for high-performance supercapacitor electrodes. J. Alloys Compd. 2019, 786, 764–769. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Sumesh, C.K. Enhanced electrocatalytic hydrogen evolution reaction by injection of photogenerated electrons in Ag/WS2 nanohybrids. Appl. Surf. Sci. 2021, 563, 150323. [Google Scholar] [CrossRef]
- Shajaripour Jaberi, S.Y.; Ghaffarinejad, A.; Khajehsaeidi, Z.; Sadeghi, A. The synthesis, properties, and potential applications of CoS2 as a transition metal dichalcogenide (TMD). Int. J. Hydrogen Energy 2023, 48, 15831–15878. [Google Scholar] [CrossRef]
- Zhang, H.C.; Li, Y.J.; Zhang, G.X.; Wan, P.B.; Xu, T.H.; Wu, X.C.; Sun, X.M. Highly crystallized cubic cattierite CoS2 for electrochemically hydrogen evolution over wide pH range from 0 to 14. Electrochim. Acta 2014, 148, 170–174. [Google Scholar] [CrossRef]
- Guo, Y.J.; Guo, D.; Ye, F.; Wang, K.; Shi, Z.Q. Synthesis of lawn-like NiS2 nanowires on carbon fiber paper as bifunctional electrode for water splitting. Int. J. Hydrogen Energy 2017, 42, 17038–17048. [Google Scholar] [CrossRef]
- Liu, P.; Li, J.; Lu, Y.; Xiang, B. Facile synthesis of NiS2 nanowires and its efficient electrocatalytic performance for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 72–77. [Google Scholar] [CrossRef]
- Ma, Q.Y.; Hu, C.Y.; Liu, K.L.; Hung, S.F.; Ou, D.H.; Chen, H.M.; Fu, G.; Zheng, N.F. Identifying the electrocatalytic sites of nickel disulfide in alkaline hydrogen evolution reaction. Nano Energy 2017, 41, 148–153. [Google Scholar] [CrossRef]
- Li, W.; Bi, R.; Liu, G.X.; Tian, Y.; Zhang, L. 3D Interconnected MoS2 with enlarged interlayer spacing grown on carbon nanofibers as a flexible anode toward superior sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 26982–26989. [Google Scholar] [CrossRef]
- Zhao, L.P.; Qi, L.; Wang, H. MoS2–C/graphite, an electric energy storage device using Na+-based organic electrolytes. RSC Adv. 2015, 5, 15431–15437. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Park, M.S.; Nagaraju, G.; Kim, D.W. Unraveling the Na-ion storage performance of a vertically aligned interlayer-expanded two-dimensional MoS2@C@MoS2 heterostructure. J. Mater. Chem. A 2019, 7, 24557–24568. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.W. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Liu, Q.L.; Shi, H.D.; Yang, T.Y.; Yang, Y.; Wu, Z.S.; Yu, J.Q.; Silva, S.R.P.; Liu, J. Sequential growth of hierarchical N-doped carbon-MoS2 nanocomposites with variable nanostructures. J. Mater. Chem. A 2019, 7, 6197–6204. [Google Scholar] [CrossRef]
- Gong, F.L.; Ye, S.; Liu, M.M.; Zhang, J.W.; Gong, L.H.; Zeng, G.; Meng, E.; Su, P.; Xie, K.F.; Zhang, Y.H.; et al. Boosting electrochemical oxygen evolution over yolk-shell structured O–MoS2 nanoreactors with sulfur vacancy and decorated Pt nanoparticles. Nano Energy 2020, 78, 105284. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, B.; Wang, Z.; Wang, C.; Wilkinson, D.P. MoS2 Nanosheets: A designed structure with high active site density for the hydrogen evolution reaction. Acs Catal. 2013, 3, 2101–2107. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, K.; Yang, G.; Li, Y.; Liu, X.; Chang, K.; Xuan, Y.; Ye, J. Ultrafine nano 1T-MoS2 monolayers with NiOx as dual co-catalysts over TiO2 photoharvester for efficient photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 279, 119387. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, Y.; Wang, H.; Chen, X.; Liu, K.; Zhang, K.; Wei, Q.; Wang, B. Study on preparation, photocatalytic performance and degradation mechanism of polymeric carbon nitride/Pt/nano-spherical MoS2 composite. J. Phys. Chem. Solids 2022, 166, 110700. [Google Scholar] [CrossRef]
- Yu, Y.F.; Huang, S.Y.; Li, Y.P.; Steinmann, S.N.; Yang, W.; Cao, L.Y. Layer-dependent electrocatalysis of MoS2 for hydrogen evolution. Nano Lett. 2014, 14, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, F.; Song, S. Recent advances in structural engineering of molybdenum disulfide for electrocatalytic hydrogen evolution reaction. Chem. Eng. J. 2021, 405, 127013. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Luyen Doan, T.L.; Prabhakaran, S.; Tran, D.T.; Kim, D.H.; Lee, J.H.; Kim, N.H. Hierarchical Co and Nb dual-doped MoS2 nanosheets shelled micro-TiO2 hollow spheres as effective multifunctional electrocatalysts for HER, OER, and ORR. Nano Energy 2021, 82, 105750. [Google Scholar] [CrossRef]
- Tang, B.S.; Yu, Z.G.; Seng, H.L.; Zhang, N.D.; Liu, X.X.; Zhang, Y.W.; Yang, W.F.; Gong, H. Simultaneous edge and electronic control of MoS2 nanosheets through Fe doping for an efficient oxygen evolution reaction. Nanoscale 2018, 10, 20113–20119. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jian, K.; Lv, Z.; Li, D.; Fan, G.; Zhang, R.; Dang, J. MoS2/Co9S8/MoC heterostructure connected by carbon nanotubes as electrocatalyst for efficient hydrogen evolution reaction. J. Mater. Sci. Technol. 2021, 79, 29–34. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Gao, J.H.; Ding, Y.N.; Kou, K.K. Oxidative modification of graphite felts for efficient H2O2 electrogeneration: Enhancement mechanism and long-term stability. J. Electroanal. Chem. 2019, 833, 258–268. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, G.; Zhu, X.; Guo, Y. Ultrasensitive NO2 gas sensing based on rGO/MoS2 nanocomposite film at low temperature. Sens. Actuat. B Chem. 2017, 251, 280–290. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Rowley-Neale, S.J.; Brownson, D.A.C.; Smith, G.C.; Sawtell, D.A.G.; Kelly, P.J.; Banks, C.E. 2D nanosheet molybdenum disulphide (MoS2) modified electrodes explored towards the hydrogen evolution reaction. Nanoscale 2015, 7, 18152–18168. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Patel, P.P.; Velikokhatnyi, O.I.; Ghadge, S.D.; Hanumantha, P.J.; Datta, M.K.; Kuruba, R.; Gattu, B.; Shanthi, P.M.; Kumta, P.N. Electrochemically active and robust cobalt doped copper phosphosulfide electro-catalysts for hydrogen evolution reaction in electrolytic and photoelectrochemical water splitting. Int. J. Hydrogen Energy 2018, 43, 7855–7871. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Ali, M.; Karuppasamy, K.; Santhoshkumar, P.; Hwang, J.H.; Jung, J.; Kim, H.S. Theoretical evaluation and experimental investigation of layered 2H/1T-phase MoS2 and its reduced graphene-oxide hybrids for hydrogen evolution reactions. J. Alloys Compd. 2021, 868, 159272. [Google Scholar] [CrossRef]
- Shilpa, R.; Sibi, K.S.; Pai, R.K.; Sarath Kumar, S.R.; Rakhi, R.B. Electrocatalytic water splitting for efficient hydrogen evolution using molybdenum disulfide nanomaterials. Mater. Sci. Eng. B 2022, 285, 115930. [Google Scholar] [CrossRef]
- Lu, H.; Tian, K.; Bu, L.M.; Huang, X.; Li, X.Y.; Zhao, Y.; Wang, F.; Bai, J.; Gao, L.; Zhao, J.Q. Synergistic effect from coaxially integrated CNTs@MoS2/MoO2 composite enables fast and stable lithium storage. J. Energy Chem. 2021, 55, 449–458. [Google Scholar] [CrossRef]
- Guan, X.B.; Zhao, L.P.; Zhang, P.; Liu, J.; Song, X.F.; Gao, L. Electrode material of core-shell hybrid MoS2@C/CNTs with carbon intercalated few-layer MoS2 nanosheets. Mater. Today Energy 2020, 16, 100379. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Y.; Lu, W.; Feng, Z.; Xi, B.; Kai, S.; Zhang, J.; Feng, J.; Xiong, S. Rationally incorporated MoS2/SnS2 nanoparticles on graphene sheets for lithium-ion and sodium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 27697–27706. [Google Scholar] [CrossRef]
- Hong, Z.A.; Hong, W.T.; Wang, B.C.; Cai, Q.; He, X.; Liu, W. STable 1T –2H MoS2 heterostructures for efficient electrocatalytic hydrogen evolution. Chem. Eng. J. 2023, 460, 141858. [Google Scholar] [CrossRef]
- Wang, D.Z.; Su, B.Y.; Jiang, Y.; Li, L.; Ng, B.K.; Wu, Z.Z.; Liu, F. Polytype 1T/2H MoS2 heterostructures for efficient photoelectrocatalytic hydrogen evolution. Chem. Eng. J. 2017, 330, 102–108. [Google Scholar] [CrossRef]
- Xu, Y.; Qu, J.T.; Li, Y.; Zhu, M.Y.; Liu, Y.; Zheng, R.; Cairney, J.M.; Li, W.X. Bridging metal-ion induced vertical growth of MoS2 and overall fast electron transfer in (C, P)3N4-M (Ni2+, Co2+)-MoS2 electrocatalyst for efficient hydrogen evolution reaction. Sustain. Mater. Techno. 2020, 25, e00172. [Google Scholar] [CrossRef]
- Wu, L.Q.; Xu, X.B.; Zhao, Y.Q.; Zhang, K.Y.; Sun, Y.; Wang, T.T.; Wang, Y.Q.; Zhong, W.; Du, Y. Mn doped MoS2/reduced graphene oxide hybrid for enhanced hydrogen evolution. Appl. Surf. Sci. 2017, 425, 470–477. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. How properly are we interpreting the Tafel lines in energy conversion electrocatalysis? Mater. Today Energy 2022, 29, 101123. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, Y.; Zhang, J.; Chen, Y.J.; Sun, K.; Liu, Y.; Liu, C. Graphene oxide co-doped with nitrogen and sulfur and decorated with cobalt phosphide nanorods: An efficient hybrid catalyst for electrochemical hydrogen evolution. Electrochim. Acta 2016, 222, 246–256. [Google Scholar] [CrossRef]
- Ye, J.B.; Yu, Z.T.; Chen, W.X.; Chen, Q.N.; Xu, S.R.; Liu, R. Facile synthesis of molybdenum disulfide/nitrogen-doped graphene composites for enhanced electrocatalytic hydrogen evolution and electrochemical lithium storage. Carbon 2016, 107, 711–722. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Feng, J.H.; Zhou, H.; Wang, J.P.; Bian, T.; Shao, J.X.; Yuan, A.H. MoS2 supported on MOF-derived carbon with core-shell structure as efficient electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 20538–20545. [Google Scholar] [CrossRef]
- Lin, Z.; Feng, T.; Ma, X.; Liu, G. Fe/Ni bi-metallic organic framework supported 1T/2H MoS2 heterostructures as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution. Fuel 2023, 339, 127395. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Templated electrochemical fabrication of hollow molybdenum sulfide microstructures and nanostructures with catalytic properties for hydrogen production. ACS Catal. 2016, 6, 3985–3993. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, X.; Yan, S.; Xiao, H.; Li, Y.; Hu, T.; Zheng, Z.; Jia, J.; Wu, H. Solvothermal synthesis of oxygen-incorporated MoS2-x nanosheets with abundant undercoordinated Mo for efficient hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 19133–19143. [Google Scholar] [CrossRef]
- Kang, H.; Youn, J.S.; Oh, I.; Manavalan, K.; Jeon, K.J. Controllable atomic-ratio of CVD-grown MoS2-MoO2 hybrid catalyst by soft annealing for enhancing hydrogen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 1399–1408. [Google Scholar] [CrossRef]
- Wu, C.L.; Huang, P.C.; Brahma, S.; Huang, J.L.; Wang, S.C. MoS2-MoO2 composite electrocatalysts by hot-injection method for hydrogen evolution reaction. Ceram. Int. 2017, 43, S621–S627. [Google Scholar] [CrossRef]
- Wang, H.B.; Zhu, H.; Sun, Y.S.; Ma, F.; Chen, Y.Z.; Zeng, D.J.; Zhou, L.; Ma, D.Y. Ultra-thin pine tree-like MoS2 nanosheets with maximally exposed active edges terminated at side surfaces on stainless steel fiber felt for hydrogen evolution reaction. J. Alloys Compd. 2021, 876, 160163. [Google Scholar] [CrossRef]
- Cao, K.; Sun, S.; Song, A.; Ba, J.; Lin, H.; Yu, X.; Xu, C.; Jin, B.; Huang, J.; Fan, D. Increased 1T-MoS2 in MoS2@CoS2/G composite for high-efficiency hydrogen evolution reaction. J. Alloys Compd. 2022, 907, 164539. [Google Scholar] [CrossRef]

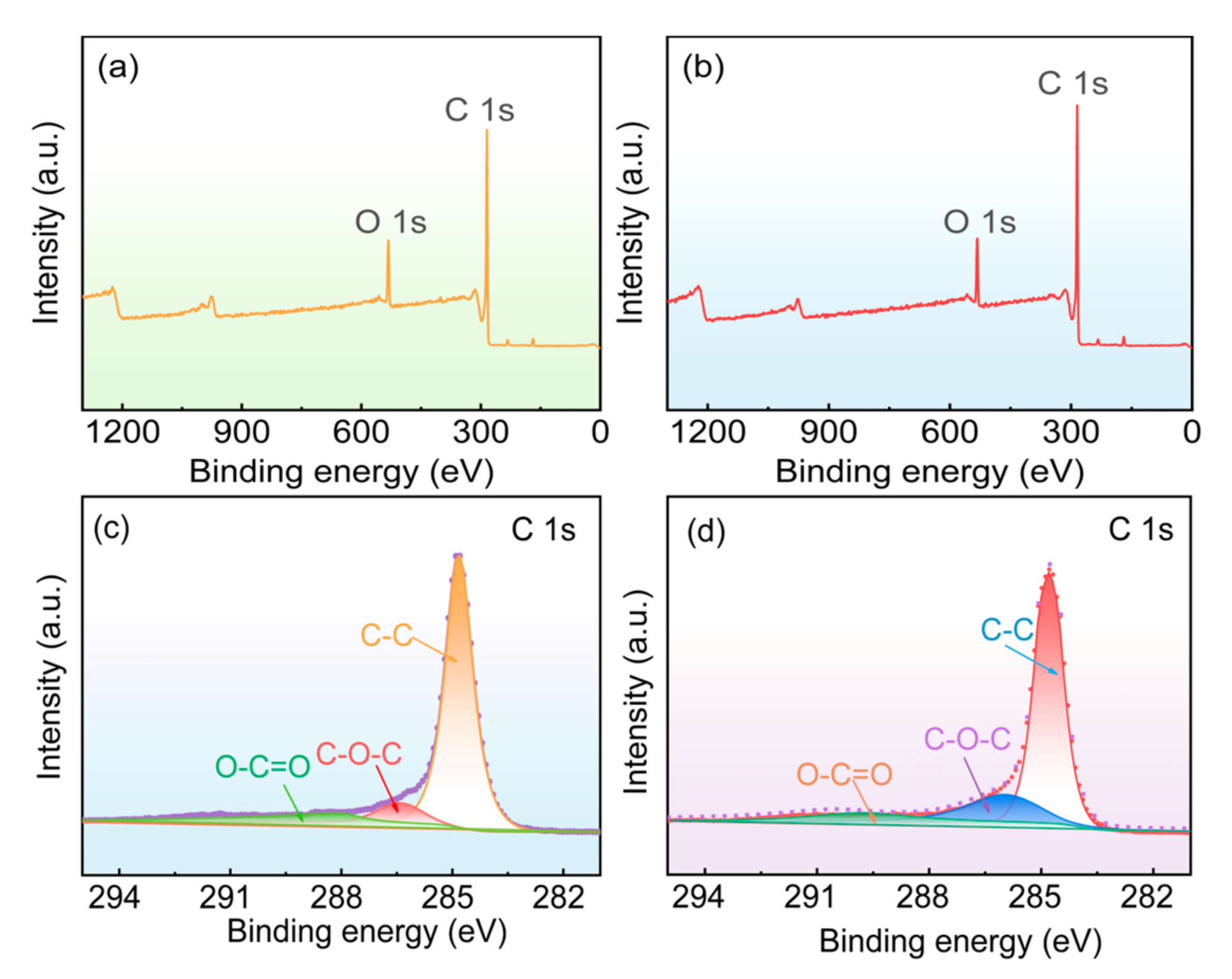

| Sample | Area Percentage (%) | ||
|---|---|---|---|
| C-C | C-O-C | O-C=O | |
| Polished graphite substrate | 82.24 | 9.33 | 8.43 |
| Acid-corroded graphite substrate | 69.04 | 21.58 | 9.38 |
| Catalyst | Synthesis Approach | Nafion | η10 (mV vs. RHE) | Tafel (mV·dec−1) | Ref. |
|---|---|---|---|---|---|
| MoS2@Fe/Ni-MOF600 | Hydrothermal | Yes | 214 | 170.7 | [62] |
| GC/MoS2 film | Electrodeposition | No | 202 | 48 | [63] |
| MoS2−x | Solvothermal | Yes | 191 | 67 | [64] |
| MoS2-MoO2 | CVD | No | 198 | 66.8 | [65] |
| MoS2-MoO2 | Hot-injection method | Yes | 210 | 129 | [66] |
| MoS2/SSF | Hydrothermal | No | 151 | 55.7 | [67] |
| MoS2/G | Sulfurization treatment | Yes | 208 | 59 | [68] |
| MoS2/Gr | Hydrothermal | No | 196 | 54.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, M.; Zhao, H.; Zeng, Z.; Xia, C.; Yang, T. In Situ Growth of Nano-MoS2 on Graphite Substrates as Catalysts for Hydrogen Evolution Reaction. Materials 2023, 16, 4627. https://doi.org/10.3390/ma16134627
Zhao Y, Zhang M, Zhao H, Zeng Z, Xia C, Yang T. In Situ Growth of Nano-MoS2 on Graphite Substrates as Catalysts for Hydrogen Evolution Reaction. Materials. 2023; 16(13):4627. https://doi.org/10.3390/ma16134627
Chicago/Turabian StyleZhao, Yifan, Mingyang Zhang, Huimin Zhao, Zhiqiang Zeng, Chaoqun Xia, and Tai Yang. 2023. "In Situ Growth of Nano-MoS2 on Graphite Substrates as Catalysts for Hydrogen Evolution Reaction" Materials 16, no. 13: 4627. https://doi.org/10.3390/ma16134627
APA StyleZhao, Y., Zhang, M., Zhao, H., Zeng, Z., Xia, C., & Yang, T. (2023). In Situ Growth of Nano-MoS2 on Graphite Substrates as Catalysts for Hydrogen Evolution Reaction. Materials, 16(13), 4627. https://doi.org/10.3390/ma16134627








