Figure 1.
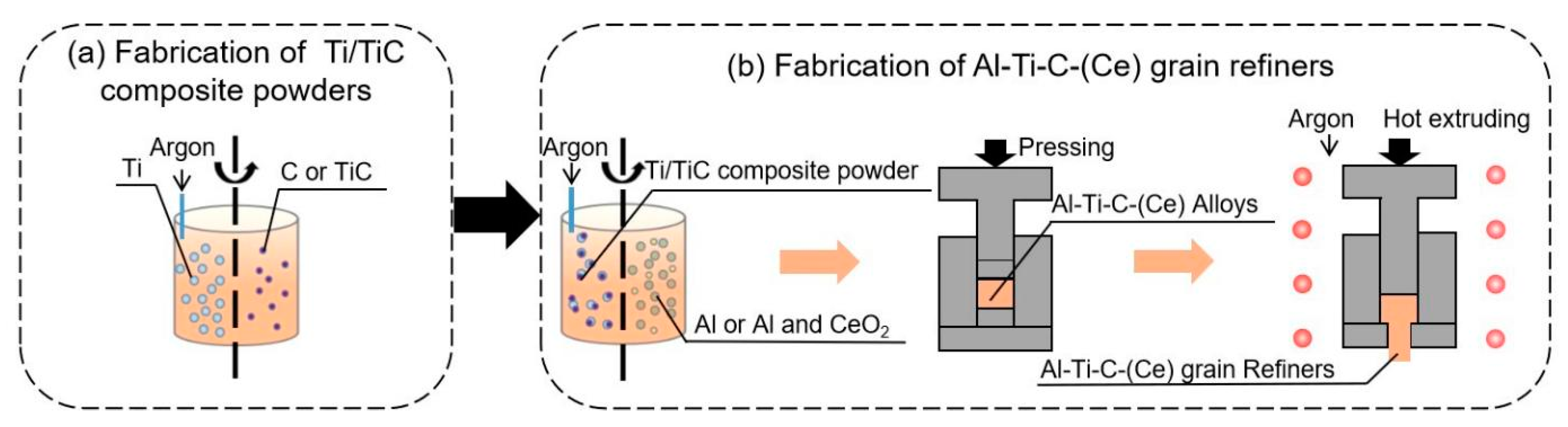
Flow diagram for the preparation of Ti/TiC composite powder and Al-Ti-C-(Ce) grain refiners: (a) fabrication of ball milling loaded and in-situ reaction Ti/TiC composite powder, (b) fabrication of Al-Ti-C-(Ce) grain refiners.
Figure 1.
Flow diagram for the preparation of Ti/TiC composite powder and Al-Ti-C-(Ce) grain refiners: (a) fabrication of ball milling loaded and in-situ reaction Ti/TiC composite powder, (b) fabrication of Al-Ti-C-(Ce) grain refiners.
Figure 2.
KBI ring model experiment was used to test the refining performance of pure aluminum grains.
Figure 2.
KBI ring model experiment was used to test the refining performance of pure aluminum grains.
Figure 3.
Microstructure and EDS images of ball milling loaded Ti/TiC composite powder: (a) ball milling loaded Ti/TiC composite powder (1#), (b) ball milling loaded Ti/TiC single particle, (c) point 1 EDS analysis.
Figure 3.
Microstructure and EDS images of ball milling loaded Ti/TiC composite powder: (a) ball milling loaded Ti/TiC composite powder (1#), (b) ball milling loaded Ti/TiC single particle, (c) point 1 EDS analysis.
Figure 4.
TEM and SAED images of ball milling loaded Ti/TiC composite powder: (a) ball milling loaded Ti/TiC composite powder (1#), (b) ball milling Ti/TiC single particle, (c) analysis of SAED patterns in region 1, (d) analysis of SAED patterns in region 2.
Figure 4.
TEM and SAED images of ball milling loaded Ti/TiC composite powder: (a) ball milling loaded Ti/TiC composite powder (1#), (b) ball milling Ti/TiC single particle, (c) analysis of SAED patterns in region 1, (d) analysis of SAED patterns in region 2.
Figure 5.
The XRD pattern of in-situ reaction Ti/TiC composite powder in high-energy ball milling for 0–15 h.
Figure 5.
The XRD pattern of in-situ reaction Ti/TiC composite powder in high-energy ball milling for 0–15 h.
Figure 6.
The Raman spectrums of in-situ reaction Ti/TiC composite powder: (a) high-energy ball milling for 12 h, (b) high-energy ball milling for 15 h.
Figure 6.
The Raman spectrums of in-situ reaction Ti/TiC composite powder: (a) high-energy ball milling for 12 h, (b) high-energy ball milling for 15 h.
Figure 7.
TEM and SAED images of in-situ reaction Ti/TiC composite powder: (a) in-situ reaction Ti/TiC composite powder (2#), (b) in-situ reaction Ti/TiC single particle, (c) analysis of HRTEM, (d) analysis of SAED patterns.
Figure 7.
TEM and SAED images of in-situ reaction Ti/TiC composite powder: (a) in-situ reaction Ti/TiC composite powder (2#), (b) in-situ reaction Ti/TiC single particle, (c) analysis of HRTEM, (d) analysis of SAED patterns.
Figure 8.
STEM and EDS images with Ti and C elements of ball milling loaded and in-situ reaction Ti/TiC composite powder: (a–c) ball milling loaded Ti/TiC composite powder (1#), (d–f) in-situ reaction Ti/TiC composite powder (2#).
Figure 8.
STEM and EDS images with Ti and C elements of ball milling loaded and in-situ reaction Ti/TiC composite powder: (a–c) ball milling loaded Ti/TiC composite powder (1#), (d–f) in-situ reaction Ti/TiC composite powder (2#).
Figure 9.
Microstructure of pure aluminum before and after being refined by different Al-Ti-C grain refiners (add 1 wt.%): (a) no grain refiner, (b) ball milling loaded Al-Ti-C grain refiner (3#), (c) in-situ reaction Al-Ti-C grain refiner (4#).
Figure 9.
Microstructure of pure aluminum before and after being refined by different Al-Ti-C grain refiners (add 1 wt.%): (a) no grain refiner, (b) ball milling loaded Al-Ti-C grain refiner (3#), (c) in-situ reaction Al-Ti-C grain refiner (4#).
Figure 10.
Microstructure and EDS images of in-situ reaction Al-Ti-C grain refiners with various extrusion ratios: (a–c) extrusion ratio 13 grain refiner (4#), (d–f) extrusion ratio 20 grain refiner (5#), (g–i) extrusion ratio 30 grain refiner (6#).
Figure 10.
Microstructure and EDS images of in-situ reaction Al-Ti-C grain refiners with various extrusion ratios: (a–c) extrusion ratio 13 grain refiner (4#), (d–f) extrusion ratio 20 grain refiner (5#), (g–i) extrusion ratio 30 grain refiner (6#).
Figure 11.
Microstructure of pure aluminum after being refined by Al-Ti-C grain refiners with various extrusion ratios (add 1 wt.%): (a) extrusion ratio 13 grain refiner (4#), (b) extrusion ratio 20 grain refiner (5#), (c) extrusion ratio 30 grain refiner (6#).
Figure 11.
Microstructure of pure aluminum after being refined by Al-Ti-C grain refiners with various extrusion ratios (add 1 wt.%): (a) extrusion ratio 13 grain refiner (4#), (b) extrusion ratio 20 grain refiner (5#), (c) extrusion ratio 30 grain refiner (6#).
Figure 12.
Microstructure of pure aluminum after being refined by extrusion ratio 30 Al-Ti-C grain refiner with different addition amounts: (a) 1.0 wt.% grain refiner (6#), (b) 3.0 wt.% grain refiner (6#), (c) 5.5 wt.% grain refiner (6#), (d) 6.0 wt.% grain refiner (6#).
Figure 12.
Microstructure of pure aluminum after being refined by extrusion ratio 30 Al-Ti-C grain refiner with different addition amounts: (a) 1.0 wt.% grain refiner (6#), (b) 3.0 wt.% grain refiner (6#), (c) 5.5 wt.% grain refiner (6#), (d) 6.0 wt.% grain refiner (6#).
Figure 13.
Microstructure of pure aluminum after being refined by Al-Ti-C-Ce grain refiners (add 5.5 wt.%) with different CeO2 content: (a) 0 wt.% CeO2 grain refiner (6#), (b) 0.25 wt.% CeO2 grain refiner (7#), (c) 0.50 wt.% CeO2 grain refiner (8#), (d) 0.75 wt.% CeO2 grain refiner (9#), (e) 1.0 wt.% CeO2 grain refiner (10#), (f) 2.0 wt.% CeO2 grain refiner (11#).
Figure 13.
Microstructure of pure aluminum after being refined by Al-Ti-C-Ce grain refiners (add 5.5 wt.%) with different CeO2 content: (a) 0 wt.% CeO2 grain refiner (6#), (b) 0.25 wt.% CeO2 grain refiner (7#), (c) 0.50 wt.% CeO2 grain refiner (8#), (d) 0.75 wt.% CeO2 grain refiner (9#), (e) 1.0 wt.% CeO2 grain refiner (10#), (f) 2.0 wt.% CeO2 grain refiner (11#).
Figure 14.
Microstructure and EDS images of Al-Ti-C-Ce grain refiners with different CeO2 contents: (a–c) 0.50 wt.% CeO2 grain refiner (8#), (d–f) 2.0 wt.% CeO2 grain refiner (11#).
Figure 14.
Microstructure and EDS images of Al-Ti-C-Ce grain refiners with different CeO2 contents: (a–c) 0.50 wt.% CeO2 grain refiner (8#), (d–f) 2.0 wt.% CeO2 grain refiner (11#).
Figure 15.
Microstructure of pure aluminum after being refined by Al-Ti-C and Al-Ti-C-Ce grain refiners (add 5.5 wt.%) with different holding times: (a–d) by Al-Ti-C grain refiner (6#) with 1, 3, 5, and 10 min, (e–h) by Al-Ti-C-Ce grain refiner (8#) with 1, 3, 5, and 10 min.
Figure 15.
Microstructure of pure aluminum after being refined by Al-Ti-C and Al-Ti-C-Ce grain refiners (add 5.5 wt.%) with different holding times: (a–d) by Al-Ti-C grain refiner (6#) with 1, 3, 5, and 10 min, (e–h) by Al-Ti-C-Ce grain refiner (8#) with 1, 3, 5, and 10 min.
Figure 16.
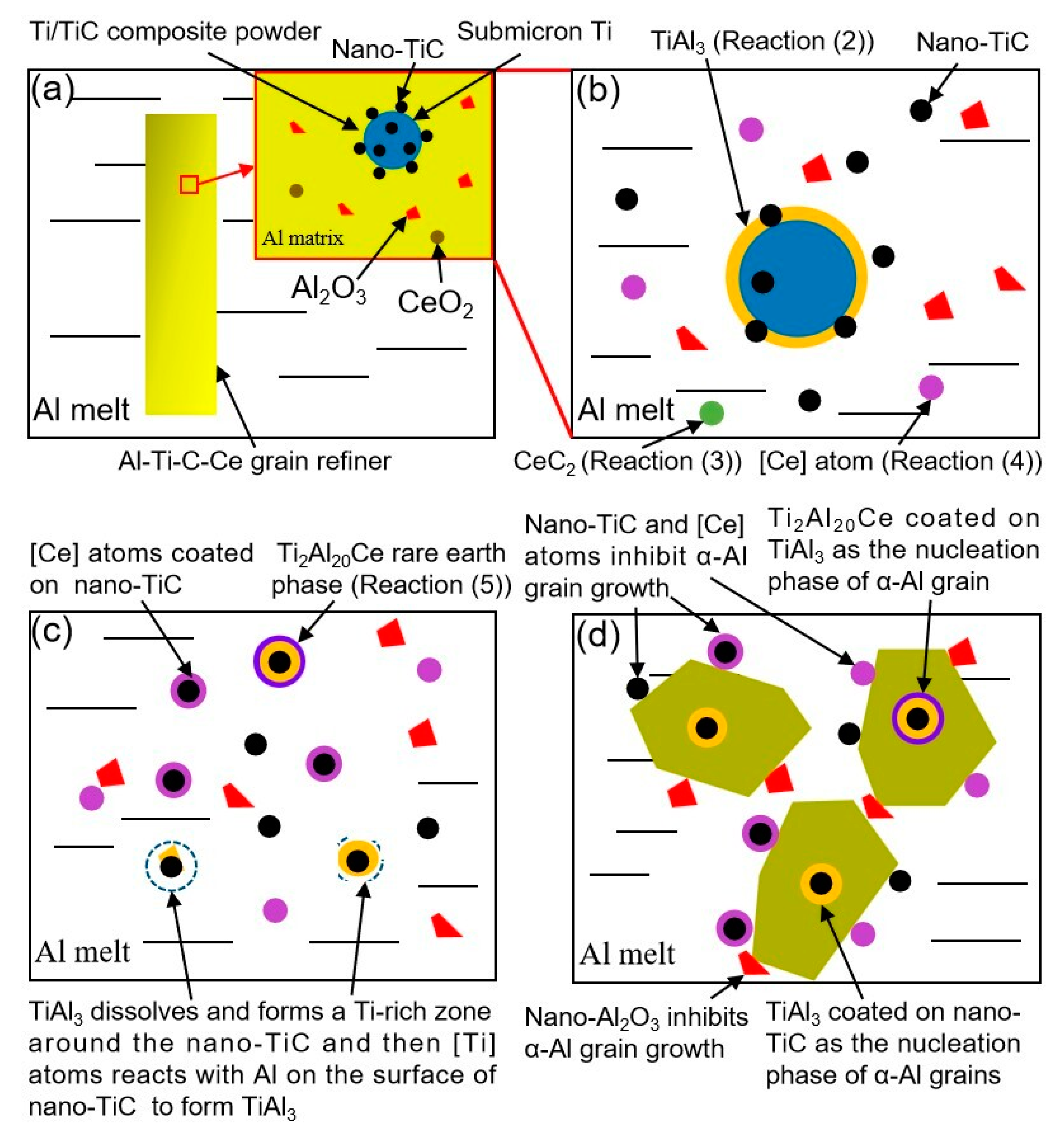
Schematic diagram of refining mechanism of the Al-Ti-C-Ce grain refiner: (a) schematic diagram of grain refiner and its local microstructure, (b) reactions of the grain refiner in Al melt to form TiAl3 and [Ce] atoms, (c) formation of Ti-rich zones and TiAl3 thin layers, adsorption of [Ce] atoms, and formation of Ti2Al20Ce rare earth phases, (d) α-Al grain nucleation and growth.
Figure 16.
Schematic diagram of refining mechanism of the Al-Ti-C-Ce grain refiner: (a) schematic diagram of grain refiner and its local microstructure, (b) reactions of the grain refiner in Al melt to form TiAl3 and [Ce] atoms, (c) formation of Ti-rich zones and TiAl3 thin layers, adsorption of [Ce] atoms, and formation of Ti2Al20Ce rare earth phases, (d) α-Al grain nucleation and growth.
Table 1.
Preparation parameters of Ti/TiC composite powder and Al-Ti-C-(Ce) grain refiners.
Table 1.
Preparation parameters of Ti/TiC composite powder and Al-Ti-C-(Ce) grain refiners.
| No. | Name | Composition of Powder |
|---|
| Al/wt.% | Ti/wt.% | TiC/wt.% | C/wt.% | CeO2/wt.% |
|---|
| 1# | Ball milling loaded Ti/TiC composite powder | - | 8.0 | 0.8 | - | - |
| 2# | In-situ reaction Ti/TiC composite powder | - | 6.8 | - | 2.0 | - |
| 3# | Extrusion ratio 13 and ball milling loaded Al-Ti-C grain refiner | 91.2 | 8.0 | 0.8 | - | - |
| 4# | Extrusion ratio 13 and in-situ reaction Al-Ti-C grain refiner | 91.2 | 6.8 | - | 2.0 | - |
| 5# | Extrusion ratio 20 and in-situ reaction Al-Ti-C grain refiner | 91.2 | 6.8 | - | 2.0 | - |
| 6# | Extrusion ratio 30 and in-situ reaction Al-Ti-C grain refiner | 91.2 | 6.8 | - | 2.0 | - |
| 7# | Extrusion ratio 30, 0.25 wt.% CeO2 and in-situ reaction Al-Ti-C-Ce grain refiner | 90.95 | 6.8 | - | 2.0 | 0.25 |
| 8# | Extrusion ratio 30, 0.50 wt.% CeO2 and in-situ reaction Al-Ti-C-Ce grain refiner | 90.7 | 6.8 | - | 2.0 | 0.50 |
| 9# | Extrusion ratio 30, 0.75 wt.% CeO2 and in-situ reaction Al-Ti-C-Ce grain refiner | 90.45 | 6.8 | - | 2.0 | 0.75 |
| 10# | Extrusion ratio 30, 1.0 wt.% CeO2 and in-situ reaction Al-Ti-C-Ce grain refiner | 90.2 | 6.8 | - | 2.0 | 1.0 |
| 11# | Extrusion ratio 30, 2.0 wt.% CeO2 and in-situ reaction Al-Ti-C-Ce grain refiner | 89.2 | 6.8 | - | 2.0 | 2.0 |
Table 2.
Average size of pure aluminum grains before and after being refined by different Al-Ti-C grain refiners (add 1 wt.%).
Table 2.
Average size of pure aluminum grains before and after being refined by different Al-Ti-C grain refiners (add 1 wt.%).
| Grain Refiner | Average Grain Size/μm |
|---|
| No Grain Refiner | 1912.4 |
| Ball Milling Loaded Al-Ti-C Grain Refiner (3#) | 580.2 |
| In-Situ Reaction Al-Ti-C Grain Refiner (4#) | 504.8 |
Table 3.
Average size of pure aluminum grains after being refined by Al-Ti-C grain refiners with different extrusion ratios (add 1 wt.%).
Table 3.
Average size of pure aluminum grains after being refined by Al-Ti-C grain refiners with different extrusion ratios (add 1 wt.%).
| Grain Refiner | Extrusion Ratio | Average Grain Size/μm |
|---|
| Grain Refiner (4#) | 13 | 504.8 |
| Grain Refiner (5#) | 20 | 492.4 |
| Grain Refiner (6#) | 30 | 470.8 |
Table 4.
Average size of pure aluminum grains after being refined by extrusion ratio 30 Al-Ti-C grain refiner (6#) with different addition amounts.
Table 4.
Average size of pure aluminum grains after being refined by extrusion ratio 30 Al-Ti-C grain refiner (6#) with different addition amounts.
| Grain Refiner | Content of Grain Refiner/wt.% | Average Grain Size/μm |
|---|
| Grain Refiner (6#) | 1.0 | 504.8 |
| Grain Refiner (6#) | 3.0 | 124.6 |
| Grain Refiner (6#) | 5.5 | 52.4 |
| Grain Refiner (6#) | 6.0 | 54.6 |
Table 5.
Average size of pure aluminum grains after being refined by Al-Ti-C-Ce grain refiners (add 5.5 wt.%) with different CeO2 contents.
Table 5.
Average size of pure aluminum grains after being refined by Al-Ti-C-Ce grain refiners (add 5.5 wt.%) with different CeO2 contents.
| Grain Refiner | CeO2 Content in Grain Refiner/wt.% | Average Grain Size/μm |
|---|
| Grain Refiner (6#) | 0 | 52.4 |
| Grain Refiner (7#) | 0.25 | 50.8 |
| Grain Refiner (8#) | 0.50 | 48.4 |
| Grain Refiner (9#) | 0.75 | 48.6 |
| Grain Refiner (10#) | 1.0 | 48.7 |
| Grain Refiner (11#) | 2.0 | 48.6 |
Table 6.
Average size of pure aluminum grains after being refined by Al-Ti-C and Al-Ti-C-Ce grain refiners (add 5.5 wt.%) with different holding times.
Table 6.
Average size of pure aluminum grains after being refined by Al-Ti-C and Al-Ti-C-Ce grain refiners (add 5.5 wt.%) with different holding times.
| Grain Refiner | CeO2 Content in Grain Refiner/wt.% | Holding Time/min | Average Grain Size/μm |
|---|
| Grain Refiner (6#) | 0 | 1 | 62.5 |
| Grain Refiner (6#) | 0 | 3 | 52.4 |
| Grain Refiner (6#) | 0 | 5 | 64.8 |
| Grain Refiner (6#) | 0 | 10 | 116.4 |
| Grain Refiner (8#) | 0.50 | 1 | 54.6 |
| Grain Refiner (8#) | 0.50 | 3 | 48.4 |
| Grain Refiner (8#) | 0.50 | 5 | 48.8 |
| Grain Refiner (8#) | 0.50 | 10 | 68.4 |
Table 7.
Gibbs free energy of the above reactions at the experimental temperature of 1003 K.
Table 7.
Gibbs free energy of the above reactions at the experimental temperature of 1003 K.
| Reaction | G/KJ·mol−1 |
|---|
| (2) | −119 |
| (3) | 476 |
| (4) | −394 |
| (5) | - |