The Relationship between the Structural Characteristics of α-Fe2O3 Catalysts and Their Lattice Oxygen Reactivity Regarding Hydrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Characterization Techniques
2.3. Examination of the State Surface of Calcined α-Fe2O3 Samples
2.4. Temperature-Programmed Reduction of α-Fe2O3 Samples by Hydrogen
3. Results and Discussion
3.1. Reactivity of Calcined α-Fe2O3 Samples in the Temperature-Programmed Reduction by Hydrogen
3.2. Examination of Calcined α-Fe2O3 Samples using Raman Spectroscopy
3.3. Study of the α-Fe2O3 Surface in Calcined Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, M.; Wachs, I.E. Iron-Based Catalysts for the High Temperature Water-Gas Shift (HT-WGS) Reaction: A Review. ACS Catal. 2016, 6, 722–732. [Google Scholar] [CrossRef]
- Wagloehner, S.; Reichert, D.; Leon-Sorzano, D.; Balle, P.; Geiger, B.; Kureti, S. Kinetic modeling of the oxidation of CO on Fe2O3 catalyst in excess of O2. J. Catal. 2008, 260, 305–314. [Google Scholar] [CrossRef]
- Wagloehner, S.; Kureti, S. Study on the mechanism of the oxidation of soot on Fe2O3 catalyst. Appl. Catal. B Environ. 2012, 125, 158–165. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-based oxygen carriers in chemical looping conversions: A review. Carbon Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Cheng, Z.; Qin, L.; Guo, M.; Xu, M.; Fan, J.A.; Fan, L. Oxygen Vacancy Promoted Methane Partial Oxidation over Iron Oxide Oxygen Carrier in Chemical Looping Process. Phys. Chem. Chem. Phys. 2016, 18, 32418–32428. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Cheng, Z.; Guo, M.; Xu, M.; Fan, J.A.; Fan, L.-S. Impact of 1% Lanthanum Dopant on Carbonaceous Fuel Redox Reactions with an Iron-Based Oxygen Carrier in Chemical Looping Processes. ACS Energy Lett. 2017, 2, 70–74. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H. Hydrogen production from water decomposition by redox of Fe2O3 modified with single-or double-metal additives. J. Solid State Chem. 2010, 183, 1075–1082. [Google Scholar] [CrossRef]
- Bora, D.K.; Braun, A.; Erat, S.; Safonova, O.; Graule, T.; Constable, E.C. Evolution of structural properties of iron oxide nano particles during temperature treatment from 250 °C-900 °C: X-ray diffraction and Fe K-shell pre-edge X-ray absorption study. Curr. Appl. Phys. 2012, 12, 817–825. [Google Scholar] [CrossRef]
- Kryukova, G.N.; Tsybulya, S.V.; Solovyeva, L.P.; Sadykov, V.A.; Litvak, G.S.; Andrianova, M.P. Effect of heat treatment on microstructure evolution of haematite derived from synthetic goethite. Mater. Sci. Eng. A 1991, 149, 121–127. [Google Scholar] [CrossRef]
- Breault, R.W.; Yarrington, C.S.; Weber, J.M. The Effect of Thermal Treatment of Hematite Ore for Chemical Looping Combustion of Methane. J. Energy Resour. Technol. 2016, 138, 042202-1–042202-8. [Google Scholar] [CrossRef]
- Zhang, J.; Tao He, T.; Wang, Z.; Zhu, M.; Zhang, K.; Li, B.; Wu, J. The search of proper oxygen carriers for chemical looping partial oxidation of carbon. Appl. Energy 2017, 190, 1119–1125. [Google Scholar] [CrossRef]
- Riley, J.; Siriwardane, R.; Tian, H.; Benincosa, W.; Poston, J. Kinetic analysis of the interactions between calcium ferrite and coal char for chemical looping gasification applications: Identifying reduction routes and modes of oxygen transfer. Appl. Energy 2017, 201, 94–110. [Google Scholar] [CrossRef]
- Cheng, Z.; Qin, L.; Fan, J.A.; Fan, L.-S. New Insight into the Development of Oxygen Carrier Materials for Chemical Looping Systems. Engineering 2018, 4, 343–351. [Google Scholar] [CrossRef]
- Zhu, W.; Winterstein, J.; Maimon, I.; Yin, Q.; Yuan, L.; Kolmogorov, A.N.; Sharma, R.; Zhou, G. Atomic Structural Evolution during the Reduction of α-Fe2O3 Nanowires. J. Phys. Chem. C 2016, 120, 14854–14862. [Google Scholar] [CrossRef] [PubMed]
- Schöttner, L.; Nefedov, A.; Yang, C.; Heissler, S.; Wang, Y.; Wöll, C. Structural Evolution of α-Fe2O3(0001) Surfaces Under Reduction Conditions Monitored by Infrared Spectroscopy. Front. Chem. 2019, 7, 451. [Google Scholar] [CrossRef] [PubMed]
- Shimokawabe, M.; Furuichi, R.; Ishii, T. Influence of the preparation history of α-Fe2O3 on its reactivity for hydrogen reduction. Thermochim. Acta 1979, 28, 287–305. [Google Scholar] [CrossRef]
- Lamberov, A.A.; Dementieva, E.V.; Kuzmina, O.V.; Khazeev, B.R. Transformation of the structure of iron oxide (III) during thermal heating in air. Bull. Kazan Technol. Univ. 2013, 16, 37–41. (In Russian) [Google Scholar]
- Chen, Z.; Dang, J.; Hu, X.; Yan, H. Reduction Kinetics of Hematite Powder in Hydrogen Atmosphere at Moderate Temperatures. Metals 2018, 8, 751. [Google Scholar] [CrossRef]
- Kirik, N.P.; Yumashev, V.V.; Solovyov, L.A.; Rabchevskii, E.V.; Shishkina, N.N.; Anshits, A.G. Influence of Temperature and Duration of α-Fe2O3Calcination on Reactivity in Hydrogen Oxidation. J. Sib. Fed. Univ. Chem. 2023, 16, 66–77. [Google Scholar]
- Rouquerol, J.; Rouquerol, F.; Grillet, Y.; Triaca, M. Quasi-equilibrium nitrogen adsorption gravimetry: Comparison with volumetry for the determination of surface areas and pore size distributions. Thermochim. Acta 1986, 10, 89–96. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size, and Density; Springer Science + Business Media: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- de Boer, J.H.; Lippens, B.C.; Lippens, B.G.; Broekhoff, J.C.P.; van den Heuvel, A.; Osinga, T.V. The t-curve of multimolecular N2-adsorption. J. Colloid Interface Sci. 1966, 21, 405–414. [Google Scholar] [CrossRef]
- Solovyov, L.A. Full-profile refinement by derivative difference minimization. J. Appl. Crystallogr. 2004, 37, 743–749. [Google Scholar] [CrossRef]
- Anshits, A.G.; Bayukov, O.A.; Kondratenko, E.V.; Anshits, N.N.; Pletnev, O.N.; Rabchevskii, E.V.; Solovyov, L.A. Catalytic properties and nature of active centers of ferrospheres in oxidative coupling of methane. Appl. Catal. A General 2016, 524, 192–199. [Google Scholar] [CrossRef]
- Knyazev, Y.V.; Tarasov, A.S.; Platunov, M.S.; Trigub, A.L.; Bayukov, O.A.; Boronin, A.I.; Solovyov, L.A.; Rabchevskii, E.V.; Shishkina, N.N.; Anshits, A.G. Structural and electron transport properties of CaFe2O4 synthesized in air and in helium atmosphere. J. Alloys Compd. 2020, 820, 153073. [Google Scholar] [CrossRef]
- Seah, M.P. Quantification of AES and XPS. In Practical Surface Analysis, 2nd ed.; Briggs, D., Seah, M.P., Eds.; John Wiley & Sons: Chichester, UK, 1990; Volume 1, pp. 201–255. [Google Scholar]
- Wagner, C.D. Handbook of X-ray Photoelectron Spectroscopy; Wagner, C.D., Riggs, W.M., Davis, L.E., Moulder, J.F., Muilenberg, G.E., Eds.; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979; 190p. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1992; Corpus ID: 133719866. [Google Scholar]
- Baklanova, N.I.; Zima, T.M.; Boronin, A.I.; Kosheev, S.V.; Titov, A.T.; Isaeva, N.V.; Graschenkov, D.V.; Solntsev, S.S. Protective ceramic multilayer coatings for carbon fibers. Surf. Coat. Technol. 2006, 201, 2313–2319. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Slavinskaya, E.M.; Gulyaev, R.V.; Zaikovskii, V.I.; Stonkus, O.A.; Danilova, I.G.; Plyasova, L.M.; Polukhina, I.A.; Boronin, A.I. Metal-support interactions in Pt/Al2O3 and Pd/Al2O3 catalysts for CO oxidation. Appl. Catal. B Environ. 2010, 97, 57–71. [Google Scholar] [CrossRef]
- DIN 51007:1994-06; Thermal Analysis; Differential Thermal Analysis. Principles, Deutsches Institut für Normung e. V.: Berlin, Germany, 1994.
- Sun, L.; Liang, X.; Liu, H.; Cao, H.; Liu, X.; Jin, Y.; Li, X.; Chen, S.; Wu, X. Activation of Co-O bond in (110) facet exposed Co3O4 by Cu doping for the boost of propane catalytic oxidation. J. Hazard. Mater. 2023, 15, 131319. [Google Scholar] [CrossRef]
- Massey, M.J.; Baier, U.; Merlin, R.; Weber, W.H. Effects of pressure and isotopic substitution on the Raman spectrum of a-Fe2O3: Identification of two-magnon scattering. Phys. Rev. B 1990, 41, 7822–7827. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- and Raman-Active Phonons of Magnetite, Maghemite, and Hematite: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hochella, M.F., Jr.; Madden, A.S. Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys. Chem. Chem. Phys. 2007, 9, 1736–1750. [Google Scholar] [CrossRef]
- Jubb, A.M.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Montenero, A. Micro-Raman Investigation of Iron Oxide Films and Powders Produced by Sol–Gel Syntheses. J. Raman Spectrosc. 1999, 30, 355–360. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Liu, C.; Gao, K. Formation mechanism and protective property of corrosion product scale on X70 steel under supercritical CO2 environment. Corros. Sci. 2015, 100, 404–420. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-Ray Photoelectron Spectroscopic Characterization of Iron Oxide Nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef]
- Deckmann, R. Point defects and transport in haematite (Fe2O3-Ɛ). Philos. Mag. 1993, 68, 725–745. [Google Scholar] [CrossRef]
- Lee, J.; Han, S. Thermodynamics of native point defects in α-Fe2O3: An ab initio study. Phys. Chem. Chem. Phys. 2013, 15, 18906–18914. [Google Scholar] [CrossRef]
- Warschkow, O.; Ellis, D.E. Defects and Charge Transport near the Hematite (0001) Surface: An Atomistic Study of Oxygen Vacancies. J. Am. Ceram. Soc. 2002, 85, 213–220. [Google Scholar] [CrossRef]
- Gieitzer, C.; Nowotny, J.; Rekas, M. Surface and Bulk Electrical Properties of the Hematite Phase Fe2O3. Appl. Phys. A 1991, 53, 310–316. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Reddy, J.; Wang, C.; Zhang, J.Z.; Li, Y. The Influence of Oxygen Content on the Thermal Activation of Hematite Nanowires. Angew. Chem. 2012, 124, 4150–4155. [Google Scholar] [CrossRef]
- Ovcharenko, R.; Voloshina, E.; Sauer, J. Water adsorption and O-defect formation on Fe2O3 (0001) surfaces. Phys. Chem. Chem. Phys. 2016, 18, 25560–25568. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kendelewicz, T.; Newberg, J.T.; Ketteler, G.; Starr, D.E.; Mysak, E.R.; Andersson, K.J.; Ogasawara, H.; Bluhm, H.; Salmeron, M.; et al. Water Adsorption on α-Fe2O3(0001) at near Ambient Conditions. J. Phys. Chem. C 2010, 114, 2256–2266. [Google Scholar] [CrossRef]
- Ismail, H.M.; Cadenhead, D.A.; Zaki, M.I. Surface Reactivity of Iron Oxide Pigmentary Powders toward Atmospheric Components: XPS, FESEM, and Gravimetry of CO and CO2 Adsorption. J. Colloid Interface Sci. 1997, 194, 482–488. [Google Scholar] [CrossRef]
- Ferretto, L.; Glisenti, A. Study of the surface acidity of an hematite powder. J. Mol. Catal. A Chem. 2002, 187, 119–128. [Google Scholar] [CrossRef]
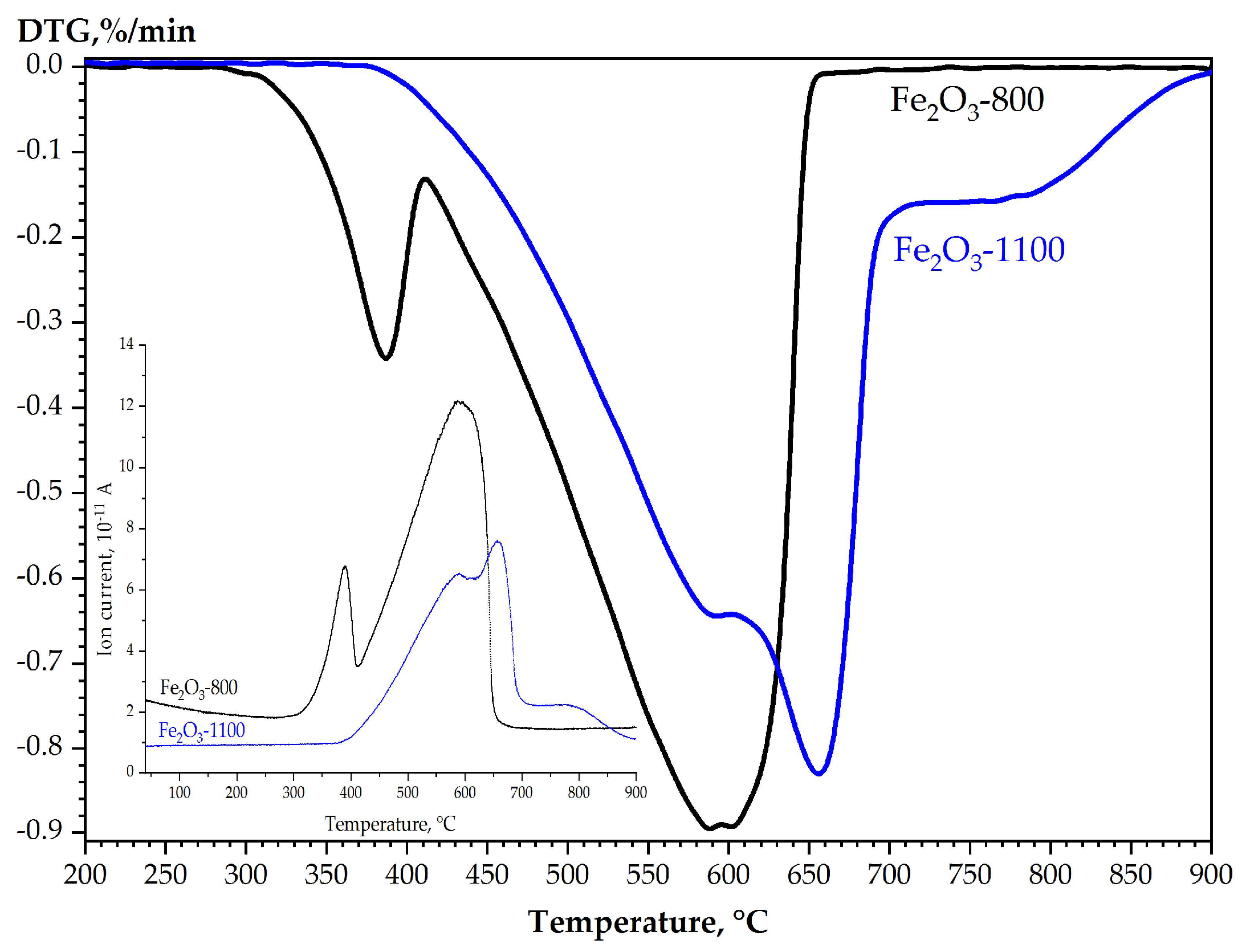
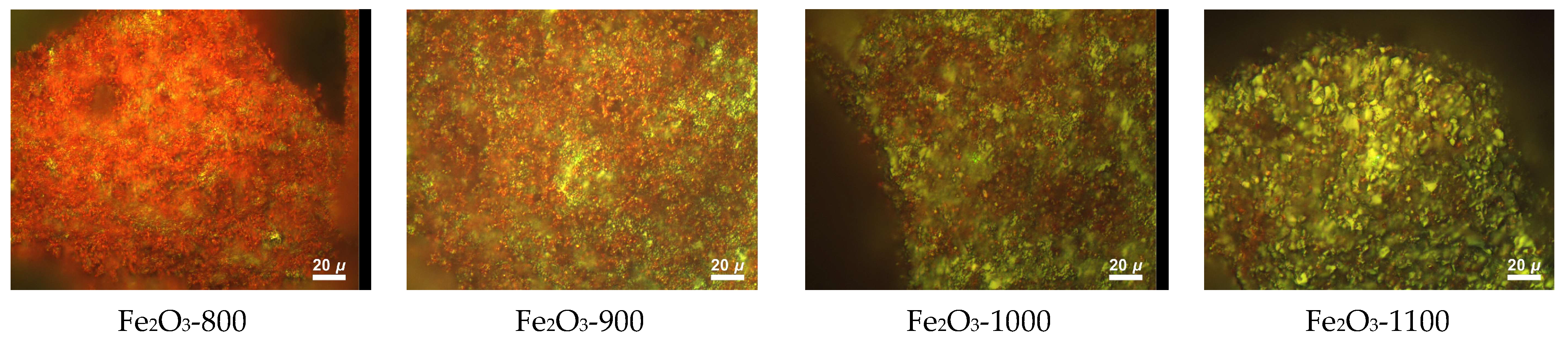
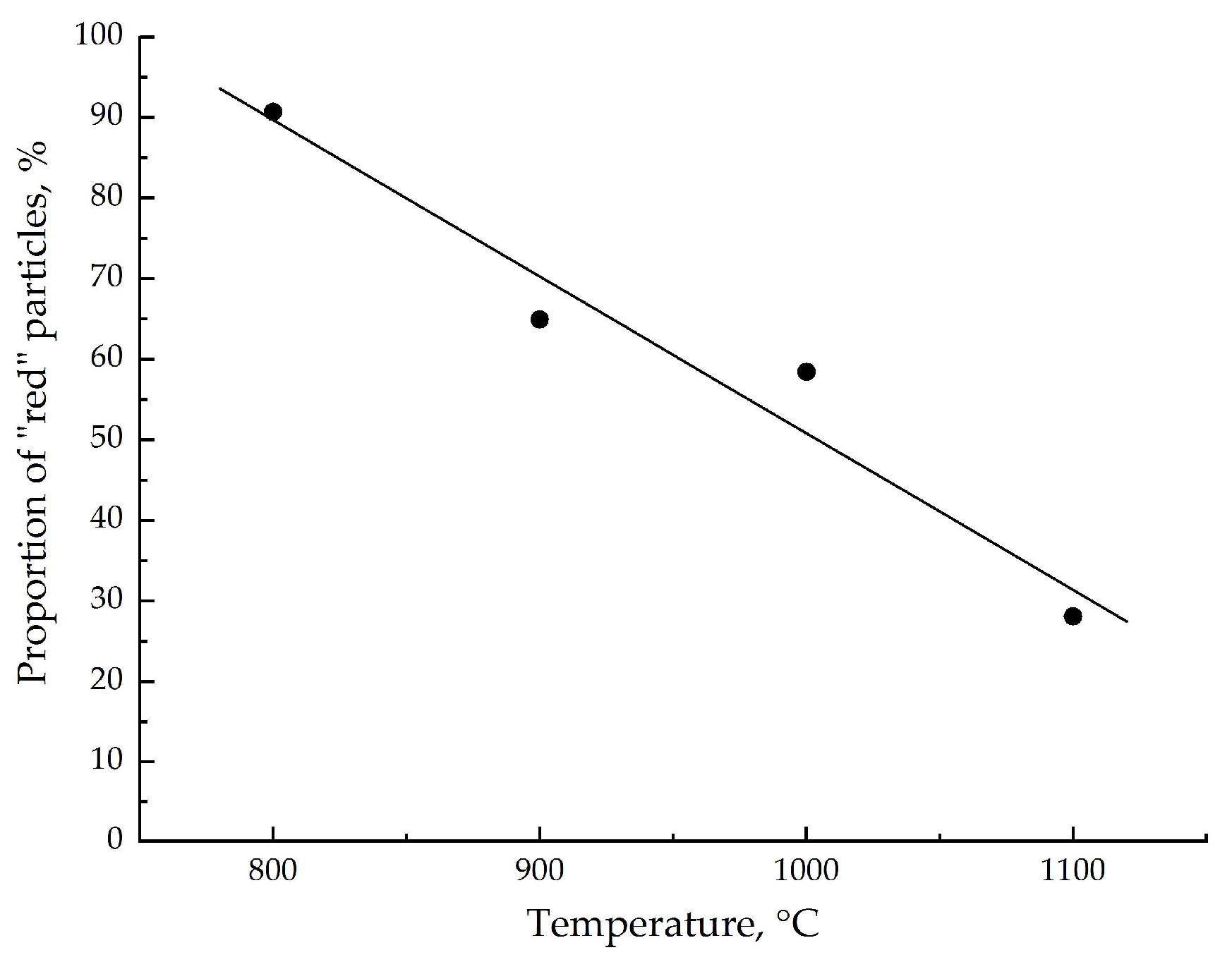
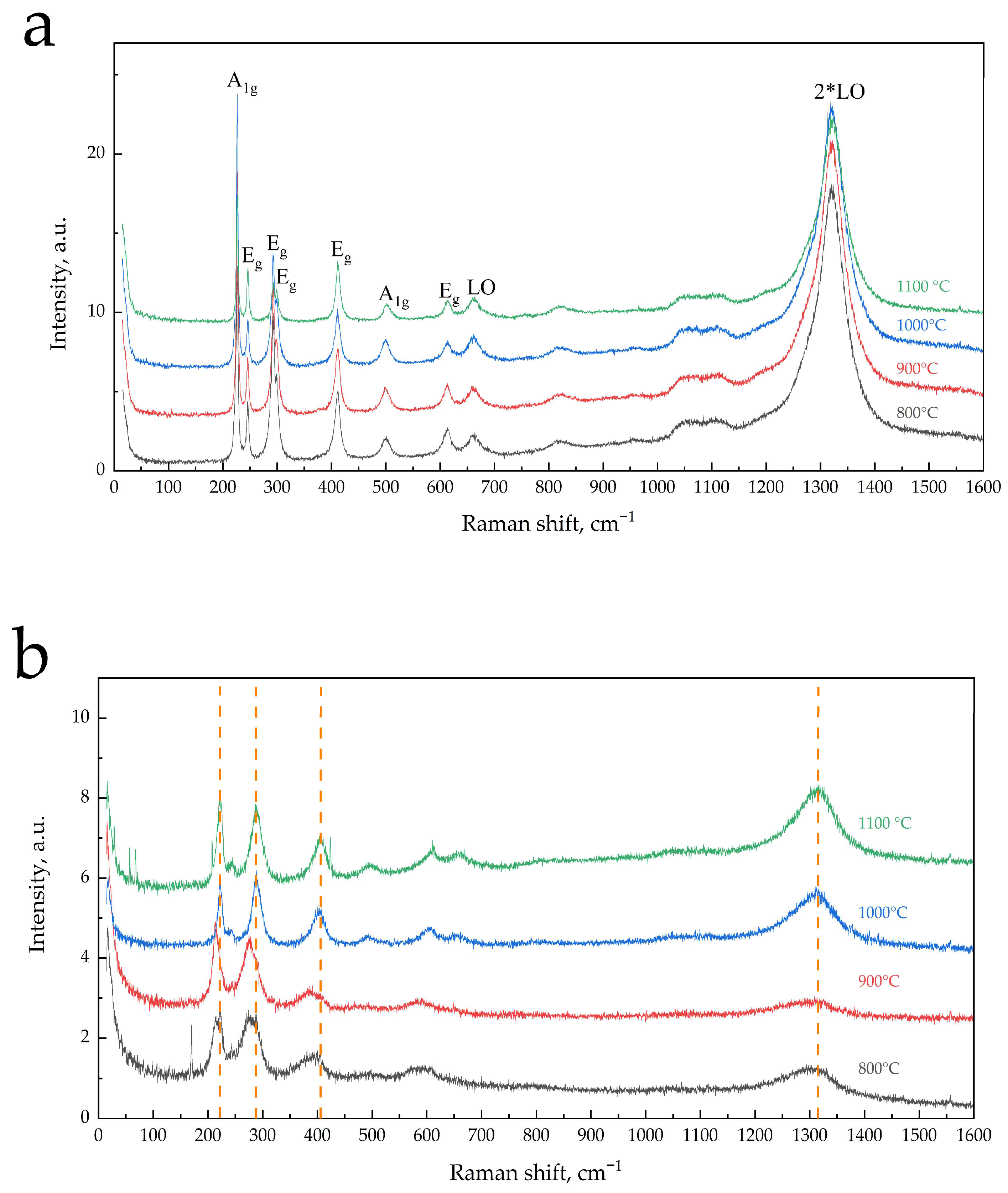
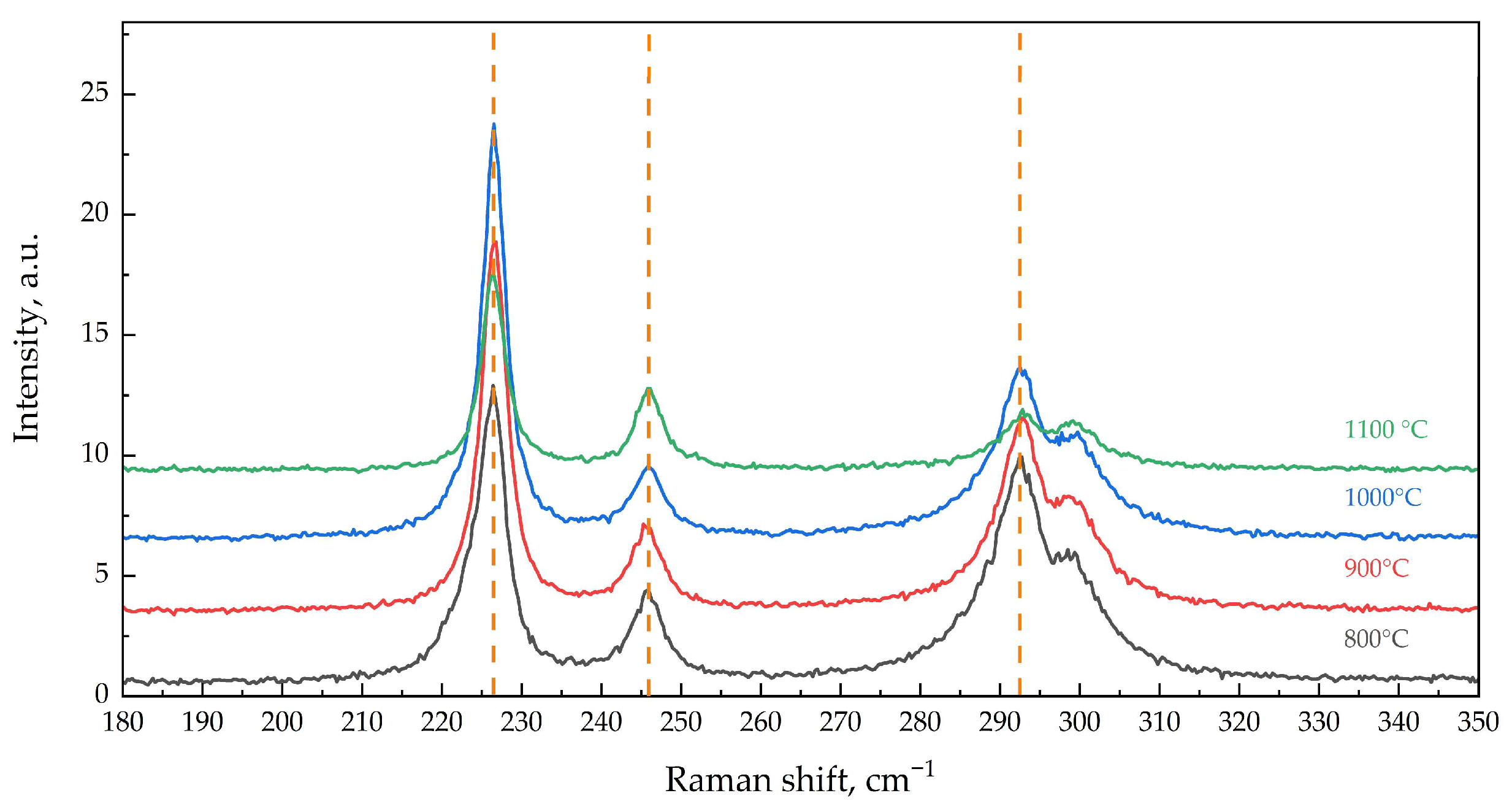
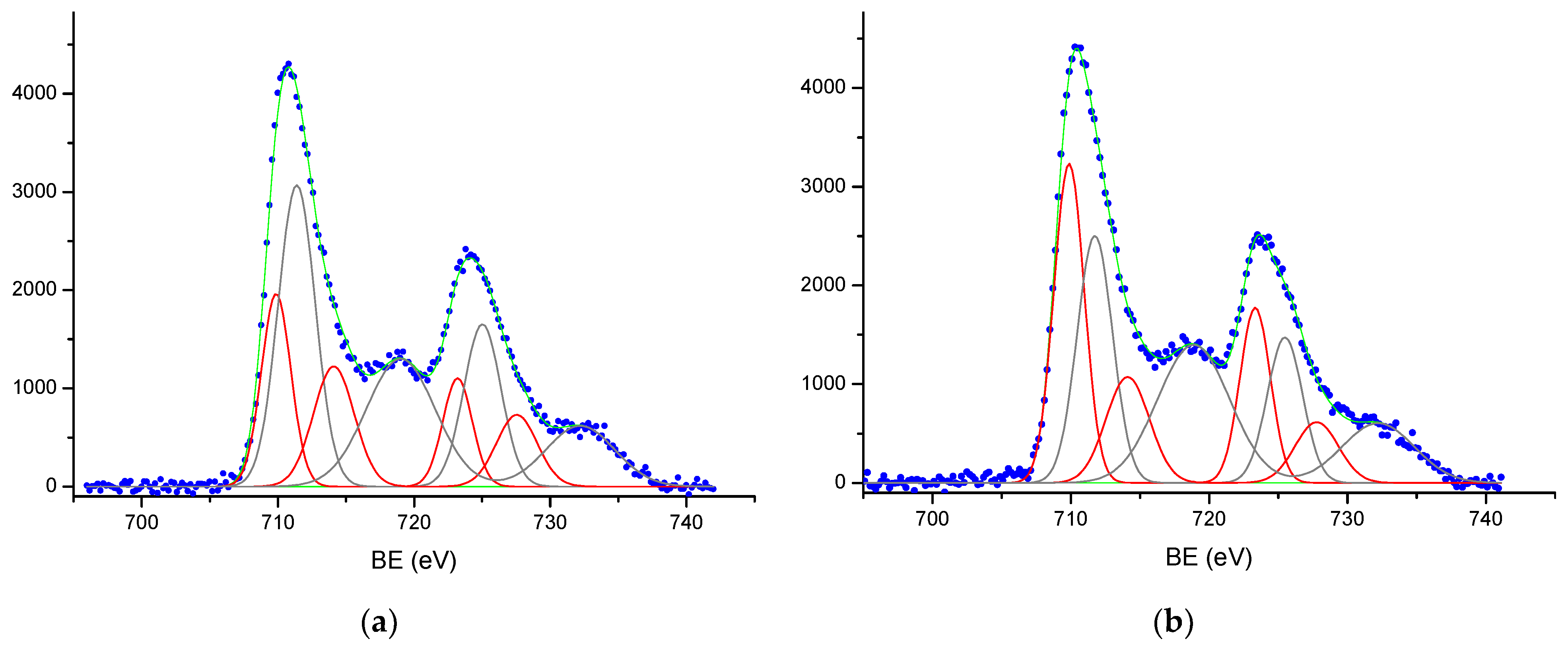
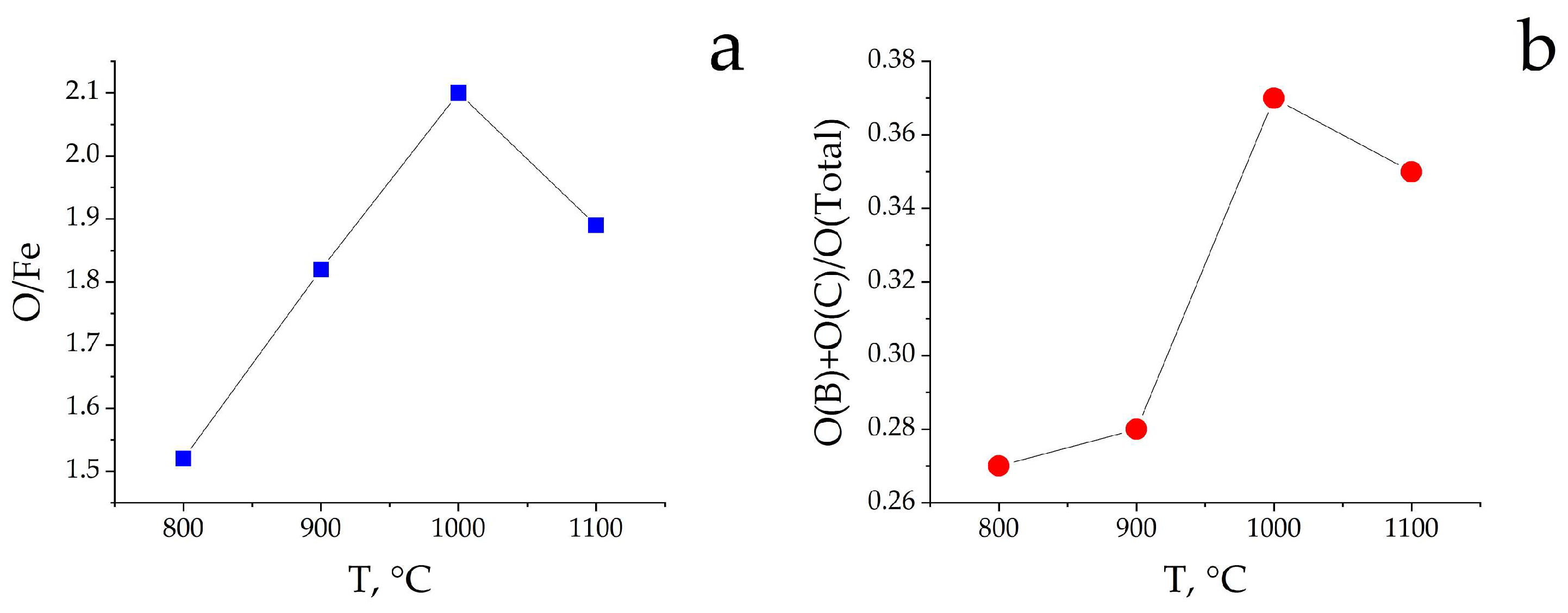
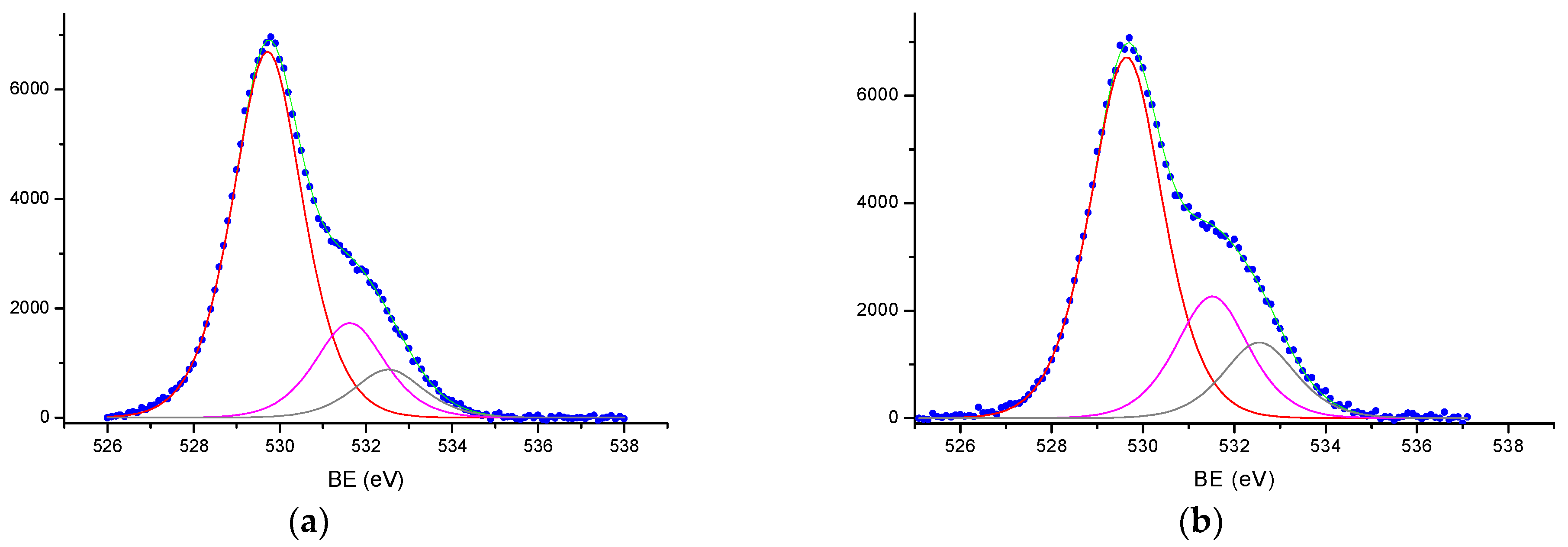
| Tcal, °C | SSA, m2/g | Vpore, × 10−4 cm3/g | Dav, μm | DX, g/cm3 | Tin., °C | T95%, °C |
|---|---|---|---|---|---|---|
| 800 | 2.10 | 27.4 | ~0.2 | 5.2704(1) | 337 | 630 |
| 900 | 0.81 | 11.5 | - | 5.2707(1) | 385 | 668 |
| 1000 | 0.21 | 3.1 | - | 5.2711(1) | 429 | 737 |
| 1100 | 0.09 | 1.6 | ~2.5 | 5.2713(1) | 435 | 812 |
| Samples | Concentration of Elements, Atomic Ratios | ||||
|---|---|---|---|---|---|
| CΣ | C(CO3) | OΣ | Fe | Si * | |
| Fe2O3-800 | 1.21 | 0.04 | 1.52 | 1 | 0.08 |
| Fe2O3-900 | 1.77 | 0.13 | 1.82 | 1 | 0.24 |
| Fe2O3-1000 | 2.23 | 0.17 | 2.10 | 1 | 0.21 |
| Fe2O3-1100 | 1.59 | 0.14 | 1.89 | 1 | 0.10 |
| Samples | Fe2p3/2 | O1s | C1s | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe2+ | Fe3+ | A | B | C | A | B | C | |||
| Fe2O3-800 | 709.9 | 714.1 | 711.5 | 719.0 | 529.7 | 531.6 | 532.6 | 285.0 | 286 | 288.5 |
| Fe2O3-800 | 709.9 | 714.1 | 711.4 | 710.0 | 529.7 | 531.6 | 532.5 | 285.0 | - | 288.6 |
| Fe2O3-800 | 709.9 | 714.1 | 711.5 | 718.8 | 529.7 | 531.6 | 532.7 | 284.9 | - | 288.6 |
| Fe2O3-1100 | 709.8 | 714.1 | 711.6 | 718.9 | 529.6 | 531.5 | 532.7 | 285.0 | - | 288.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirik, N.; Krylov, A.; Boronin, A.; Koshcheev, S.; Solovyov, L.; Rabchevskii, E.; Shishkina, N.; Anshits, A. The Relationship between the Structural Characteristics of α-Fe2O3 Catalysts and Their Lattice Oxygen Reactivity Regarding Hydrogen. Materials 2023, 16, 4466. https://doi.org/10.3390/ma16124466
Kirik N, Krylov A, Boronin A, Koshcheev S, Solovyov L, Rabchevskii E, Shishkina N, Anshits A. The Relationship between the Structural Characteristics of α-Fe2O3 Catalysts and Their Lattice Oxygen Reactivity Regarding Hydrogen. Materials. 2023; 16(12):4466. https://doi.org/10.3390/ma16124466
Chicago/Turabian StyleKirik, Nadezhda, Alexander Krylov, Andrey Boronin, Sergey Koshcheev, Leonid Solovyov, Evgenii Rabchevskii, Nina Shishkina, and Alexander Anshits. 2023. "The Relationship between the Structural Characteristics of α-Fe2O3 Catalysts and Their Lattice Oxygen Reactivity Regarding Hydrogen" Materials 16, no. 12: 4466. https://doi.org/10.3390/ma16124466
APA StyleKirik, N., Krylov, A., Boronin, A., Koshcheev, S., Solovyov, L., Rabchevskii, E., Shishkina, N., & Anshits, A. (2023). The Relationship between the Structural Characteristics of α-Fe2O3 Catalysts and Their Lattice Oxygen Reactivity Regarding Hydrogen. Materials, 16(12), 4466. https://doi.org/10.3390/ma16124466







