Heterogeneous Catalysts in N-Heterocycles and Aromatics as Liquid Organic Hydrogen Carriers (LOHCs): History, Present Status and Future
Abstract
1. Introduction
2. Single-Function Catalysts of LOHCs
2.1. Hydrogenation Catalysts of LOHCs
2.1.1. Monometallic Hydrogenation Catalysts
2.1.2. Bimetallic Hydrogenation Catalysts
2.1.3. Other Hydrogenation Catalysts
2.2. Dehydrogenation Catalysts of LOHCs
2.2.1. Monometallic Dehydrogenation Catalysts
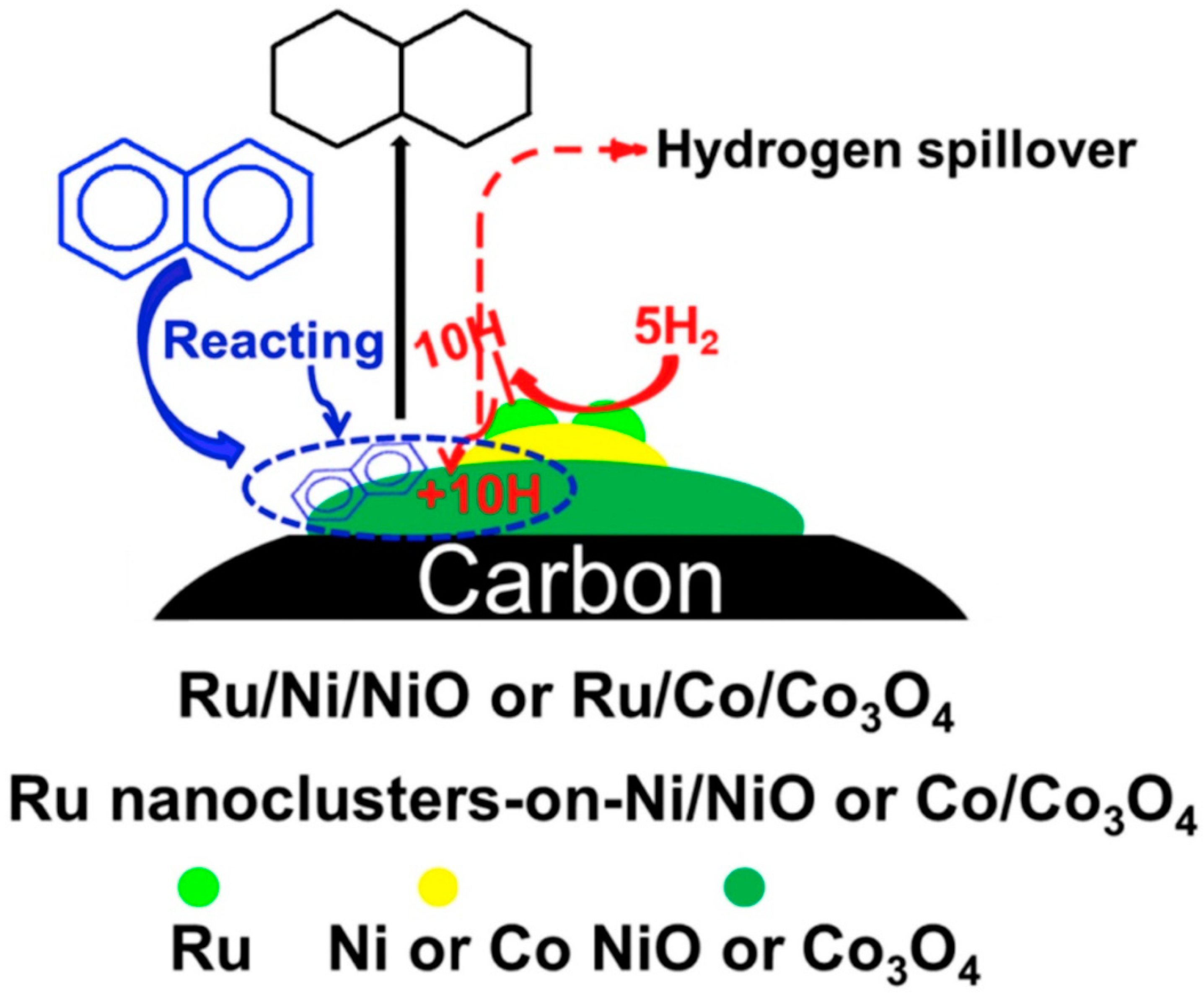
2.2.2. Bimetallic Dehydrogenation Catalysts
3. Bifunctional Catalysts of LOHCs
4. Conclusions and Outlook
- (1)
- Compared with monometallic catalysts, catalysts with an appropriate bimetallic combination and proportion have achieved better catalytic performance. On this basis, we can try to develop tri-metal or even high-entropy alloy catalysts to achieve the goals of better economic efficiency and catalytic performance. Researchers need to explore different metal compositions, screen out excellent metal combinations and proportions and construct a positive synergy between multiple metals.
- (2)
- It is an effective way to develop efficient and multifunctional catalysts by using catalyst carriers with excellent characteristics (such as rapid hydrogen transfer capability or the activation ability of hydrogen or LOHCs). Additionally, improving existing, commonly used carriers can also effectively improve catalyst performance. In this respect, using defect engineering to construct carrier surface defects to anchor active metals or adding promotive compounds (such as CeO2) to enhance the interaction between active metals and carriers have been proven to be effective methods for improving carriers.
- (3)
- It is necessary to develop hydrogenation and dehydrogenation bifunctional catalysts to satisfy the application requirements of fuel cells, renewable energy storage systems and other fields. The bifunctional catalysts can effectively improve the efficiency and economy of the entire system, representing future development requirements.
- (4)
- The reaction mechanism of LOHCs, the reaction pathway on different catalysts and the catalytic properties of noble metals can be further revealed using theoretical calculation. The theoretical calculation results can provide a theoretical guide for the combination of multiple metals, the optimization of ratio and the selection of excellent carriers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | activated carbon |
| ACC | activated carbon cloth |
| ACD | acridine |
| Al2O3 | alumina |
| Ben | benzene |
| BNPs | bimetallic nanoparticles |
| CNTs | carbon nanotubes |
| co– SEA | simultaneous strong electrostatic adsorption |
| CYC | cyclohexane |
| DBT | dibenzyltoluene |
| DFT | Density functional theory |
| HBEA | zeolite beta in proton form |
| KIT-6 | a typical ordered mesoporous SiO2 |
| LDH | Layered double hydroxides |
| LDH-CNT | Layered double hydroxides–carbon nanotubes composites |
| LDHs-us | Cl-intercalated MgAl LDHs |
| LDOs | hydrotalcite-derived oxides |
| LHSV | liquid hourly space velocities |
| LOHCs | liquid organic hydrogen carriers |
| MBP | 2-(N-methylbenzyl)-pyridine |
| MCH | methylcyclohexane |
| MWNTs | carboxylate functionalized multi-walled carbon nanotubes |
| NAP | naphthalene |
| NEC | N-ethylcarbazole |
| NEID | N-ethylindole |
| NGC | N-doped graphitized carbon |
| NPC | N-propylcarbazole |
| NPs | nanoparticles |
| pg-BC | partially graphitized biocarbon |
| P25 | rutile/anatase~1/4 mixture |
| rGO | reduced graphene oxide |
| TOF | turnover frequency |
| TOL | toluene |
| TiO2 | titanium dioxide |
| 1-MID | 1-methylindole |
| 1,2-DMID | 1,2-dimethylindole |
| 2-MID | 2-methylindole |
| 7-EID | 7-ethylindole |
| 12H-NEC | dodecahydro-N-ethylcarbazole |
| 12H-NPC | perhydro-N-propylcarbazole |
References
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Pingkuo, L.; Xue, H. Comparative analysis on similarities and differences of hydrogen energy development in the World’s top 4 largest economies: A novel framework. Int. J. Hydrog. Energy 2022, 47, 9485–9503. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment based on chemical and economic properties. Int. J. Hydrog. Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Muttaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrog. Energy 2022, 47, 17285–17312. [Google Scholar] [CrossRef]
- Razi, F.; Dincer, I. Renewable energy development and hydrogen economy in MENA region: A review. Renew. Sust. Energ. Rev. 2022, 168, 112763. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The future of hydrogen energy: Bio-hydrogen production technology. Int. J. Hydrog. Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Catumba, B.D.; Sales, M.B.; Borges, P.T.; Ribeiro Filho, M.N.; Lopes, A.A.S.; Sousa Rios, M.A.d.; Desai, A.S.; Bilal, M.; Santos, J.C.S.d. Sustainability and challenges in hydrogen production: An advanced bibliometric analysis. Int. J. Hydrog. Energy 2023, 48, 7975–7992. [Google Scholar] [CrossRef]
- Rasul, M.G.; Hazrat, M.A.; Sattar, M.A.; Jahirul, M.I.; Shearer, M.J. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Hannan, M.A.; Abu, S.M.; Al-Shetwi, A.Q.; Mansor, M.; Ansari, M.N.M.; Muttaqi, K.M.; Dong, Z.Y. Hydrogen energy storage integrated battery and supercapacitor based hybrid power system: A statistical analysis towards future research directions. Int. J. Hydrog. Energy 2022, 47, 39523–39548. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Li, L.; Zhu, T.; Chen, X.; Cheng, H. Study on reversible hydrogen uptake and release of 1,2-dimethylindole as a new liquid organic hydrogen carrier. Int. J. Hydrog. Energy 2019, 44, 4919–4929. [Google Scholar] [CrossRef]
- Bayrakdar Ates, E.; Calik, E. Public awareness of hydrogen energy: A comprehensive evaluation based on statistical approach. Int. J. Hydrog. Energy 2023, 48, 8756–8767. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. An overview of conventional and non-conventional hydrogen production methods. Mater. Today Proc. 2021, 46, 5353–5359. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Mazzeo, D.; Herdem, M.S.; Matera, N.; Wen, J.Z. Green hydrogen production: Analysis for different single or combined large-scale photovoltaic and wind renewable systems. Renew. Energy 2022, 200, 360–378. [Google Scholar] [CrossRef]
- Nami, H.; Rizvandi, O.B.; Chatzichristodoulou, C.; Hendriksen, P.V.; Frandsen, H.L. Techno-economic analysis of current and emerging electrolysis technologies for green hydrogen production. Energy Convers. Manag. 2022, 269, 116162. [Google Scholar] [CrossRef]
- Zhang, C.; Song, P.; Zhang, Y.; Xiao, L.; Hou, J.; Wang, X. Technical and cost analysis of imported hydrogen based on MCH-TOL hydrogen storage technology. Int. J. Hydrog. Energy 2022, 47, 27717–27732. [Google Scholar] [CrossRef]
- Li, Q. The view of technological innovation in coal industry under the vision of carbon neutralization. Int. J. Coal Sci. Technol. 2021, 8, 1197–1207. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Oda, T.; Purnomo, C.W.; Kashiwagi, T.; Aziz, M. Liquid hydrogen, methylcyclohexane, and ammonia as potential hydrogen storage: Comparison review. Int. J. Hydrog. Energy 2019, 44, 15026–15044. [Google Scholar] [CrossRef]
- Wulf, C.; Zapp, P. Assessment of system variations for hydrogen transport by liquid organic hydrogen carriers. Int. J. Hydrog. Energy 2018, 43, 11884–11895. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Source 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrog. Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Song, P.; Sui, Y.; Shan, T.; Hou, J.; Wang, X. Assessment of hydrogen supply solutions for hydrogen fueling station: A Shanghai case study. Int. J. Hydrog. Energy 2020, 45, 32884–32898. [Google Scholar] [CrossRef]
- Avargani, V.M.; Zendehboudi, S.; Saady, N.M.C.; Dusseault, M.B. A comprehensive review on hydrogen production and utilization in North America: Prospects and challenges. Energy Convers. Manag. 2022, 269, 115927. [Google Scholar] [CrossRef]
- Jiang, Z.; Pan, Q.; Xu, J.; Fang, T. Current situation and prospect of hydrogen storage technology with new organic liquid. Int. J. Hydrog. Energy 2014, 39, 17442–17451. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Gupta, N.; Zhang, K.; van Doorne, C.; Fesmire, J.; Dindoruk, B.; Balakotaiah, V. Hydrogen supply chain and challenges in large-scale LH2 storage and transportation. Int. J. Hydrog. Energy 2021, 46, 24149–24168. [Google Scholar] [CrossRef]
- Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global hydrogen development—A technological and geopolitical overview. Int. J. Hydrog. Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Jensen, J.O.; Vestbø, A.P.; Li, Q.; Bjerrum, N.J. The energy efficiency of onboard hydrogen storage. J. Alloys Compd. 2007, 446–447, 723–728. [Google Scholar] [CrossRef]
- Nagar, R.; Srivastava, S.; Hudson, S.L.; Amaya, S.L.; Tanna, A.; Sharma, M.; Achayalingam, R.; Sonkaria, S.; Khare, V.; Srinivasan, S.S. Recent developments in state-of-the-art hydrogen energy technologies—Review of hydrogen storage materials. Solar Compass 2023, 5, 100033. [Google Scholar] [CrossRef]
- Yoo, B.-H.; Wilailak, S.; Bae, S.-H.; Gye, H.-R.; Lee, C.-J. Comparative risk assessment of liquefied and gaseous hydrogen refueling stations. Int. J. Hydrog. Energy 2021, 46, 35511–35524. [Google Scholar] [CrossRef]
- Salehi, F.; Abbassi, R.; Asadnia, M.; Chan, B.; Chen, L. Overview of safety practices in sustainable hydrogen economy—An Australian perspective. Int. J. Hydrog. Energy 2022, 47, 34689–34703. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Fursikov, P.V.; Volodin, A.A.; Bocharnikov, M.S.; Shimkus, Y.Y.; Kashin, A.M.; Yartys, V.A.; Chidziva, S.; Pasupathi, S.; Lototskyy, M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. Int. J. Hydrog. Energy 2021, 46, 13647–13657. [Google Scholar] [CrossRef]
- Galey, B.; Auroux, A.; Sabo-Etienne, S.; Dhaher, S.; Grellier, M.; Postole, G. Improved hydrogen storage properties of Mg/MgH2 thanks to the addition of nickel hydride complex precursors. Int. J. Hydrog. Energy 2019, 44, 28848–28862. [Google Scholar] [CrossRef]
- Li, R.; Han, X.; Liu, Q.; Qian, A.; Shen, H.; Liu, J.; Pu, X.; Xu, H.; Mu, B. Porous carbon materials with improved hydrogen storage capacity by carbonizing Zn(BDC)TED0.5. J. Solid State Chem. 2022, 314, 123409. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Dong, H.; Zheng, M.; Liu, Y. Polyacrylonitrile-based highly porous carbon materials for exceptional hydrogen storage. Int. J. Hydrog. Energy 2019, 44, 23210–23215. [Google Scholar] [CrossRef]
- Baricco, M.; Bang, M.; Fichtner, M.; Hauback, B.; Linder, M.; Luetto, C.; Moretto, P.; Sgroi, M. SSH2S: Hydrogen storage in complex hydrides for an auxiliary power unit based on high temperature proton exchange membrane fuel cells. J. Power Sources 2017, 342, 853–860. [Google Scholar] [CrossRef]
- Kiruthika, S.; Sundar, P.; Ravindran, P. Structural phase stability and thermodynamical properties of transition metal complex hydrides Na2MgTMH7 (TM=Sc−Cu) for hydrogen storage applications. J. Solid State Chem. 2023, 321, 123867. [Google Scholar] [CrossRef]
- Shin, B.S.; Yoon, C.W.; Kwak, S.K.; Kang, J.W. Thermodynamic assessment of carbazole-based organic polycyclic compounds for hydrogen storage applications via a computational approach. Int. J. Hydrog. Energy 2018, 43, 12158–12167. [Google Scholar] [CrossRef]
- Shi, L.; Qi, S.; Qu, J.; Che, T.; Yi, C.; Yang, B. Integration of hydrogenation and dehydrogenation based on dibenzyltoluene as liquid organic hydrogen energy carrier. Int. J. Hydrog. Energy 2019, 44, 5345–5354. [Google Scholar] [CrossRef]
- Safronov, S.P.; Vostrikov, S.V.; Samarov, A.A.; Wasserscheid, P.; Müller, K.; Verevkin, S.P. Comprehensive thermodynamic study of substituted indoles/perhydro indoles as potential liquid organic hydrogen carrier system. Fuel 2023, 331, 125764. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrog. Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Fei, S.; Han, B.; Li, L.; Mei, P.; Zhu, T.; Yang, M.; Cheng, H. A study on the catalytic hydrogenation of N-ethylcarbazole on the mesoporous Pd/MoO3 catalyst. Int. J. Hydrog. Energy 2017, 42, 25942–25950. [Google Scholar] [CrossRef]
- Byun, M.; Choe, C.; Cheon, S.; Lee, A.; Lim, H. Statistical and stochastic feasibility studies of potential liquid organic hydrogen carriers in a membrane reactor for simultaneous hydrogen storage and production: Technical, economic, and environmental aspects. Renew. Energy 2022, 195, 1393–1411. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Samoilov, V.O.; Sultanova, M.U.; Borisov, R.S.; Kozhevnikov, A.A.; Lavrentyev, V.A.; Utepbergenova, A.S.; Maximov, A.L. The production and the properties of the liquid organic hydrogen carrier (LOHC) obtained from the light cycle oil (LCO). Fuel Process. Technol. 2023, 240, 107576. [Google Scholar] [CrossRef]
- Sultan, O.; Shaw, H. Study of automotive storage of hydrogen using recyclable liquid chemical carriers. Recon. Tech. Rep. N 1975, 76, 33642. [Google Scholar]
- Choi, I.Y.; Shin, B.S.; Kwak, S.K.; Kang, K.S.; Yoon, C.W.; Kang, J.W. Thermodynamic efficiencies of hydrogen storage processes using carbazole-based compounds. Int. J. Hydrog. Energy 2016, 41, 9367–9373. [Google Scholar] [CrossRef]
- Yang, M.; Xing, X.; Zhu, T.; Chen, X.; Dong, Y.; Cheng, H. Fast hydrogenation kinetics of acridine as a candidate of liquid organic hydrogen carrier family with high capacity. J. Energy Chem. 2020, 41, 115–119. [Google Scholar] [CrossRef]
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H. Hydrogen Storage Reversible Hydrogenated of Pi-Conjugated Substrates. U.S. Patent 20040223907, 11 November 2004. [Google Scholar]
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H. Hydrogen Storage Reversible Hydrogenated of Pi-Conjugated Substrates. U.S. Patent 20050002857, 4 January 2005. [Google Scholar]
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H. Hydrogen Storage Reversible Hydrogenated of Pi-Conjugated Substrates. U.S. Patent 7101530, 5 September 2006. [Google Scholar]
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H. Hydrogen Storage Reversible Hydrogenated of Pi-Conjugated Substrates. U.S. Patent 7351395, 1 April 2008. [Google Scholar]
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H. Hydrogen Storage Reversible Hydrogenated of Pi-Conjugated Substrates. U.S. Patent 20080260630, 23 October 2008. [Google Scholar]
- Liu, H.; Xue, J.; Yu, P.; Zhang, Y.; Wang, J.; Che, D. Hydrogenation of N-ethylcarbazole with Hydrogen-Methane mixtures for hydrogen storage. Fuel 2023, 331, 125920. [Google Scholar] [CrossRef]
- Kiermaier, S.; Lehmann, D.; Bösmann, A.; Wasserscheid, P. Dehydrogenation of perhydro-N-ethylcarbazole under reduced total pressure. Int. J. Hydrog. Energy 2021, 46, 15660–15670. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.; Pan, Q.; Ni, G.; Han, C.; Ke, H.; Fang, Q.; Cheng, H. Hydrogenation of N-propylcarbazole over supported ruthenium as a new prototype of liquid organic hydrogen carriers (LOHC). RSC Adv. 2013, 3, 24877–24881. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Zhu, T.; Chen, X.; Cheng, G.; Ke, H.; Cheng, H. Fast Dehydrogenation Kinetics of Perhydro-N-propylcarbazole over a Supported Pd Catalyst. ACS Appl. Energy Mater. 2018, 1, 4285–4292. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, G.; Xie, D.; Zhu, T.; Dong, Y.; Ke, H.; Cheng, H. Study of hydrogenation and dehydrogenation of 1-methylindole for reversible onboard hydrogen storage application. Int. J. Hydrog. Energy 2018, 43, 8868–8876. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Dong, Y.; Mei, P.; Cheng, H. Hydrogen storage and release from a new promising Liquid Organic Hydrogen Storage Carrier (LOHC): 2-methylindole. Int. J. Hydrog. Energy 2016, 41, 16129–16134. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Yang, Z.; Ke, H.; Cheng, H. Catalytic hydrogenation and dehydrogenation of N-ethylindole as a new heteroaromatic liquid organic hydrogen carrier. Int. J. Hydrog. Energy 2015, 40, 10918–10922. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Zhu, T.; Chen, X.; Li, C.; Ke, H.; Cheng, H. Hydrogenation Kinetics of N-Ethylindole on a Supported Ru Catalyst. Energy Technol. 2018, 6, 558–562. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, M.; Zhu, T.; Zhang, Z.; Chen, X.; Liu, Z.; Dong, Y.; Cheng, G.; Cheng, H. 7-ethylindole: A new efficient liquid organic hydrogen carrier with fast kinetics. Int. J. Hydrog. Energy 2018, 43, 12688–12696. [Google Scholar] [CrossRef]
- Oh, J.; Jeong, K.; Kim, T.W.; Kwon, H.; Han, J.W.; Park, J.H.; Suh, Y.W. 2-(N-Methylbenzyl)pyridine: A Potential Liquid Organic Hydrogen Carrier with Fast H(2) Release and Stable Activity in Consecutive Cycles. ChemSusChem 2018, 11, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, N.; Obesser, K.; Bosmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of industrially applied heat-transfer fluids as liquid organic hydrogen carrier systems. ChemSusChem 2014, 7, 229–235. [Google Scholar] [CrossRef]
- Heller, A.; Rausch, M.H.; Schulz, P.S.; Wasserscheid, P.; Fröba, A.P. Binary Diffusion Coefficients of the Liquid Organic Hydrogen Carrier System Dibenzyltoluene/Perhydrodibenzyltoluene. J. Chem. Eng. Data 2015, 61, 504–511. [Google Scholar] [CrossRef]
- Ali, A.; Rohini, A.K.; Lee, H.J. Dehydrogenation of perhydro-dibenzyltoluene for hydrogen production in a microchannel reactor. Int. J. Hydrog. Energy 2022, 47, 20905–20914. [Google Scholar] [CrossRef]
- Gambini, M.; Guarnaccia, F.; Di Vona, M.L.; Manno, M.; Vellini, M. Liquid organic hydrogen carriers: Development of a thermodynamic and kinetic model for the assessment of hydrogenation and dehydrogenation processes. Int. J. Hydrog. Energy 2022, 47, 28034–28045. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, M.; Chen, X.; Dong, Y.; Zhang, Z.; Cheng, H. A highly active bifunctional Ru–Pd catalyst for hydrogenation and dehydrogenation of liquid organic hydrogen carriers. J. Catal. 2019, 378, 382–391. [Google Scholar] [CrossRef]
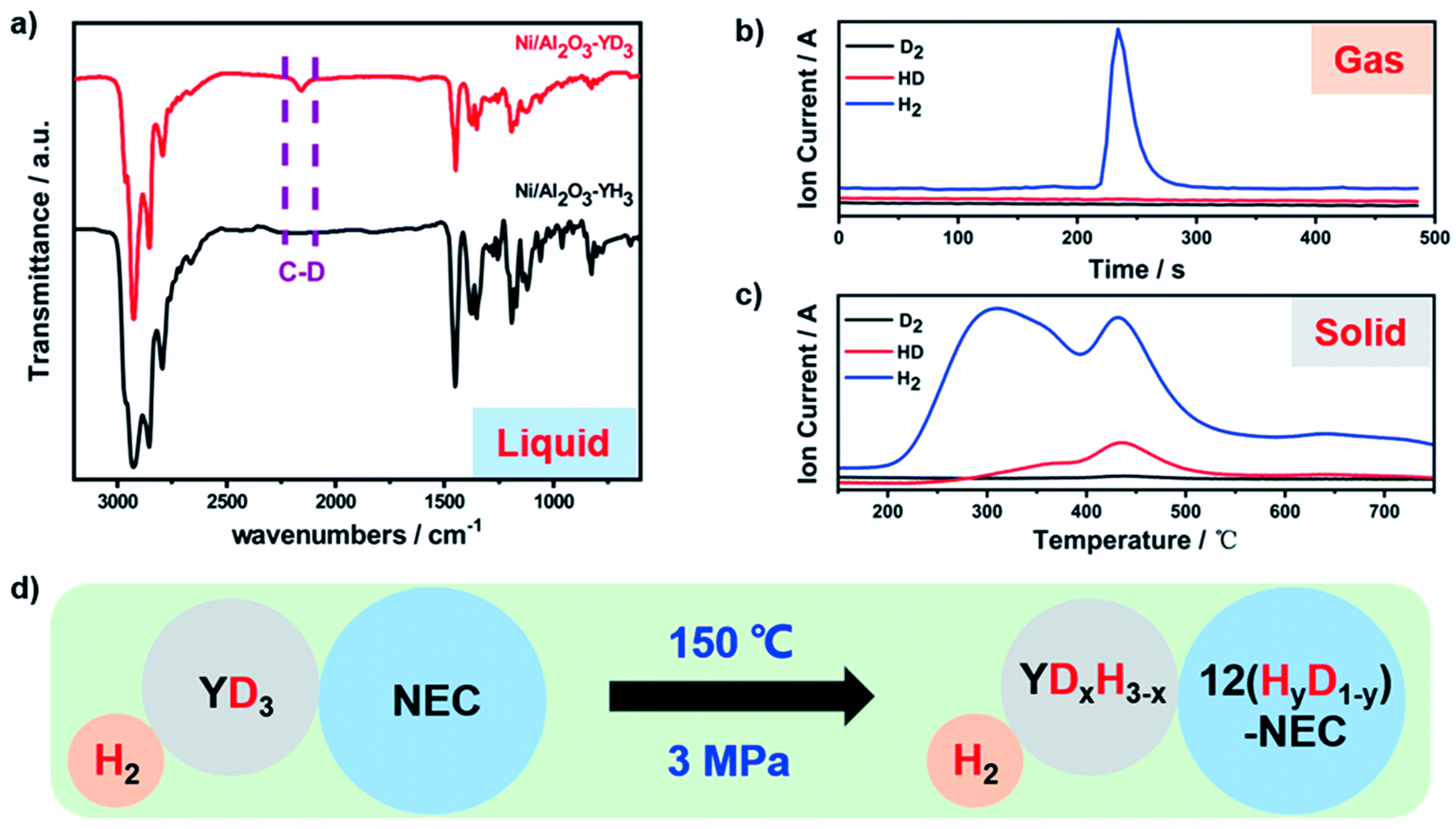
- Wu, Y.; Guo, Y.; Yu, H.; Jiang, X.; Zhang, Y.; Qi, Y.; Fu, K.; Xie, L.; Li, G.; Zheng, J.; et al. Nonstoichiometric Yttrium Hydride–Promoted Reversible Hydrogen Storage in a Liquid Organic Hydrogen Carrier. CCS Chem. 2021, 3, 974–984. [Google Scholar] [CrossRef]
- Salman, M.S.; Rambhujun, N.; Pratthana, C.; Srivastava, K.; Aguey-Zinsou, K.-F. Catalysis in Liquid Organic Hydrogen Storage: Recent Advances, Challenges, and Perspectives. Ind. Eng. Chem. Res. 2022, 61, 6067–6105. [Google Scholar] [CrossRef]
- Amende, M.; Gleichweit, C.; Schernich, S.; Hofert, O.; Lorenz, M.P.; Zhao, W.; Koch, M.; Obesser, K.; Papp, C.; Wasserscheid, P.; et al. Size and Structure Effects Controlling the Stability of the Liquid Organic Hydrogen Carrier Dodecahydro-N-ethylcarbazole during Dehydrogenation over Pt Model Catalysts. J. Phys. Chem. Lett. 2014, 5, 1498–1504. [Google Scholar] [CrossRef]
- Amende, M.; Gleichweit, C.; Werner, K.; Schernich, S.; Zhao, W.; Lorenz, M.P.; Hofert, O.; Papp, C.; Koch, M.; Wasserscheid, P.; et al. Model Catalytic Studies of Liquid Organic Hydrogen Carriers: Dehydrogenation and Decomposition Mechanisms of Dodecahydro-N-ethylcarbazole on Pt(111). ACS Catal. 2014, 4, 657–665. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, T.; Han, B.; Wu, W.; Liu, Z.; Xie, Z.; Zhang, J. A Novel Method to Immobilize Ru Nanoparticles on SBA-15 Firmly by Ionic Liquid and Hydrogenation of Arene. Catal. Lett. 2005, 103, 59–62. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Zhao, L.; Smith, K.J. Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst. Appl. Catal. A-Gen. 2009, 362, 155–162. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, T.; Wang, F.; Zhang, Z.; Dong, Y.; Yang, M.; Cheng, G.; Ke, H.; Cheng, H. High active pd@mil-101 catalyst for dehydrogenation of liquid organic hydrogen carrier. Int. J. Hydrog. Energy 2020, 45, 16144–16152. [Google Scholar] [CrossRef]
- Tang, C.; Feng, Z.; Bai, X. In situ preparation of Pd nanoparticles on N-doped graphitized carbon derived from ZIF-67 by nitrogen glow-discharge plasma for the catalytic dehydrogenation of dodecahydro-N-ethylcarbazole. Fuel 2021, 302, 121186. [Google Scholar] [CrossRef]
- Jorschick, H.; Preuster, P.; Dürr, S.; Seidel, A.; Müller, K.; Bösmann, A.; Wasserscheid, P. Hydrogen storage using a hot pressure swing reactor. Energy Environ. Sci 2017, 10, 1652–1659. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, X.; Tang, J.; Zhang, J.; Liu, C.; Huang, J. Supported mesoporous Pt catalysts with excellent performance for toluene hydrogenation under low reaction pressure. Mol. Catal. 2022, 524, 112341. [Google Scholar] [CrossRef]
- Patil, S.P.; Bindwal, A.B.; Pakade, Y.B.; Biniwale, R.B. On H2 supply through liquid organic hydrides—Effect of functional groups. Int. J. Hydrog. Energy 2017, 42, 16214–16224. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Tam, K.; Tsang, S.C.E. Comparison of catalytic performance of supported ruthenium and rhodium for hydrogenation of 9-ethylcarbazole for hydrogen storage applications. Energy Environ. Sci. 2012, 5, 8621–8630. [Google Scholar] [CrossRef]
- Pande, J.V.; Bindwal, A.B.; Pakade, Y.B.; Biniwale, R.B. Application of microwave synthesized Ag-Rh nanoparticles in cyclohexane dehydrogenation for enhanced H2 delivery. Int. J. Hydrog. Energy 2018, 43, 7411–7423. [Google Scholar] [CrossRef]
- Wang, W.; Miao, L.; Wu, K.; Chen, G.; Huang, Y.; Yang, Y. Hydrogen evolution in the dehydrogenation of methylcyclohexane over Pt/Ce Mg Al O catalysts derived from their layered double hydroxides. Int. J. Hydrog. Energy 2019, 44, 2918–2925. [Google Scholar] [CrossRef]
- Liu, X.; Bai, X.; Wu, W. Ultrasound-assisted green synthesis of Ru supported on LDH-CNT composites as an efficient catalyst for N-ethylcarbazole hydrogenation. Ultrason. Sonochem. 2022, 91, 106227. [Google Scholar] [CrossRef]
- Liu, X.; Shi, J.; Bai, X.; Wu, W. Ultrasound-excited hydrogen radical from NiFe layered double hydroxide for preparation of ultrafine supported Ru nanocatalysts in hydrogen storage of N-ethylcarbazole. Ultrason. Sonochem. 2021, 81, 105840. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, X.; Ding, J.; Qi, Z.; Wang, M.; Sun, S.; Bao, J.; Gao, C. Mechanism Study of Photocatalytic Degradation of Gaseous Toluene on TiO2 with Weak-Bond Adsorption Analysis Using In Situ Far Infrared Spectroscopy. Catal. Lett. 2014, 144, 995–1000. [Google Scholar] [CrossRef]
- Liu, H.; Fang, R.; Li, Z.; Li, Y. Solventless hydrogenation of benzene to cyclohexane over a heterogeneous Ru–Pt bimetallic catalyst. Chem. Eng. Sci. 2015, 122, 350–359. [Google Scholar] [CrossRef]
- Atsumi, R.; Kobayashi, K.; Xieli, C.; Nanba, T.; Matsumoto, H.; Matsuda, K.; Tsujimura, T. Effects of steam on toluene hydrogenation over a Ni catalyst. Appl. Catal. A Gen. 2020, 590, 117374. [Google Scholar] [CrossRef]
- Jing, J.-Y.; Yang, Z.-F.; Wang, J.-Z.; Liu, D.-C.; Feng, J.; Li, W.-Y. Effect of preparation methods on the structure and naphthalene hydrogenation performance of Ni2P/SiO2 catalyst. J. Fuel Chem. Technol. 2020, 48, 842–851. [Google Scholar] [CrossRef]
- Zhao, W.; Chizallet, C.; Sautet, P.; Raybaud, P. Dehydrogenation mechanisms of methyl-cyclohexane on γ-Al2O3 supported Pt13: Impact of cluster ductility. J. Catal. 2019, 370, 118–129. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, B.-B.; Niu, Y.-F.; Liang, Y.; Liu, L.; Dong, J.-X.; Tang, M.-X.; Li, X.-K. Hydrogenation of naphthalene to decalin catalyzed by Pt supported on WO3 of different crystallinity at low temperature. J. Fuel Chem. Technol. 2021, 49, 1181–1189. [Google Scholar] [CrossRef]
- Zhang, M.; Song, Q.; He, Z.; Wang, Q.; Wang, L.; Zhang, X.; Li, G. Tuning the mesopore-acid-metal balance in Pd/HY for efficient deep hydrogenation saturation of naphthalene. Int. J. Hydrog. Energy 2022, 47, 20881–20893. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Y.; Li, Z.-L.; Peng, Z.; Yang, J.-H.; Liu, B.; Liu, Z. Bimetallic Pd-M (M = Pt, Ni, Cu, Co) nanoparticles catalysts with strong electrostatic metal-support interaction for hydrogenation of toluene and benzene. Mol. Catal. 2020, 492, 110992. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Song, J.; Li, J.; Liu, J.; Wu, T.; Zhang, P.; Han, B. Ru nanoparticles immobilized on metal–organic framework nanorods by supercritical CO2-methanol solution: Highly efficient catalyst. Green Chem. 2011, 13, 2078–2082. [Google Scholar] [CrossRef]
- Su, F.; Lv, L.; Lee, F.Y.; Liu, T.; Cooper, A.I.; Zhao, X.S. Thermally reduced ruthenium nanoparticles as a highly active heterogeneous catalyst for hydrogenation of monoaromatics. J. Am. Chem. Soc. 2007, 129, 14213–14223. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Özkar, S. Intrazeolite Ruthenium(0) Nanoclusters: A Superb Catalyst for the Hydrogenation of Benzene and the Hydrolysis of Sodium Borohydride. Langmuir 2008, 24, 7065–7067. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, Y.; Cheng, Y.; Wang, L.; Li, X. Biosynthesized ruthenium nanoparticles supported on carbon nanotubes as efficient catalysts for hydrogenation of benzene to cyclohexane: An eco-friendly and economical bioreduction method. Appl. Catal. A Gen. 2014, 484, 154–160. [Google Scholar] [CrossRef]
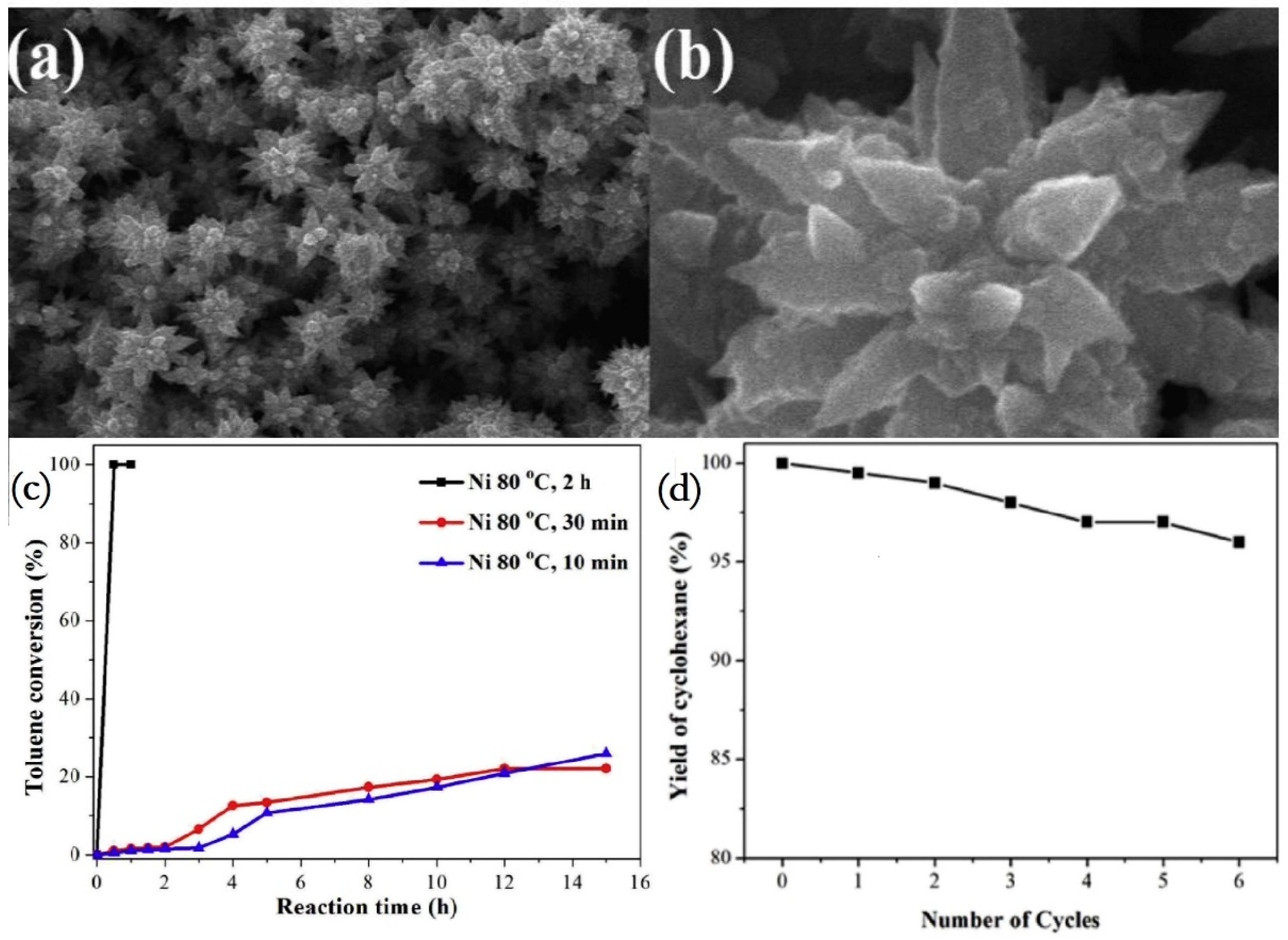
- Song, H.; Wang, T.; Jia, X.; Zhu, Y. Nickel hierarchical flower-like nanostructures: Controlled synthesis and catalytic hydrogenation of toluene. J. Alloys Compd. 2018, 747, 523–529. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Guo, Y.; Zhang, Y.; Jiang, X.; Sun, B.; Fu, K.; Chen, J.; Qi, Y.; Zheng, J.; et al. Promoting hydrogen absorption of liquid organic hydrogen carriers by solid metal hydrides. J. Mater. Chem. A 2019, 7, 16677–16684. [Google Scholar] [CrossRef]
- Ding, Y.; Dong, Y.; Zhang, H.; Zhao, Y.; Yang, M.; Cheng, H. A highly adaptable Ni catalyst for Liquid Organic Hydrogen Carriers hydrogenation. Int. J. Hydrog. Energy 2021, 46, 27026–27036. [Google Scholar] [CrossRef]
- Jorschick, H.; Bulgarin, A.; Alletsee, L.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Charging a Liquid Organic Hydrogen Carrier with Wet Hydrogen from Electrolysis. ACS Sustain. Chem. Eng. 2019, 7, 4186–4194. [Google Scholar] [CrossRef]
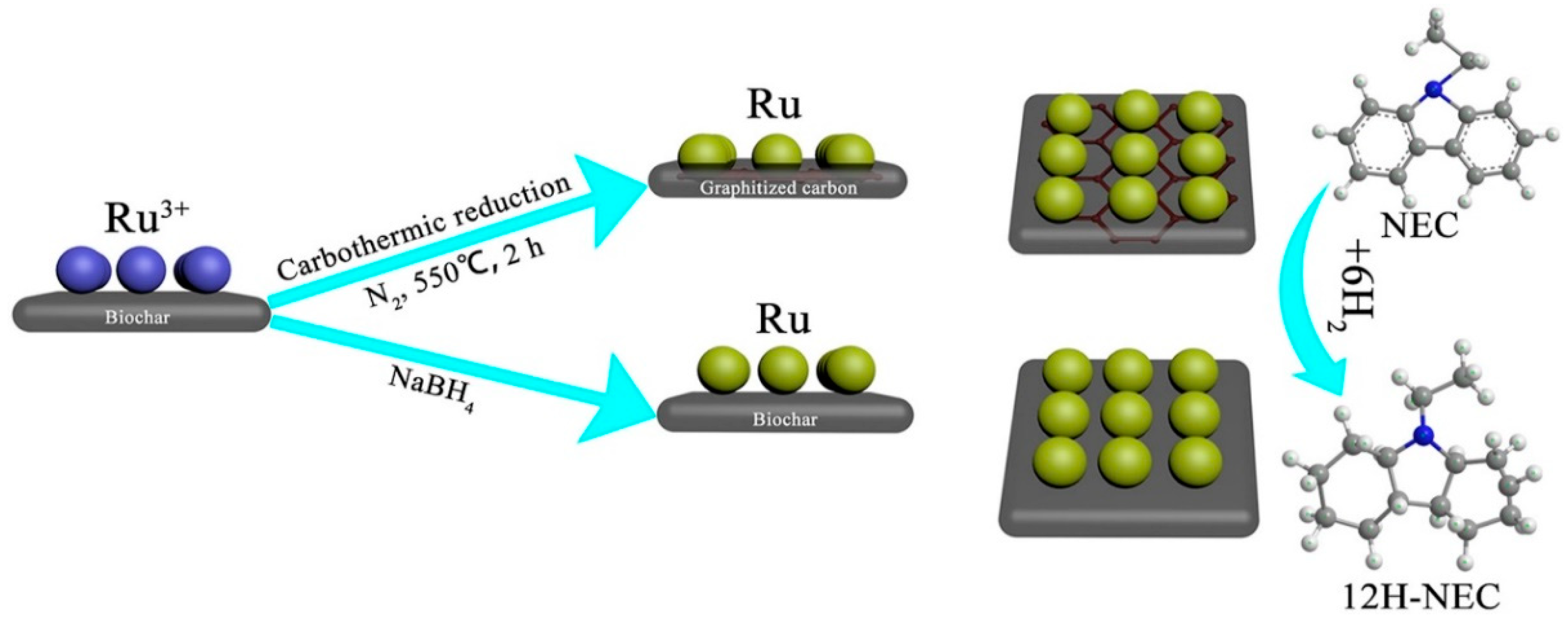
- Qin, Y.; Shi, J.; Bai, X. Preparing ultra-stable Ru nanocatalysts supported on partially graphitized biochar via carbothermal reduction for hydrogen storage of N-ethylcarbazole. Int. J. Hydrog. Energy 2021, 46, 25543–25554. [Google Scholar] [CrossRef]
- Sun, F.; An, Y.; Lei, L.; Wu, F.; Zhu, J.; Zhang, X. Identification of the starting reaction position in the hydrogenation of (N-ethyl)carbazole over Raney-Ni. J. Energy Chem. 2015, 24, 219–224. [Google Scholar] [CrossRef]
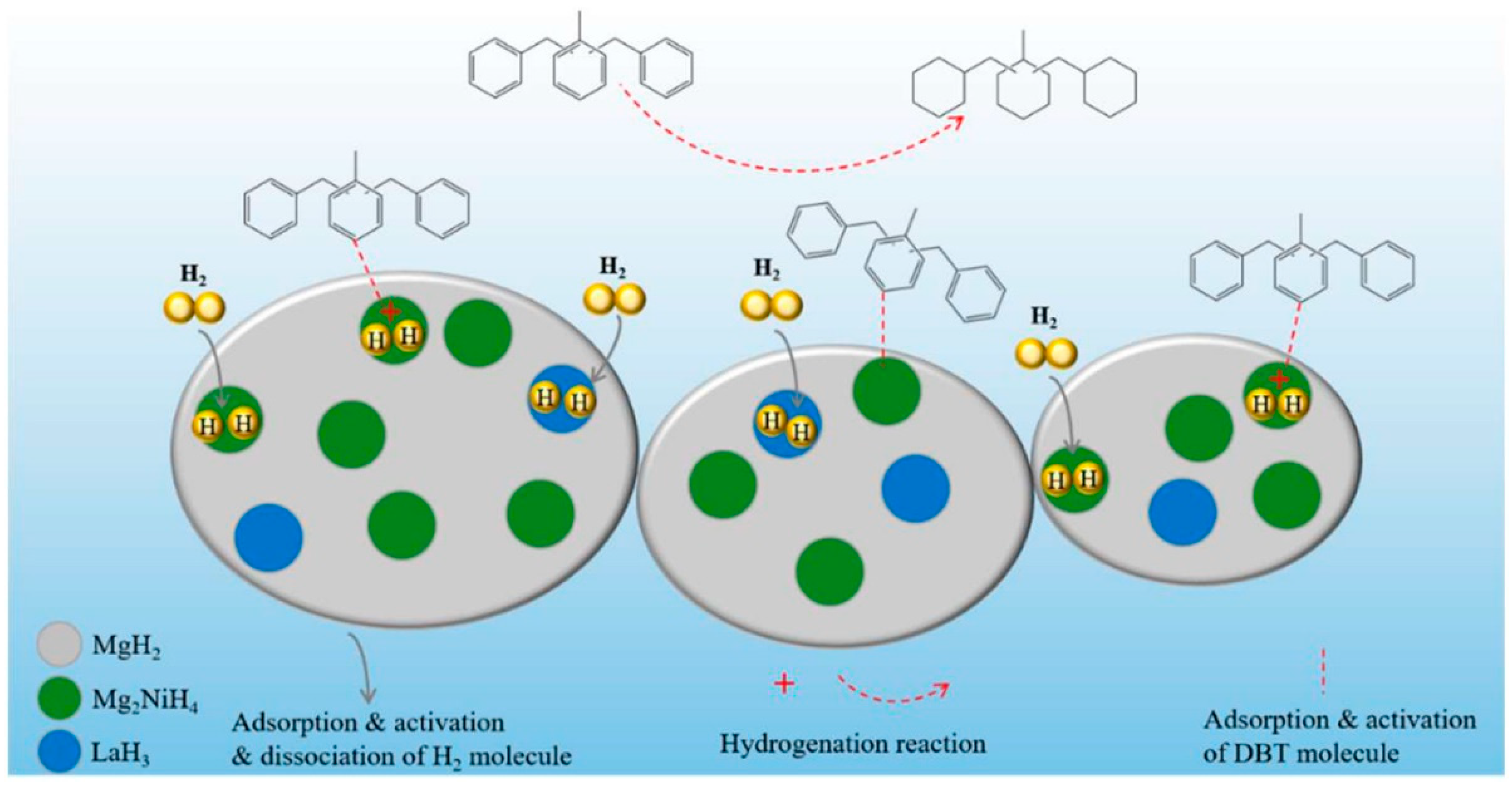
- Wu, Y.; Yu, H.; Guo, Y.; Jiang, X.; Qi, Y.; Sun, B.; Li, H.; Zheng, J.; Li, X. A rare earth hydride supported ruthenium catalyst for the hydrogenation of N-heterocycles: Boosting the activity via a new hydrogen transfer path and controlling the stereoselectivity. Chem. Sci. 2019, 10, 10459–10465. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Rentsch, D.; Friedrichs, O.; Remhof, A.; Zuettel, A.; Ramirez-Cuesta, A.J.; Tsang, S.C. Hydrogenation of 9-ethylcarbazole as a prototype of a liquid hydrogen carrier. Int. J. Hydrog. Energy 2010, 35, 11609–11621. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.-Y.; Lu, S.-H.; Li, P.-Y.; Fang, T. Trace ultrafine Ru nanoparticles on Ni/Al hydrotalcite-derived oxides supports as extremely active catalysts for N-ethylcarbazole hydrogenation. Fuel 2023, 339, 127338. [Google Scholar] [CrossRef]
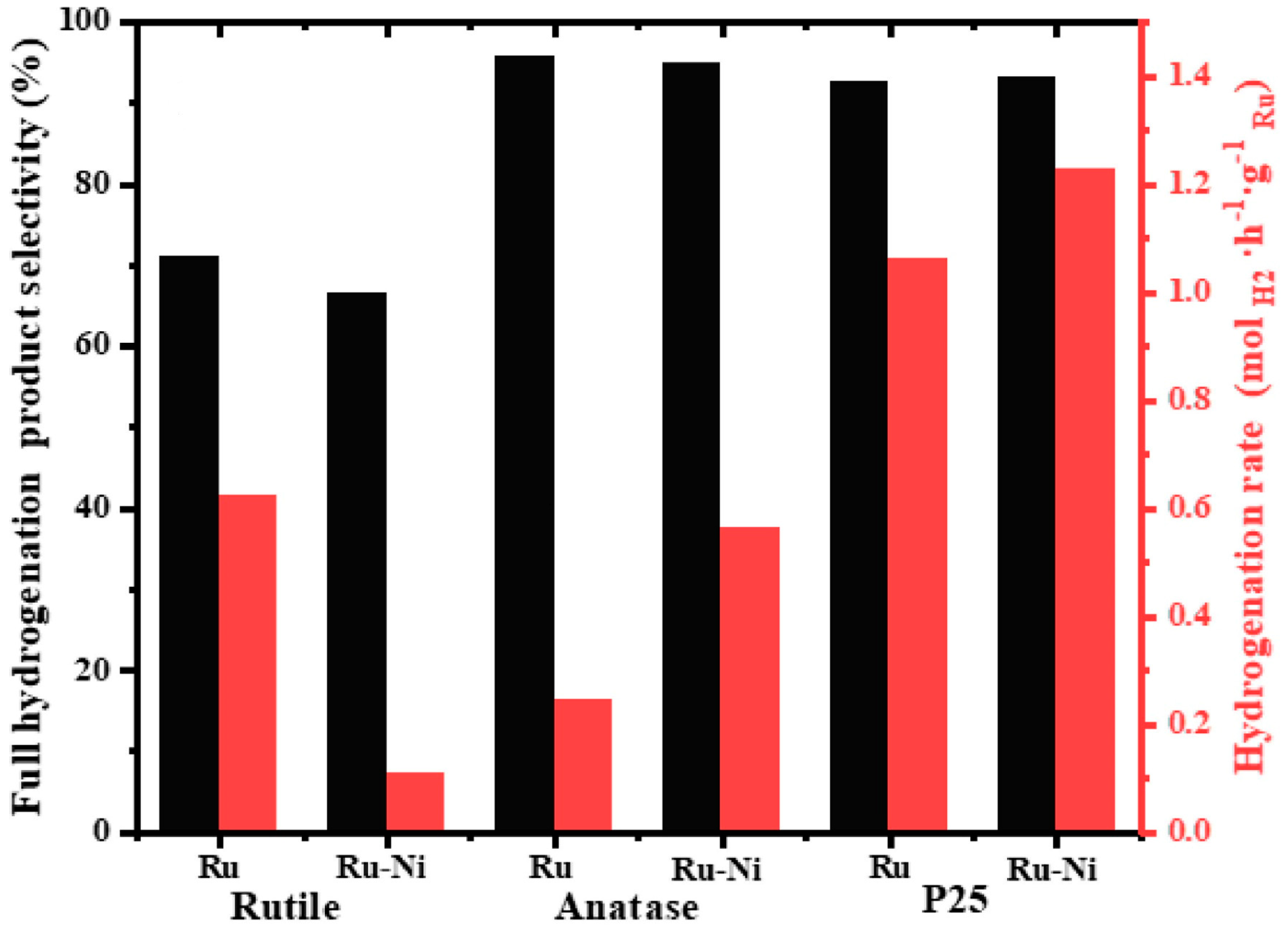
- Yu, H.; Yang, X.; Wu, Y.; Guo, Y.; Li, S.; Lin, W.; Li, X.; Zheng, J. Bimetallic Ru-Ni/TiO2 catalysts for hydrogenation of N-ethylcarbazole: Role of TiO2 crystal structure. J. Energy Chem. 2020, 40, 188–195. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Tam, K.; Yu, K.M.K.; Tsang, S.C.E. Comparative Study of Catalytic Hydrogenation of 9-Ethylcarbazole for Hydrogen Storage over Noble Metal Surfaces. J. Phys. Chem. C 2012, 116, 7421–7429. [Google Scholar] [CrossRef]
- Truszkiewicz, E.; Kowalczyk, K.; Dębska, A.; Wojda, D.; Iwanek, E.; Kępiński, L.; Mierzwa, B. Methanation of CO on Ru/graphitized-carbon catalysts: Effects of the preparation method and the carbon support structure. Int. J. Hydrog. Energy 2020, 45, 31985–31999. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni–Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Hoffer, B.; Dickvanlangeveld, A.; Janssens, J.; Bonne, R.; Lok, C.; Moulijn, J. Stability of highly dispersed Ni/AlO catalysts: Effects of pretreatment. J. Catal. 2000, 192, 432–440. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, L.; Du, K.; Fu, H.; Li, Y.; You, G.; Chen, B.H. An efficient and stable Ru–Ni/C nano-bimetallic catalyst with a comparatively low Ru loading for benzenehydrogenation under mild reaction conditions. RSC Adv. 2013, 3, 713–719. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Wei, Y.; Liu, J.; Zhao, Z. Research advances of rare earth catalysts for catalytic purification of vehicle exhausts—Commemorating the 100th anniversary of the birth of Academician Guangxian Xu. J. Rare Earths 2021, 39, 1151–1180. [Google Scholar] [CrossRef]
- Xu, L.; Wen, X.; Xu, C.; Bian, Y.; Chen, M.; Cheng, G.; Wu, C.-E.; Qiu, J.; Chen, B.; Hu, X. Rare earths modified highly dispersed fibrous Ni/KCC-1 nanosphere catalysts with superb low-temperature CO2 methanation performances. Appl. Surf. Sci. 2023, 608, 155258. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, H.; Fu, H.; Zheng, J.; Zhang, N.; Li, Y.; Chen, B.H. Effect of ruthenium nickel bimetallic composition on the catalytic performance for benzene hydrogenation to cyclohexane. Appl. Catal. A Gen. 2015, 499, 124–132. [Google Scholar] [CrossRef]
- Loiha, S.; Föttinger, K.; Zorn, K.; Klysubun, W.; Rupprechter, G.; Wittayakun, J. Catalytic enhancement of platinum supported on zeolite beta for toluene hydrogenation by addition of palladium. J. Ind. Eng. Chem. 2009, 15, 819–823. [Google Scholar] [CrossRef]
- Pan, H.-B.; Wai, C.M. Facile sonochemical synthesis of carbon nanotube-supported bimetallic Pt–Rh nanoparticles for room temperature hydrogenation of arenes. New J. Chem. 2011, 35, 1649–1660. [Google Scholar] [CrossRef]
- Zhu, L.; Shan, S.; Petkov, V.; Hu, W.; Kroner, A.; Zheng, J.; Yu, C.; Zhang, N.; Li, Y.; Luque, R.; et al. Ruthenium–nickel–nickel hydroxide nanoparticles for room temperature catalytic hydrogenation. J. Mater. Chem. A 2017, 5, 7869–7875. [Google Scholar] [CrossRef]
- Deng, X.; Zhu, L.; Zhang, H.; Kroner, A.; Zheng, J.; Zhang, N.; He, J.; Chen, B.H. Ruthenium stabilized on transition metal-on-transition metal oxide nanoparticles for naphthalene hydrogenation. Int. J. Hydrog. Energy 2018, 43, 15055–15063. [Google Scholar] [CrossRef]
- Qin, Y.; Bai, X. Hydrogenation of N-ethylcarbazole over Ni-Ru alloy nanoparticles loaded on graphitized carbon prepared by carbothermal reduction. Fuel 2022, 307, 121921. [Google Scholar] [CrossRef]
- Qin, Y.; Bai, X. N-doped graphitized carbon supported Co@Ru core–shell bimetallic catalyst for hydrogen storage of N-ethylcarbazole. Catal. Sci. Technol. 2022, 12, 2829–2836. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, X. Efficient catalytic hydrogen storage of N-ethylcarbazole over RuNi alloy nanoparticles loaded on SBA-15 prepared by electrostatic adsorption. Fuel 2023, 331, 125709. [Google Scholar] [CrossRef]
- Li, C.; Yang, M.; Liu, Z.; Zhang, Z.; Zhu, T.; Chen, X.; Dong, Y.; Cheng, H. Ru–Ni/Al2O3 bimetallic catalysts with high catalytic activity for N-propylcarbazole hydrogenation. Catal. Sci. Technol. 2020, 10, 2268–2276. [Google Scholar] [CrossRef]
- Feng, D.; Zhou, D.; Zhao, Z.; Zhai, T.; Yuan, Z.; Sun, H.; Ren, H.; Zhang, Y. Progress of graphene and loaded transition metals on Mg-based hydrogen storage alloys. Int. J. Hydrog. Energy 2021, 46, 33468–33485. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides. Energy Environ. Sci 2021, 14, 2302–2313. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L.; Li, Z.; Wang, S.; Ye, J.; Wu, Y.; Yuan, B. Boosting the hydrogenation activity of dibenzyltoluene catalyzed by Mg-based metal hydrides. Int. J. Hydrog. Energy 2022, 47, 23994–24003. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, H.; Zhong, L.; Zheng, J.; Yu, C.; Zhang, N.; Chen, B.H. RuNiCo-based nanocatalysts with different nanostructures for naphthalene selective hydrogenation. Fuel 2018, 216, 208–217. [Google Scholar] [CrossRef]
- Xia, Z.; Lu, H.; Liu, H.; Zhang, Z.; Chen, Y. Cyclohexane dehydrogenation over Ni-Cu/SiO2 catalyst: Effect of copper addition. Catal. Commun. 2017, 90, 39–42. [Google Scholar] [CrossRef]
- Jiang, N.; Rao, K.S.R.; Jin, M.-J.; Park, S.-E. Effect of hydrogen spillover in decalin dehydrogenation over supported Pt catalysts. Appl. Catal. A Gen. 2012, 425–426, 62–67. [Google Scholar] [CrossRef]
- Shinohara, C.; Kawakami, S.; Moriga, T.; Hayashi, H.; Hodoshima, S.; Saito, Y.; Sugiyama, S. Local structure around platinum in Pt/C catalysts employed for liquid-phase dehydrogenation of decalin in the liquid-film state under reactive distillation conditions. Appl. Catal. A Gen. 2004, 266, 251–255. [Google Scholar] [CrossRef]
- Wang, B.; Froment, G.; Goodman, D. CO-free hydrogen production via dehydrogenation of a Jet A hydrocarbon mixture. J. Catal. 2008, 253, 239–243. [Google Scholar] [CrossRef]
- Shukla, A.; Pande, J.V.; Biniwale, R.B. Dehydrogenation of methylcyclohexane over Pt/V2O5 and Pt/Y2O3 for hydrogen delivery applications. Int. J. Hydrog. Energy 2012, 37, 3350–3357. [Google Scholar] [CrossRef]
- Sugiura, Y.; Nagatsuka, T.; Kubo, K.; Hirano, Y.; Nakamura, A.; Miyazawa, K.; Iizuka, Y.; Furuta, S.; Iki, H.; Higo, T.; et al. Dehydrogenation of Methylcyclohexane over Pt/TiO2-Al2O3 Catalysts. Chem. Lett. 2017, 46, 1601–1604. [Google Scholar] [CrossRef]
- Lee, G.; Jeong, Y.; Kim, B.-G.; Han, J.S.; Jeong, H.; Na, H.B.; Jung, J.C. Hydrogen production by catalytic decalin dehydrogenation over carbon-supported platinum catalyst: Effect of catalyst preparation method. Catal. Commun. 2015, 67, 40–44. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, W.; Wang, S.; Wang, G.; Jiang, L.; Li, H.; Sun, G.; Xin, Q. Preparation of highly active 40 wt% Pt/C cathode electrocatalysts for DMFC via different routes. Catal. Today 2004, 93–95, 523–528. [Google Scholar] [CrossRef]
- Sinfelt, J.H. The turnover frequency of methylcyclohexane dehydrogenation to toluene on a Pt reforming catalyst. J. Mol. Catal. A Chem. 2000, 163, 123–128. [Google Scholar] [CrossRef]
- Hodoshima, S. Catalytic decalin dehydrogenation/naphthalene hydrogenation pair as a hydrogen source for fuel-cell vehicle. Int. J. Hydrog. Energy 2003, 28, 1255–1262. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Kariya, N.; Ichikawa, M. Dehydrogenation of Cyclohexane Over Ni Based Catalysts Supported on Activated Carbon using Spray-pulsed Reactor and Enhancement in Activity by Addition of a Small Amount of Pt. Catal. Lett. 2005, 105, 83–87. [Google Scholar] [CrossRef]
- Shukla, A.A.; Gosavi, P.V.; Pande, J.V.; Kumar, V.P.; Chary, K.V.R.; Biniwale, R.B. Efficient hydrogen supply through catalytic dehydrogenation of methylcyclohexane over Pt/metal oxide catalysts. Int. J. Hydrog. Energy 2010, 35, 4020–4026. [Google Scholar] [CrossRef]
- Boufaden, N.; Akkari, R.; Pawelec, B.; Fierro, J.L.G.; Said Zina, M.; Ghorbel, A. Dehydrogenation of methylcyclohexane to toluene over partially reduced Mo–SiO2 catalysts. Appl. Catal. A Gen. 2015, 502, 329–339. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V. Cyclohexane photocatalytic oxidative dehydrogenation to benzene on sulphated titania supported MoOx. In Studies in Surface Science and Catalysis; Gamba, A., Colella, C., Coluccia, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 155, pp. 179–187. [Google Scholar]
- Qi, S.; Yue, J.; Li, Y.; Huang, J.; Yi, C.; Yang, B. Replacing Platinum with Tungsten Carbide for Decalin Dehydrogenation. Catal. Lett. 2014, 144, 1443–1449. [Google Scholar] [CrossRef]
- Yolcular, S. Organic chemical hydride dehydrogenation over nickel catalysts supported with SiO2 for hydrogen recovery. Energy Sources Part A 2016, 38, 2031–2034. [Google Scholar] [CrossRef]
- Yolcular, S.; Olgun, Ö. Ni/Al2O3 catalysts and their activity in dehydrogenation of methylcyclohexane for hydrogen production. Catal. Today 2008, 138, 198–202. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, G.; An, Y.; Chen, C.; Wang, Q. Dehydrogenation of methyl-cyclohexane under multiphase reaction conditions. Int. J. Hydrog. Energy 2006, 31, 2250–2255. [Google Scholar] [CrossRef]
- Al-ShaikhAli, A.H.; Jedidi, A.; Cavallo, L.; Takanabe, K. Non-precious bimetallic catalysts for selective dehydrogenation of an organic chemical hydride system. Chem. Commun. 2015, 51, 12931–12934. [Google Scholar] [CrossRef]
- Gobara, H.M.; Mohamed, R.S.; Aboutaleb, W.A. A facile synthesis of 3D pyramidal hierarchical CeO2–Al2O3 nanocomposites supported nickel catalyst for cyclohexane dehydrogenation. Microporous Mesoporous Mater. 2021, 323, 111151. [Google Scholar] [CrossRef]
- He, T.; Pei, Q.; Chen, P. Liquid organic hydrogen carriers. J. Energy Chem. 2015, 24, 587–594. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sust. Energ. Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.C.; Biga, R.; Kolisnichenko, A.; Marocco, P.; Monteverde, A.H.A.; Santarelli, M. Potential and technical challenges of on-board hydrogen storage technologies coupled with fuel cell systems for aircraft electrification. J. Power Sources 2023, 555, 232397. [Google Scholar] [CrossRef]
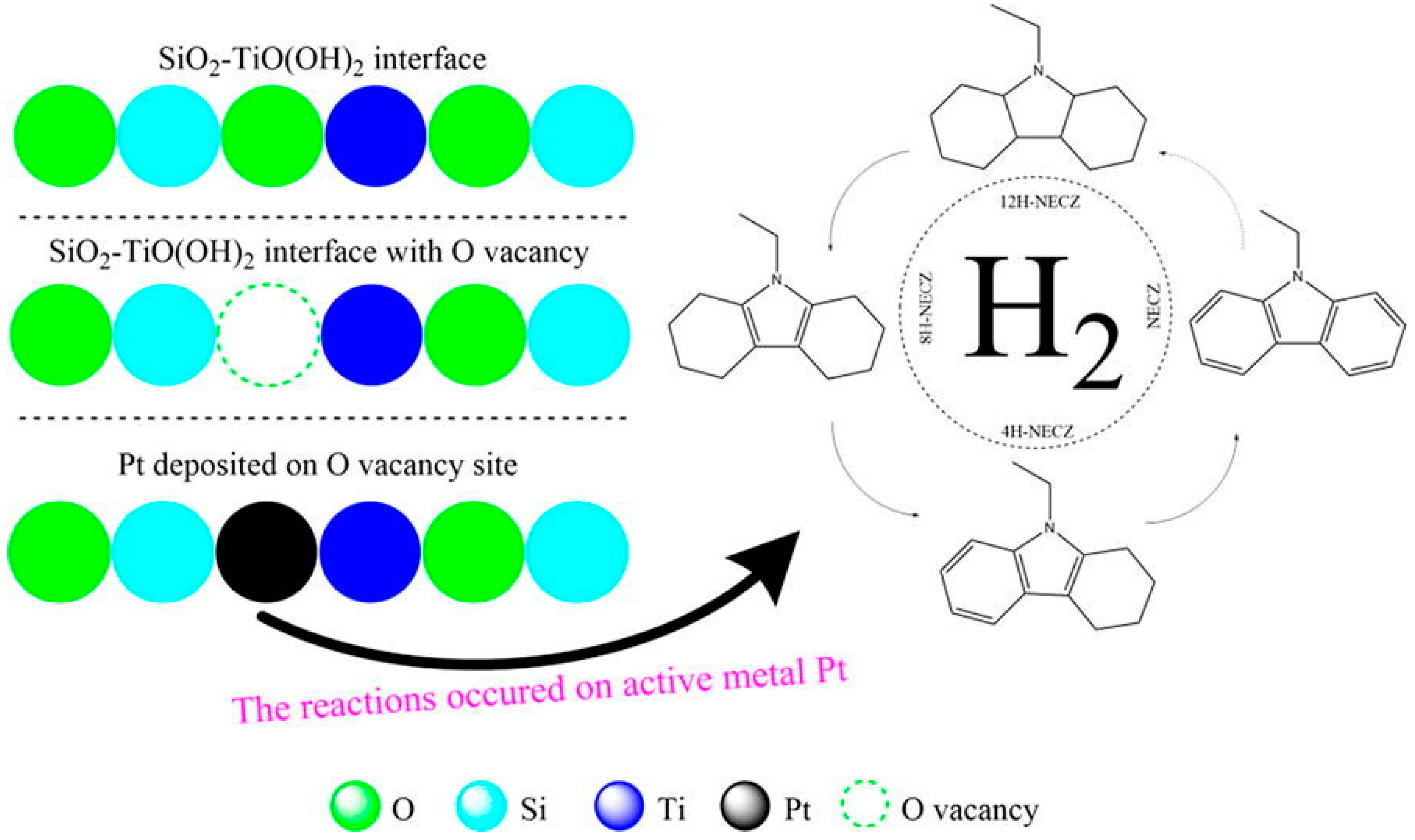
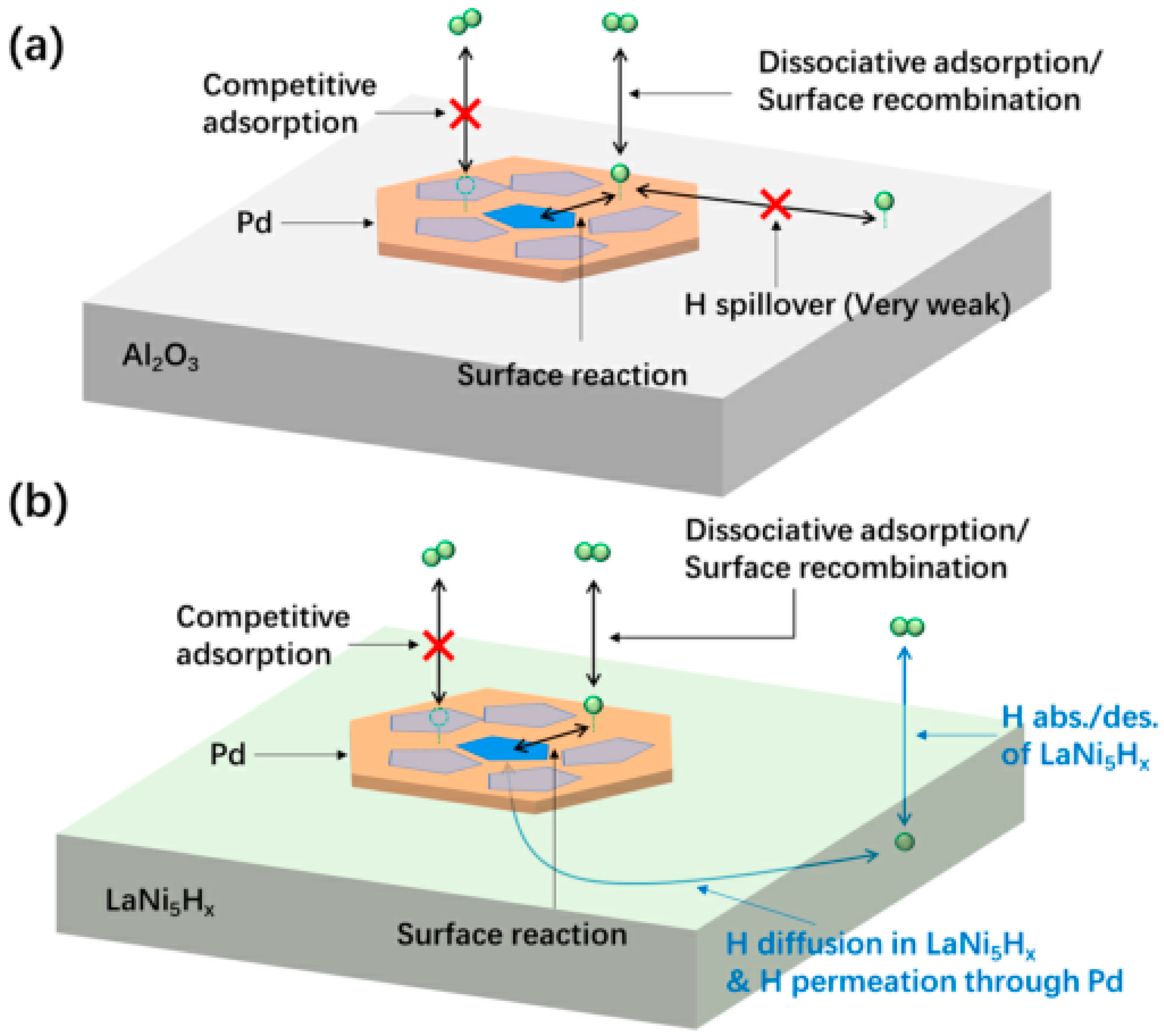
- Yang, Z.; Gong, X.; Li, L.; Jiang, Z.; Zhang, R.; Fang, T. Strengthening the metal-support interaction over Pt/SiO2-TiO(OH)2 by defect engineering for efficient dehydrogenation of dodecahydro-N-ethylcarbazole. Fuel 2023, 334, 126733. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Bai, X.; Wu, W. Ultrasonic-assisted preparation of ultrafine Pd nanocatalysts loaded on Cl(-)-intercalated MgAl layered double hydroxides for the catalytic dehydrogenation of dodecahydro-N-ethylcarbazole. Ultrason Sonochem 2022, 88, 106097. [Google Scholar] [CrossRef]
- Feng, Z.; Bai, X. 3D-mesoporous KIT-6 supported highly dispersed Pd nanocatalyst for dodecahydro-N-ethylcarbazole dehydrogenation. Microporous Mesoporous Mater. 2022, 335, 111789. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.; Ke, H.; Cheng, H. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts. Int. J. Hydrog. Energy 2014, 39, 18976–18983. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Smith, K.J. Structure sensitivity of dodecahydro-N-ethylcarbazole dehydrogenation over Pd catalysts. J. Catal. 2011, 279, 36–47. [Google Scholar] [CrossRef]
- Wang, B.; Yan, T.; Chang, T.; Wei, J.; Zhou, Q.; Yang, S.; Fang, T. Palladium supported on reduced graphene oxide as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole. Carbon 2017, 122, 9–18. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, Z.; Fang, T. Enhancing selectivity and reducing cost for dehydrogenation of dodecahydro-N-ethylcarbazole by supporting platinum on titanium dioxide. Int. J. Hydrog. Energy 2020, 45, 6838–6847. [Google Scholar] [CrossRef]
- Wang, B.; Chang, T.-Y.; Jiang, Z.; Wei, J.-J.; Zhang, Y.-H.; Yang, S.; Fang, T. Catalytic dehydrogenation study of dodecahydro-N-ethylcarbazole by noble metal supported on reduced graphene oxide. Int. J. Hydrog. Energy 2018, 43, 7317–7325. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, M.; Hua, J.; Yang, M.; Dong, Y.; Cheng, H. Remarkable activity of Pd catalyst supported on alumina synthesized via a hydrothermal route for hydrogen release of perhydro-N-propylcarbazole. Int. J. Hydrog. Energy 2021, 46, 9718–9729. [Google Scholar] [CrossRef]
- Jiang, Z.; Gong, X.; Wang, B.; Wu, Z.; Fang, T. A experimental study on the dehydrogenation performance of dodecahydro-N-ethylcarbazole on M/TiO2 catalysts. Int. J. Hydrog. Energy 2019, 44, 2951–2959. [Google Scholar] [CrossRef]
- Wang, B.; Chen, Y.-T.; Chang, T.-Y.; Jiang, Z.; Huang, Z.-Q.; Wang, S.-Y.; Chang, C.-R.; Chen, Y.-S.; Wei, J.-J.; Yang, S.; et al. Facet-dependent catalytic activities of Pd/rGO: Exploring dehydrogenation mechanism of dodecahydro-N-ethylcarbazole. Appl. Catal. B 2020, 266, 118658. [Google Scholar] [CrossRef]
- Ouma, C.N.M.; Modisha, P.M.; Bessarabov, D. Catalytic dehydrogenation of the liquid organic hydrogen carrier octahydroindole on Pt (111) surface: Ab initio insights from density functional theory calculations. Appl. Surf. Sci. 2019, 471, 1034–1040. [Google Scholar] [CrossRef]
- Ouma, C.N.M.; Obodo, K.O.; Modisha, P.M.; Rhyman, L.; Ramasami, P.; Bessarabov, D. Effect of chalcogen (S, Se and Te) surface additives on the dehydrogenation of a liquid organic hydrogen carrier system, octahydroindole–indole, on a Pt (1 1 1) surface. Appl. Surf. Sci. 2021, 566, 150636. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. The effect of the N atom on the dehydrogenation of heterocycles used for hydrogen storage. Appl. Catal. A Gen. 2012, 419–420, 67–72. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. Dehydrogenation kinetics and catalysis of organic heteroaromatics for hydrogen storage. Int. J. Hydrog. Energy 2012, 37, 2715–2722. [Google Scholar] [CrossRef]
- Saputera, W.H.; Tan, T.H.; Lovell, E.C.; Rawal, A.; Aguey-Zinsou, K.-F.; Friedmann, D.; Amal, R.; Scott, J.A. Modulating catalytic oxygen activation over Pt–TiO2/SiO2 catalysts by defect engineering of a TiO2/SiO2 support. Catal. Sci. Technol. 2022, 12, 1049–1059. [Google Scholar] [CrossRef]
- Punde, S.S.; Tatarchuk, B.J. Pt-CeO2/SiO2 catalyst for CO oxidation in humid air at ambient temperature. Chin. J. Catal. 2017, 38, 475–488. [Google Scholar] [CrossRef]
- Patil, S.P.; Pande, J.V.; Biniwale, R.B. Non-noble Ni–Cu/ACC bimetallic catalyst for dehydrogenation of liquid organic hydrides for hydrogen storage. Int. J. Hydrog. Energy 2013, 38, 15233–15241. [Google Scholar] [CrossRef]
- Pande, J.V.; Shukla, A.; Biniwale, R.B. Catalytic dehydrogenation of cyclohexane over Ag-M/ACC catalysts for hydrogen supply. Int. J. Hydrog. Energy 2012, 37, 6756–6763. [Google Scholar] [CrossRef]
- Miao, L.; Yan, J.; Wang, W.; Huang, Y.; Li, W.; Yang, Y. Dehydrogenation of methylcyclohexane over Pt supported on Mg–Al mixed oxides catalyst: The effect of promoter Ir. Chin. J. Chem. Eng. 2020, 28, 2337–2342. [Google Scholar] [CrossRef]
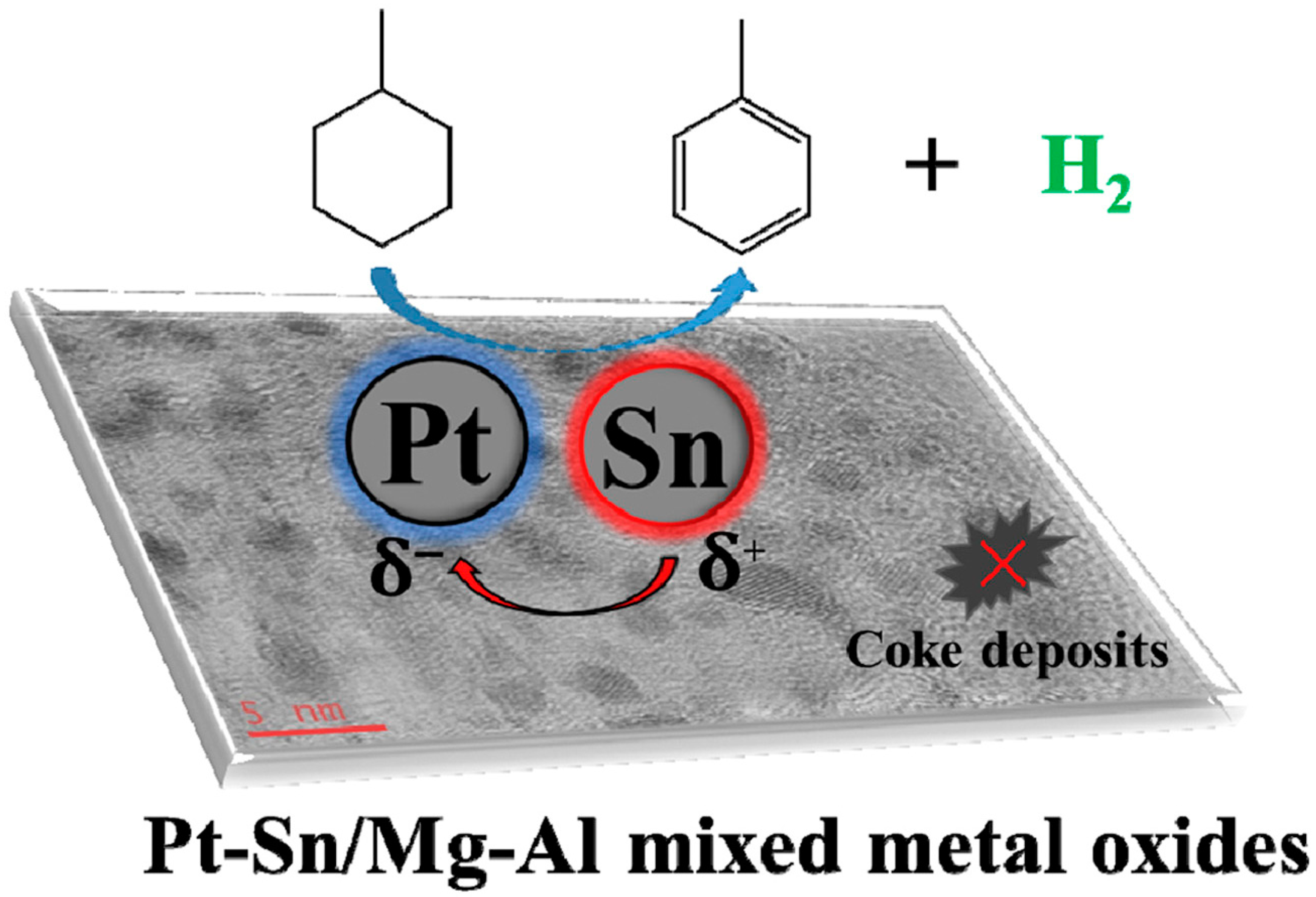
- Yan, J.; Wang, W.; Miao, L.; Wu, K.; Chen, G.; Huang, Y.; Yang, Y. Dehydrogenation of methylcyclohexane over Pt Sn supported on Mg Al mixed metal oxides derived from layered double hydroxides. Int. J. Hydrog. Energy 2018, 43, 9343–9352. [Google Scholar] [CrossRef]
- Zhang, X.; He, N.; Lin, L.; Zhu, Q.; Wang, G.; Guo, H. Study of the carbon cycle of a hydrogen supply system over a supported Pt catalyst: Methylcyclohexane–toluene–hydrogen cycle. Catal. Sci. Technol. 2020, 10, 1171–1181. [Google Scholar] [CrossRef]
- Tang, C.; Feng, Z.; Bai, X. Magnetic N-doped partially graphitized carbon-loaded Pd-Co alloy nanoparticles for efficient hydrogen production. Colloids Surf. 2022, 648, 129348. [Google Scholar] [CrossRef]
- Jiang, Z.; Gong, X.; Guo, S.; Bai, Y.; Fang, T. Engineering PdCu and PdNi bimetallic catalysts with adjustable alloying degree for the dehydrogenation reaction of dodecahydro-N-ethylcarbazole. Int. J. Hydrog. Energy 2021, 46, 2376–2389. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, S.; Fang, T. Enhancing the Catalytic Activity and Selectivity of PdAu/SiO2 Bimetallic Catalysts for Dodecahydro-N-ethylcarbazole Dehydrogenation by Controlling the Particle Size and Dispersion. ACS Appl. Energy Mater. 2019, 2, 7233–7243. [Google Scholar] [CrossRef]
- Wang, B.; Chang, T.-Y.; Gong, X.; Jiang, Z.; Yang, S.; Chen, Y.-S.; Fang, T. One-Pot Synthesis of Au/Pd Core/Shell Nanoparticles Supported on Reduced Graphene Oxide with Enhanced Dehydrogenation Performance for Dodecahydro-N-ethylcarbazole. ACS Sustain. Chem. Eng. 2018, 7, 1760–1768. [Google Scholar] [CrossRef]
- Gong, X.; Guo, S.; Jiang, Z.; Yang, B.; Fang, T. Tuning the alloy degree for Pd-M/Al2O3 (M=Co/Ni/Cu) bimetallic catalysts to enhance the activity and selectivity of dodecahydro-N-ethylcarbazole dehydrogenation. Int. J. Hydrog. Energy 2021, 46, 33835–33848. [Google Scholar] [CrossRef]
- Feng, Z.; Bai, X. Enhanced activity of bimetallic Pd-Ni nanoparticles on KIT-6 for production of hydrogen from dodecahydro-N-ethylcarbazole. Fuel 2022, 329, 125473. [Google Scholar] [CrossRef]
- Wang, B.; Chang, T.-Y.; Jiang, Z.; Wei, J.-J.; Fang, T. Component controlled synthesis of bimetallic PdCu nanoparticles supported on reduced graphene oxide for dehydrogenation of dodecahydro-N-ethylcarbazole. Appl. Catal. B 2019, 251, 261–272. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Gao, M.; Dong, Y.; Yang, M.; Cheng, H. Wet-impregnated bimetallic Pd-Ni catalysts with enhanced activity for dehydrogenation of perhydro-N-propylcarbazole. Int. J. Hydrog. Energy 2020, 45, 32168–32178. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Q.; Fang, W.; Xu, H. Enhanced performance of Ca-doped Pt/γ-Al2O3 catalyst for cyclohexane dehydrogenation. Int. J. Hydrog. Energy 2011, 36, 11536–11544. [Google Scholar] [CrossRef]
- Dong, A.-H.; Wang, K.; Zhu, S.-Z.; Yang, G.-B.; Wang, X.-T. Facile preparation of PtSn-La/Al2O3 catalyst with large pore size and its improved catalytic performance for isobutane dehydrogenation. Fuel Process. Technol. 2017, 158, 218–225. [Google Scholar] [CrossRef]
- Resini, C.; Lucarelli, C.; Taillades-Jacquin, M.; Liew, K.-E.; Gabellini, I.; Albonetti, S.; Wails, D.; Rozière, J.; Vaccari, A.; Jones, D. Pt–Sn/γ-Al2O3 and Pt–Sn–Na/γ-Al2O3 catalysts for hydrogen production by dehydrogenation of Jet A-1 fuel: Characterisation and preliminary activity tests. Int. J. Hydrog. Energy 2011, 36, 5972–5982. [Google Scholar] [CrossRef]
- Okada, Y.; Sasaki, E.; Watanabe, E.; Hyodo, S.; Nishijima, H. Development of dehydrogenation catalyst for hydrogen generation in organic chemical hydride method. Int. J. Hydrog. Energy 2006, 31, 1348–1356. [Google Scholar] [CrossRef]
- Deng, L.; Arakawa, T.; Ohkubo, T.; Miura, H.; Shishido, T.; Hosokawa, S.; Teramura, K.; Tanaka, T. Highly Active and Stable Pt–Sn/SBA-15 Catalyst Prepared by Direct Reduction for Ethylbenzene Dehydrogenation: Effects of Sn Addition. Ind. Eng. Chem. Res. 2017, 56, 7160–7172. [Google Scholar] [CrossRef]
- Boudart, M. Heterogeneous catalysis by metals. J. Mol. Catal. 1985, 30, 27–38. [Google Scholar] [CrossRef]
- Nakano, A.; Manabe, S.; Higo, T.; Seki, H.; Nagatake, S.; Yabe, T.; Ogo, S.; Nagatsuka, T.; Sugiura, Y.; Iki, H.; et al. Effects of Mn addition on dehydrogenation of methylcyclohexane over Pt/Al2O3 catalyst. Appl. Catal. A Gen. 2017, 543, 75–81. [Google Scholar] [CrossRef]
- Kustov, L.M.; Tarasov, A.L.; Kirichenko, O.A. Microwave-activated dehydrogenation of perhydro-N-ethylcarbazol over bimetallic Pd-M/TiO2 catalysts as the second stage of hydrogen storage in liquid substrates. Int. J. Hydrog. Energy 2017, 42, 26723–26729. [Google Scholar] [CrossRef]
- Jorschick, H.; Dürr, S.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Operational Stability of a LOHC-Based Hot Pressure Swing Reactor for Hydrogen Storage. Energy Technol. 2019, 7, 146–152. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Yu, H.; Sun, M.; Zheng, J.; Li, X.; Lin, W.; Wu, Y. A YH3 promoted palladium catalyst for reversible hydrogen storage of N-ethylcarbazole. Int. J. Hydrog. Energy 2020, 45, 33657–33662. [Google Scholar] [CrossRef]
- Yu, H.; Wu, Y.; Chen, S.; Xie, Z.; Wu, Y.; Cheng, N.; Yang, X.; Lin, W.; Xie, L.; Li, X.; et al. Pd-modified LaNi5 nanoparticles for efficient hydrogen storage in a carbazole type liquid organic hydrogen carrier. Appl. Catal. B 2022, 317, 121720. [Google Scholar] [CrossRef]
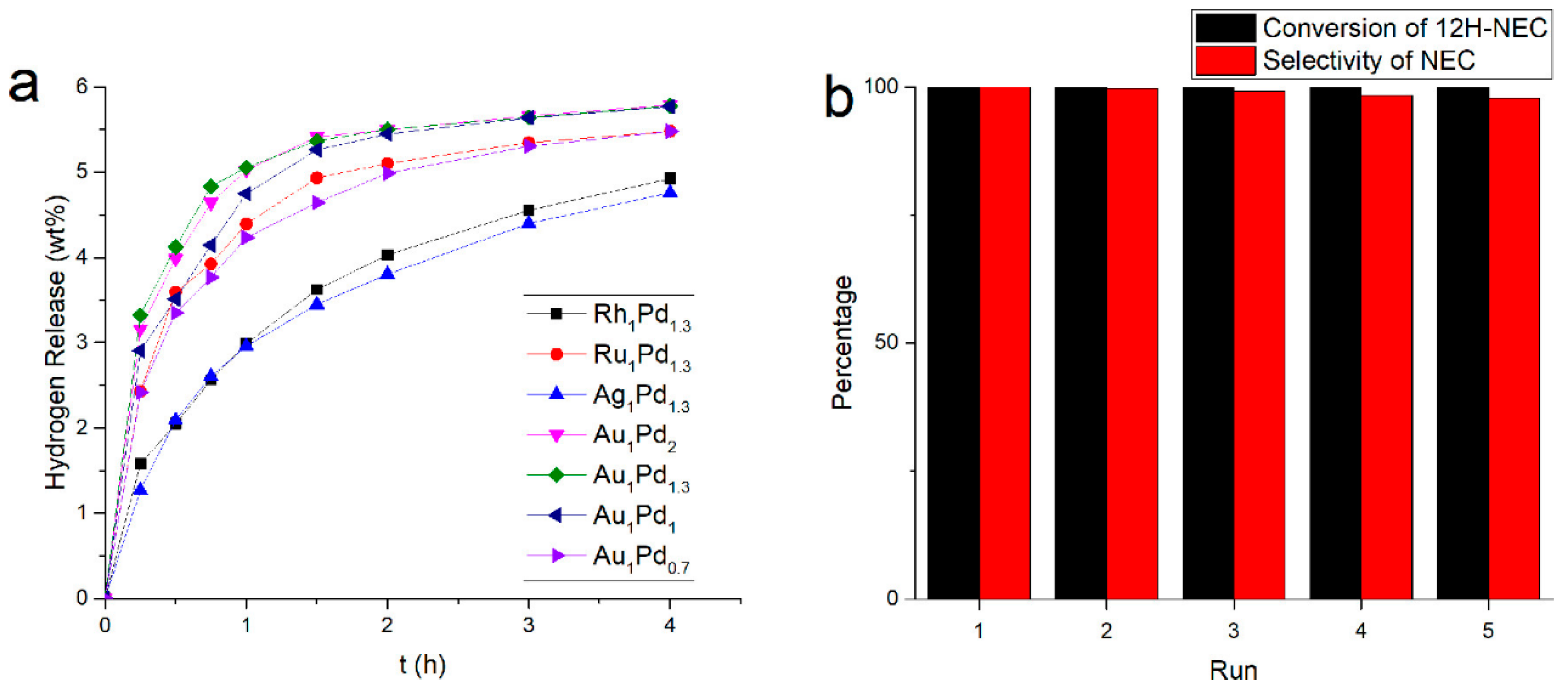
- Xue, W.; Liu, H.; Mao, B.; Liu, H.; Qiu, M.; Yang, C.; Chen, X.; Sun, Y. Reversible hydrogenation and dehydrogenation of N-ethylcarbazole over bimetallic Pd-Rh catalyst for hydrogen storage. Chem. Eng. J. 2021, 421, 127781. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Xu, Z.; Liu, L.; Zhu, T.; Chen, Z.; Dong, Y.; Yang, M. Ce-promoted highly active bifunctional Pd/Al2O3 catalyst for reversible catalytic hydrogenation and dehydrogenation of N-propylcarbazole. Int. J. Hydrog. Energy 2023, 48, 90–100. [Google Scholar] [CrossRef]
- Yu, H.; Yang, X.; Jiang, X.; Wu, Y.; Chen, S.; Lin, W.; Wu, Y.; Xie, L.; Li, X.; Zheng, J. LaNi5.5 particles for reversible hydrogen storage in N-ethylcarbazole. Nano Energy 2021, 80, 105476. [Google Scholar] [CrossRef]
- Forberg, D.; Schwob, T.; Zaheer, M.; Friedrich, M.; Miyajima, N.; Kempe, R. Single-catalyst high-weight% hydrogen storage in an N-heterocycle synthesized from lignin hydrogenolysis products and ammonia. Nat. Commun. 2016, 7, 13201. [Google Scholar] [CrossRef]
- Gong, Z.; Wenfei, W.; Zhao, Z.; Li, B. Combination of catalytic combustion and catalytic denitration on semi-coke with Fe2O3 and CeO2. Catal. Today 2018, 318, 59–65. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar] [CrossRef]
- Zhou, G.; Li, T.; Chen, J.; Deng, L.; Xie, H. Nano-Pd/CeO2 catalysts for hydrogen storage by reversible benzene hydrogenation/dehydrogenation reactions. Int. J. Hydrog. Energy 2021, 46, 14540–14555. [Google Scholar] [CrossRef]
- Chen, L.; Verma, P.; Hou, K.; Qi, Z.; Zhang, S.; Liu, Y.S.; Guo, J.; Stavila, V.; Allendorf, M.D.; Zheng, L.; et al. Reversible dehydrogenation and rehydrogenation of cyclohexane and methylcyclohexane by single-site platinum catalyst. Nat. Commun. 2022, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ge, C.; An, D.; Tong, Q.; Gao, F.; Dong, L. Review on characterization methods of oxygen vacancy in rare earth cerium-based catalysts. CIESC J. 2020, 71, 3403–3415. [Google Scholar]
- Yang, F.; Wang, J.; Zhang, Y.; Wu, Z.; Zhang, Z.; Zhao, F.; Huot, J.; Grobivć Novaković, J.; Novaković, N. Recent progress on the development of high entropy alloys (HEAs) for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2022, 47, 11236–11249. [Google Scholar] [CrossRef]



| LOHCs | Melting Point (°C) | Boiling Point (°C) | H2 Storage Capacity wt% | Reference |
|---|---|---|---|---|
| Benzene (Ben) | 5.5 | 80 | 7.2 | [20] |
| Toluene (TOL) | −95 | 111 | 6.2 | [20] |
| Naphthalene | 80 | 218 | 7.3 | [20] |
| Carbazole | 245 | 355 | 6.7 | [20] |
| N-ethylcarbazole (NEC) | 69 | 378 | 5.8 | [20] |
| N-propylcarbazole (NPC) | 48 | 336 | 5.43 | [55] |
| Dibenzyltoluene (DBT) | −39~−34 | 390 | 6.2 | [38] |
| 1-methylindole (1-MID) | −20 | 239 | 5.76 | [57] |
| 2-methylindole (2-MID) | 57 | 273 | 5.76 | [58] |
| 1,2-dimethylindole (1,2-DMID) | 55 | 260 | 5.23 | [10] |
| N-ethylindole (NEID) | −17.8 | 253.5 | 5.23 | [59] |
| 7-ethylindole (7-EID) | 14 | 230 | 5.23 | [61] |
| 2-(N-methylbenzyl)-pyridine (MBP) | −50.1~−40.2 | 291~293 | 6.15 | [62] |
| Acridine (ACD) | 111 | 253 | 7.25 | [47] |
| LOHCs | Catalysts | T (°C) | PH2 (MPa) | Time (h) | Conv a. (%) | Yield b (%) | TOF (h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| BEN | Ru/SBA-15 | 20 | 1 | - | 100 | 100 | 85.3 | [72] |
| BEN | Ru/MOF | 60 | 6 | 1.5 | 100 | 100 | 3200 | [92] |
| BEN | 4.2 wt% Ru/C-silica | 110 | 8 | 0.53 | 100 | 99.8 | 37,700 | [93] |
| BEN | Ru(0)-Zeolite-Y | 22 | 0.28 | 1 | 100 | 100 | 1040 | [94] |
| BEN | Ru/CNTs | 80 | 4 | 0.5 | 100 | 99.97 | 6983 | [95] |
| BEN | Pd/SiO2 (co-SEA) | 150 | 7 | 6 | 84.1 | 84.1 | - | [91] |
| TOL | Ni nanoflowers | 140 | 5 | 0.5 | 100 | 100 | - | [96] |
| TOL | Pd/SiO2 (co-SEA) | 150 | 7 | 6 | 85.4 | 85.4 | - | [91] |
| TOL | Pt (MP)/CeO2-A-400 | 100 | 0.5 | 3 | 90.8 | 90.8 | - | [77] |
| NAP | Pt/WO3-500 | 70 | 3 | 1 | 100 | 100 | - | [89] |
| NAP | Pd/HY-9.5 | 200 | 4 | 1 | 100 | 73.15 | - | [90] |
| TEN | 1 wt% Ni/Al2O3–YH3 | 150 | 10 | 5 | - | 95 | - | [97] |
| DBT | Ni70/AlSiO-1/1 | 150 | 7 | 1.5 | 100 | 100 | - | [98] |
| DBT | 0.3 wt% Pt/Al2O3 | 270 | 3 | 1.42 | - | 100 | -- | [76] |
| DBT | 5 wt% Pd/Al2O3 | 260 | 3 | 6 | - | 100 | - | [99] |
| NEC | Ru/pg-BC | 130 | 6 | 1.17 | 100 | 99.41 | - | [100] |
| NEC | Raney-Ni | 180 | 5 | 1.3 | - | 86.2 | [101] | |
| NEC | Ni70/AlSiO-1/1 | 150 | 7 | 1.5 | 100 | 100 | -- | [98] |
| NEC | 1.3 wt% Ru/YH3 | 130 | 7 | 2.5 | 100 | 100 | - | [102] |
| NEC | 5 wt% Ru/TiO2 | 130 | 7 | - | - | 95 | - | [103] |
| NEC | Ru black | 130 | 7 | - | - | 85 | - | [103] |
| NEC | 1.5 wt% Ru-Ni1Al2-LDO | 150 | 8 | 1 | 100 | 100 | - | [104] |
| NEC | 1wt%Ni/Al2O3–YH3 | 180 | 10 | 1.5 | 100 | 100 | - | [97] |
| NEC | 5 wt% Ru/LDH-3.9CNT | 120 | 6 | 0.4 | 100 | 98.31 | - | [82] |
| NEC | Ru/P25 | 150 | 7 | 24 | 100 | 92.4 | - | [105] |
| NEC | Ru/anatase | 150 | 7 | 24 | 100 | 95.7 | - | [105] |
| NEC | Ru/Ni-Fe LDH | 110 | 6 | 1.33 | - | 98.88 | - | [83] |
| NPC | 5 wt% Ru/Al2O3 | 150 | 7 | 0.5 | - | 100 | - | [55] |
| NPC | Ni70/AlSiO-1/1 | 150 | 7 | 1 | 100 | 100 | [98] |
| LOHCs | Catalysts | T (°C) | P (MPa) | Time (h) | Conv a. (%) | Yield b (%) | Ref. |
|---|---|---|---|---|---|---|---|
| BEN | Pd-Ni/SiO2 (co-SEA) | 150 | 7 | 6 | 99.9 | 99.9 | [91] |
| BEN | Pd-Pt/SiO2 (co-SEA) | 150 | 7 | 6 | 90.8 | 90.8 | [91] |
| BEN | 0.024 wt% Ru–1.00 wt% Ni/C | 60 | 700 psi | 2 | 100 | 100 | [110] |
| BEN | Ru0.56Ni0.44/C | 60 | 5.3 | 0.5 | - | 99.8 | [113] |
| BEN | 1 wt% Ru2Pt1 MIL-101 | 60 | 1 | 6 | 100 | 100 | [85] |
| TOL | Pd-Ni/SiO2 (co-SEA) | 150 | 7 | 6 | 99.9 | 99.9 | [91] |
| TOL | Pd-Pt/SiO2 (co-SEA) | 150 | 7 | 6 | 91.4 | 91.4 | [91] |
| TOL | 6 wt% Pt1Pd1/HBEA | 150 | 7–12 | 2 | 100 | 100 | [114] |
| TOL | Pt–Rh/MWNTs | 20 | 1 | 3 | 100 | 100 | [115] |
| NAP | Ru/Ni/Ni(OH)2/C | 100 | 4.48 | 1 | - | >99 | [116] |
| NAP | Ru/Ni/NiO/C | 100 | 4.5 | 1 | 100 | 100 | [117] |
| NAP | Ru/Co/Co3O4/C | 100 | 4.5 | 0.8 | 100 | 100 | [117] |
| NEC | 5.0 wt% Ni0.5Ru4.5/pg-BC | 130 | 6 | 1.17 | 100 | 99.06 | [118] |
| NEC | 5.0 wt% Co@Ru/NGC | 130 | 6 | 1 | 100 | 99.1 | [119] |
| NEC | Ru-Ni/P25 | 150 | 7 | 24 | 100 | 93 | [105] |
| NEC | Ru-Ni/anatase | 150 | 7 | 24 | 100 | 94.8 | [105] |
| NEC | Ru0.7Ni0.3/SBA15 | 100 | 5 | 1.33 | 100 | 99.82 | [120] |
| NPC | Ru2.5Ni2.5/Al2O3 | 150 | 4 | 0.5 | 100 | 100 | [121] |
| LOHCs | Catalysts | T (°C) | P (MPa) | Time (h) | Conv a. (%) | Yield b (%) | H2 Release (wt%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 12H-NEC | 5 wt% Pd/NGC | 180 | 0.1 | 10 | 100 | 98.72 | 5.76 | [75] |
| 12H-NEC | 2.5 wt% Pt/SiO2-TiO(OH)2 | 180 | 0.1 | 7 | 100 | 97.9 | 5.75 | [150] |
| 12H-NEC | 2.5 wt% Pd/LDHs-us | 180 | 0.1 | 6 | 100 | - | 5.72 | [151] |
| 12H-NEC | 1 wt% Pd-EU/KIT-6 | 190 | 0.1 | 6 | 100 | 100 | - | [152] |
| 12H-NEC | 5 wt% Pd/Al2O3 | 180 | 0.1 | 4 | 100 | 100 | - | [153] |
| 12H-NEC | 5 wt% Pt/Al2O3 | 180 | 0.1 | 5 | 100 | 100 | - | [153] |
| 12H-NEC | 4 wt% Pd/SiO2 | 170 | 1.6 | 100 | 100 | 5.8 | [154] | |
| 12H-NEC | 2.5 wt% Pd/rGO-EG | 170 | 0.1 | 12 | 100 | 84.61 | 5.49 | [155] |
| 12H-NEC | 5 wt% Pt/TiO2 | 180 | 0.1 | 6 | 100 | 79 | 5.38 | [156] |
| 12H-NEC | 2.32 wt% Pd/rGO | 180 | 0.1 | - | 100 | 97.65 | 5.74 | [157] |
| 12H-NPC | 1 wt% Pd/Al2O3-120 | 180 | 7 | 6 | 100 | 100 | 5.43 | [158] |
| 12H-NPC | 3 wt% Pd@MIL-101 | 190 | 0.1 | 4 | 100 | 100 | 5.43 | [74] |
| LOHCs | Catalysts | T (°C) | P (MPa) | Time (h) | Conv a. (%) | Yield b (%) | H2 Release (wt%) | H2 Evolution Rate mmol/gmet/min | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CYH | 10 wt% Ni0.8Cu0.2/ACC | 350 | - | 10 | 25.78 | - | - | 39.45 | [167] |
| CYH | 10 wt% Ag-1 wt% Pd/ACC | 300 | - | 7 | - | - | - | 7.5 | [168] |
| CYH | 10 wt% Ag-1 wt% Rh/ACC | 300 | - | 6 | - | - | - | 12.34 | [168] |
| CYH | 10 wt% Ag-1 wt% Pt/ACC | 300 | - | 6 | - | - | - | 13.36 | [168] |
| CYH | 5 wt% 1:4 Ag-Rh/Y2O3 | 300 | 0.1 | 4 | - | - | - | 400 | [80] |
| CYH | 5 wt%1:4 Ag-Rh/ACC | 300 | 0.1 | 5 | - | - | - | 178.7 | [80] |
| MCH | 2.5wt%Pt0.8Ir0.2/Mg-Al-O | 350 | - | 1.6 | 91.1 | 99.9 | - | 263.9 | [169] |
| MCH | 2.0 wt% Pt-0.5 wt% Sn/MgAleO-350 | 300 | - | 12 | 90.5 | - | - | 262.1 | [170] |
| MCH | Pt-Cu/S-1 | 400 | 0.1 | 6 | 92.26 | - | - | 445.3 | [171] |
| 12H-NEC | 5 wt% PdCo/NGC | 180 | 0.1 | 6 | 100 | 97.87 | 5.71 | - | [172] |
| 12H-NEC | Pd3 (3.75 wt%)-Ni1/SiO2 | 180 | 0.1 | 8 | 100 | 91.1 | 5.63 | - | [173] |
| 12H-NEC | Pd3 (3.75wt%)-Cu1/SiO2 | 180 | 0.1 | 8 | 100 | 83.11 | 5.47 | - | [173] |
| 12H-NEC | Pd3 (3.75wt%)-Au1/SiO2 | 180 | 0.1 | 8 | 100 | 94.9 | 5.7 | - | [174] |
| 12H-NEC | 0.65 mol%Pd1.3–0.52 mol% Au1/rGO | 180 | 0.1 | 4 | 100 | 100 | 5.79 | - | [175] |
| 12H-NEC | 0.58 mol%Pd1.3–0.42 mol% Ru1/rGO | 180 | 0.1 | 4 | 100 | 84.11 | 5.48 | - | [175] |
| 12H-NEC | Pd1 (2.5 wt%)-Co1/Al2O3 | 180 | 0.1 | 8 | 100 | 85.4 | 5.52 | - | [176] |
| 12H-NEC | Pd4Ni1/KIT-6 | 180 | 0.1 | 6 | - | - | 5.74 | - | [177] |
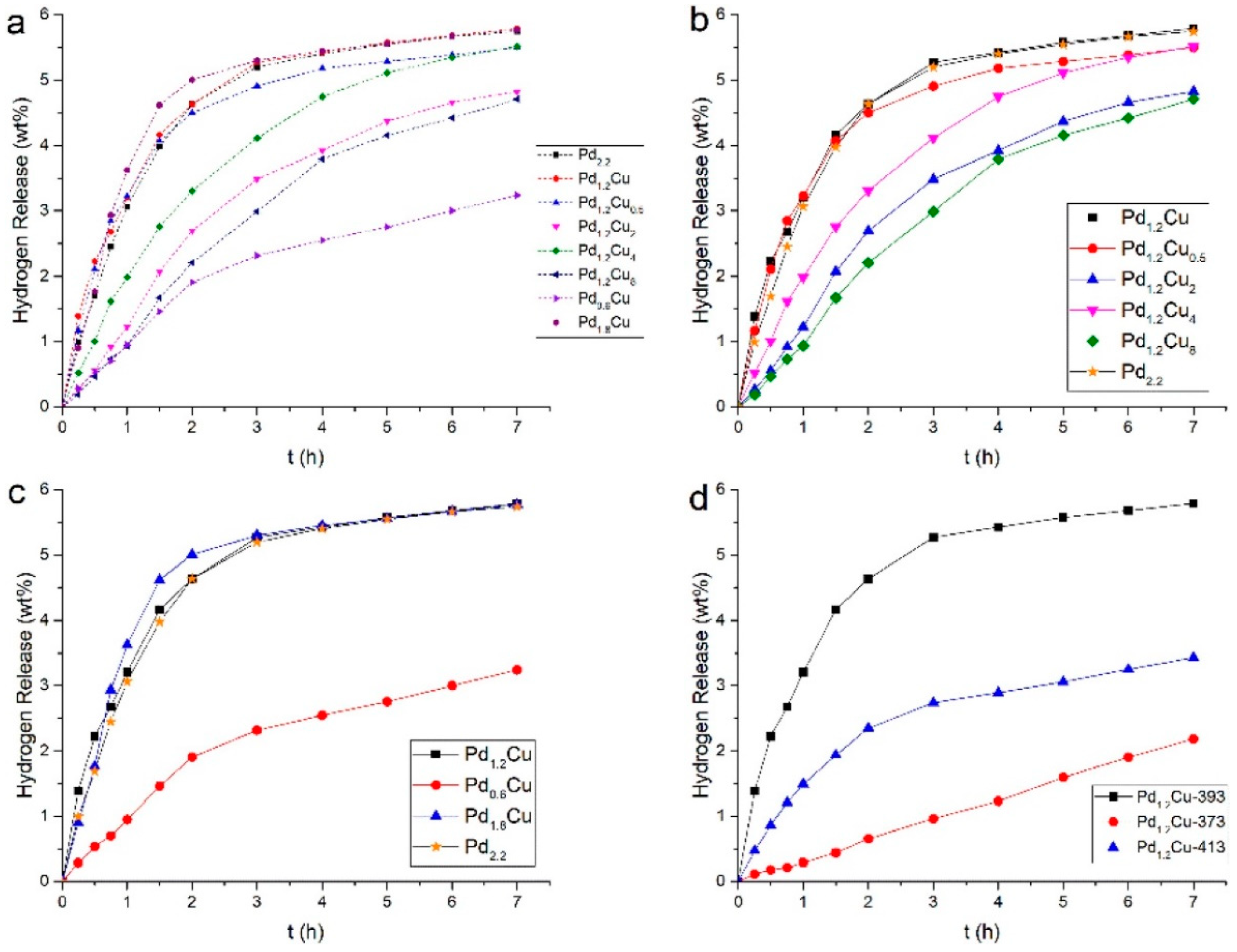
| 12H-NEC | Pd1.2Cu/rGO | 180 | 0.1 | 7 | 100 | 100 | 5.79 | - | [178] |
| 12H-NPC | 5 wt%Pd1-Ni1/Al2O3 | 180 | 6 | 7 | 100 | 100 | 5.43 | - | [179] |
| LOHCs | Catalysts | Hydrogenation Reaction | Dehydrogenation Reaction | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Reaction Conditions | Time (h) | H2 Uptake (wt%) | Reaction Conditions | Time (h) | H2 Release (wt%) | |||
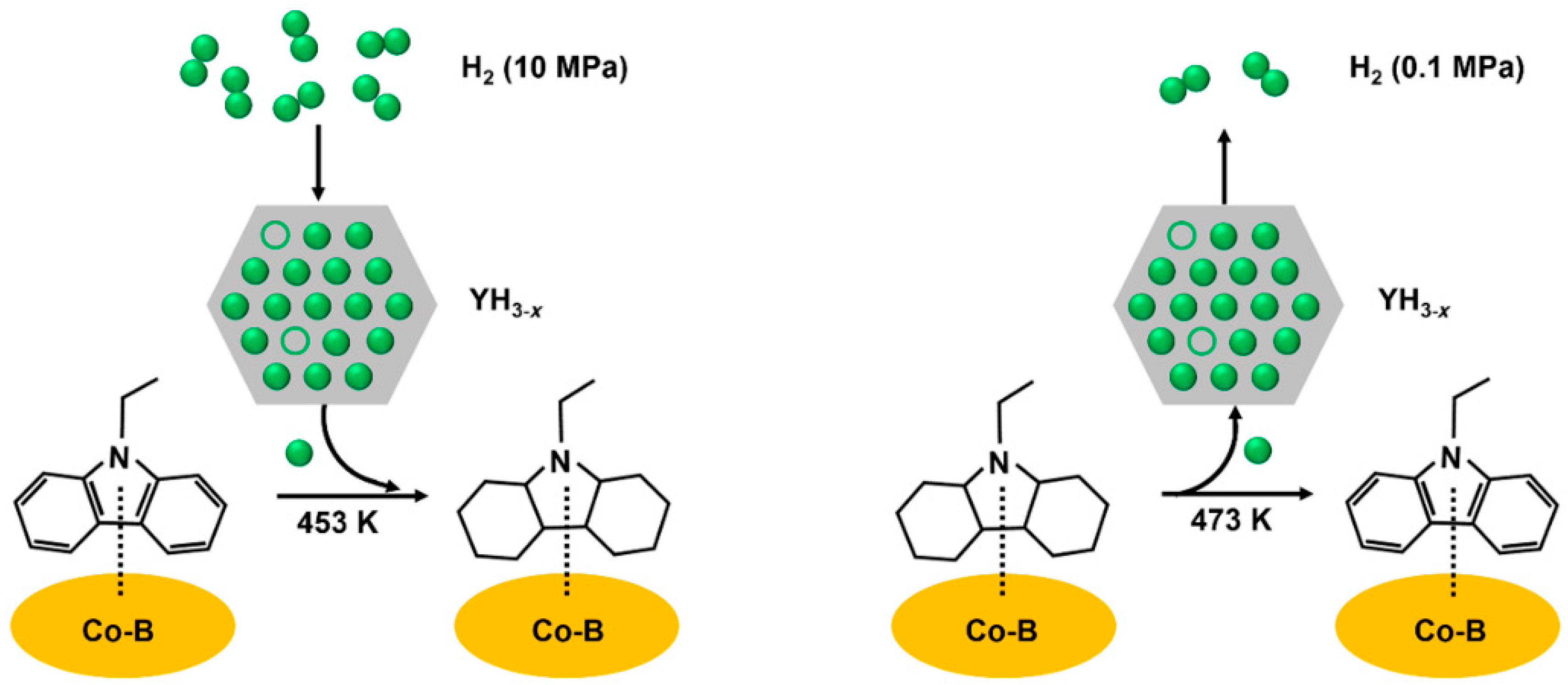
| NEC | Co-B/Al2O3-YH3 | 180 °C, 10 MPa H2 | 2 | 5.60 | 200 °C, 0.1 MPa H2 | 7 | 5.5 | [68] |
| NEC | Pd/Al2O3-YH3 | 180 °C, 10 MPa H2 | 2 | 5.5 | 200 °C, 0.1 MPa H2 | 4 | 5.5 | [189] |
| NPC | 1 wt% Pd/CeO2-Al2O3 | 150 °C, 7 MPa H2 | 3 | 5.43 | 190 °C, 0.1 MPa H2 | 3 | 5.43 | [192] |
| NEC | 10 wt% LaNi5.5 | 180 °C, 7 MPa H2 | 4.5 | 5.5 | 200 °C, 0.1 MPa H2 | 4 | 5.5 | [193] |
| NEC | 0.52 mol% Pd2Ru@SiCN | 110 °C, 2 MPa H2 | 36 | 5.68 | 180 °C, 0.1 MPa H2 | 7 | 5.51 | [194] |
| NEC | 0.6% Rh–1% Rd/γ-Al2O3 | 160 °C, 6 MPa H2 | 1 | 5.46 | 180 °C, 0.1 MPa H2 | 4 | 5.48 | [191] |
| NPC | 5 wt% Ru0.5Pd0.5/Al2O3 | 150 °C, 7 MPa H2 | 7 | 5.41 | 180 °C, 0.1 MPa H2 | 4 | 5.38 | [67] |
| NEC | 1 wt% Pd/LaNi5 | 180 °C, 7 MPa H2 | 0.7 | 5.5 | 200 °C, 0.1 MPa H2 | 2.1 | 5.5 | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yang, F.; Wang, B.; Li, D.; Wei, M.; Fang, T.; Zhang, Z. Heterogeneous Catalysts in N-Heterocycles and Aromatics as Liquid Organic Hydrogen Carriers (LOHCs): History, Present Status and Future. Materials 2023, 16, 3735. https://doi.org/10.3390/ma16103735
Zhang J, Yang F, Wang B, Li D, Wei M, Fang T, Zhang Z. Heterogeneous Catalysts in N-Heterocycles and Aromatics as Liquid Organic Hydrogen Carriers (LOHCs): History, Present Status and Future. Materials. 2023; 16(10):3735. https://doi.org/10.3390/ma16103735
Chicago/Turabian StyleZhang, Jinxu, Fusheng Yang, Bin Wang, Dong Li, Min Wei, Tao Fang, and Zaoxiao Zhang. 2023. "Heterogeneous Catalysts in N-Heterocycles and Aromatics as Liquid Organic Hydrogen Carriers (LOHCs): History, Present Status and Future" Materials 16, no. 10: 3735. https://doi.org/10.3390/ma16103735
APA StyleZhang, J., Yang, F., Wang, B., Li, D., Wei, M., Fang, T., & Zhang, Z. (2023). Heterogeneous Catalysts in N-Heterocycles and Aromatics as Liquid Organic Hydrogen Carriers (LOHCs): History, Present Status and Future. Materials, 16(10), 3735. https://doi.org/10.3390/ma16103735






