Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive
Abstract
1. Introduction
2. Experimental Procedures
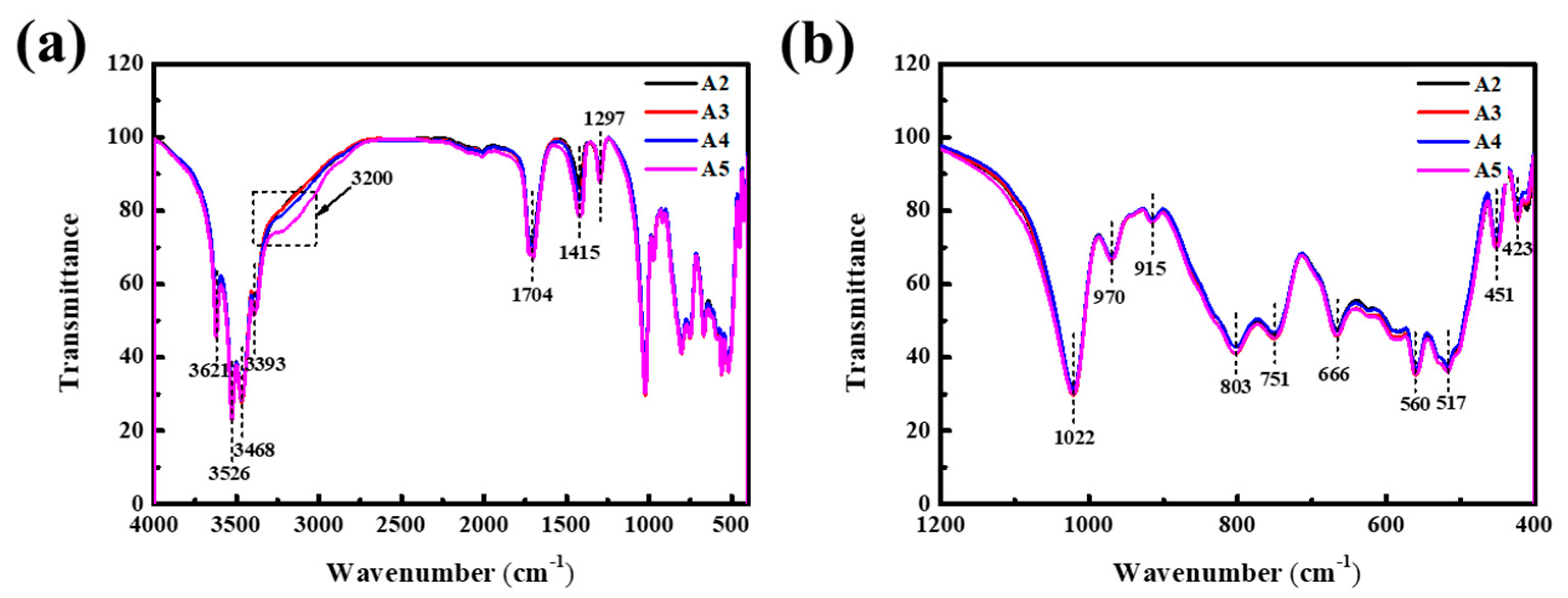
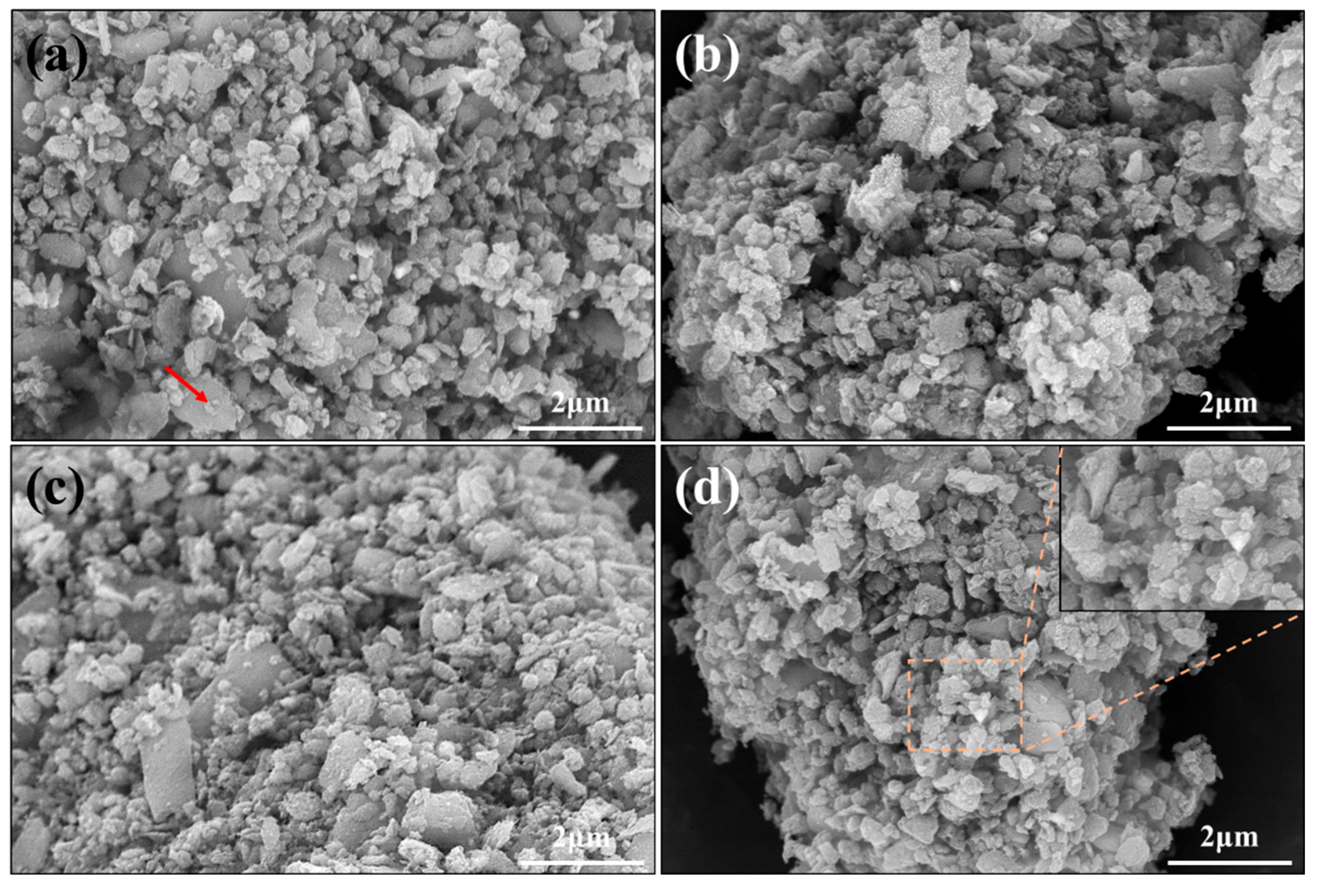
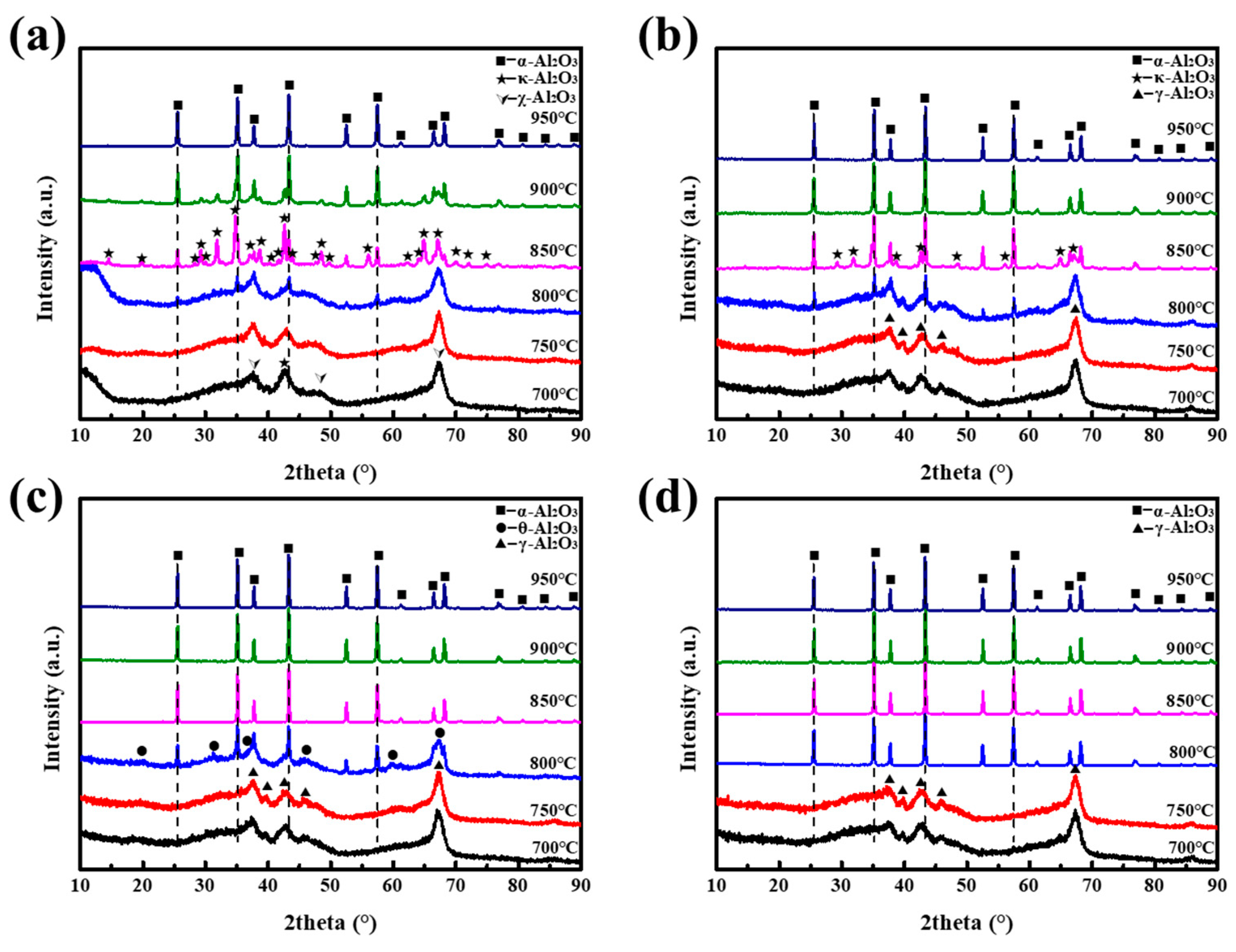
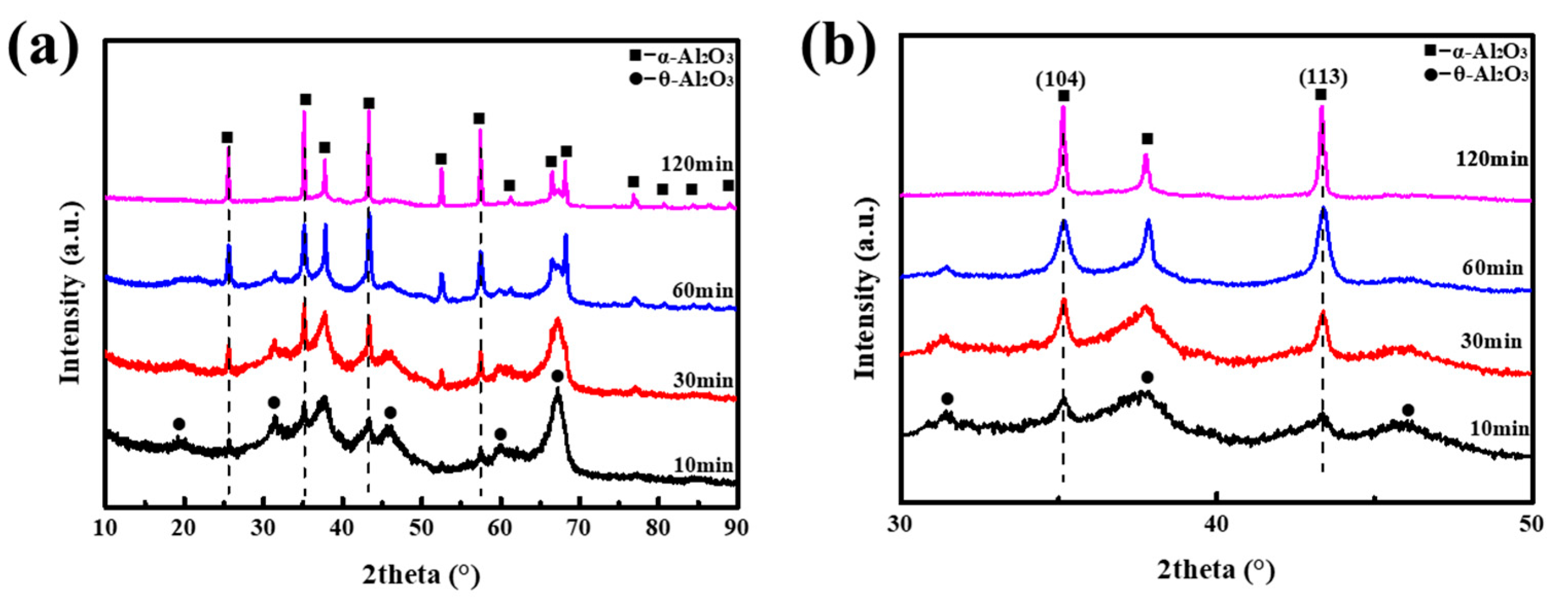
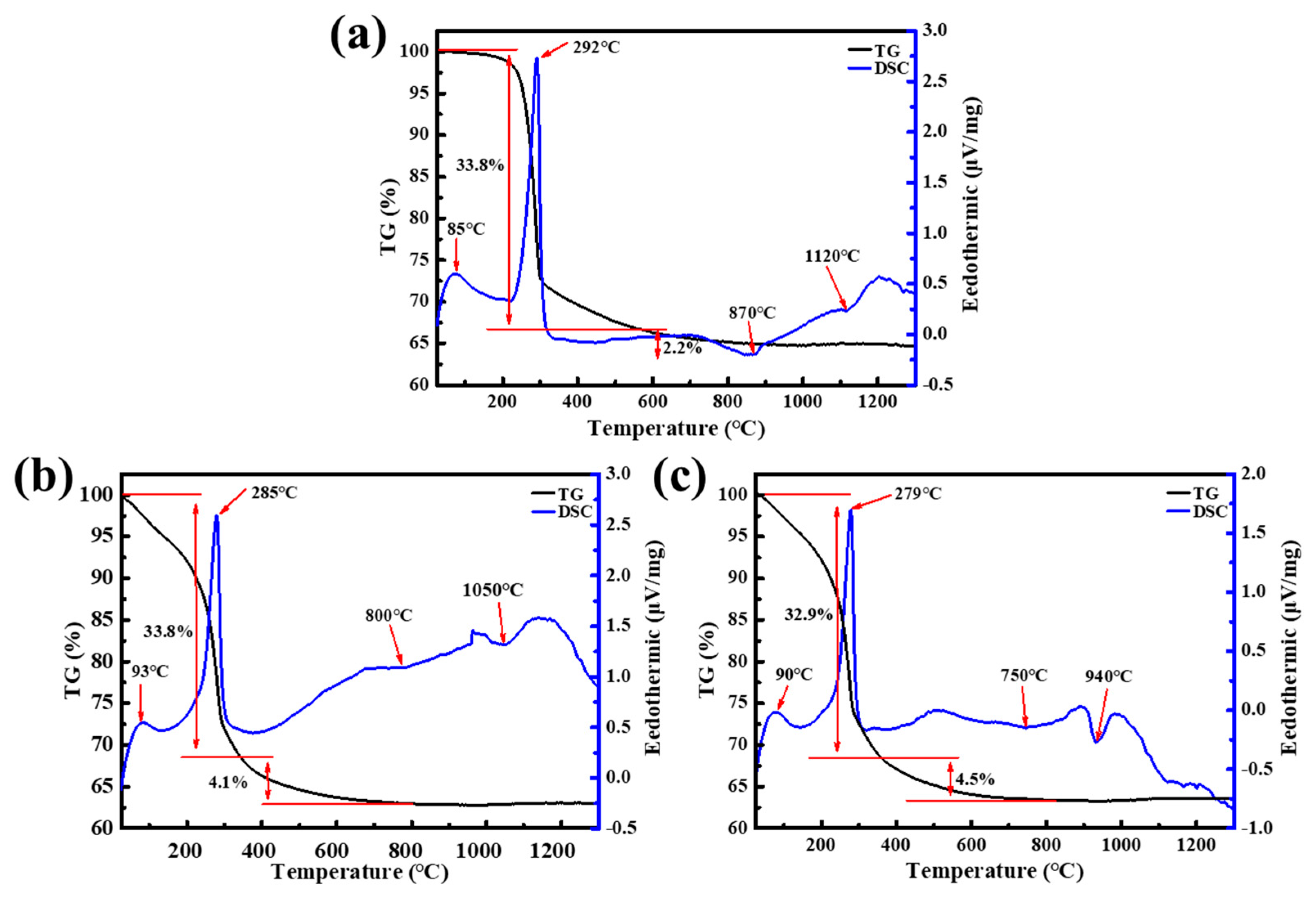
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Mai, Z.; Wu, Y.; Wang, Y.; Cao, Z.; Li, M.; Fan, B.; Shao, G.; Wang, H.; Xu, H.; et al. Preparation of spherical α-Al2O3 nanoparticles by microwave hydrothermal synthesis and addition of nano-Al seeds. J. Am. Ceram. Soc. 2022, 105, 5585–5597. [Google Scholar]
- Panasyuk, G.P.; Belan, V.N.; Voroshilov, I.L.; Kozerozhets, V.I.; Luchkov, V.; Kondakov, D.F.; Demina, L.I. The study of hydrargillite and γ-alumina conversion process in boehmite in different hydrothermal media. Theor. Found. Chem. Eng. 2013, 47, 415–421. [Google Scholar]
- Xu, X.; Zhou, S.; Wu, J.; Zhang, Q.; Zhang, Y.; Zhu, G. Preparation of highly dispersive solid microspherical α-Al2O3 powder with a hydrophobic surface for stereolithography-based 3D printing technology. Ceram. Int. 2020, 46, 1895–1906. [Google Scholar]
- Kozerozhets, I.V.; Panasyuk, G.P.; Semenov, E.A.; Danchevskaya, M.N.; Azarova, L.A.; Simonenko, N.P. Transformations of nanosized boehmite and γ-Al2O3 upon heat treatment. Russ. J. Inorg. Chem. 2020, 65, 587–591. [Google Scholar]
- Zhu, L.; Liu, L.; Sun, C.; Zhang, X.; Zhang, L.; Gao, Z.; Ye, G.; Li, H. Low temperature synthesis of polyhedral α-Al2O3 nanoparticles through two different modes of planetary ball milling. Ceram. Int. 2020, 46, 28414–28421. [Google Scholar]
- Miyake, K.; Hirata, Y.; Shimonosono, T.; Sameshima, S. The Effect of particle shape on sintering behavior and compressive strength of porous alumina. Materials 2018, 11, 1137. [Google Scholar] [CrossRef]
- Li, S.; Zhu, L.; Liu, L.; Chen, L.; Li, H.; Sun, C. Influence of high-energy ball milling and additives on the formation of sphere-like α-Al2O3 powder by high-temperature calcination. Z. Naturforsch. B 2018, 73, 589–596. [Google Scholar]
- Tian, Q.; Zhang, Y.; Yang, X.; Dai, J.; Lv, Z. Influences of NH4F on transformation and morphology of high-pure α-alumina. Mater. Res. Express 2017, 4, 105904. [Google Scholar]
- Li, X.; Su, X.; Xiao, H.; Chen, L.; Li, S.; Tang, M. Continuous α-Al2O3 fibers grown by seeding with in-situ suspension. Ceram. Int. 2020, 46, 15638–15645. [Google Scholar]
- Hill, R.; Danzer, R.; Paine, R. Synthesis of aluminum oxide platelets. J. Am. Ceram. Soc. 2001, 84, 514–520. [Google Scholar]
- Zhou, J.; Laoui, T.; Biest, O. Toughening of X-sialon with Al2O3 platelets. J. Eur. Ceram. Soc. 1995, 15, 297–305. [Google Scholar]
- Karl-Heinz Heussner, N.C. Yttria- and ceria-stabilized tetragonal zirconia polycrystals (Y-TZP, Ce-TZP) reinforced with A12O3 platelets. J. Eur. Ceram. Soc. 1989, 5, 193–200. [Google Scholar]
- Jin, X.; Gao, L. Size control of α-A12O3 platelets synthesized in molten Na2SO4 flux. J. Am. Ceram. Soc. 2004, 87, 533–540. [Google Scholar]
- Liu, L.; Zhang, X.; Zhu, L.; Wei, Y.; Guo, C.; Li, H. Preparation of hexagonal micro-sized α-A12O3 platelets from a milled Al(OH)3 precursor with NH4F and NH4Cl additives. Z. Naturforsch. B 2019, 74, 579–583. [Google Scholar]
- Yin, X.; Zhang, Q.; Liu, J.; Zhong, X.; Chai, C. Polishing property of α-Al2O3 nanoflakes prepared by self-burning method. Acta Phys-Chim. Sin. 2009, 25, 1443–1448. [Google Scholar]
- Suchanek, W.; Garcés, J.; Fulvio, P.; Jaroniec, M. Hydrothermal synthesis and surface sharacteristics of novel alpha alumina nanosheets with controlled chemical composition. Chem. Mater. 2010, 22, 6564–6574. [Google Scholar]
- Zhu, L.; Tu, R.; Huang, Q. Molten salt synthesis of α-Al2O3 platelets using NaAlO2 as raw material. Ceram. Int. 2012, 38, 901–908. [Google Scholar]
- Wei, M.; Zhi, D.; Choy, K. Novel synthesis of α-alumina hexagonal nanoplatelets using electrostatic spray assisted chemical vapour deposition. Nanotechnology 2006, 17, 181–184. [Google Scholar]
- Amrute, A.; Lodziana, Z.; Schreyer, H.; Weidenthaler, C.; Schuth, F. High-surface-area corundum by mechanochemically induced phase transformation of boehmite. Science 2019, 366, 485–489. [Google Scholar]
- Deng, B.; Advincula, P.; Luong, D.; Zhou, J.; Zhang, B.; Wang, Z.; McHugh, E.; Chen, J.; Carter, R.; Kittrell, C.; et al. High-surface-area corundum nanoparticles by resistive hotspot-induced phase transformation. Nat. Commun. 2022, 13, 5027. [Google Scholar]
- Wang, W.; Bi, J.; Qi, Y.; Zhang, Z.; Xing, Z.; Zhu, H.; Guo, D.; Xu, J.; Bai, Y. Preparation of micrometer-sized α-Al2O3 platelets by thermal decomposition of AACH. Powder Technol. 2010, 201, 273–276. [Google Scholar]
- Fu, G.; Wang, J.; Kang, J. Influence of AlF3 and hydrothermal conditions on morphologies of α-Al2O3. T. Nonferr. Metal. Soc. 2008, 18, 743–748. [Google Scholar]
- Tian, Q. The influences of fluorides on the transformation of α-alumina crystals. Ceram.-Silik. 2017, 64, 357–366. [Google Scholar]
- Riello, D.; Zetterström, C.; Parr, C.; Braulio, M.; Moreira, M.; Gallo, J.; Pandolfelli, V. AlF3 reaction mechanism and its influence on α-Al2O3 mineralization. Ceram. Int. 2016, 42, 9804–9814. [Google Scholar]
- Kim, H.; Park, N.; Lee, T.; Kang, M. Effect of AlF3 seed concentrations and calcination temperatures on the crystal growth of hexagonally shaped α-alumina powders. Ceram. Int. 2014, 40, 3813–3818. [Google Scholar]
- Kim, H.; Kang, M. Rapid crystal phase transformation into hexagonally shaped α-alumina using AlF3 seeds. J. Sol-Gel Sci. Techn. 2013, 68, 110–120. [Google Scholar]
- Shimbo, M.; Yamamoto, O.; Hayashi, S.; Nakagawa, Z. Influence of addition of AlF3 on thermal decomposition of gibbsite and phase transition of the intermediate alumina to α-Al2O3. J. Ceram. Soc. Jpn. 2007, 115, 536–540. [Google Scholar]
- Wu, Y.; Pezzotti, G.; Guo, J. Influence of AlF3 and ZnF2 on the phase transformation of gamma to alpha alumina. Mater. Lett. 2002, 52, 366–369. [Google Scholar]
- Živkovic, Ž.; Štrbac, N.; Šesták, J. Influence of fluorides on polymorphous transformation of α-Al2O3 formation. Thermochim. Acta 1995, 266, 293–300. [Google Scholar]
- Zhu, L.; Sun, C.; Chen, L.; Lu, X.; Li, S.; Ye, G.; Liu, L. Influences of NH4F additive and calcination time on the morphological evolution of α-Al2O3 from a milled γ-Al2O3 precursor. Z. Naturforsch. B 2017, 72, 665–670. [Google Scholar]
- Kang, J.; Wang, J.; Zhang, W. Influence of AlF3 additive on microstructure of alumina powder. Light Met. 2008, 3, 13–16. [Google Scholar]
- Islam, M.; Patel, R. Thermal activation of basic oxygen furnace slag and evaluation of its fluoride removal efficiency. Chem. Eng. J. 2011, 169, 68–77. [Google Scholar]
- Chen, H.; Ren, B.; Chen, L.; Zhao, F.; Bai, Q.; Chen, J.; Li, B. Preparation of equiaxed α-Al2O3 by adding oxalic acid. Ceram. Int. 2021, 47, 31512–31517. [Google Scholar]
- Ibrahim, D.; Abu-Ayana, Y. Preparation and characterization of ultrafine alumina via sol–gel polymeric route. Mater. Chem. Phys. 2008, 111, 326–330. [Google Scholar]
- Meher, T.; Basu, A.; Ghatak, S. Physicochemical characteristics of alumina gel in hydroxyhydrogel and normal form. Ceram. Int. 2005, 31, 831–838. [Google Scholar]
- Wilson, S. Phase transformations and development of microstructure in boehmite-derived transition aluminas. Proc. Brit. Ceram. Soc. 1979, 28, 281–294. [Google Scholar]
- Igor Levin, D.B. Metastable alumina polymorphs crystal structures and transition sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar]
- Brindley, G.; Choe, J. The reaction series, gibbsite→chi alumina→kappa alumina→corundum. Am. Mineral. 1961, 46, 771–785. [Google Scholar]
- Yan, F.; Huang, H. Habit evolution of alumina crystallite during κ- to α-phase transformation. Chin. J. Process Eng. 2004, 4, 366–370. [Google Scholar]
- Chang, P.; Yen, F.; Cheng, K.; Wen, H. Examinations on the critical and primary crystallite sizes during θ- to α-phase transformation of ultrafine alumina powders. Nano Lett. 2001, 1, 253–261. [Google Scholar]
- Bagwell, R.; Messing, G. Effect of seeding and water vapor on the nucleation and growth of γ-Al2O3 from α-Al2O3. J. Am. Ceram. Soc. 1999, 82, 825–832. [Google Scholar]
- Arai, H.; Machida, M. Thermal stabilization of catalyst supports and their application to high-temperature catalytic. Appl. Catal. A-Gen. 1996, 138, 161–176. [Google Scholar]
- Barth, C.; Reichling, M. Imaging the atomic arrangements on the high-temperature reconstructed α-Al2O3 (0001) surface. Nature 2001, 414, 54–57. [Google Scholar]
- Mohri, M.; Uchida, Y.; Sawabe, Y.; Watanabe, H. α-Alumina. U.S. Patent 6165437, 26 December 2000. [Google Scholar]


| Morphology | Additive | Mixing Amount | Material | Temperature/Time | Ref. |
|---|---|---|---|---|---|
| Polyhedral | AlF3 | 1 wt.% | Al(OH)3 | 910 °C/3 h | [5] |
| Sphere-like | NH4BF4 | 5 wt.% | Al(OH)3 | 1450 °C/3 h | [7] |
| Plate-like | NH4F | 5 wt.% | AlOOH | 900 °C/2.5 h | [8] |
| Plate-like | NH4F+NH4F | 5 wt.% + 5 wt.% | Al(OH)3 | 1300 °C/3 h | [14] |
| Plate-like | AlF3 | 5 wt.% | AACH | 1200 °C/1 h | [21] |
| Plate-like | AlF3 | 2 wt.% | AIP | 900 °C/3 h | [22] |
| Plate-like | AlF3 NH4F | 2.8 wt.% of F | AlOOH | 1050 °C/1 h 950 °C/1 h | [23] |
| Plate-like | AlF3 | 0.6 wt.% | t-Al2O3Al(OH)3 | 1000 °C/0.5 h 950 °C/0.5 h | [24] |
| Plate-like | AlF3 | 1 mol% | Al(OH)3 | 750 °C/10 h | [25] |
| Plate-like | AlF3 | 2 mol% | AlOOH | 750 °C/10 h | [26] |
| Plate-like | ZnF2 AlF3 | - | Al(NO3)3 | 920 °C/10 h 900 °C/1 h | [28] |
| Square-like | NH4F | 2 wt.% | γ-Al2O3 | 1300 °C/2 h | [30] |
| Plate-like | Oxalic acid + NH4F | 0.16 mol/L + 1 wt.% | Al(OH)3 | 850 °C/3 h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Li, B.; Liu, M.; Yang, X.; Liu, J.; Qin, T.; Xue, Z.; Xing, Y.; Chen, J. Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive. Materials 2023, 16, 4415. https://doi.org/10.3390/ma16124415
Chen H, Li B, Liu M, Yang X, Liu J, Qin T, Xue Z, Xing Y, Chen J. Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive. Materials. 2023; 16(12):4415. https://doi.org/10.3390/ma16124415
Chicago/Turabian StyleChen, Haiyang, Bin Li, Meng Liu, Xue Yang, Jie Liu, Tingwei Qin, Zejian Xue, Yun Xing, and Junhong Chen. 2023. "Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive" Materials 16, no. 12: 4415. https://doi.org/10.3390/ma16124415
APA StyleChen, H., Li, B., Liu, M., Yang, X., Liu, J., Qin, T., Xue, Z., Xing, Y., & Chen, J. (2023). Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive. Materials, 16(12), 4415. https://doi.org/10.3390/ma16124415








