Sol–Gel Synthesis of Translucent and Persistent Luminescent SiO2@ SrAl2O4 Eu, Dy, B Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SrAl2O4:Eu, Dy, and B/SiO2 Composite Materials
2.3. Methods of Characterization
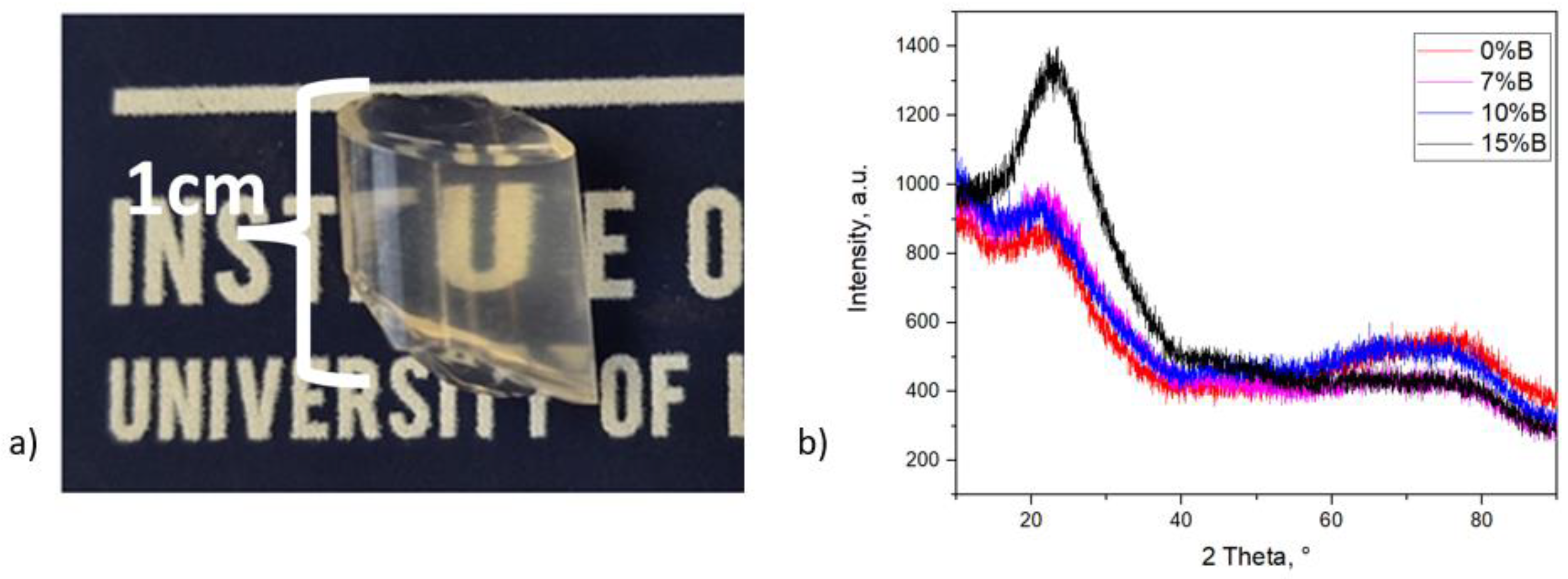
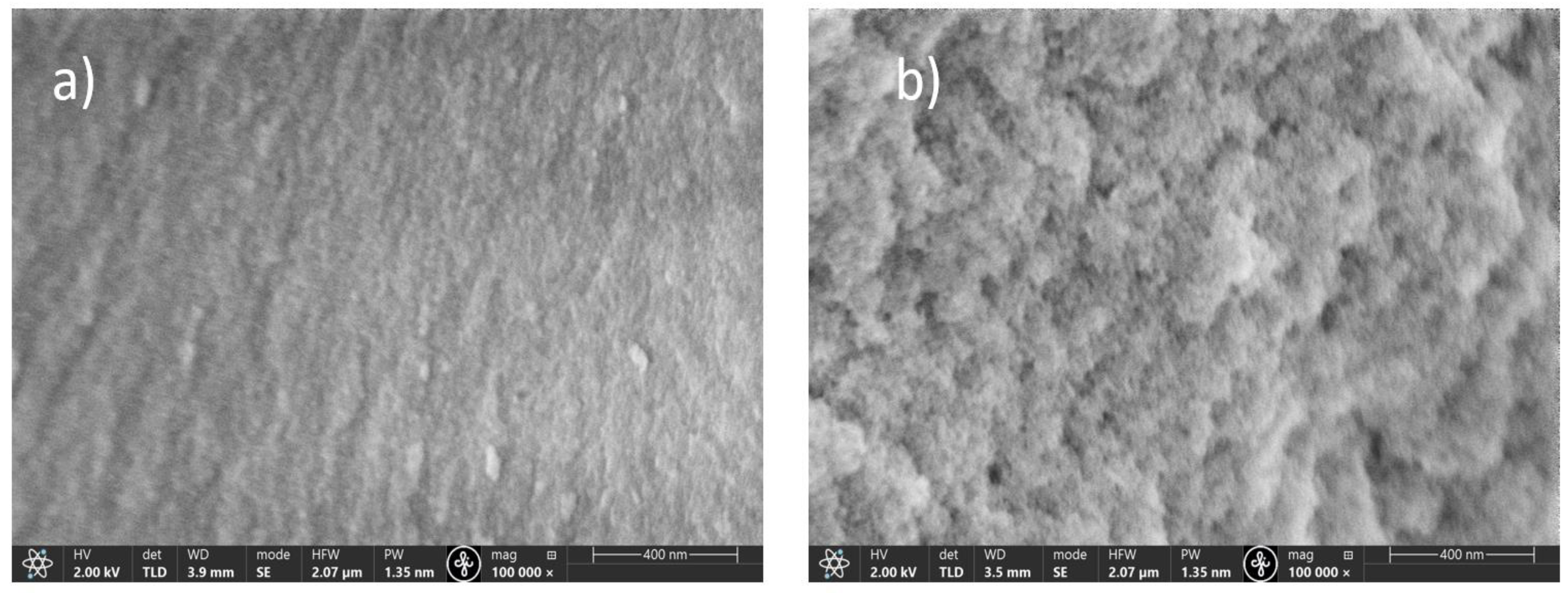
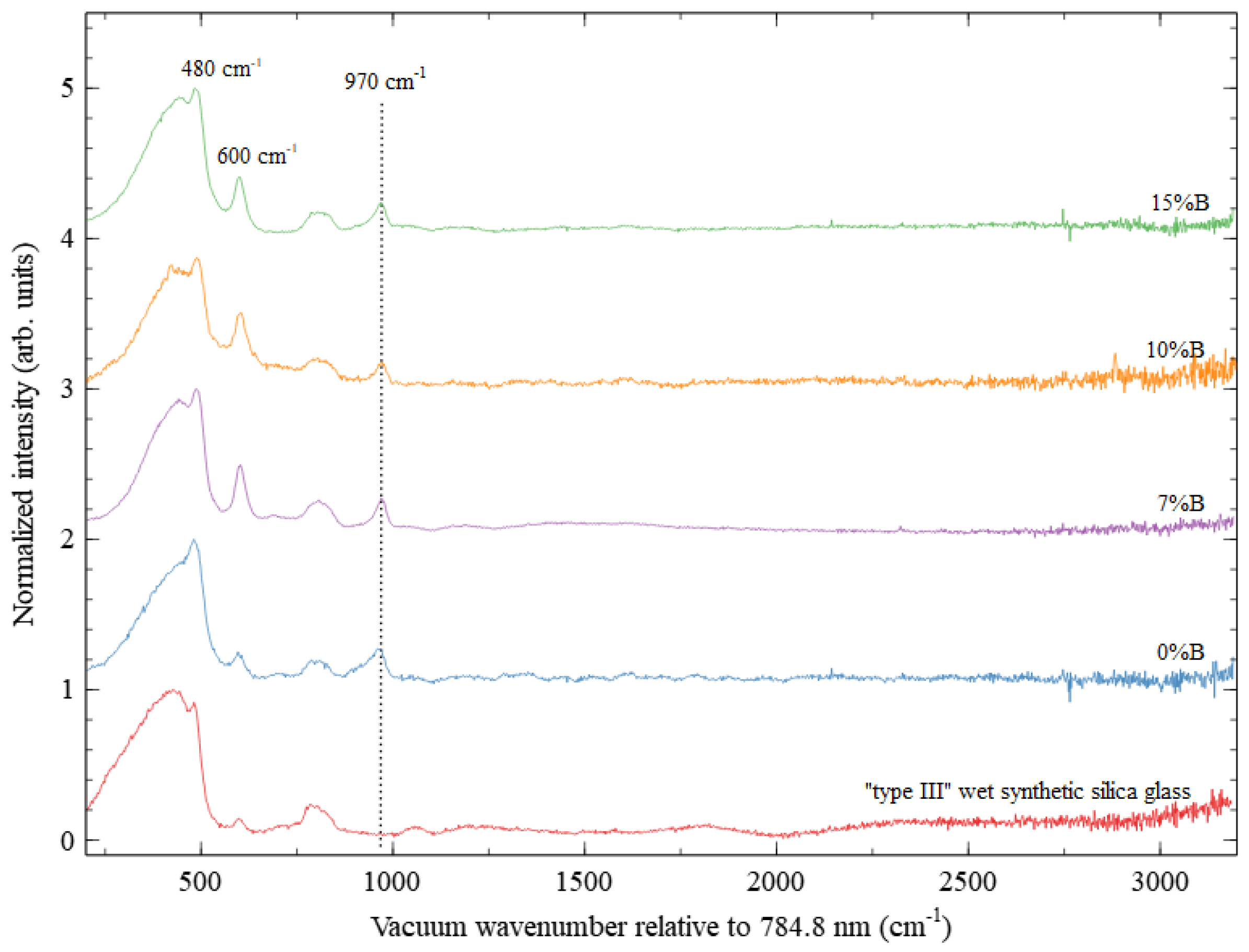
3. Results and Discussion
3.1. The Successes and Failures of PeL Silicate Glass Synthesis
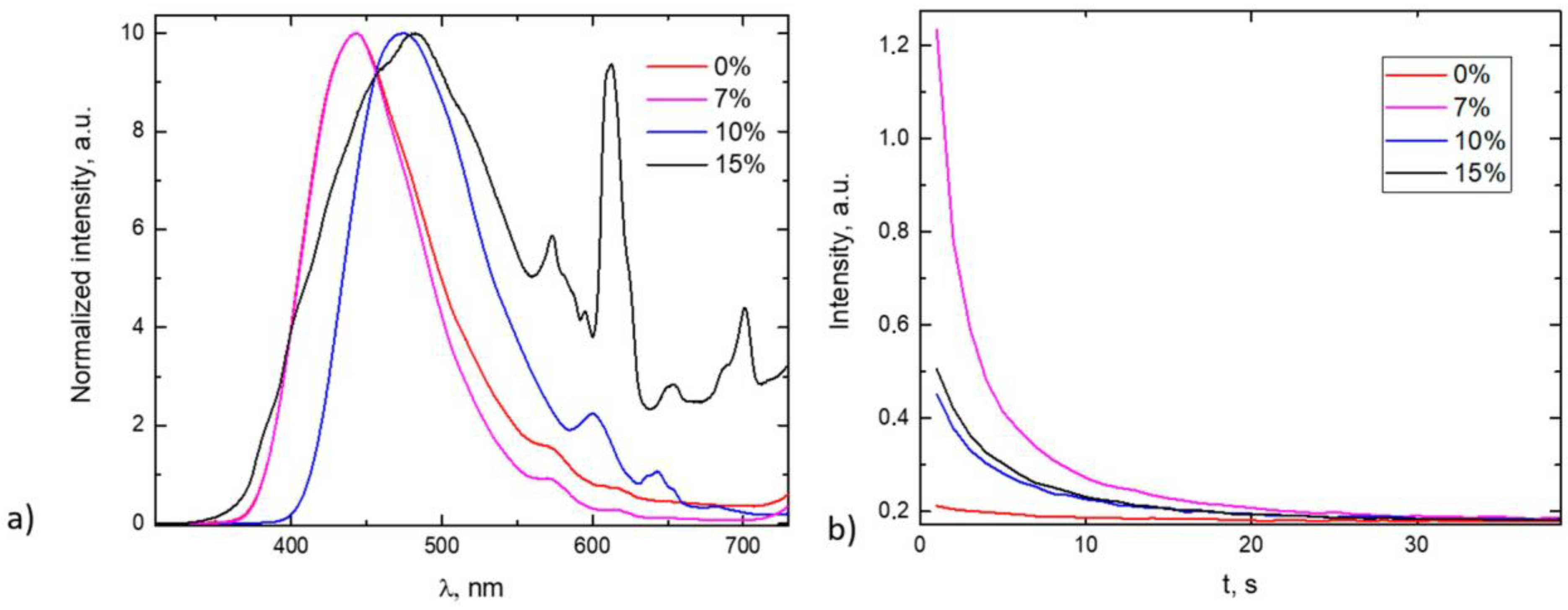
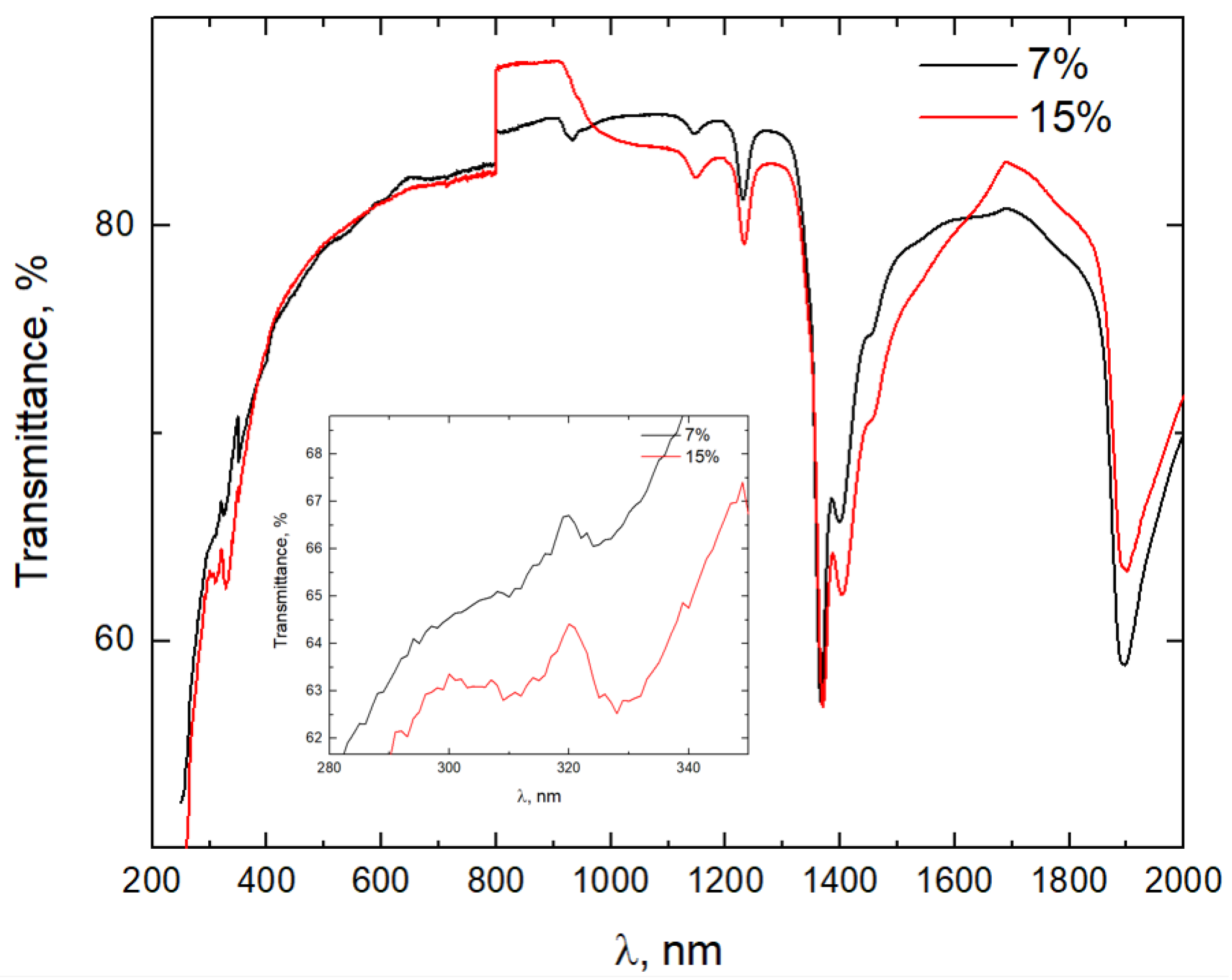
3.2. The Optical Properties of the PeL Glass
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vitola, V.; Millers, D.; Bite, I.; Smits, K.; Spustaka, A. Recent progress in understanding the persistent luminescence in SrAl2O4:Eu, Dy. Mater. Sci. Technol. 2019, 35, 1661–1677. [Google Scholar] [CrossRef]
- Van der Heggen, D.; Joos, J.J.; Feng, A.; Fritz, V.; Delgado, T.; Gartmann, N.; Walfort, B.; Rytz, D.; Hagemann, H.; Poelman, D.; et al. Persistent Luminescence in Strontium Aluminate: A Roadmap to a Brighter Future. Adv. Funct. Mater. 2022, 32, 2208809. [Google Scholar] [CrossRef]
- Einbergs, E.; Zolotarjovs, A.; Bite, I.; Vītola, V.; Spustaka, A.; Tunēns, G.; Arnautov, A. A mechanoluminescence based approach to spatial mechanical stress visualisation of additively manufactured (3D printed) parts. Materialia 2022, 24, 101516. [Google Scholar] [CrossRef]
- Rodlovskaya, E.N.; Vasnev, V.A. Cotton Materials Grapped with Luminescent Organosilazane Coatings. INEOS OPEN 2020, 3, 214–218. [Google Scholar] [CrossRef]
- Rogulis, U.; Elsts, E.; Jansons, J.; Sarakovskis, A.; Doke, G.; Stunda, A.; Smits, K. Cathodoluminescence of oxyfluoride glass-ceramics. Radiat. Meas. 2013, 56, 120–123. [Google Scholar] [CrossRef]
- Vitola, V.; Lahti, V.; Bite, I.; Spustaka, A.; Millers, D.; Lastusaari, M.; Petit, L.; Smits, K. Low temperature afterglow from SrAl2O4: Eu, Dy, B containing glass. Scr. Mater. 2021, 190, 86–90. [Google Scholar] [CrossRef]
- Liu, J.; Lama, G.C.; Recupido, F.; Santillo, C.; Gentile, G.; Buonocore, G.G.; Verdolotti, L.; Zhang, X.; Lavorgna, M. A multifunctional composite material with piezoresistivity and mechanoluminescence properties for a wearable sensor. Compos. Sci. Technol. 2023, 236, 109993. [Google Scholar] [CrossRef]
- Gencel, O.; Danish, A.; Yilmaz, M.; Erdogmus, E.; Sutcu, M.; Ozbakkaloglu, T.; Gholampour, A. Experimental evaluation of the luminescence performance of fired clay brick coated with SrAl2O4:Eu/Dy phosphor. Ceram. Int. 2022, 48, 33167–33176. [Google Scholar] [CrossRef]
- Auzins, K.; Zolotarjovs, A.; Bite, I.; Laganovska, K.; Vitola, V.; Smits, K.; Millers, D. Production of Phosphorescent Coatings on 6082 Aluminum Using Sr0.95Eu0.02Dy0.03Al2O4-δ Powder and Plasma Electrolytic Oxidation. Coatings 2019, 9, 865. [Google Scholar] [CrossRef]
- Hölsä, J.; Laamanen, T.; Lastusaari, M.; Niittykoski, J.; Novák, P. Electronic structure of the SrAl2O4:Eu2+ persistent luminescence material. J. Rare Earths 2009, 27, 550–554. [Google Scholar] [CrossRef]
- Duan, X.; Huang, S.; You, F.; Xu, Z.; Teng, F.; Yi, L. Electrooptical characteristics of nanoscale and bulk long persistent phosphor SrAl2O4:Eu, Dy. J. Exp. Nanosci. 2009, 4, 169–176. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Y.; Yin, L.; Zhang, M.; Li, Y.; Townsend, P.D.; Poelman, D. Structural and optical properties of iron ions doped near-infrared persistent spinel-type phosphors. J. Lumin. 2023, 258, 119822. [Google Scholar] [CrossRef]
- Luchechko, A.; Zhydachevskyy, Y.; Ubizskii, S.; Kravets, O.; Popov, A.I.; Rogulis, U.; Elsts, E.; Bulur, E.; Suchocki, A. Afterglow, TL and OSL properties of Mn2+-doped ZnGa2O4 phosphor. Sci. Rep. 2019, 9, 9544. [Google Scholar] [CrossRef]
- Haranath, D.; Shanker, V.; Chander, H.; Sharma, P. Tuning of emission colours in strontium aluminate long persisting phosphor. J. Phys. D Appl. Phys. 2003, 36, 2244–2248. [Google Scholar] [CrossRef]
- Van den Eeckhout, K.; Smet, P.F.; Poelman, D. Persistent luminescene in Eu2+-doped compounds: A review. Materials 2010, 3, 2536–2566. [Google Scholar] [CrossRef]
- Xu, J.; Ueda, J.; Kuroishi, K.; Tanabe, S. Fabrication of Ce3+–Cr3+ co-doped yttrium aluminium gallium garnet transparent ceramic phosphors with super long persistent luminescence. Scr. Mater. 2015, 102, 47–50. [Google Scholar] [CrossRef]
- Delgado, T.; Rytz, D.; Cai, G.; Allix, M.; Veron, E.; Di Carlo, I.; Viana, B. Highly transparent Ce3+,Cr3+ co-doped GYAGG single crystals with enhanced persistent luminescence. Ceram. Int. 2023, in press. [Google Scholar] [CrossRef]
- Nakanishi, T.; Watanabe, K.; Ueda, J.; Fushimi, K.; Tanabe, S.; Hasegawa, Y. Enhanced Light Storage of SrAl2O4 Glass-Ceramics Controlled by Selective Europium Reduction. J. Am. Ceram. Soc. 2015, 98, 423–429. [Google Scholar] [CrossRef]
- Nakanishi, T.; Katayama, Y.; Ueda, J.; Honma, T.; Tanabe, S.; Komatsu, T. Fabrication of Eu:SrAl2O4-based glass ceramics using Frozen sorbet method. J. Ceram. Soc. Jpn. 2011, 119, 609–615. [Google Scholar] [CrossRef]
- Ohja, N.; Tuomisto, M.; Lastusaari, M.; Petit, L. Phosphate glasses with persistent luminescence prepared using the direct doping method. Opt. Mater. 2019, 87, 151–156. [Google Scholar]
- Massera, J.; Głuchowski, P.; Lastusaari, M.; Rodrigues, L.C.V.; Petit, L.; Hölsä, J.; Hupa, L.; Hupa, M. New alternative route for the preparation of phosphate glasses with persistent luminescence properties. J. Eur. Ceram. Soc. 2015, 35, 1255–1261. [Google Scholar] [CrossRef]
- Skuja, L.; Leimane, M.; Bite, I.; Millers, D.; Zolotarjovs, A.; Vitola, V.; Smits, K. Ultraviolet luminescence of polycyclic aromatic hydrocarbons in partially consolidated sol-gel silica glasses. J. Non-Cryst. Solids 2022, 577, 121325. [Google Scholar] [CrossRef]
- Kaneko, K.; Kajihara, K.; Kanamura, K. Cosolvent-free sol–gel synthesis of rare-earth and aluminum codoped monolithic silica glasses. J. Ceram. Soc. Jpn. 2013, 121, 299–302. [Google Scholar] [CrossRef]
- Kajihara, K.; Kuwatani, S.; Kanamura, K. Sol-Gel synthesis of rare-earth and phosphorus codoped monolithic silica glasses from a cosolvent-free phase-separating system. Appl. Phys. Express 2012, 5, 012601. [Google Scholar] [CrossRef]
- Kajihara, K.; Nakagawa, S.; Iwasaki, R. Energy Transfer and Quenching in Sol–Gel-Derived Silica Glass Green Phosphors Doped with Tb3+ and Ce3+ Ions: Distinct Difference between P- and Al-Codoped Glasses. Phys. Status Solidi A 2022, 219, 2100494. [Google Scholar] [CrossRef]
- Vitola, V.; Bite, I.; Millers, D.; Zolotarjovs, A.; Laganovska, K.; Smits, K.; Spustaka, A. The boron effect on low temperature luminescence of SrAl2O4:Eu, Dy. Ceram. Int. 2020, 46, 26377–26381. [Google Scholar] [CrossRef]
- Irshidat, M.R.; Al-Saleh, M.H.; Sanad, S. Effect of Nanoclay on the Expansive Potential of Cement Mortar due to Alkali-Silica Reaction. ACI Mater. J. 2015, 112, 801–808. [Google Scholar] [CrossRef]
- Jena, H.; Raghavan, S.; Pogaku, V.; Bandi, P.R.; Vadakkapet, G.K.K. Removal of Ru from Simulated High-Level Waste Prior to the Final Vitrification into Borosilicate Glass Using Tin as the Alloying Element: Feasibility Study. J. Hazard. Toxic Radioact. Waste 2018, 22, 04018018. [Google Scholar] [CrossRef]
- Dung, C.T.M.; Van Hieu, L.; Vinh, L.Q.; Van, T.T.T. Remarkable enhancement of Er3+ emission at 1.54 μm in Er/Yb co-doped SiO2-SnO2 glass-ceramics. J. Alloys Compd. 2018, 757, 489–495. [Google Scholar] [CrossRef]
- Nagayama, S.; Kajihara, K.; Kanamura, K. Synthesis of nanocrystalline LaF3 doped silica glasses by hydrofluoric acid catalyzed sol–gel process. Mater. Sci. Eng. B 2012, 177, 510–514. [Google Scholar] [CrossRef]
- Dung, C.T.M.; Giang, L.T.T.; Binh, D.H.; Van Hieu, L.; Van, T.T.T. Understanding up and down-conversion luminescence for Er3+/Yb3+ co-doped SiO2-SnO2 glass-ceramics. J. Alloys Compd. 2021, 870, 159405. [Google Scholar] [CrossRef]
- Zhu, Y.; Zeng, J.; Li, W.; Xu, L.; Guan, Q.; Liu, Y. Encapsulation of strontium aluminate phosphors to enhance water resistance and luminescence. Appl. Surf. Sci. 2009, 255, 7580–7585. [Google Scholar] [CrossRef]
- Kajihara, K.; Yamaguchi, S.; Kaneko, K.; Kanamura, K. Highly transparent, bright green, sol–gel-derived monolithic silica-(Tb,Ce)PO4 glass-ceramic phosphors. RSC Adv. 2014, 4, 26692–26696. [Google Scholar] [CrossRef]
- Kajihara, K.; Hirano, M.; Hosono, H. Sol-gel synthesis of monolithic silica gels and glasses from phase-separating tetraethoxysilane–water binary system. Chem. Commun. 2009, 18, 2580. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, K. Recent advances in sol–gel synthesis of monolithic silica and silica-based glasses. J. Asian Ceram. Soc. 2013, 1, 121–133. [Google Scholar] [CrossRef]
- Paterson, A.S.; Raja, B.; Garvey, G.; Kolhatkar, A.; Hagström, A.E.; Kourentzi, K.; Lee, T.R.; Willson, R.C. Persistent Luminescence Strontium Aluminate Nanoparticles as Reporters in Lateral Flow Assays. Anal. Chem. 2014, 86, 9481–9488. [Google Scholar] [CrossRef] [PubMed]
- El Hamzaoui, H.; Courthéoux, L.; Nguyen, V.N.; Berrier, E.; Favre, A.; Bigot, L.; Bouazaoui, M.; Capoen, B. From porous silica xerogels to bulk optical glasses: The control of densification. Mater. Chem. Phys. 2010, 121, 83–88. [Google Scholar] [CrossRef]
- Nevolina, L.A.; Koroleva, O.N.; Tyurnina, N.G.; Tyurnina, Z.G. Study of Alkaline Earth Borosilicate Glass by Raman Spectroscopy. Glass Phys. Chem. 2021, 47, 24–29. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Berrier, E.; Zoller, C.; Beclin, F.; Turell, S.; Bouazaoui, M.; Capoen, B. Microstructures and Structural Properties of Sol−Gel Silica Foams. J. Phys. Chem. B 2005, 109, 22799–22807. [Google Scholar] [CrossRef]
- González, P.; Serra, J.; Liste, S.; Chiussi, S.; León, B.; Pérez-Amor, M. Raman spectroscopic study of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 320, 92–99. [Google Scholar] [CrossRef]
- Ashwini, K.R.; Premkumar, H.B.; Darshan, G.P.; Basavaraj, R.B.; Nagabhushana, H.; Prasad, B.D. Near UV-light excitable SrAl2O4:Eu3+ nanophosphors for display device applications. J. Sci. Adv. Mater. Devices 2020, 5, 111–118. [Google Scholar] [CrossRef]
- Yoon, S.; Bierwagen, J.; Trottmann, M.; Walfort, B.; Gartmann, N.; Weidenkaff, A.; Hagemann, H.; Pokrant, S. The influence of boric acid on improved persistent luminescence and thermal oxidation resistance of SrAl2O4:Eu2+. J. Lumin. 2015, 167, 126–131. [Google Scholar] [CrossRef]
- Bierwagen, J.; Delgado, T.; Jiranek, G.; Yoon, S.; Gartmann, N.; Walfort, B.; Pollnau, M.; Hagemann, H. Probing traps in the persistent phosphor SrAl2O4:Eu2+,Dy3+,B3+—A wavelength, temperature and sample dependent thermoluminescence investigation. J. Lumin. 2020, 222, 117113. [Google Scholar] [CrossRef]
- Botterman, J.; Joos, J.J.; Smet, P.F. Trapping and detrapping in SrAl2O4:Eu, Dy persistent phosphors: Influence of excitation wavelength and temperature. Phys. Rev. B Condens. Matter. Mater. Phys. 2014, 90, 085147. [Google Scholar] [CrossRef]
- Trukhin, A.N. Luminescence of localized states in oxidized and fluorinated silica glass. J. Non-Cryst. Solids 2019, 521, 119525. [Google Scholar] [CrossRef]
- Skuja, L.; Kajihara, K.; Ikuta, Y.; Hirano, M.; Hosono, H. Urbach absorption edge of silica: Reduction of glassy disorder by fluorine doping. J. Non-Cryst. Solids 2004, 345–346, 328–331. [Google Scholar] [CrossRef]
- Efimov, A.M.; Pogareva, V.G. IR absorption spectra of vitreous silica and silicate glasses: The nature of bands in the 1300 to 5000 cm−1 region. Chem. Geol. 2006, 229, 198–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leimane, M.; Krizmane, K.; Bite, I.; Grube, J.; Vitola, V. Sol–Gel Synthesis of Translucent and Persistent Luminescent SiO2@ SrAl2O4 Eu, Dy, B Materials. Materials 2023, 16, 4416. https://doi.org/10.3390/ma16124416
Leimane M, Krizmane K, Bite I, Grube J, Vitola V. Sol–Gel Synthesis of Translucent and Persistent Luminescent SiO2@ SrAl2O4 Eu, Dy, B Materials. Materials. 2023; 16(12):4416. https://doi.org/10.3390/ma16124416
Chicago/Turabian StyleLeimane, Madara, Katrina Krizmane, Ivita Bite, Jurgis Grube, and Virginija Vitola. 2023. "Sol–Gel Synthesis of Translucent and Persistent Luminescent SiO2@ SrAl2O4 Eu, Dy, B Materials" Materials 16, no. 12: 4416. https://doi.org/10.3390/ma16124416
APA StyleLeimane, M., Krizmane, K., Bite, I., Grube, J., & Vitola, V. (2023). Sol–Gel Synthesis of Translucent and Persistent Luminescent SiO2@ SrAl2O4 Eu, Dy, B Materials. Materials, 16(12), 4416. https://doi.org/10.3390/ma16124416