High-Entropy Perovskite Thin Film in the Gd-Nd-Sm-La-Y-Co System: Deposition, Structure and Optoelectronic Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-Entropy Alloy: Challenges and Prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and Microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 High Entropy Oxide Characterized by Spinel Structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent Equiatomic Rare Earth Oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-Entropy Ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Reece, M.J. Review of High Entropy Ceramics: Design, Synthesis, Structure and Properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Bała, P.; Górecki, K.; Bednarczyk, W.; Wątroba, M.; Lech, S.; Kawałko, J. Effect of High-Temperature Exposure on the Microstructure and Mechanical Properties of the Al5Ti5Co35Ni35Fe20 High-Entropy Alloy. J. Mater. Res. Technol. 2020, 9, 551–559. [Google Scholar] [CrossRef]
- High-Entropy Alloys—2nd Edition. Available online: https://www.elsevier.com/books/high-entropy-alloys/murty/978-0-12-816067-1 (accessed on 13 May 2023).
- Rost, C.M. Entropy-Stabilized Oxides: Explorations of a Novel Class of Multicomponent Materials. Ph.D. thesis, North Carolina State University, Raleigh, NC, USA, 2016. [Google Scholar]
- Zhang, Y.; Zhang, Z.; Wang, X.; Yao, W.; Liang, X. Structure and Properties of High-Entropy Amorphous Thin Films: A Review. JOM 2022, 74, 794–807. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, M.; Zhang, W.; Yi, D.; Lan, J.; Nan, C.-W.; Lin, Y.-H. Electrical and Thermal Transport Behaviours of High-Entropy Perovskite Thermoelectric Oxides. J. Adv. Ceram. 2021, 10, 377–384. [Google Scholar] [CrossRef]
- Ferro, S.M.; Wobben, M.; Ehrler, B. Rare-Earth Quantum Cutting in Metal Halide Perovskites—A Review. Mater. Horiz. 2021, 8, 1072–1083. [Google Scholar] [CrossRef]
- Dhole, S.; Chen, A.; Nie, W.; Park, B.; Jia, Q. Strain Engineering: A Pathway for Tunable Functionalities of Perovskite Metal Oxide Films. Nanomaterials 2022, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Corey, Z.J.; Lu, P.; Zhang, G.; Sharma, Y.; Rutherford, B.X.; Dhole, S.; Roy, P.; Wang, Z.; Wu, Y.; Wang, H.; et al. Structural and Optical Properties of High Entropy (La,Lu,Y,Gd,Ce)AlO3 Perovskite Thin Films. Adv. Sci. 2022, 9, 2202671. [Google Scholar] [CrossRef]
- Uehara, M.; Mori, S.; Chen, C.-L.; Cheong, S.-W. Percolative Phase Separation Underlies Colossal Magnetoresistance in Mixed-Valent Manganites. Nature 1999, 399, 560–563. [Google Scholar] [CrossRef]
- Dagotto, E.; Hotta, T.; Moreo, A. Colossal Magnetoresistant Materials: The Key Role of Phase Separation. Phys. Rep. 2001, 344, 1–153. [Google Scholar] [CrossRef]
- Bresolin, B.-M.; Park, Y.; Bahnemann, D.W. Recent Progresses on Metal Halide Perovskite-Based Material as Potential Photocatalyst. Catalysts 2020, 10, 709. [Google Scholar] [CrossRef]
- Tabish, A.; Varghese, A.M.; Wahab, M.A.; Karanikolos, G.N. Perovskites in the Energy Grid and CO2 Conversion: Current Context and Future Directions. Catalysts 2020, 10, 95. [Google Scholar] [CrossRef]
- Górecki, K.; Bała, P.; Bednarczyk, W.; Kawałko, J. Cryogenic Behaviour of the Al5Ti5Co35Ni35Fe20 Multi-Principal Component Alloy. Mater. Sci. Eng. A 2019, 745, 346–352. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Li, H.-B.; Hattori, A.N.; Tanaka, H. Comprehensive Determination of Proton Diffusion in Protonated NdNiO3 Thin Film by a Combination of Electrochemical Impedance Spectroscopy and Optical Observation. Appl. Phys. Express 2023, 16, 035501. [Google Scholar] [CrossRef]
- Qi, H.; Chen, L.; Deng, S.; Chen, J. High-Entropy Ferroelectric Materials. Nat. Rev. Mater. 2023, 8, 355–356. [Google Scholar] [CrossRef]
- Kante, M.V.; Weber, M.L.; Ni, S.; van den Bosch, I.C.G.; van der Minne, E.; Heymann, L.; Falling, L.J.; Gauquelin, N.; Tsvetanova, M.; Cunha, D.M.; et al. A High-Entropy Oxide as High-Activity Electrocatalyst for Water Oxidation. ACS Nano 2023, 17, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Esquius, J.R.; Liu, L. High Entropy Materials as Emerging Electrocatalysts for Hydrogen Production through Low-Temperature Water Electrolysis. Mater. Futures 2023, 2, 022102. [Google Scholar] [CrossRef]
- Tu, J.; Ding, J.; Xi, G.; Li, H.; Yang, Q.; Tian, J.; Zhang, L. Controllable Chemical Composition in Double-Perovskite Bi0.5Sm0.5FeO3 Epitaxial Thin Films for Ferroelectric, Photovoltaic, and Ferromagnetic Properties. Chem. Eng. J. 2023, 453, 139726. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Z.; Song, Y.; Yang, G.; Qian, W.; Yang, M.; Zhu, Y.; Ran, R.; Wang, W.; Zhou, W.; et al. High-Entropy Perovskite Oxide: A New Opportunity for Developing Highly Active and Durable Air Electrode for Reversible Protonic Ceramic Electrochemical Cells. Nano-Micro Lett. 2022, 14, 217. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zhivulin, V.E.; Trofimov, E.A.; Gudkova, S.A.; Punda, A.Y.; Valiulina, A.N.; Gavrilyak, M.; Zaitseva, O.V.; Taskaev, S.V.; Khandaker, M.U.; et al. A-Site Cation Size Effect on Structure and Magnetic Properties of Sm(Eu,Gd)Cr0.2Mn0.2Fe0.2Co0.2Ni0.2O3 High-Entropy Solid Solutions. Nanomaterials 2022, 12, 36. [Google Scholar] [CrossRef]
- Goko, T.; Arguello, C.J.; Hamann, A.; Wolf, T.; Lee, M.; Reznik, D.; Maisuradze, A.; Khasanov, R.; Morenzoni, E.; Uemura, Y.J. Restoration of Quantum Critical Behavior by Disorder in Pressure-Tuned (Mn,Fe)Si. npj Quantum Mater. 2017, 2, 44. [Google Scholar] [CrossRef]
- Sales, B.C.; Jin, K.; Bei, H.; Stocks, G.M.; Samolyuk, G.D.; May, A.F.; McGuire, M.A. Quantum Critical Behavior in a Concentrated Ternary Solid Solution. Sci. Rep. 2016, 6, 26179. [Google Scholar] [CrossRef]
- Chen, Q.; Han, T.; Zeng, J.; He, Z.; Liu, Y.; Sun, J.; Tang, M.; Zhang, Z.; Gao, P.; Liu, G. Perovskite-Based Memristor with 50-Fold Switchable Photosensitivity for In-Sensor Computing Neural Network. Nanomaterials 2022, 12, 2217. [Google Scholar] [CrossRef]
- Zeng, F.; Guo, Y.; Hu, W.; Tan, Y.; Zhang, X.; Feng, J.; Tang, X. Opportunity of the Lead-Free All-Inorganic Cs3Cu2I5 Perovskite Film for Memristor and Neuromorphic Computing Applications. ACS Appl. Mater. Interfaces 2020, 12, 23094–23101. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Han, H.; Park, C. Nanostructured Perovskites for Retina-Inspired Structurally Tunable Synapse. In Proceedings of the International Conference on Perovskite Memristors and Electronics 2021 (ICPME2021), Online, 13–14 December 2021. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Y.; Liu, Y. Perovskite Photodetectors and Their Application in Artificial Photonic Synapses. Chem. Commun. 2021, 57, 11429–11442. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, X.; Liu, Q.; Jiang, Y.; Zhong, W.; Luo, F. An Artificial Synapse Based on CsPbI3 Thin Film. Micromachines 2022, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Han, J.S.; Hong, K.; Kim, S.Y.; Jang, H.W. Organic–Inorganic Hybrid Halide Perovskites for Memories, Transistors, and Artificial Synapses. Adv. Mater. 2018, 30, 1704002. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Ojha, S.K.; Kumar, S.; Saha, A.; Mandal, P.; Freeland, J.W.; Middey, S. Epitaxial Stabilization of Ultra Thin Films of High Entropy Perovskite. Appl. Phys. Lett. 2020, 116, 071601. [Google Scholar] [CrossRef]
- Son, Y.; Zhu, W.; Trolier-McKinstry, S.E. Electrocaloric Effect of Perovskite High Entropy Oxide Films. Adv. Electron. Mater. 2022, 8, 2200352. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A New Class of High-Entropy Perovskite Oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Kotsonis, G.N.; Rost, C.M.; Harris, D.T.; Maria, J.-P. Epitaxial Entropy-Stabilized Oxides: Growth of Chemically Diverse Phases via Kinetic Bombardment. MRS Commun. 2018, 8, 1371–1377. [Google Scholar] [CrossRef]
- Schlom, D.G.; Chen, L.-Q.; Pan, X.; Schmehl, A.; Zurbuchen, M.A. A Thin Film Approach to Engineering Functionality into Oxides. J. Am. Ceram. Soc. 2008, 91, 2429–2454. [Google Scholar] [CrossRef]
- Krawczyk, P.A.; Jurczyszyn, M.; Pawlak, J.; Salamon, W.; Baran, P.; Kmita, A.; Gondek, Ł.; Sikora, M.; Kapusta, C.; Strączek, T.; et al. High-Entropy Perovskites as Multifunctional Metal Oxide Semiconductors: Synthesis and Characterization of (Gd0.2Nd0.2La0.2Sm0.2Y0.2)CoO3. ACS Appl. Electron. Mater. 2020, 2, 3211–3220. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-Component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Jędrusik, M.; Cieniek, Ł.; Kopia, A.; Turquat, C.; Leroux, C. Structural Characterization of LaCoO3 Thin Films Grown by Pulsed Laser Deposition. Arch. Metall. Mater. 2020, 65, 793–797. [Google Scholar] [CrossRef]
- Schwarcz, D.; Burov, S. The Effect of Disordered Substrate on Crystallization in 2D. J. Phys. Condens. Matter 2019, 31, 445401. [Google Scholar] [CrossRef] [PubMed]
- Salaheldeen, M.; Garcia, A.; Corte-Leon, P.; Ipatov, M.; Zhukova, V.; Zhukov, A. Unveiling the Effect of Annealing on Magnetic Properties of Nanocrystalline Half-Metallic Heusler Co2FeSi Alloy Glass-Coated Microwires. J. Mater. Res. Technol. 2022, 20, 4161–4172. [Google Scholar] [CrossRef]
- Biesinger, M.; Payne, B.; Grosvenor, A.; Lau, L.; Gerson, A.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Okamoto, Y.; Adachi, T.; Maezawa, A.; Imanaka, T. Effect of ZnO Addition on Cobalt–Alumina Interaction Species. Bull. Chem. Soc. Jpn. 1991, 64, 236–242. [Google Scholar] [CrossRef]
- Dash, K.; Folkesson, B.; Larsson, R.; Mohapatra, M. An XPS Investigation on a Series of Schiff Base Dioxime Ligands and Cobalt Complexes. J. Electron Spectrosc. Relat. Phenom. 1989, 49, 343–357. [Google Scholar] [CrossRef]
- Wagner, A.; Naumkin, A.; Kraut-Vass, A.; Allison, J.; Powell, C.; Rumble, J. NIST X-Ray Photoelectron Spectroscopy Database 1, Version 2; Natl Std. Ref. Data Series (NIST NSRDS); National Institute of Standards and Technology: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Wandelt, K.; Brundle, C. The Interaction of Oxygen with Gadolinium: UPS and XPS Studies. Surf. Sci. 1985, 157, 162–182. [Google Scholar] [CrossRef]
- Uwamino, Y.; Ishizuka, T.; Yamatera, H. X-Ray Photoelectron Spectroscopy of Rare-Earth Compounds. J. Electron Spectrosc. Relat. Phenom. 1984, 34, 67–78. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Mazur, Ł.; Borkowski, M.; Maćkosz, K.; Giżyński, K.; Mazur, T. Enhanced Assembly of Ag Nanoparticles for Surface-Independent Fabrication of Conductive Patterns. ACS Appl. Nano Mater. 2022, 5, 12711–12719. [Google Scholar] [CrossRef]
- Wolski, K.; Szuwarzyński, M.; Zapotoczny, S. A Facile Route to Electronically Conductive Polyelectrolyte Brushes as Platforms of Molecular Wires. Chem. Sci. 2015, 6, 1754–1760. [Google Scholar] [CrossRef]
- Jarosiński, Ł.; Pawlak, J.; Al-Ani, S.K.J. Inverse Logarithmic Derivative Method for Determining the Energy Gap and the Type of Electron Transitions as an Alternative to the Tauc Method. Opt. Mater. 2019, 88, 667–673. [Google Scholar] [CrossRef]
- Cisneros, J.I. Optical Characterization of Dielectric and Semiconductor Thin Films by Use of Transmission Data. Appl. Opt. 1998, 37, 5262–5270. [Google Scholar] [CrossRef]
- Al-Ani, S.K.J.; Hogarth, C.A. The Optical Properties of Amorphous V2O5 and SiO Thin Films and of the Mixed Dielectric System SiO/V2O5. J. Mater. Sci. 1985, 20, 1185–1192. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Kats, M.A.; Capasso, F. Optical Absorbers Based on Strong Interference in Ultra-Thin Films. Laser Photonics Rev. 2016, 10, 735–749. [Google Scholar] [CrossRef]
- Babbe, F.; Sutter-Fella, C.M. Optical Absorption-Based In Situ Characterization of Halide Perovskites. Adv. Energy Mater. 2020, 10, 1903587. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Gustafson, W.J.; Murray, S.E.; Shoemaker, D.P.; Ertekin, E.; Krogstad, J.A.; Perry, N.H. Perovskite Na-Ion Conductors Developed from Analogous Li3xLa2/3-xTiO3 (LLTO): Chemo-Mechanical and Defect Engineering. J. Mater. Chem. A 2021, 9, 21241–21258. [Google Scholar] [CrossRef]
- Katzbaer, R.R.; dos Santos Vieira, F.M.; Dabo, I.; Mao, Z.; Schaak, R.E. Band Gap Narrowing in a High-Entropy Spinel Oxide Semiconductor for Enhanced Oxygen Evolution Catalysis. J. Am. Chem. Soc. 2023, 145, 6753–6761. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Li, Z.; Zhang, G.; Yang, G.-J. Lead-Free Perovskite [H3NC6H4NH3]CuBr4 with Both a Bandgap of 1.43 EV and Excellent Stability. J. Mater. Chem. A 2020, 8, 5484–5488. [Google Scholar] [CrossRef]
- Vashishtha, P.; Bishnoi, S.; Li, C.-H.A.; Jagadeeswararao, M.; Hooper, T.J.N.; Lohia, N.; Shivarudraiah, S.B.; Ansari, M.S.; Sharma, S.N.; Halpert, J.E. Recent Advancements in Near-Infrared Perovskite Light-Emitting Diodes. ACS Appl. Electron. Mater. 2020, 2, 3470–3490. [Google Scholar] [CrossRef]
- Ding, N.; Xu, W.; Zhou, D.; Pan, G.; Li, D.; Ji, Y.; Chen, X.; Yang, D.; Bai, X.; Ma, C.-G.; et al. Upconversion Ladder Enabled Super-Sensitive Narrowband near-Infrared Photodetectors Based on Rare Earth Doped Florine Perovskite Nanocrystals. Nano Energy 2020, 76, 105103. [Google Scholar] [CrossRef]
- Alarifi, I.M. Advanced Selection Materials in Solar Cell Efficiency and Their Properties—A Comprehensive Review. Mater. Today Proc. 2023, 81, 403–414. [Google Scholar] [CrossRef]
- Milot, R.L.; Eperon, G.E.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Temperature-Dependent Charge-Carrier Dynamics in CH3NH3PbI3 Perovskite Thin Films. Adv. Funct. Mater. 2015, 25, 6218–6227. [Google Scholar] [CrossRef]
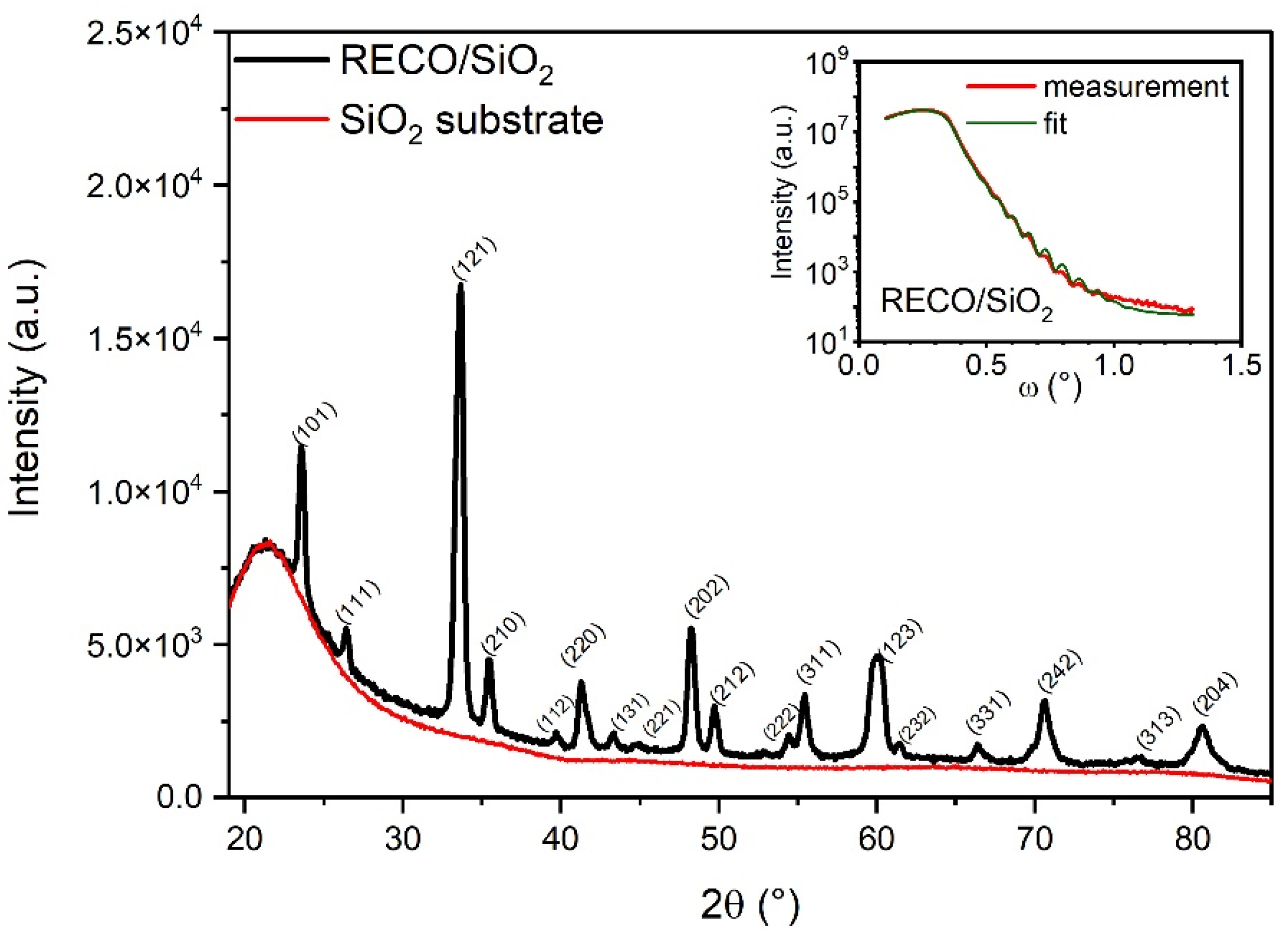
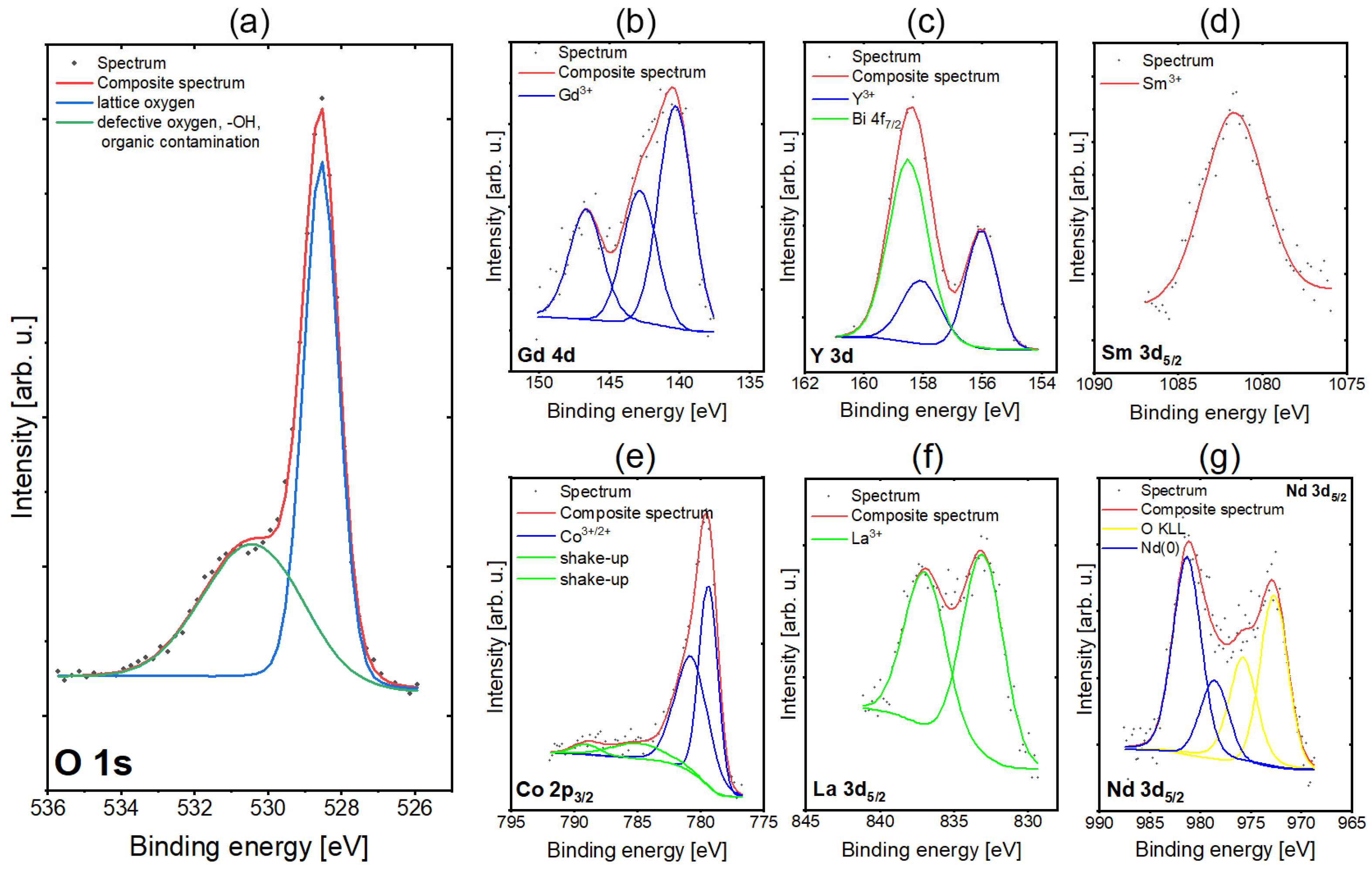
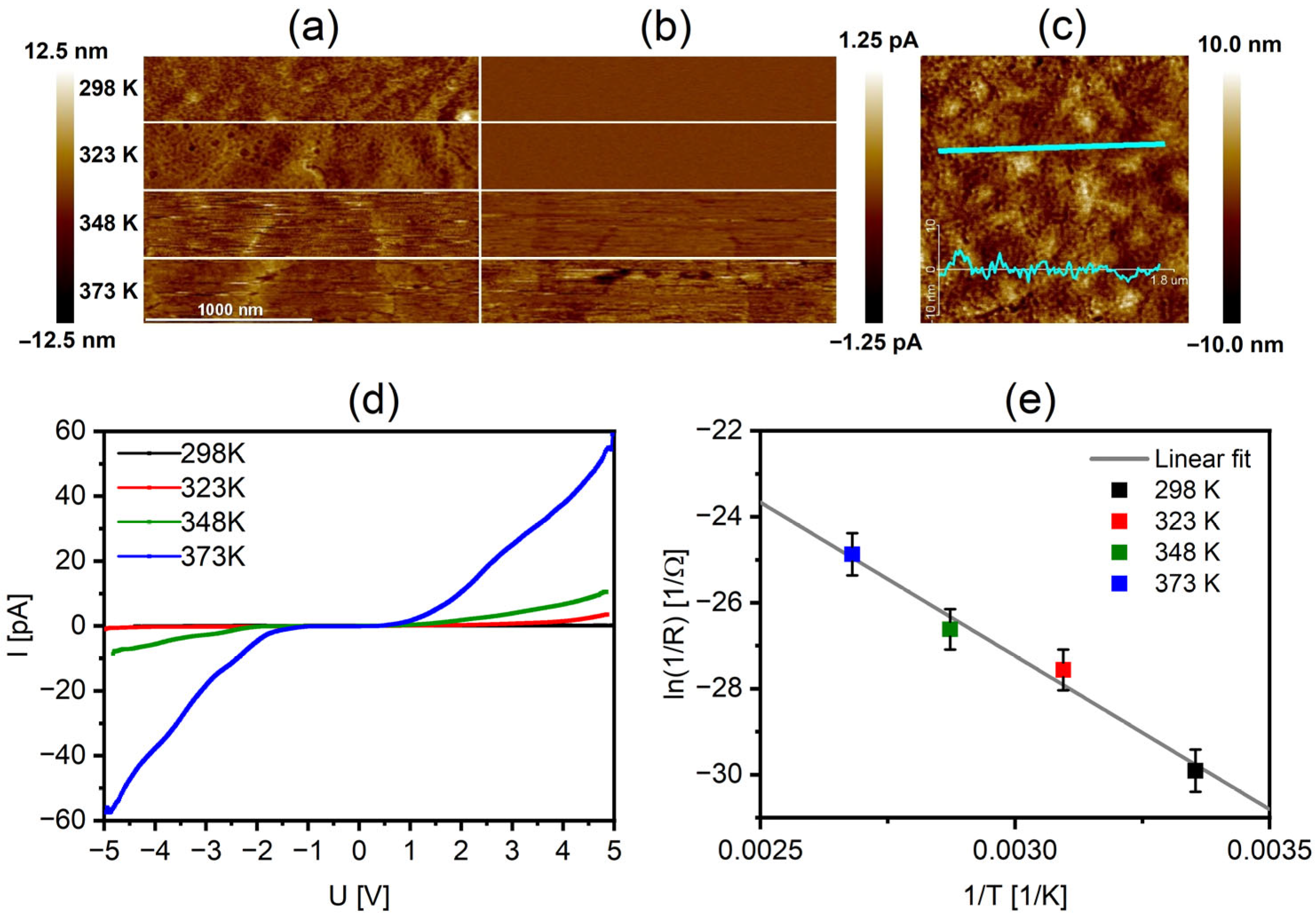

| Layer | Density [g cm−1] | Thickness [nm] | Roughness RMS [nm] |
|---|---|---|---|
| quartz substrate | 2.6 ± 0.2 | - | 1.6 ± 0.2 |
| RECO thin film | 8.0 ± 0.9 | 59.0 ± 0.5 | 2.4 ± 0.4 |
| Element | O | Co | Y | La | Nd | Sm | Gd |
|---|---|---|---|---|---|---|---|
| at-% | |||||||
| Surface | 61.1 ± 6.4 | 17.8 ± 2.1 | 5.3 ± 0.2 | 4.0 ± 0.3 | 3.3 ± 0.3 | 3.4 ± 0.2 | 4.8 ± 0.3 |
| Theoretical | 60.0 | 20.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Method | Energy Gap [eV] | R2 [−] |
|---|---|---|
| AFM U-I | 1.23 ± 0.12 | 0.9810 |
| FPRM | 0.93 ± 0.01 | 0.9995 |
| UV/VIS | 0.88 ± 0.02 | 0.9873 |
| 1.98 ± 0.04 | 0.9934 | |
| 2.66 ± 0.07 | 0.9899 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, P.A.; Salamon, W.; Marzec, M.; Szuwarzyński, M.; Pawlak, J.; Kanak, J.; Dziubaniuk, M.; Kubiak, W.W.; Żywczak, A. High-Entropy Perovskite Thin Film in the Gd-Nd-Sm-La-Y-Co System: Deposition, Structure and Optoelectronic Properties. Materials 2023, 16, 4210. https://doi.org/10.3390/ma16124210
Krawczyk PA, Salamon W, Marzec M, Szuwarzyński M, Pawlak J, Kanak J, Dziubaniuk M, Kubiak WW, Żywczak A. High-Entropy Perovskite Thin Film in the Gd-Nd-Sm-La-Y-Co System: Deposition, Structure and Optoelectronic Properties. Materials. 2023; 16(12):4210. https://doi.org/10.3390/ma16124210
Chicago/Turabian StyleKrawczyk, Pawel A., Wojciech Salamon, Mateusz Marzec, Michał Szuwarzyński, Jakub Pawlak, Jarosław Kanak, Małgorzata Dziubaniuk, Władyslaw W. Kubiak, and Antoni Żywczak. 2023. "High-Entropy Perovskite Thin Film in the Gd-Nd-Sm-La-Y-Co System: Deposition, Structure and Optoelectronic Properties" Materials 16, no. 12: 4210. https://doi.org/10.3390/ma16124210
APA StyleKrawczyk, P. A., Salamon, W., Marzec, M., Szuwarzyński, M., Pawlak, J., Kanak, J., Dziubaniuk, M., Kubiak, W. W., & Żywczak, A. (2023). High-Entropy Perovskite Thin Film in the Gd-Nd-Sm-La-Y-Co System: Deposition, Structure and Optoelectronic Properties. Materials, 16(12), 4210. https://doi.org/10.3390/ma16124210









