The Impact of Commercially Available Dry Mouth Products on the Corrosion Resistance of Common Dental Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Electrochemical Characterization
2.4. Surface Morphology Analysis
2.5. X-ray Diffraction
3. Results and Discussion
3.1. Open Circuit Potential Measurements
3.2. Electrochemical Impedance Measurement
3.3. Corrosion Analysis
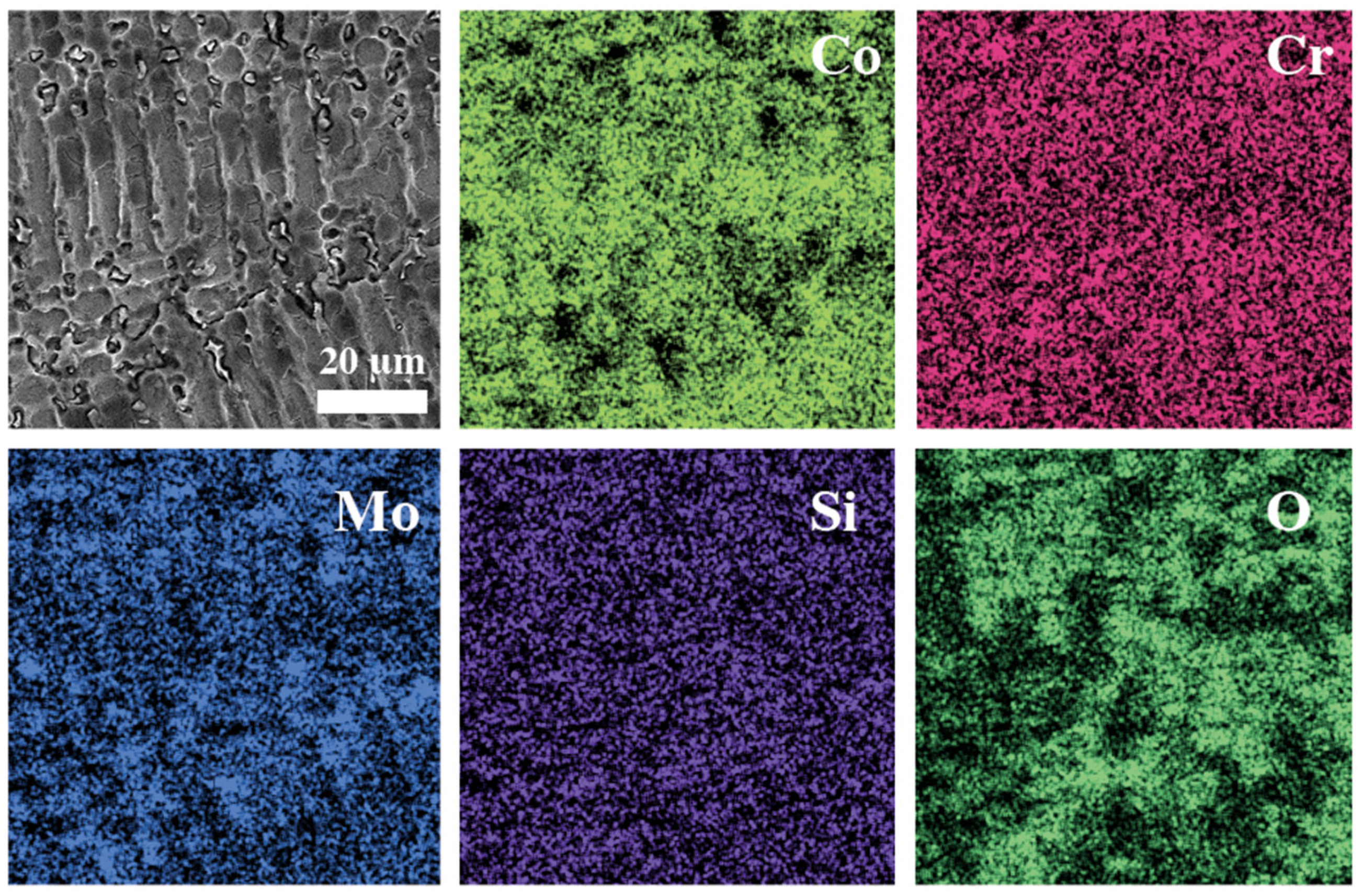
3.4. Surface Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, P.E.; Ogawa, H. Promoting Oral Health and Quality of Life of Older People—The Need for Public Health Action. Oral Health Prev. Dent. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Elagra, M.E.I.; Alhayek, A.I.A.; Al-Mutairi, B.F.M.; Aljohar, N.A.; Aladwani, R.A. Changing Trends of Prosthetic Rehabilitation of Partially Edentulous Patients Visiting a Tertiary Care Dental Hospital. J. Fam. Med. Prim. Care 2019, 8, 1914–1918. [Google Scholar] [CrossRef]
- Caracaş, A.M.; Manolea, H.O.; Mitruţ, I.; Caracaş, R.E.; Ţârtea, D.A.; Drăghici, M.A.; Nistor, C.M.L.; Alexandru, D.O. The Frequency of Dental Materials Use for Fixed Prostheses in a General Dental Practice. Curr. Health Sci. J. 2021, 47, 393–397. [Google Scholar] [CrossRef]
- Alnazzawi, A. Effect of Fixed Metallic Oral Appliances on Oral Health. J. Int. Soc. Prev. Community Dent. 2018, 8, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.M.S.; Elias, C.N.; Monteiro, E.S.; Coimbra, M.E.R.; Santana, A.I.C. Galvanic Corrosion of Ti Dental Implants Coupled to CoCrMo Prosthetic Component. Int. J. Biomater. 2021, 2021, e1313343. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; Muñoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, L.; Lin, H.; Cheng, H. Cell Death Affected by Dental Alloys: Modes and Mechanisms. Dent. Mater. J. 2017, 36, 82–87. [Google Scholar] [CrossRef]
- Martin, S.F. T Lymphocyte-Mediated Immune Responses to Chemical Haptens and Metal Ions: Implications for Allergic and Autoimmune Disease. Int. Arch. Allergy Immunol. 2004, 134, 186–198. [Google Scholar] [CrossRef]
- Amine, M.; Merdma, W.; El Boussiri, K. Electrogalvanism in Oral Implantology: A Systematic Review. Int. J. Dent. 2022, 2022, 4575416. [Google Scholar] [CrossRef]
- Gaur, S.; Agnihotri, R.; Albin, S. Bio-Tribocorrosion of Titanium Dental Implants and Its Toxicological Implications: A Scoping Review. Sci. World J. 2022, 2022, 4498613. [Google Scholar] [CrossRef]
- Opydo-Szymaczek, J.; Opydo, J.; Opydo, W. The Effects of Conductivity and PH of Saliva on Electrochemical Potentials of Metallic Dental Materials. Comput. Appl. Electr. Eng. 2015, 13, 143–152. [Google Scholar]
- Lu, Y.-P.; Huang, J.-W.; Lee, I.-N.; Weng, R.-C.; Lin, M.-Y.; Yang, J.-T.; Lin, C.-T. A Portable System to Monitor Saliva Conductivity for Dehydration Diagnosis and Kidney Healthcare. Sci. Rep. 2019, 9, 14771. [Google Scholar] [CrossRef]
- Spirk, C.; Hartl, S.; Pritz, E.; Gugatschka, M.; Kolb-Lenz, D.; Leitinger, G.; Roblegg, E. Comprehensive Investigation of Saliva Replacement Liquids for the Treatment of Xerostomia. Int. J. Pharm. 2019, 571, 118759. [Google Scholar] [CrossRef]
- Winter, C.; Keimel, R.; Gugatschka, M.; Kolb, D.; Leitinger, G.; Roblegg, E. Investigation of Changes in Saliva in Radiotherapy-Induced Head Neck Cancer Patients. Int. J. Environ. Res. Public. Health 2021, 18, 1629. [Google Scholar] [CrossRef]
- Thomas, A.; Sridhar, S.; Aghyarian, S.; Watkins-Curry, P.; Chan, J.Y.; Pozzi, A.; Rodrigues, D.C. Corrosion Behavior of Zirconia in Acidulated Phosphate Fluoride. J. Appl. Oral Sci. 2016, 24, 52–60. [Google Scholar] [CrossRef]
- Anwar, E.M.; Kheiralla, L.S.; Tammam, R.H. Effect of Fluoride on the Corrosion Behavior of Ti and Ti6Al4V Dental Implants Coupled with Different Superstructures. J. Oral Implantol. 2011, 37, 309–317. [Google Scholar] [CrossRef]
- Erdogan, A.T.; Nalbantgil, D.; Ulkur, F.; Sahin, F. Metal Ion Release from Silver Soldering and Laser Welding Caused by Different Types of Mouthwash. Angle Orthod. 2015, 85, 665–672. [Google Scholar] [CrossRef]
- Fais, L.M.G.; Fernandes-Filho, R.B.; Pereira-da-Silva, M.A.; Vaz, L.G.; Adabo, G.L. Titanium Surface Topography after Brushing with Fluoride and Fluoride-Free Toothpaste Simulating 10 Years of Use. J. Dent. 2012, 40, 265–275. [Google Scholar] [CrossRef]
- Polychronis, G.; Al Jabbari, Y.S.; Eliades, T.; Zinelis, S. Galvanic Coupling of Steel and Gold Alloy Lingual Brackets with Orthodontic Wires: Is Corrosion a Concern? Angle Orthod. 2018, 88, 450–457. [Google Scholar] [CrossRef]
- Pupim, D.; Peixoto, R.F.; Macedo, A.P.; Palma-Dibb, R.G.; de Mattos, M.d.G.C.; Galo, R. Influence of the Commercial Mouthwashes on the Corrosion Behaviour of Dental Alloy. Mater. Res. 2022, 25, e20210385. [Google Scholar] [CrossRef]
- Jafari, K.; Rahimzadeh, S.; Hekmatfar, S. Nickel Ion Release from Dental Alloys in Two Different Mouthwashes. J. Dent. Res. Dent. Clin. Dent. Prospects 2019, 13, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rincic Mlinaric, M.; Karlovic, S.; Ciganj, Z.; Acev, D.P.; Pavlic, A.; Spalj, S. Oral Antiseptics and Nickel-Titanium Alloys: Mechanical and Chemical Effects of Interaction. Odontology 2019, 107, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pavlic, A.; Perissinotto, F.; Turco, G.; Contardo, L.; Spalj, S. Do Chlorhexidine and Probiotics Solutions Provoke Corrosion of Orthodontic Mini-Implants? An In Vitro Study. Int. J. Oral Maxillofac. Implants 2019, 33, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.-A.; Silvestre, F.-J.; Barrios, R.; Silvestre-Rangil, J. Treatment of Xerostomia and Hyposalivation in the Elderly: A Systematic Review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e355–e366. [Google Scholar] [CrossRef]
- Lung, C.B.; Watson, G.E.; Verma, S.; Feng, C.; Saunders, R.H. Duration of Effect of Biotène Spray in Patients with Symptomatic Dry Mouth: A Pilot Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 415–421. [Google Scholar] [CrossRef]
- Barbe, A.G.; Ludwar, L.; Hamacher, S.; Noack, M.J. Efficacy of a Newly Developed Mouth Gel for Xerostomia Relief-A Randomized Double-Blind Trial. Oral Dis. 2019, 25, 1519–1529. [Google Scholar] [CrossRef]
- Qureshi, S.; Milić, L.; Petrović, B.; Vejin, M.; Kojić, S.; Jarić, S.; Stojanović, G. The Measurement of Contact Angle, PH, and Conductivity of Artificial Saliva and Mouthwashes on Enamel, Glass-Ionomer, and Composite Dental Materials. Materials 2022, 15, 4533. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023. [Google Scholar] [CrossRef]
- Shalnova, S.A.; Gushchina, M.O.; Strekalovskaya, D.A.; Alekseeva, E.L.; Klimova-Korsmik, O.G. Electrochemical Properties of the Heat-Treated Ti-6Al-4V Alloy Manufactured by Direct Energy Deposition. J. Alloys Compd. 2022, 899, 163226. [Google Scholar] [CrossRef]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular Analysis of Chromium and Cobalt-Related Toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef]
- Posada, O.M.; Tate, R.J.; Grant, M.H. Toxicity of Cobalt–Chromium Nanoparticles Released from a Resurfacing Hip Implant and Cobalt Ions on Primary Human Lymphocytes in Vitro. J. Appl. Toxicol. 2015, 35, 614–622. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Kurz, S.; Virtanen, S.; Fervel, V.; Olsson, C.-O.A.; Mischler, S. Passive and Transpassive Behaviour of CoCrMo in Simulated Biological Solutions. Electrochim. Acta 2004, 49, 2167–2178. [Google Scholar] [CrossRef]
- Pound, B.G. Corrosion Behavior of Metallic Materials in Biomedical Applications. II. Stainless Steels and Co-Cr Alloys. Corros. Rev. 2014, 32, 21–41. [Google Scholar] [CrossRef]
- Li, K.C.; Prior, D.J.; Waddell, J.N.; Swain, M.V. Comparison of the Microstructure and Phase Stability of As-Cast, CAD/CAM and Powder Metallurgy Manufactured Co–Cr Dental Alloys. Dent. Mater. 2015, 31, e306–e315. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; van Engen, P.G.; Jongebreur, R. Magneto-Optical Properties of Metallic Ferromagnetic Materials. J. Magn. Magn. Mater. 1983, 38, 1–22. [Google Scholar] [CrossRef]
- Rideout, S.; Manly, W.D.; Kamen, E.L.; Lement, B.S.; Beck, P.A. Intermediate Phases in Ternary Alloy Systems of Transition Elements. JOM 1951, 3, 872–876. [Google Scholar] [CrossRef]







| Studied Solution | Manufacturer | Composition | pH |
|---|---|---|---|
| Fusayama Meyer’s artificial saliva (AS) | The solution was prepared just before the experiment following the formulation | NaCl—0.4 g, KCl—0.4 g, CaCl2·2H2O—0.8 g, Na2HPO4—0.7 g, Urea 1.0 g, and distilled water up to 1.0 L | 7.0 |
| Buccotherm Fresh Breath Spray | LaboratoireOdost, France | Castera-Verduzan thermal water, Alcohol, Xylitol, Glycerin, Camelia sinesis leaf water, Mentha piperita leaf water, Aroma, Limonene, Benzyl alcohol, and Dehydroacetic acid | 7.0 |
| Buccotherm Dental Spray | LaboratoireOdost, France | 100% Castéra-Verduzan Thermal Spring water | 6.9 |
| Xerostom Mouth Spray | Biocosmetics Laboratories, Spain | Xylitol, Glycerin, Betaine, Panthenol, Carum Petroselinum (Parsley Oil), Calcium Lactate, PEG-40 Hydrogenated Castor Oil, Allantoin, Olea Europaea (Virgin Olive Oil), Tocopheryl Acetate, Aqua, Propylene Glycol, Aroma, D-limonene, Lactic acid, Sodium Methylparaben, Sodium Propylparaben, and Diazolidinyl Urea | 7.0 |
| Aquamed mundspray | Hager&Werken GmbH, Germany | Xylitol, Eriodictyon californicum flower/leaf/stem extract, PEG-40 Hydrogenated Castor Oil, Dipotassium phosphate, Lysozyme hydrochloride, Mentha arvensis leaf oil, Magnesium chloride, Calcium chloride, Aqua, Butylene glycol, Aroma, Limonene, Potassium sorbate, Sodium Benzoate, Citric acid, Sodium chloride, and Xanthan gum | 6.8 |
| GC Dry Mouth | GC, USA | Diglycerin, Sodium Citrate, Aqua, Aroma, Ethylparaben, Benzyl Alcohol, Cellulose Gum, and Carrageenan | 6.86 |
| Xerostom gel | Biocosmetics Laboratories, Spain | Xylitol, Glycerin, Betaine, Panthenol, Potassium Citrate, Potassium phosphate, Calcium Lactate, Tetrapotassium Pyrophosphate, Olea Europaea Fruit Oil (Extra Virgin Olive Oil/Aceite de Oliva Virgen Extra), Tocopheryl Acetate, Aqua, Aroma, Sodium Propylparaben, Sodium Benzoate, Carbomer, and Xanthan Gum | 7.03 |
| Electrode | Solution | Ecorr, V | icorr, A cm−2 | ipass, A cm−2 | Epitt, V | ipitt, A cm2 |
|---|---|---|---|---|---|---|
| Ti64 | Fusayama Meyer’s artificial saliva (AS) | −0.66 | 4.0 × 10−7 | 3.4 × 10−5 | >5 V | N/A |
| Buccoterm Fresh Breath Spray | −0.06 | 1.2 × 10−6 | 4.3 × 10−6 | >5 V | N/A | |
| Buccoterm Dental Spray | −0.26 | 4.1 × 10−6 | 1.3 × 10−5 | >5 V | N/A | |
| Aquamed mundspray | −0.03 | 6.1 × 10−6 | 8.7 × 10−5 | >5 V | N/A | |
| CoCr | Fusayama Meyer’s artificial saliva (AS) | −0.82 | 3.9 × 10−7 | 9.8 × 10−7 | 0.7 | 2.1 × 10−5 |
| Buccoterm Fresh Breath Spray | −0.07 | 8.4 × 10−6 | 6.3 × 10−6 | 1.4 | 1.2 × 10−5 | |
| Buccoterm Dental Spray | −0.11 | 3.3 × 10−5 | 7.1 × 10−5 | 1.5 | 1.5 × 10−4 | |
| Aquamed mundspray | 0.11 | 3.5 × 10−5 | 1.1 × 10−4 | 1.4 | 1.3 × 10−4 | |
| -Ti64+CoCr | Fusayama Meyer’s artificial saliva (AS) | −0.11 | 6.3 × 10−7 | 1.1 × 10−6 | 1.44 | 1.9 × 10−6 |
| +Ti64-CoCr | Fusayama Meyer’s artificial saliva (AS) | −0.01 | 3.3 × 10−7 | 3.0 × 10−5 | >5 V | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkina, A.Y.; Makeeva, I.M.; Dubinin, O.N.; Bondareva, J.V.; Chernodoubov, D.A.; Shibalova, A.A.; Arzukanyan, A.V.; Antoshin, A.A.; Timashev, P.S.; Evlashin, S.A. The Impact of Commercially Available Dry Mouth Products on the Corrosion Resistance of Common Dental Alloys. Materials 2023, 16, 4195. https://doi.org/10.3390/ma16114195
Turkina AY, Makeeva IM, Dubinin ON, Bondareva JV, Chernodoubov DA, Shibalova AA, Arzukanyan AV, Antoshin AA, Timashev PS, Evlashin SA. The Impact of Commercially Available Dry Mouth Products on the Corrosion Resistance of Common Dental Alloys. Materials. 2023; 16(11):4195. https://doi.org/10.3390/ma16114195
Chicago/Turabian StyleTurkina, Anna Yu., Irina M. Makeeva, Oleg N. Dubinin, Julia V. Bondareva, Daniil A. Chernodoubov, Anastasia A. Shibalova, Alina V. Arzukanyan, Artem A. Antoshin, Peter S. Timashev, and Stanislav A. Evlashin. 2023. "The Impact of Commercially Available Dry Mouth Products on the Corrosion Resistance of Common Dental Alloys" Materials 16, no. 11: 4195. https://doi.org/10.3390/ma16114195
APA StyleTurkina, A. Y., Makeeva, I. M., Dubinin, O. N., Bondareva, J. V., Chernodoubov, D. A., Shibalova, A. A., Arzukanyan, A. V., Antoshin, A. A., Timashev, P. S., & Evlashin, S. A. (2023). The Impact of Commercially Available Dry Mouth Products on the Corrosion Resistance of Common Dental Alloys. Materials, 16(11), 4195. https://doi.org/10.3390/ma16114195






