A Facile and Eco-Friendly Hydrothermal Synthesis of High Tetragonal Barium Titanate with Uniform and Controllable Particle Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of BT Powders
2.3. Materials Characterization
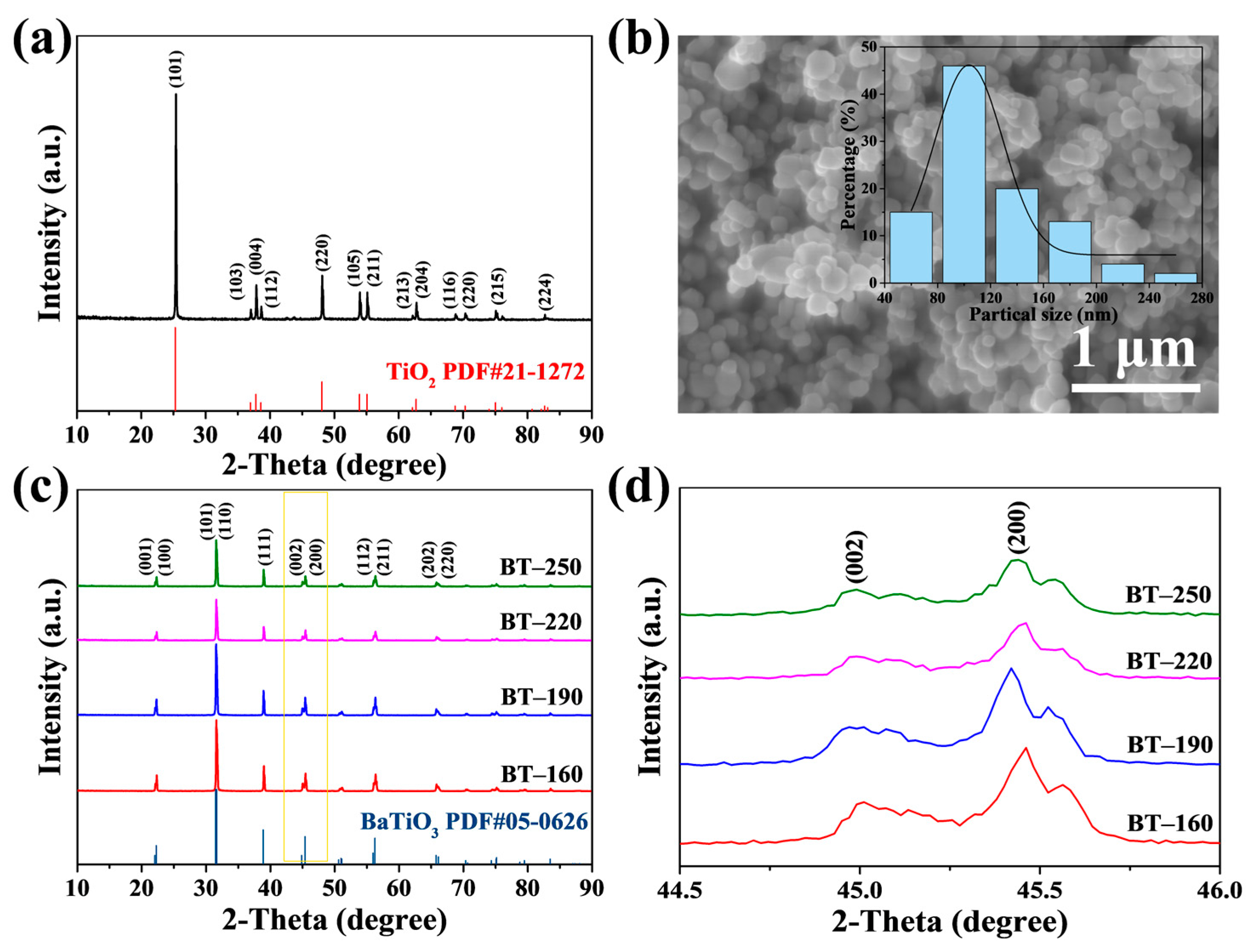
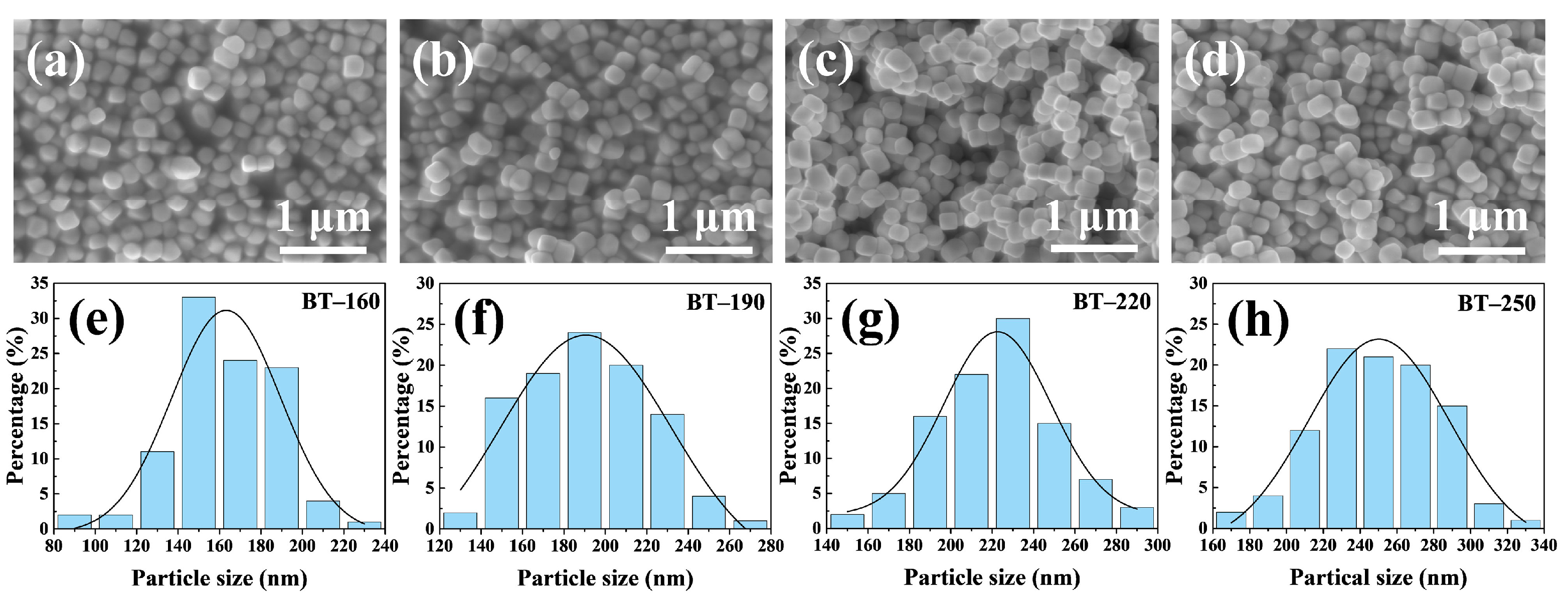
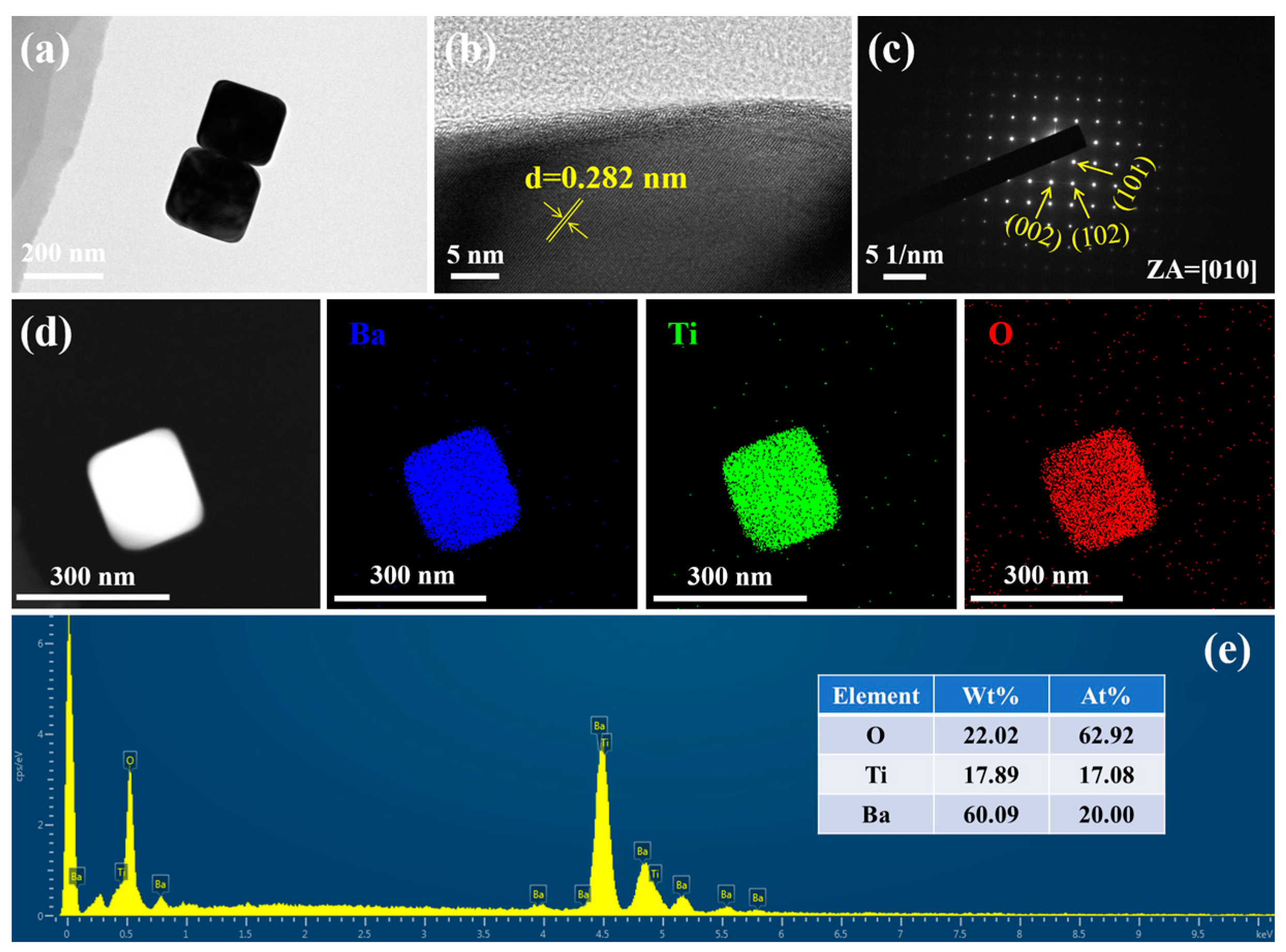
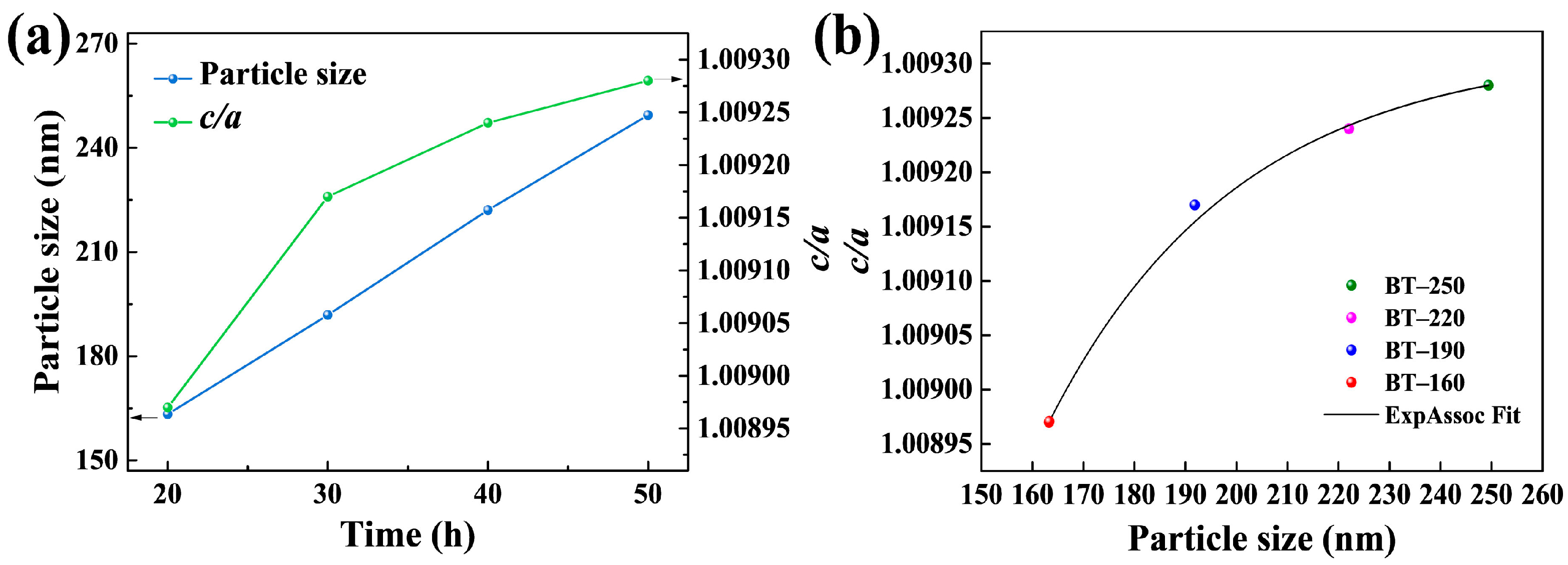
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, E.; Kim, D.H.; Jeong, E.J.; Choi, M.; Kim, Y.; Jung, H.J.; Choi, M.Y. Effects of Particle Size and Polymorph Type of TiO2 on the Properties of BaTiO3 Nanopowder Prepared by Solid-State Reaction. Environ. Res. 2021, 202, 111668. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Fang, L.; Xie, W.; Zhou, H.; Wang, Y.; Peng, C. Study on the Hydrothermal Synthesis of Barium Titanate Nano-Powders and Calcination Parameters. J. Mater. Sci. Mater. Electron. 2015, 26, 8555–8562. [Google Scholar] [CrossRef]
- Zhu, C.; Cai, Z.; Guo, L.; Li, L.; Wang, X. Grain Size Engineered High-Performance Nanograined BaTiO3-Based Ceramics: Experimental and Numerical Prediction. J. Am. Ceram. Soc. 2020, 104, 273–283. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, J.; Zhao, Y.; Yan, Z.; Zhu, G.; Liu, L.; Xu, H.; Yu, A. Synthesis of Tetragonal BaTiO3 Nano-Particle Via a Novel Tartaric Acid Co-Precipitation Process. Ceram. Int. 2021, 47, 7263–7267. [Google Scholar] [CrossRef]
- Usher, T.-M.; Kavey, B.; Caruntu, G.; Page, K. Effect of BaCO3 Impurities on the Structure of BaTiO3 Nanocrystals: Implications for Multilayer Ceramic Capacitors. ACS Appl. Nano Mater. 2020, 3, 9715–9723. [Google Scholar] [CrossRef]
- Gromada, M.; Biglar, M.; Trzepieciński, T.; Stachowicz, F. Characterization of BaTiO3 Piezoelectric Perovskite Material for Multilayer Actuators. Bull. Mat. Sci. 2017, 40, 759–771. [Google Scholar] [CrossRef]
- On, D.V.; Vuong, L.D.; Chuong, T.V.; Quang, D.A.; Tung, V.T. Study on the Synthesis and Application of BaTiO3 Nanospheres. Int. J. Mater. Res. 2021, 112, 448–456. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Zhu, G.; Yu, A. Synthesis and Characterization of Tetragonal Barium Titanate Powders by an Ion-Exchange and Hydrothermal Process. J. Ceram. Process. Res. 2015, 16, 372–375. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef]
- Cui, X.C.; Wang, J.; Zhang, X.Y.; Wang, Q.; Song, M.M.; Chai, J.L. Preparation of Nano-TiO2 by a Surfactant-Free Microemulsion–Hydrothermal Method and Its Photocatalytic Activity. Langmuir 2019, 35, 9255–9263. [Google Scholar] [CrossRef]
- Özen, M.; Mertens, M.; Snijkers, F.; Cool, P. Hydrothermal Synthesis and Formation Mechanism of Tetragonal Barium Titanate in a Highly Concentrated Alkaline Solution. Ceram. Int. 2016, 42, 10967–10975. [Google Scholar] [CrossRef]
- Ding, S.S.; Chen, L.; Liao, J.; Huo, Q.; Wang, Q.; Tian, G.; Yin, W.Y. Harnessing Hafnium-Based Nanomaterials for Cancer Diagnosis and Therapy. Small 2023, 2300341. [Google Scholar] [CrossRef]
- Hao, Y.; Feng, Z.; Banerjee, S.; Wang, X.; Billinge, S.J.L.; Wang, J.; Jin, K.; Bi, K.; Li, L. Ferroelectric State and Polarization Switching Behaviour of Ultrafine BaTiO3 Nanoparticles with Large-Scale Size Uniformity. J. Mater. Chem. C 2021, 9, 5267–5276. [Google Scholar] [CrossRef]
- Su, C.Y.; Otsuka, Y.; Huang, C.Y.; Hennings, D.F.; Pithan, C.; Shiao, F.T.; Waser, R. Grain Growth and Crystallinity of Ultrafine Barium Titanate Particles Prepared by Various Routes. Ceram. Int. 2013, 39, 6673–6680. [Google Scholar] [CrossRef]
- Li, R.J.; Wei, W.X.; Hai, J.L.; Gao, L.X.; Gao, Z.W.; Fan, Y.Y. Preparation and Electric-Field Response of Novel Tetragonal Barium Titanate. J. Alloy. Compd. 2013, 574, 212–216. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, H.; Ma, S. Effects of Annealing Temperature on Structural Phase Transition and Microstructure Evolution of Hydrothermally Synthesized Barium Titanate Nanoparticles. Mater. Res. Express 2022, 9, 065001. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Q.; Hosono, E.; Asakura, D.; Miyawaki, J.; Harada, Y. Tetragonal Distortion of a BaTiO3/Bi0.5Na0.5TiO3 Nanocomposite Responsible for Anomalous Piezoelectric and Ferroelectric Behaviors. ACS Omega 2020, 5, 22800–22807. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, X.; Jia, Y.; Huang, M.; Wang, F.; Zhang, X.; Bai, Y.; Yuan, G.; Wang, Y. Piezo-Catalysis for Nondestructive Tooth Whitening. Nat. Commun. 2020, 11, 1328. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, D.; Gong, S.; Luo, W. Multishell Structure and Size Effect of Barium Titanate Nanoceramics Induced by Grain Surface Effects. Phys. Status Solidi B 2010, 247, 219–224. [Google Scholar] [CrossRef]
- Nakashima, K.; Onagi, K.; Kobayashi, Y.; Ishigaki, T.; Ishikawa, Y.; Yoneda, Y.; Yin, S.; Kakihana, M.; Sekino, T. Stabilization of Size-Controlled BaTiO3 Nanocubes Via Precise Solvothermal Crystal Growth and Their Anomalous Surface Compositional Reconstruction. ACS Omega 2021, 6, 9410–9425. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, H.; Shi, F.; Wang, J. Effect of Polyethylene Glycol on BaTiO3 Nanoparticles Prepared by Hydrothermal Preparation. IET Nanodielectr. 2020, 3, 69–73. [Google Scholar] [CrossRef]
- Maček Kržmanc, M.; Bračko, I.; Budič, B.; Suvorov, D.; Parans Paranthaman, M. The Morphology Control of BaTiO3 Particles Synthesized in Water and a Water/Ethanol Solvent. J. Am. Ceram. Soc. 2013, 96, 3401–3409. [Google Scholar] [CrossRef]
- Habib, A.; Stelzer, N.; Angerer, P.; Haubner, R. Effect of Temperature and Time on Solvothermal Synthesis of Tetragonal BaTiO3. Bull. Mat. Sci. 2011, 34, 19–23. [Google Scholar] [CrossRef]
- Pang, X.X.; Wang, T.T.; Liu, B.; Fan, X.Y.; Liu, X.R.; Shen, J.; Zhong, C.; Hu, W.B. Effect of Solvents on the Morphology and Structure of Barium Titanate Synthesized by a One-Step Hydrothermal Method. Int. J. Miner., Metall. Mater. 2023, 30, 1407–1416. [Google Scholar] [CrossRef]
- Pfaff, G. BaTiO3 Preparation by Reaction of TiO2 with Ba(OH)2. J. Eur. Ceram. Soc. 1991, 8, 35–39. [Google Scholar] [CrossRef]
- Jain, S.; Khire, V.H.; Kandasubramanian, B. Barium Titanate: A Novel Perovskite Oxide Burning Rate Modifier for Htpb/Ap/Al Based Composite Propellant Formulations. Propellants Explos. Pyrotech. 2019, 44, 505–512. [Google Scholar] [CrossRef]
- Han, M.; Rong, Y.; Li, Q.; Xing, X.; Kang, L. Thermal Expansion of Nano-Sized BaTiO3. CrystEngComm 2015, 17, 1944–1951. [Google Scholar] [CrossRef]
- Baek, C.; Yun, J.H.; Wang, H.S.; Wang, J.E.; Park, H.; Park, K.-I.; Kim, D.K. Enhanced Output Performance of a Lead-Free Nanocomposite Generator Using BaTiO3 Nanoparticles and Nanowires Filler. Appl. Surf. Sci. 2018, 429, 164–170. [Google Scholar] [CrossRef]
- Hayashi, H.; Ebina, T. Effect of Hydrothermal Temperature on the Tetragonality of BaTiO3 Nanoparticles and in-Situ Raman Spectroscopy under Tetragonal Cubic Transformation. J. Ceram. Soc. Jpn. 2018, 126, 214–220. [Google Scholar] [CrossRef]
- Baek, C.; Wang, J.E.; Moon, S.; Choi, C.-H.; Kim, D.K.; Riman, R. Formation and Accumulation of Intragranular Pores in the Hydrothermally Synthesized Barium Titanate Nanoparticles. J. Am. Ceram. Soc. 2016, 99, 3802–3808. [Google Scholar] [CrossRef]
- Noma, T.; Wada, S.; Yano, M.; Suzuki, T. Analysis of Lattice Vibration in Fine Particles of Barium Titanate Single Crystal Including the Lattice Hydroxyl Group. J. Appl. Phys. 1996, 80, 5223–5233. [Google Scholar] [CrossRef]
- Ji, X.; Zhu, Y.; Lian, X.; Fan, B.; Liu, X.; Xiao, P.; Zhang, Y. Hydroxylation Mechanism of Phase Regulation of Nanocrystal BaTiO3 Synthesized by a Hydrothermal Method. Ceram. Int. 2022, 48, 2281–2288. [Google Scholar] [CrossRef]
- Venkateswaran, U.D.; Naik, V.M.; Naik, R. High-Pressure Raman Studies of Polycrystalline BaTiO3. Phys. Rev. B 1998, 58, 14256–14260. [Google Scholar] [CrossRef]
- DiDomenico, M.; Wemple, S.H.; Porto, S.P.S.; Bauman, R.P. Raman Spectrum of Single-Domain BaTiO3. Phys. Rev. 1968, 174, 522–530. [Google Scholar] [CrossRef]
- Shi, T.; Xie, L.; Gu, L.; Zhu, J. Why Sn Doping Significantly Enhances the Dielectric Properties of Ba(Ti1−X Snx)O3. Sci. Rep. 2015, 5, 8606. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, K.; Wang, J.; Gu, Q.; Cao, Y.; Zheng, H.; Liu, J.; Qiu, J. Microwave-Assisted Sol–Hydrothermal Synthesis of Tetragonal Barium Titanate Nanoparticles with Hollow Morphologies. J. Mater. Sci. Mater. Electron. 2015, 26, 1597–1601. [Google Scholar] [CrossRef]
- Wang, L.; Lv, J.; Shi, F.; Song, K.; Lei, W.; Zhou, H.; Qi, Z.-M.; Wang, J. Intrinsic Dielectric Properties and Lattice Vibrational Characteristics of Single Phase BaTiO3 Ceramic. J. Mater. Sci. Mater. Electron. 2021, 32, 24041–24049. [Google Scholar] [CrossRef]
- Küçük, Ö.; Teber, S.; Cihan Kaya, İ.; Akyıldız, H.; Kalem, V. Photocatalytic Activity and Dielectric Properties of Hydrothermally Derived Tetragonal BaTiO3 Nanoparticles Using TiO2 Nanofibers. J. Alloy. Compd. 2018, 765, 82–91. [Google Scholar] [CrossRef]
- Amaechi, I.C.; Katoch, R.; Kolhatkar, G.; Sun, S.; Ruediger, A. Particle Size Effect on the Photocatalytic Kinetics of Barium Titanate Powders. Catal. Sci. Technol. 2020, 10, 6274–6284. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, K.; Liu, J.; Qiu, J. Fabrication of BaTiO3 Nanoparticles and Its Formation Mechanism Using the High Temperature Mixing Method under Hydrothermal Conditions. Adv. Powder Technol. 2014, 25, 853–858. [Google Scholar] [CrossRef]
- Sonkusare, V.N.; Chaudhary, R.G.; Bhusari, G.S.; Mondal, A.; Potbhare, A.K.; Mishra, R.K.; Juneja, H.D.; Abdala, A.A. Mesoporous Octahedron-Shaped Tricobalt Tetroxide Nanoparticles for Photocatalytic Degradation of Toxic Dyes. ACS Omega 2020, 5, 7823–7835. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xia, H.; Fu, Y.; Xu, Z.; Ni, Q.-Q. Controlled Hydrothermal Synthesis of Different Sizes of BaTiO3 Nano-Particles for Microwave Absorption. Mater. Res. Express 2020, 6, 1250i3. [Google Scholar] [CrossRef]
- Potbhare, A.K.; Chaudhary, R.G.; Chouke, P.B.; Yerpude, S.; Mondal, A.; Sonkusare, V.N.; Rai, A.R.; Juneja, H.D. Phytosynthesis of Nearly Monodisperse Cuo Nanospheres Using Phyllanthus Reticulatus/Conyza Bonariensis and Its Antioxidant/Antibacterial Assays. Mater. Sci. Eng. C 2019, 99, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Chouke, P.B.; Potbhare, A.K.; Meshram, N.P.; Rai, M.M.; Dadure, K.M.; Chaudhary, K.; Rai, A.R.; Desimone, M.F.; Chaudhary, R.G.; Masram, D.T. Bioinspired Nio Nanospheres: Exploring in Vitro Toxicity Using Bm-17 and L. Rohita Liver Cells, DNA Degradation, Docking, and Proposed Vacuolization Mechanism. ACS Omega 2022, 7, 6869–6884. [Google Scholar] [CrossRef]
- Taheri, M.; Zanca, B.; Dolgos, M.; Bryant, S.; Trudel, S. Water-Dispersible and Ferroelectric Pegylated Barium Titanate Nanoparticles. Mater. Adv. 2021, 2, 5089–5095. [Google Scholar] [CrossRef]
- Yaseen, H.; Baltianski, S.; Tsur, Y. Effect of Incorporating Method of Niobium on the Properties of Doped Barium Titanate Ceramics. J. Am. Ceram. Soc. 2006, 89, 1584–1589. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Li, W.L.; Xu, L.L.; Zhu, Y.F. High Photocatalytic Oxygen Evolution Via Strong Built-in Electric Field Induced by High Crystallinity of Perylene Imide Supramolecule. Adv. Mater. 2022, 34, e2102354. [Google Scholar] [CrossRef]
- Choi, I.; Lee, S.J.; Kim, J.C.; Kim, Y.G.; Hyeon, D.Y.; Hong, K.S.; Suh, J.; Shin, D.; Jeong, H.Y.; Park, K.I. Piezoelectricity of Picosecond Laser-Synthesized Perovskite BaTiO3 Nanoparticles. Appl. Surf. Sci. 2020, 511, 145614. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, D.; Gong, S. Core-Shell Structure and Size Effect in Barium Titanate Nanoparticle. Phys. B 2011, 406, 1317–1322. [Google Scholar] [CrossRef]
- Pasuk, I.; Neatu, F.; Neatu, S.; Florea, M.; Istrate, C.M.; Pintilie, I.; Pintilie, L. Structural Details of BaTiO3 Nano-Powders Deduced from the Anisotropic XRD Peak Broadening. Nanomaterials 2021, 11, 1121. [Google Scholar] [CrossRef]
- Zamperlin, N.; Ceccato, R.; Fontana, M.; Pegoretti, A.; Chiappini, A.; Dire, S. Effect of Hydrothermal Treatment and Doping on the Microstructural Features of Sol-Gel Derived BaTiO3 Nanoparticles. Materials 2021, 14, 4345. [Google Scholar] [CrossRef]
- Meng, H.; Chen, Z.; Lu, Z.; Wang, X.; Fu, X. Hydrothermal Synthesis of Tetragonal Barium Titanate Nanopowders under Moderate Conditions. Process. Appl. Ceram. 2021, 15, 179–183. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Yin, X.; Xing, C.; Li, J.; Qiao, H.; Shi, F. Hydrothermal Synthesis of BaTiO3 Nanoparticles and Role of Pva Concentration in Preparation. Mater. Res. Express 2019, 6, 055028. [Google Scholar] [CrossRef]
- Li, J.; Inukai, K.; Tsuruta, A.; Takahashi, Y.; Shin, W. Synthesis of Highly Disperse Tetragonal BaTiO3 Nanoparticles with Core-Shell by a Hydrothermal Method. J. Asian. Ceram. Soc. 2018, 5, 444–451. [Google Scholar] [CrossRef]
- Huang, Y.A.; Lu, B.; Li, D.D.; Tang, Z.H.; Yao, Y.B.; Tao, T.; Liang, B.; Lu, S.G. Control of Tetragonality Via Dehydroxylation of BaTiO3 Ultrafine Powders. Ceram. Int. 2017, 43, 16462–16466. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Pang, X.; Liu, B.; Liu, J.; Shen, J.; Zhong, C. A Facile and Eco-Friendly Hydrothermal Synthesis of High Tetragonal Barium Titanate with Uniform and Controllable Particle Size. Materials 2023, 16, 4191. https://doi.org/10.3390/ma16114191
Wang T, Pang X, Liu B, Liu J, Shen J, Zhong C. A Facile and Eco-Friendly Hydrothermal Synthesis of High Tetragonal Barium Titanate with Uniform and Controllable Particle Size. Materials. 2023; 16(11):4191. https://doi.org/10.3390/ma16114191
Chicago/Turabian StyleWang, Tingting, Xiaoxiao Pang, Bin Liu, Jie Liu, Jing Shen, and Cheng Zhong. 2023. "A Facile and Eco-Friendly Hydrothermal Synthesis of High Tetragonal Barium Titanate with Uniform and Controllable Particle Size" Materials 16, no. 11: 4191. https://doi.org/10.3390/ma16114191
APA StyleWang, T., Pang, X., Liu, B., Liu, J., Shen, J., & Zhong, C. (2023). A Facile and Eco-Friendly Hydrothermal Synthesis of High Tetragonal Barium Titanate with Uniform and Controllable Particle Size. Materials, 16(11), 4191. https://doi.org/10.3390/ma16114191







