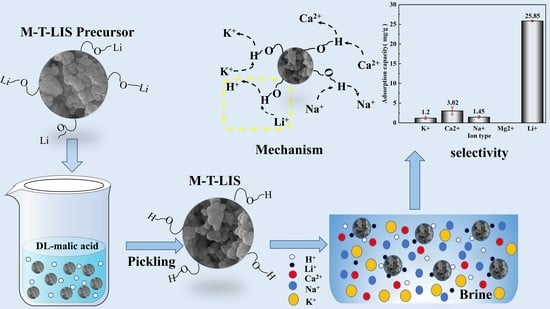
Manganese-Titanium Mixed Ion Sieves for the Selective Adsorption of Lithium Ions from an Artificial Salt Lake Brine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of M-T-LIS
2.3. Material Characterization
2.3.1. Lithium Adsorptive Experiments
2.3.2. Equilibrium Isotherm Studies
2.3.3. Adsorption Kinetics Studies
2.3.4. Recyclability of the Adsorbents
2.3.5. Selective Experiment
3. Results and Discussion
3.1. TGA-DSC and XRD Analysis
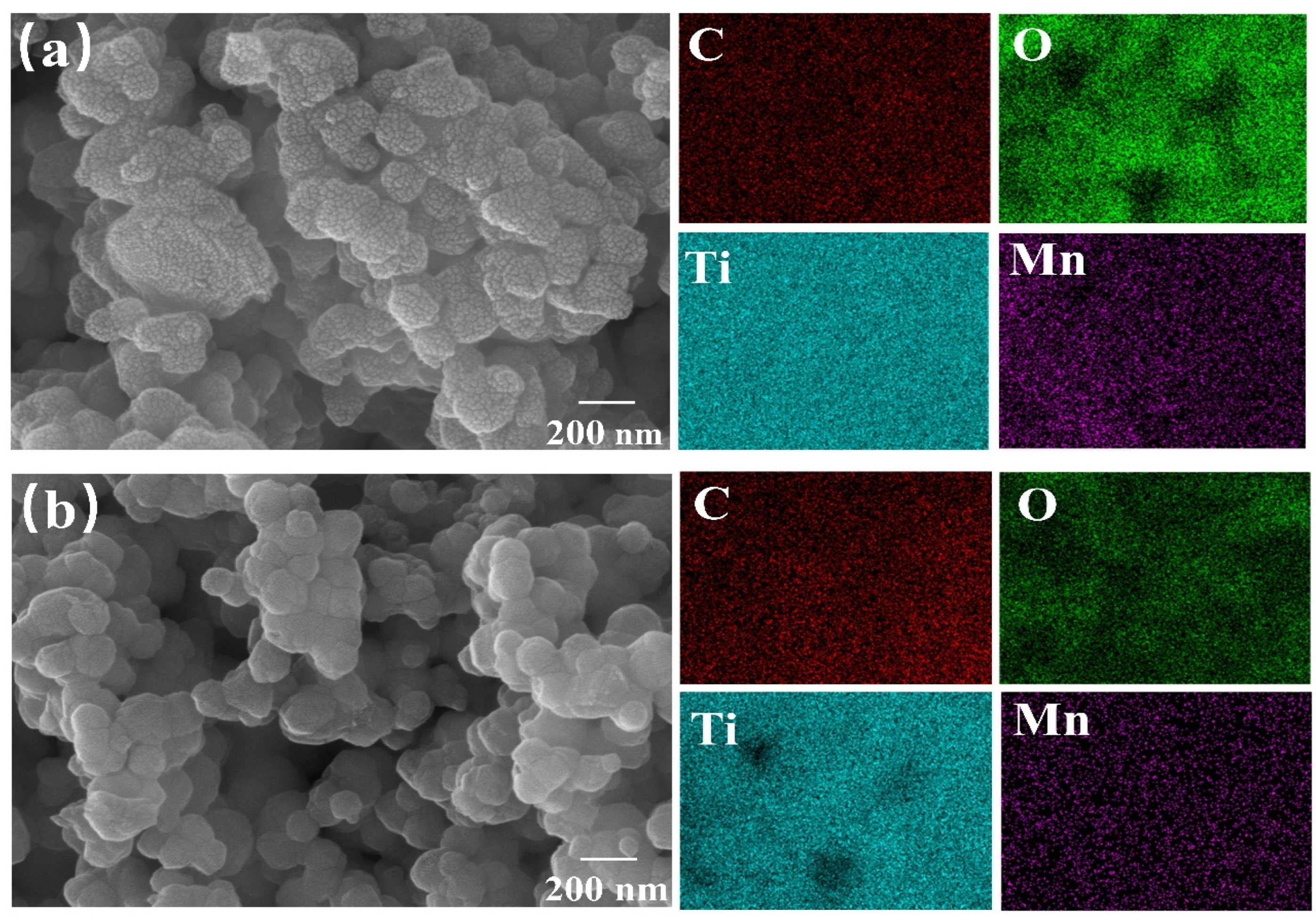
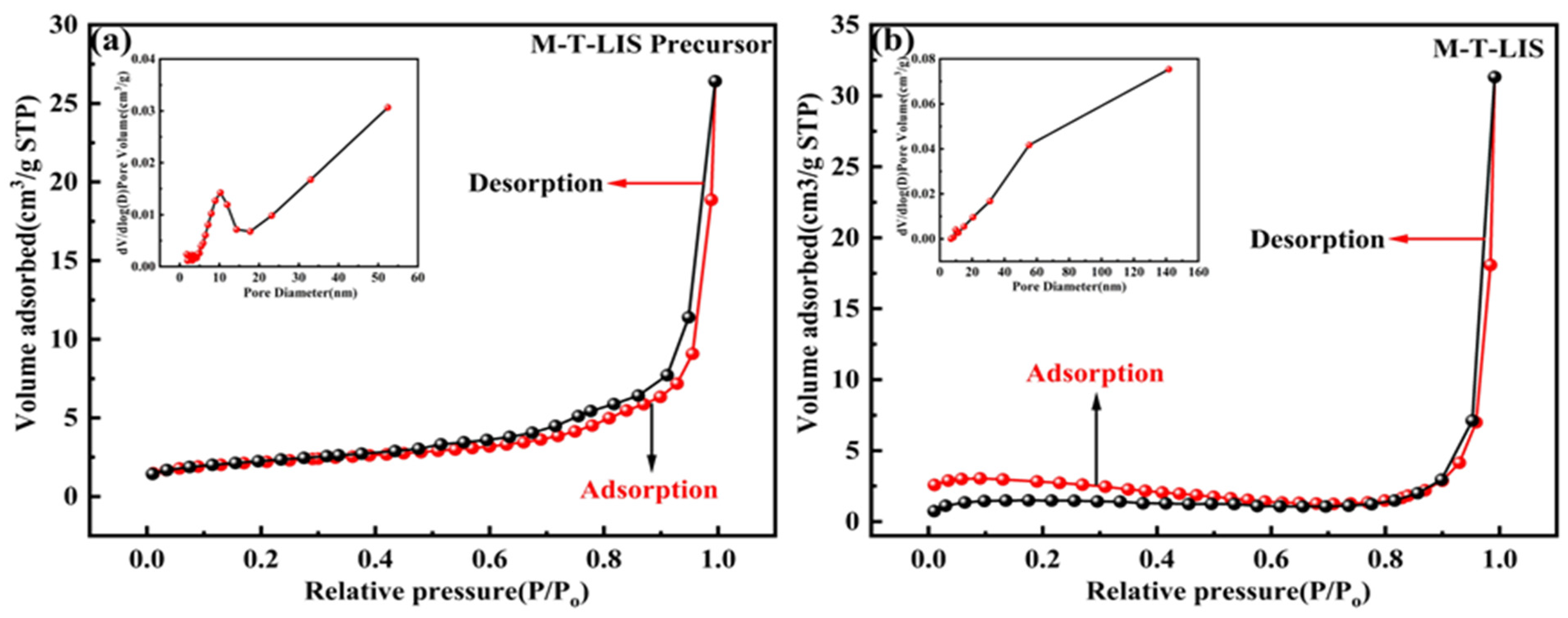
3.2. SEM and BET Analysis
3.3. Lithium Adsorptive Experiments
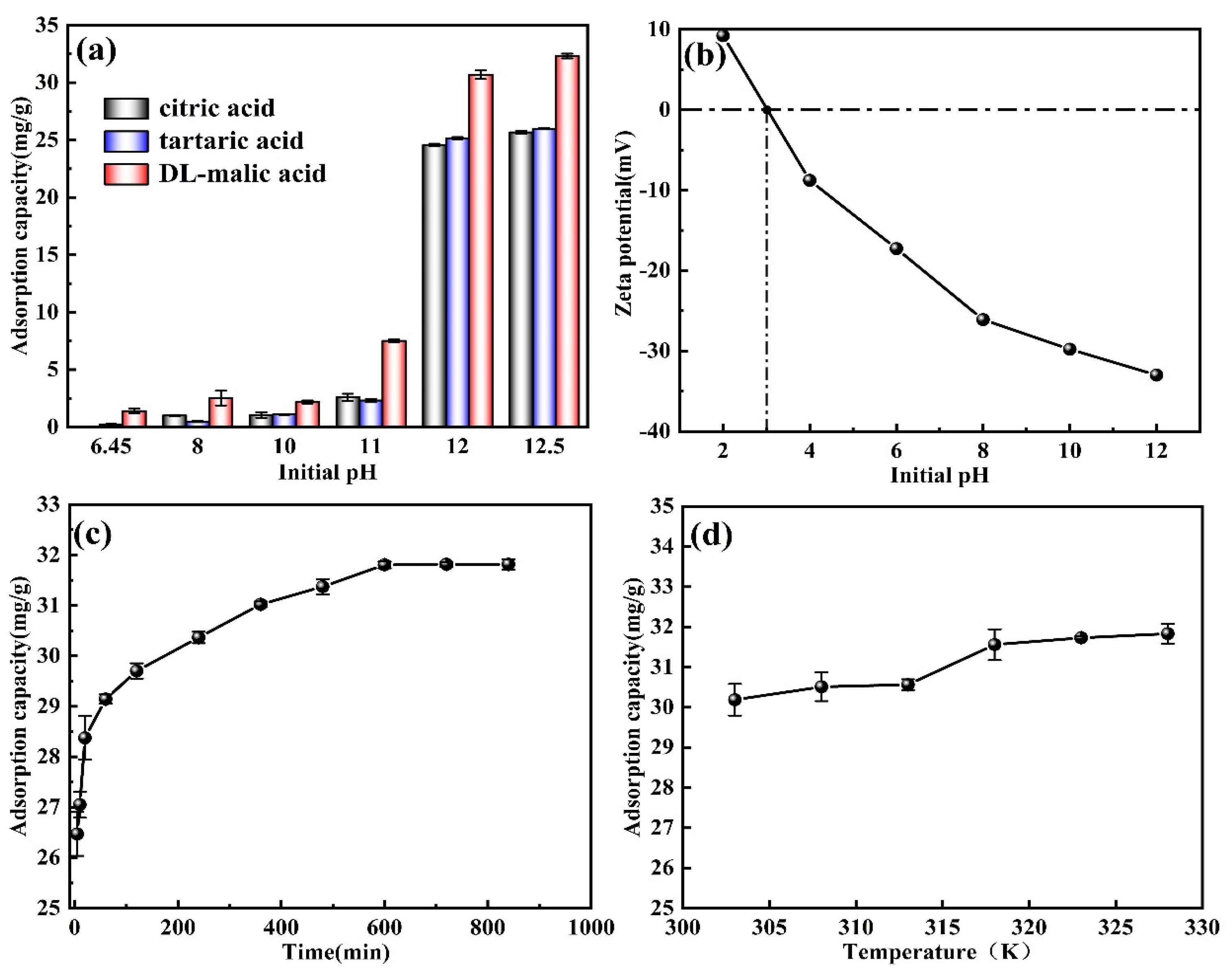
3.3.1. Influence of the Initial pH
3.3.2. Influence of Time
3.3.3. Influence of Temperature
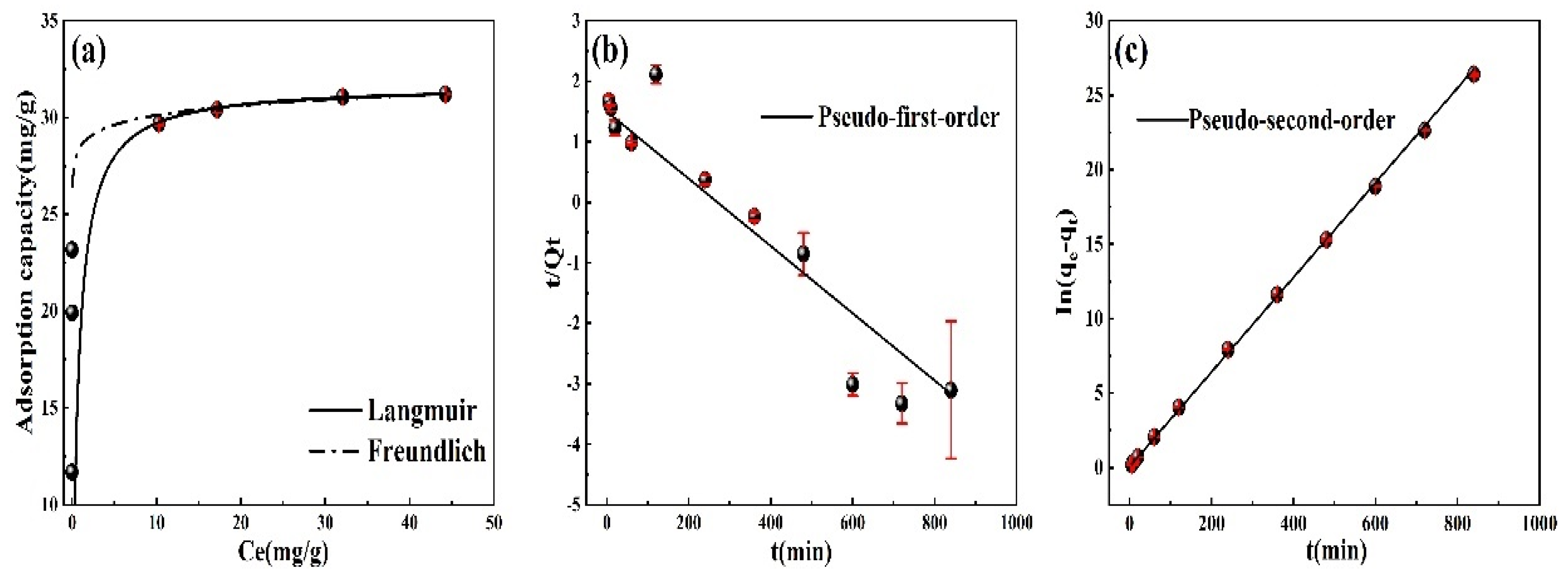
3.4. Adsorption Isotherm and Kinetics
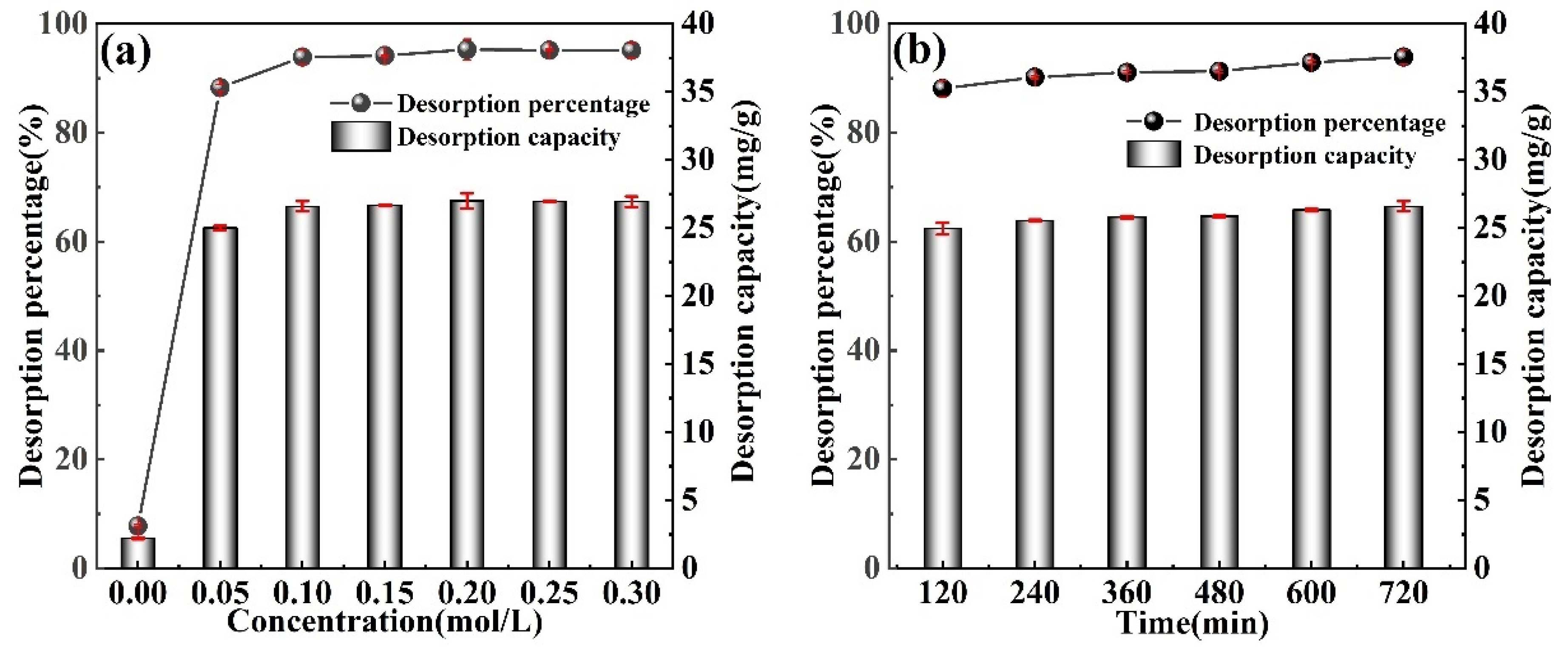
3.5. Lithium Desorption Experiments
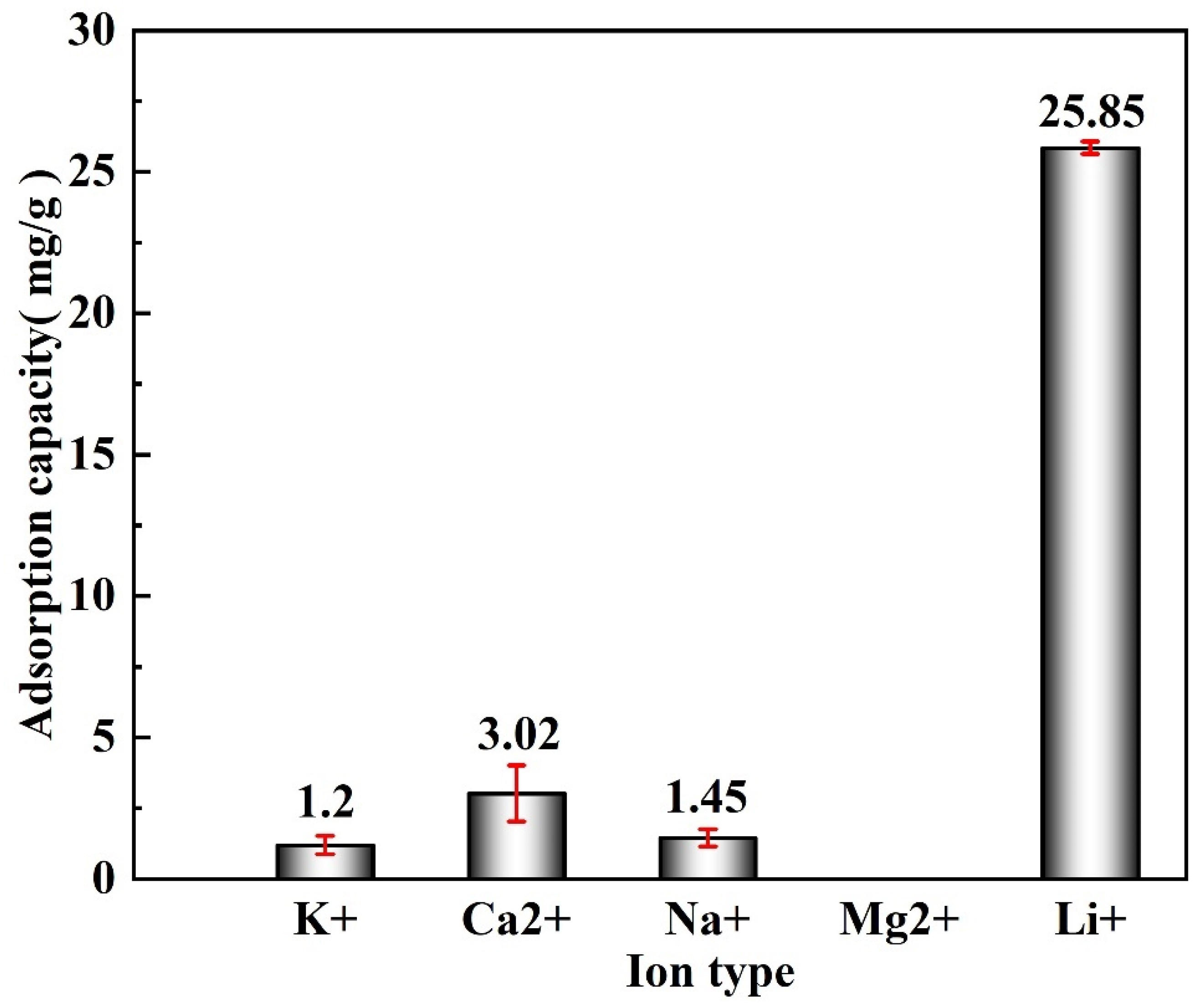
3.6. Selective Experiment
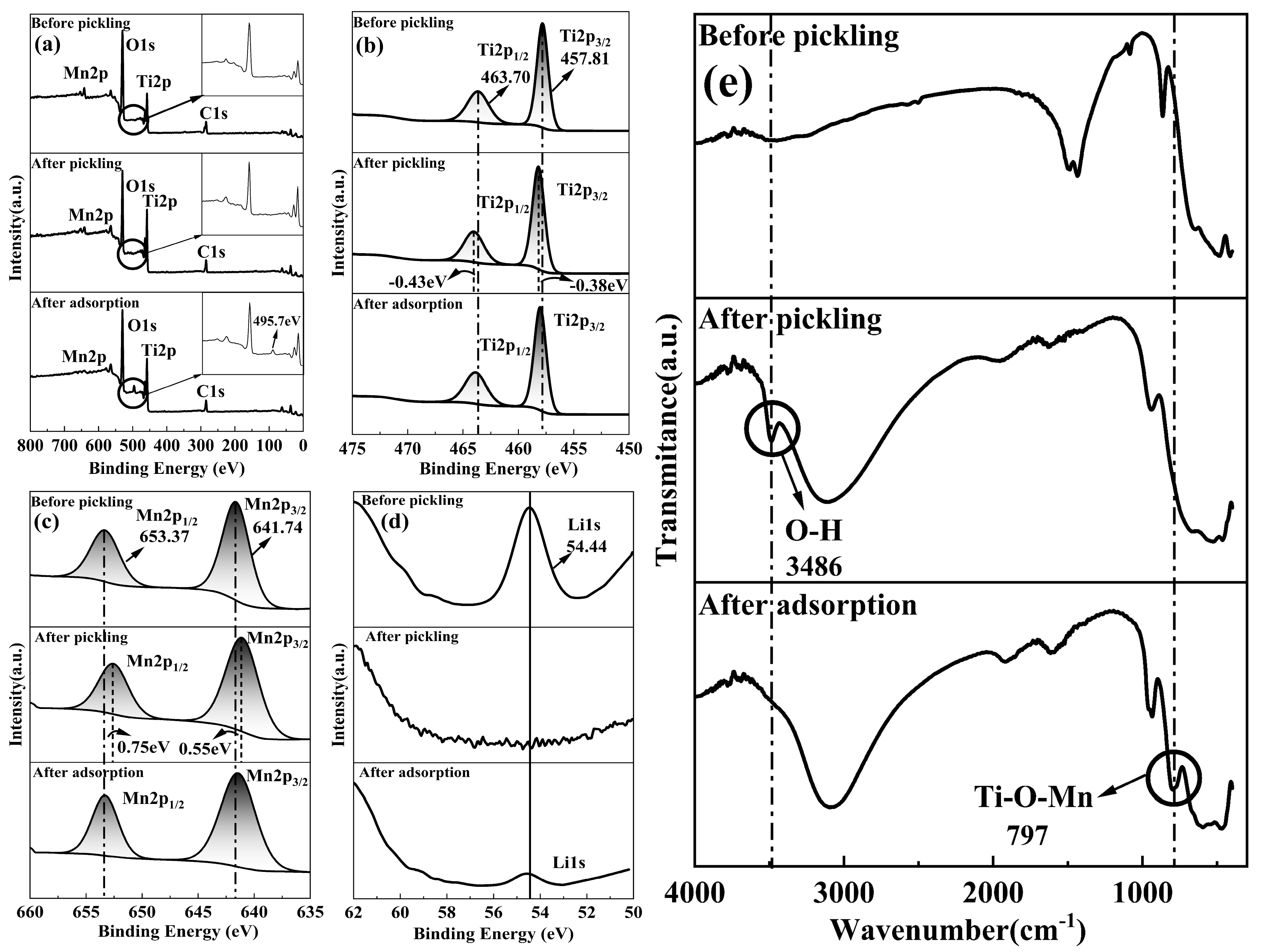
4. Adsorption Mechanism
5. Adsorption Performance
6. Conclusions
- Evaluated the adsorption performance of M-T-LIS precursor after organic acid treatment. After the precursor of M-T-LIS pickling by citric acid, tartaric acid, and DL-malic acid, the adsorption capacity of M-T-LIS for Li+ was compared under the optimal adsorption conditions: DL-malic acid 32.32 mg/g, citric acid 25.67 mg/g, and tartaric acid 25.99 mg/g. This indicated that DL-malic acid had a more significant elution performance on the M-T-LIS precursor. At the same time, the adsorption data of M-T-LIS were fitted with a first-order kinetic model and Langmuir isotherm model, and it was found that M-T-LIS belonged to single-layer chemical adsorption.
- The Li+ selectivity of M-T-LIS was evaluated in an artificial salt lake saline solution. The (Kd) and separation factors(α) of the M-T-LIS for K+, Ca2+, Na+, and Li+ in an artificial salt lake brine were calculated. The results indicated that M-T-LIS had satisfactory leaching Li+ selectivity, making it well utilized in the study of lithium extraction from salt lake brine. By simulating the adsorption performance of M-T-LIS in actual salt lake brine, the adsorption capacity of M-T-LIS in artificial salt lake brine was 25.85 mg/g.
- The mechanism of M-T-LIS adsorbing Li+ ion in artificial salt lake brine was analyzed using XPS and FT-IR. XPS and FT-IR analysis indicated that M-T-LIS adsorption was carried out through ion exchange between H+ and Li+.
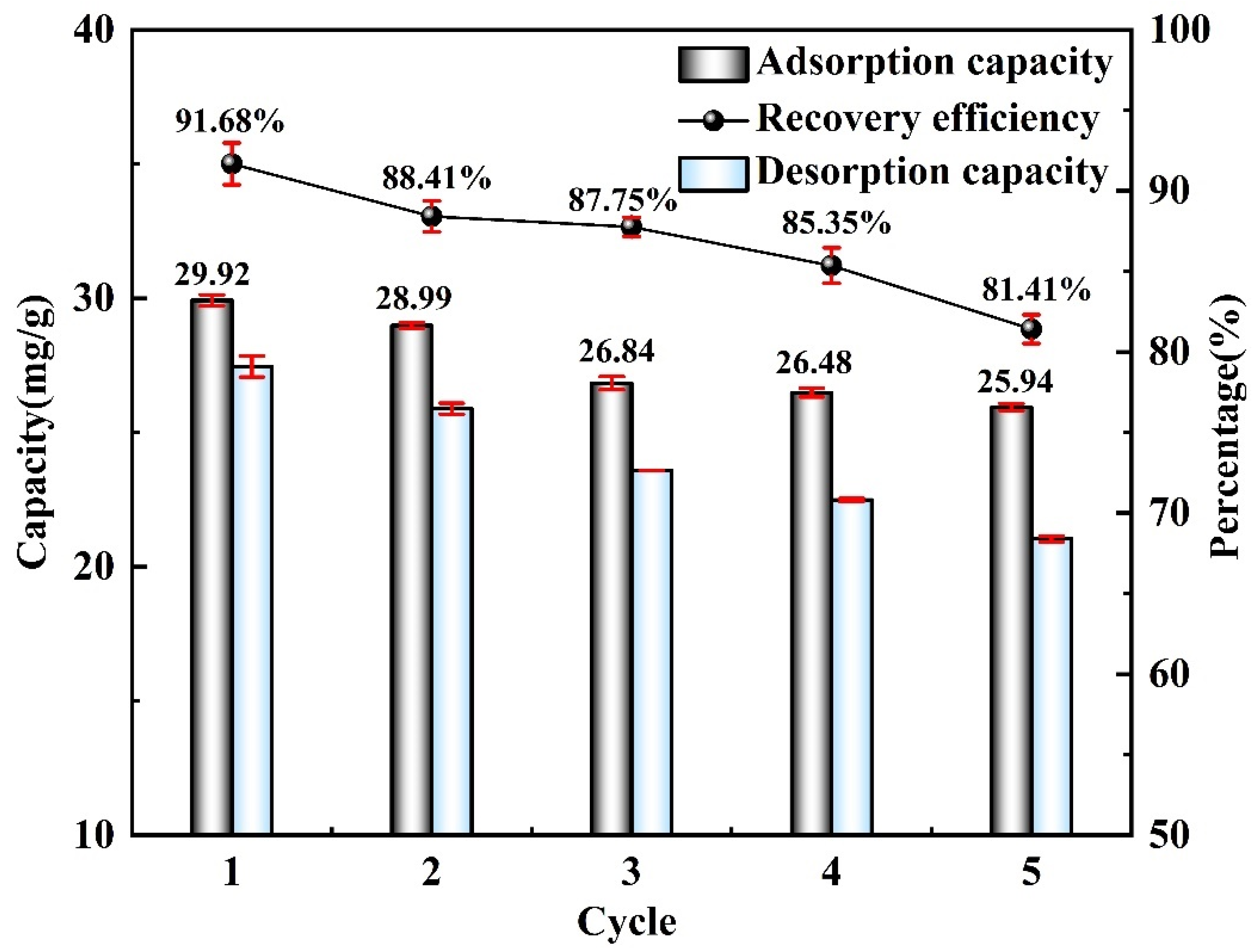
- The cycling performance of materials is also one of the important indicators for measuring material stability. The M-T-LIS exhibited significant Li+ adsorption and desorption properties after five adsorption–desorption cycles. In the fifth adsorption cycle, a high adsorption capacity of more than 20 mg/g (25.90 mg/g) was maintained, and the recovery rate of Li+ was 81.41%, which demonstrated the Li+ recovery efficiency and structural stability of M-T-LIS. Therefore, the method combining DL-malic acid and M-T-LIS to recover Li+ from salt lake brine had a good application prospect. This study would be used in the application of actual brine water.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Hua, H.; Zhang, L.; Liu, X.; Wu, H.; Yuan, Z. Environmental impacts of hydrometallurgical recycling and reusing for manufacturing of lithium-ion traction batteries in China. Sci. Total Environ. 2022, 811, 152224. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, L.; Sun, W. Systematic review of lithium extraction from salt-lake brines via precipitation approaches. Miner. Eng. 2019, 139, 105868. [Google Scholar] [CrossRef]
- Naeem, A.; Aslam, M.; Saifullah; Muhling, K.H. Lithium: Perspectives of nutritional beneficence, dietary intake, biogeochemistry, and biofortification of vegetables and mushrooms. Sci. Total Environ. 2021, 798, 149249. [Google Scholar] [CrossRef] [PubMed]
- Nygren, R.E.; Youchison, D.L.; Michael, J.R.; Puskar, J.D.; Lutz, T.J. Failure of a lithium-filled target and some implications for fusion components. Fusion Eng. Des. 2021, 171, 112664. [Google Scholar] [CrossRef]
- Baumgartner, S.; Gmeiner, R.; Schönherr, J.A.; Stampfl, J. Stereolithography-based additive manufacturing of lithium disilicate glass ceramic for dental applications. Mater. Sci. Eng. C 2020, 116, 111180. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Feng, Y.; Wang, C.; Fang, D.; Yi, G.; Gao, Z.; Shao, P.; Liu, C.; Luo, X.; Luo, S. Closed-loop regeneration of battery-grade FePO4 from lithium extraction slag of spent Li-ion batteries via phosphoric acid mixture selective leaching. Chem. Eng. J. 2022, 431, 133232. [Google Scholar] [CrossRef]
- Choubey, P.K.; Chung, K.-S.; Kim, M.-S.; Lee, J.-C.; Srivastava, R.R. Advance review on the exploitation of the prominent energy-storage element Lithium. Part II: From sea water and spent lithium ion batteries (LIBs). Miner. Eng. 2017, 110, 104–121. [Google Scholar] [CrossRef]
- Raggam, S.; Mohammad, M.; Choo, Y.; Naidu, G.; Zargar, M.; Shon, H.K.; Razmjou, A. Advances in metal organic framework (MOF)—Based membranes and adsorbents for lithium-ion extraction. Sep. Purif. Technol. 2023, 307, 122628. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, T.; Pan, X.; Zhang, J. Eco-friendly extraction of magnesium and lithium from salt lake brine for lithium-ion battery. J. Clean. Prod. 2021, 327, 129481. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. Selective recovery and efficient separation of lithium, rubidium, and cesium from lepidolite ores. Sep. Purif. Technol. 2022, 288, 120667. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, Z.; Yu, Z.; Wang, C.; Zhong, X.; Wei, L.; Yao, Y.; Sui, X.; Han, D.S.; Chen, Y. Thermally assisted efficient electrochemical lithium extraction from simulated seawater. Water Res. 2022, 223, 118969. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Wan, Y.; Guo, Y.; Deng, T.; Yu, X. Synthesis of functional ionic liquids with high extraction rate and electroconductivity for lithium-magnesium separation and metallic magnesium production from salt lake brine. Chem. Eng. J. 2023, 452, 139610. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Liu, R.; Zhou, Y.; Zhang, Y.; Ji, L.; Li, L. Recovery of lithium from salt lake brine with high Na/Li ratio using solvent extraction. J. Mol. Liq. 2022, 362, 119667. [Google Scholar] [CrossRef]
- Lee, J.; Chung, E. Lithium recovery from a simulated geothermal fluid by a combined selective precipitation and solvent extraction method. Geothermics 2022, 102, 102388. [Google Scholar] [CrossRef]
- Çelebi, E.E.; Öncel, M.S.; Kobya, M.; Bayramoğlu, M. Extraction of lithium from wastewaters using a synergistic solvent extraction system consisting of Mextral EOL and Cyanex 923. Hydrometallurgy 2019, 185, 46–54. [Google Scholar] [CrossRef]
- Bai, R.; Wang, J.; Wang, D.; Cui, J.; Zhang, Y. Recovery of lithium from high Mg/Li ratio salt-lake brines using ion-exchange with NaNTf2 and TBP. Hydrometallurgy 2022, 213, 105914. [Google Scholar] [CrossRef]
- Nie, X.-Y.; Sun, S.-Y.; Song, X.; Yu, J.-G. Further investigation into lithium recovery from salt lake brines with different feed characteristics by electrodialysis. J. Membr. Sci. 2017, 530, 185–191. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, T.; Ren, X.-K.; Wang, J.; Wang, Z.; Zhao, S. Nanofiltration membrane with crown ether as exclusive Li+ transport channels achieving efficient extraction of lithium from salt lake brine. Chem. Eng. J. 2022, 438, 135658. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Z.; Qin, X.; Gao, X.; Wang, M.; Xiang, X. Recent advances in lithium extraction from salt lake brine using coupled and tandem technologies. Desalination 2023, 547, 116225. [Google Scholar] [CrossRef]
- Luo, G.; Li, X.; Chen, L.; Chao, Y.; Zhu, W. Electrochemical lithium ion pumps for lithium recovery: A systematic review and influencing factors analysis. Desalination 2023, 548, 116228. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhou, Y.; Qi, G.; Zeng, J.; Sun, Y.; Shen, Y.; Li, X.; Ren, X.; Dong, S.; et al. Practical synthesis of manganese oxide MnO2·0.5H2O for an advanced and applicable lithium ion-sieve. J. Solid. State Chem. 2021, 293, 121768. [Google Scholar] [CrossRef]
- Chen, C.W.; Chen, P.A.; Wei, C.J.; Huang, H.L.; Jou, C.J.; Wei, Y.L.; Wang, H.P. Lithium recovery with LiTi(2)O(4) ion-sieves. Mar. Pollut. Bull. 2017, 124, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, H.; Zhang, Y.; Cao, D.; Zhao, X. Lithium extraction from seawater by manganese oxide ion sieve MnO2·0.5H2O. Colloids Surf. A Physicochem. Eng. Asp. 2015, 468, 280–284. [Google Scholar] [CrossRef]
- Li, H.; Chen, T.; Jiang, J.; Gao, G.; Wang, C.; Lu, Z. Prelithiation-derived hierarchical TiO2 sieve with metal-organic framework gate for selective lithium recovery. Chem. Eng. J. 2023, 451, 138662. [Google Scholar] [CrossRef]
- Lin, S.; Pan, Y.; Du, J.; Yang, Y.; Su, H.; Yu, J. Double-edged role of interlayer water on Li(+) extraction from ultrahigh Mg(2+)/Li(+) ratio brines using Li/Al-LDHs. J. Colloid Interface Sci. 2022, 627, 872–879. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Chen, X.; Fu, H.; Chen, Y.; Ning, S.; Fujita, T.; Wei, Y.; Wang, X. Layered ammonium vanadate nanobelt as efficient adsorbents for removal of Sr(2+) and Cs(+) from contaminated water. J. Colloid Interface Sci. 2022, 615, 110–123. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Z.; Wang, H.; Huang, D.; He, Y.-b.; Zhao, S.-X. Mg2+ doping into Li sites to improve anionic redox reversibility and thermal stability of lithium-rich manganese-based oxides cathode. Mater. Today Energy 2022, 29, 101116. [Google Scholar] [CrossRef]
- Tian, L.; Ma, W.; Han, M. Adsorption behavior of Li+ onto nano-lithium ion sieve from hybrid magnesium/lithium manganese oxide. Chem. Eng. J. 2010, 156, 134–140. [Google Scholar] [CrossRef]
- Pulido, R.; Naveas, N.; Martín-Palma, R.J.; Graber, T.; Brito, I.; Hernández-Montelongo, J.; Manso Silván, M. Experimental and density functional theory study of the Li+ desorption in spinel/layered lithium manganese oxide nanocomposites using HCl. Chem. Eng. J. 2022, 441, 136019. [Google Scholar] [CrossRef]
- Yu, Q.; Sasaki, K. Microwave-assisted hydrothermal synthesis of nanocrystalline lithium-ion sieve from biogenic manganese oxide, its characterization and lithium sorption studies. Hydrometallurgy 2016, 165, 118–124. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Zhang, Y.; Zhang, Y.; Qiao, S.; Zheng, S. Application of citric acid as eluting medium for titanium type lithium ion sieve. Hydrometallurgy 2019, 183, 166–174. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, Y.; Ye, Z.; Wang, N.; He, C.; Zhu, Y.; Fujita, T.; Wu, H.; Wang, X. Three-dimension titanium phosphate aerogel for selective removal of radioactive strontium(II) from contaminated waters. J. Environ. Manag. 2023, 325, 116424. [Google Scholar] [CrossRef]
- Wang, L.; Meng, C.G.; Han, M.; Ma, W. Lithium uptake in fixed-pH solution by ion sieves. J. Colloid Interface Sci. 2008, 325, 31–40. [Google Scholar] [CrossRef]
- He, C.; Yang, Y.; Qi, M.; Jiang, Y.; Wei, Y.; Fujita, T.; Wang, G.; Ma, S.; Yang, W. Separation and recovery of indium from solution in a sulfite-sulfuric acid system. J. Environ. Chem. Eng. 2023, 11, 109372. [Google Scholar] [CrossRef]
- Meng, J.; He, C.; Li, Y.; Zhou, J.; Li, J.; Zheng, C.; Zhao, J.; Fujita, T.; Ning, S.; Wei, Y. Enhanced adsorption and separation of gallium using silica-based P507-TBP/SiO2–P adsorbent from sulfuric acid solution. Microporous Mesoporous Mater. 2021, 314, 110859. [Google Scholar] [CrossRef]
- Ning, P.; Meng, Q.; Dong, P.; Duan, J.; Xu, M.; Lin, Y.; Zhang, Y. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system. Waste Manag. 2020, 103, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chu, G.-W.; Li, S.-C.; Bai, S.; Luo, Y.; Sun, B.-C.; Chen, J.-F. Preparation of lithium carbonate by thermal decomposition in a rotating packed bed reactor. Chem. Eng. J. 2019, 377, 119929. [Google Scholar] [CrossRef]
- Haidry, A.A.; Fatima, Q.; Wang, Z.; Wang, Y.; Ji, Y.; Raza, A. Optimization of the specific surface area of ordered mesoporous TiO2 yields a high response to humidity. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131371. [Google Scholar] [CrossRef]
- de Oliveira Demarco, J.; Stefanello Cadore, J.; da Silveira de Oliveira, F.; Hiromitsu Tanabe, E.; Assumpção Bertuol, D. Recovery of metals from spent lithium-ion batteries using organic acids. Hydrometallurgy 2019, 190, 105169. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Huang, Y.; Luo, G.; Tao, D.; Yu, J.; Chen, L.; Chao, Y.; Zhu, W. Preparation of high hydrophilic H2TiO3 ion sieve for lithium recovery from liquid lithium resources. Chem. Eng. J. 2023, 453, 139485. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yang, Y.; Lin, S.; Li, P. Preparation of granular titanium-type lithium-ion sieves and recyclability assessment for lithium recovery from brines with different pH value. Sep. Purif. Technol. 2021, 267, 118613. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, H.; Wen, H.; Yang, Y. Lithium extraction from clay-type lithium resource using ferric sulfate solutions via an ion-exchange leaching process. Hydrometallurgy 2021, 206, 105759. [Google Scholar] [CrossRef]
- Xu, S.; Ning, S.; Wang, Y.; Wang, X.; Dong, H.; Chen, L.; Yin, X.; Fujita, T.; Wei, Y. Precise separation and efficient enrichment of palladium from wastewater by amino-functionalized silica adsorbent. J. Clean. Prod. 2023, 396, 136479. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, R.; Wang, L.; Sun, W. Separation of magnesium from lithium in salt-lake brine through struvite precipitation. Miner. Eng. 2022, 180, 107468. [Google Scholar] [CrossRef]
- Han, N.; Gao, R.; Peng, H.; He, Q.; Miao, Z.; Wan, K. Selective recovery of lithium ions from acidic medium based on capacitive deionization-enhanced imprinted polymers. J. Clean. Prod. 2022, 373, 133773. [Google Scholar] [CrossRef]
- Park, J.; Lee, M.-Y.; Han, S.; Lee, K.-Y.; Kang, S. Selective removal of Na+ by NaTi2(PO4)3-MWCNT composite hollow-fiber membrane electrode in capacitive deionization. Npj Clean Water 2022, 5, 14. [Google Scholar] [CrossRef]
- Kim, N.; Su, X.; Kim, C. Electrochemical lithium recovery system through the simultaneous lithium enrichment via sustainable redox reaction. Chem. Eng. J. 2021, 420, 127715. [Google Scholar] [CrossRef]
- Nie, X.-Y.; Sun, S.-Y.; Sun, Z.; Song, X.; Yu, J.-G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Cherian, A.M.; Joseph, J.; Nair, M.B.; Nair, S.V.; Vijayakumar, M.; Menon, D. Coupled benefits of nanotopography and titania surface chemistry in fostering endothelialization and reducing in-stent restenosis in coronary stents. Biomater. Adv. 2022, 142, 213149. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Lu, J.; Chen, J.; Gao, L.; Guo, S.; Omran, M.; Chen, G. Mechanism of enhanced enrichment manganese from manganese ore-pyrite under microwave heating: Process optimization and kinetic studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130534. [Google Scholar] [CrossRef]
- Slama de Freitas, A.L.; Subburaj, J.; Navarro, J.C.; Khan, H.A.; Kashif, T.A.; Hakimov, K.; Ruiz-Martinez, J.; Farooq, A. Shockwave impact on the stability of anatase titania nanoparticles. Mater. Today Commun. 2022, 32, 104031. [Google Scholar] [CrossRef]
- Wei, S.; Wei, Y.; Chen, T.; Liu, C.; Tang, Y. Porous lithium ion sieves nanofibers: General synthesis strategy and highly selective recovery of lithium from brine water. Chem. Eng. J. 2020, 379, 122407. [Google Scholar] [CrossRef]
- Chen, X.; Kang, D.; Cao, L.; Li, J.; Zhou, T.; Ma, H. Separation and recovery of valuable metals from spent lithium ion batteries: Simultaneous recovery of Li and Co in a single step. Sep. Purif. Technol. 2019, 210, 690–697. [Google Scholar] [CrossRef]
- Kanagathara, N.; Bhavani, R.; Lo, A.Y.; Marchewka, M.K.; Janczak, J. Structural, vibrational characterization and DFT calculations of urea: DL-malic acid (1:1)—Co-crystal. J. Mol. Struct. 2022, 1270, 133930. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, J.; Wu, J.; Zhang, G.; Yang, Y.; Tang, W.; Xue, M. Preparation of Mg-doped Li1.6Mn1.6O4 with enhanced Li+ adsorption performance and anti-dissolution properties of Mn. Hydrometallurgy 2022, 209, 105772. [Google Scholar] [CrossRef]
- Gao, J.-m.; Du, Z.; Zhao, Q.; Guo, Y.; Cheng, F. Enhanced Li+ adsorption by magnetically recyclable iron-doped lithium manganese oxide ion-sieve: Synthesis, characterization, adsorption kinetics and isotherm. J. Mater. Res. Technol. 2021, 13, 228–240. [Google Scholar] [CrossRef]
- Qian, F.; Zhao, B.; Guo, M.; Qian, Z.; Xu, N.; Wu, Z.; Liu, Z. Enhancing the Li+ adsorption and anti-dissolution properties of Li1.6Mn1.6O4 with Fe, Co doped. Hydrometallurgy 2020, 193, 105291. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhou, Y.; Qi, G.; Wu, Y.; Hai, C.; Tang, W. Synthesis of aluminum-doped ion-sieve manganese oxides powders with enhanced adsorption performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123950. [Google Scholar] [CrossRef]
- Bekhradinassab, E.; Tavakoli, A.; Haghighi, M.; Shabani, M. Catalytic biofuel production over 3D macro-structured cheese-like Mn-promoted TiO2 isotype: Mn-catalyzed microwave-combustion design. Energy Convers. Manag. 2022, 251, 114916. [Google Scholar] [CrossRef]


| BET Surface Area (m2g−1) | Average Pore Size (nm) | Pore Volume (cm3g−1) | C (wt.%) | |
|---|---|---|---|---|
| Before sintering | —— | —— | 7.75 | |
| Precursor | 7.82 | 10.16 | 0.04 | 4.39 |
| After pickling | 12.59 | 38.68 | 0.05 | 0.18 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | n | KF (L/g) | R2 |
| 31.68 | 1.51 | 0.99 | 0.01 | 28.50 | 0.85 |
| Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|
| qe1 (mg/g) | K1 (min−1) | R2 | qe2 (mg/g) | K2 (g/mg/min) | R2 |
| 4.78 | 0.01 | 0.86 | 31.55 | 0.57 | 0.99 |
| K+ | Ca2+ | Na+ | Li+ | |
|---|---|---|---|---|
| Kd (mL/g) | 0.07 | 18.69 | 0.11 | 46.47 |
| α | 674.35 | 2.49 | 441.22 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Nhung, N.T.H.; An, J.; Chen, H.; Liao, L.; He, C.; Wang, X.; Fujita, T. Manganese-Titanium Mixed Ion Sieves for the Selective Adsorption of Lithium Ions from an Artificial Salt Lake Brine. Materials 2023, 16, 4190. https://doi.org/10.3390/ma16114190
Ding Y, Nhung NTH, An J, Chen H, Liao L, He C, Wang X, Fujita T. Manganese-Titanium Mixed Ion Sieves for the Selective Adsorption of Lithium Ions from an Artificial Salt Lake Brine. Materials. 2023; 16(11):4190. https://doi.org/10.3390/ma16114190
Chicago/Turabian StyleDing, Yaxuan, Nguyen Thi Hong Nhung, Jiahao An, Hao Chen, Lianying Liao, Chunlin He, Xinpeng Wang, and Toyohisa Fujita. 2023. "Manganese-Titanium Mixed Ion Sieves for the Selective Adsorption of Lithium Ions from an Artificial Salt Lake Brine" Materials 16, no. 11: 4190. https://doi.org/10.3390/ma16114190
APA StyleDing, Y., Nhung, N. T. H., An, J., Chen, H., Liao, L., He, C., Wang, X., & Fujita, T. (2023). Manganese-Titanium Mixed Ion Sieves for the Selective Adsorption of Lithium Ions from an Artificial Salt Lake Brine. Materials, 16(11), 4190. https://doi.org/10.3390/ma16114190