Effect of Sintering Mechanism towards Crystallization of Geopolymer Ceramic—A Review
Abstract
1. Introduction
2. Geopolymer Overview
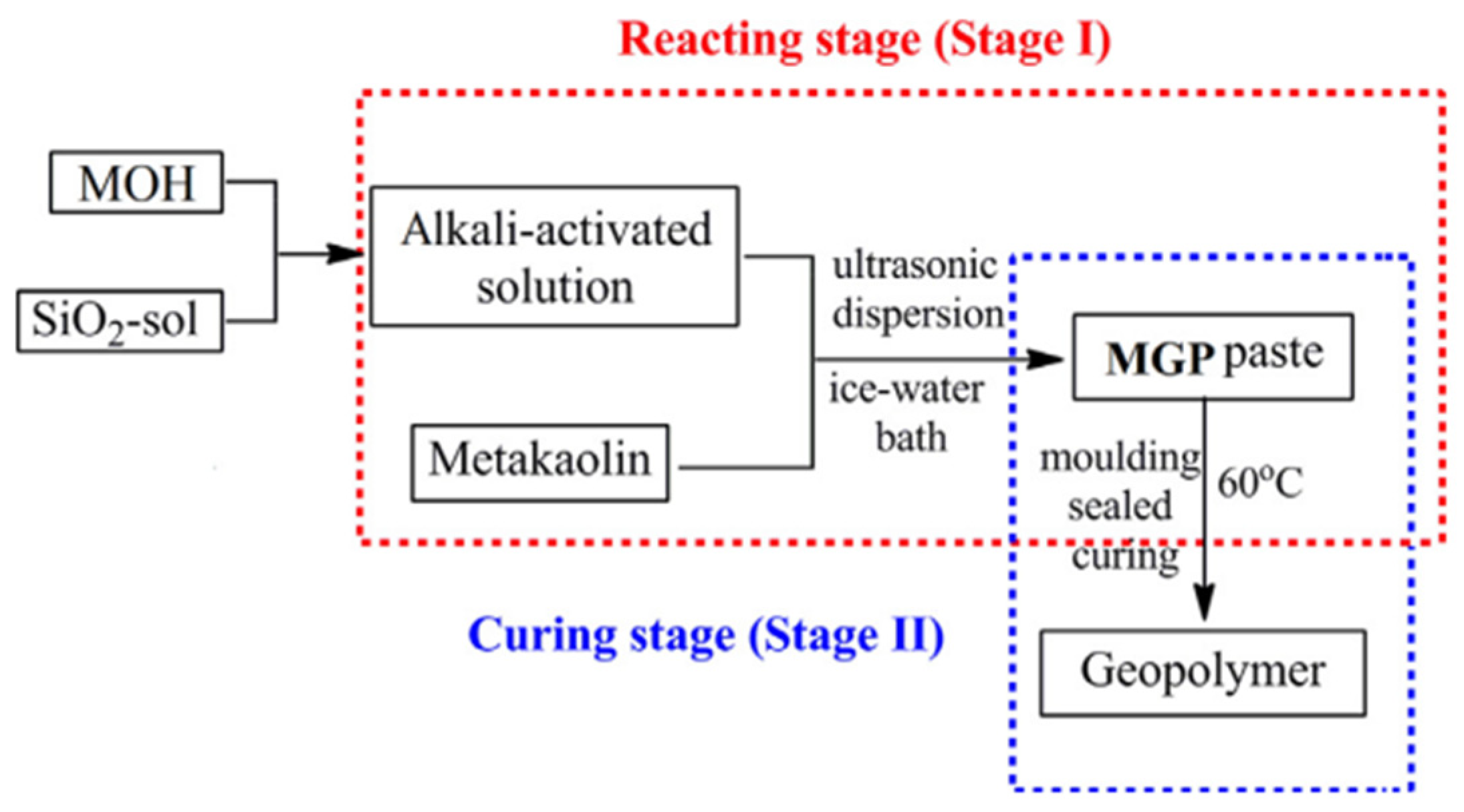
2.1. Source Materials
2.1.1. Precursor
2.1.2. Alkali Activator
2.2. Properties of Geopolymer
2.3. Geopolymer Exposure to High Temperature
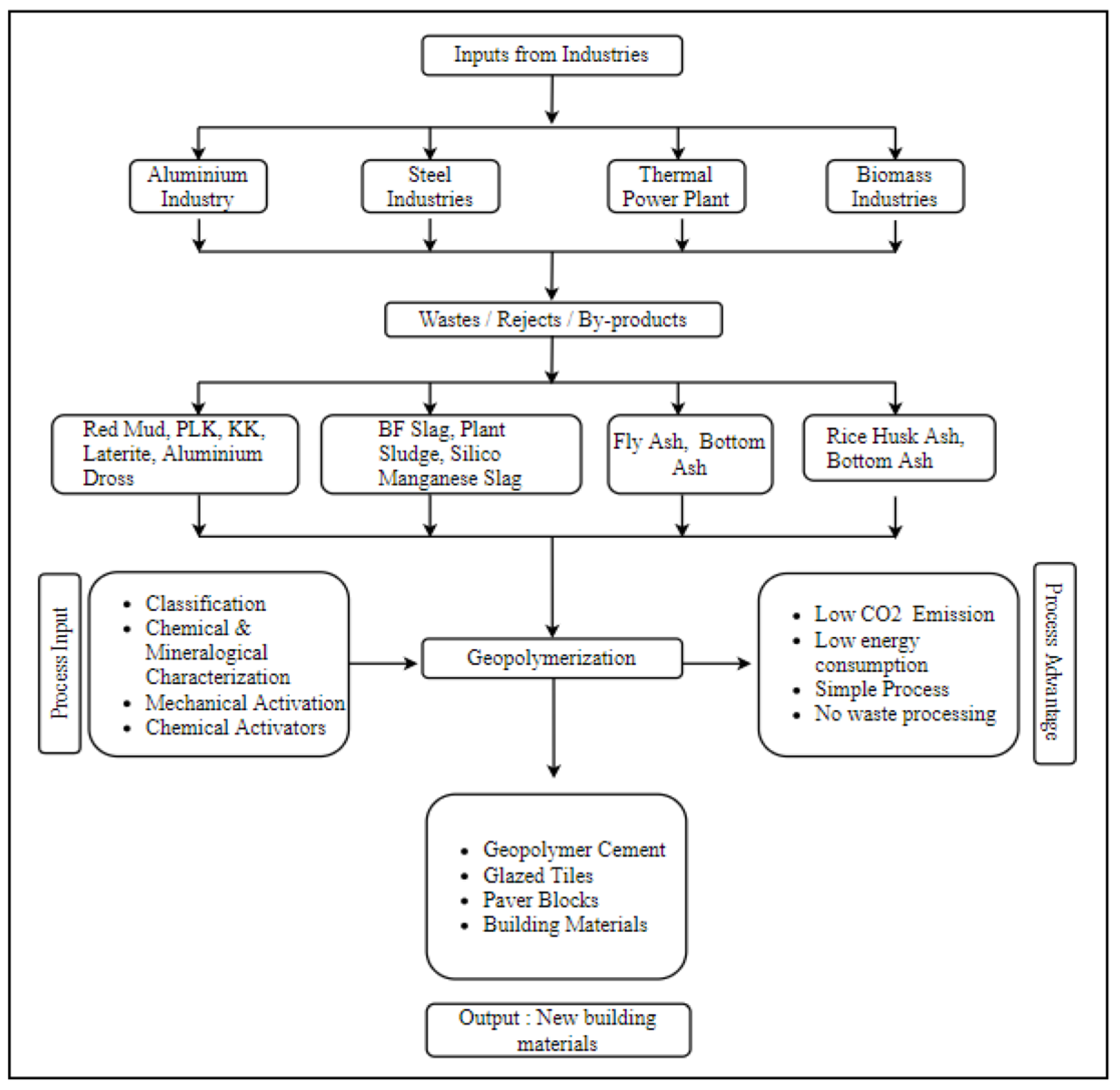
3. Geopolymer for Ceramic Application
3.1. Geopolymer Ceramics
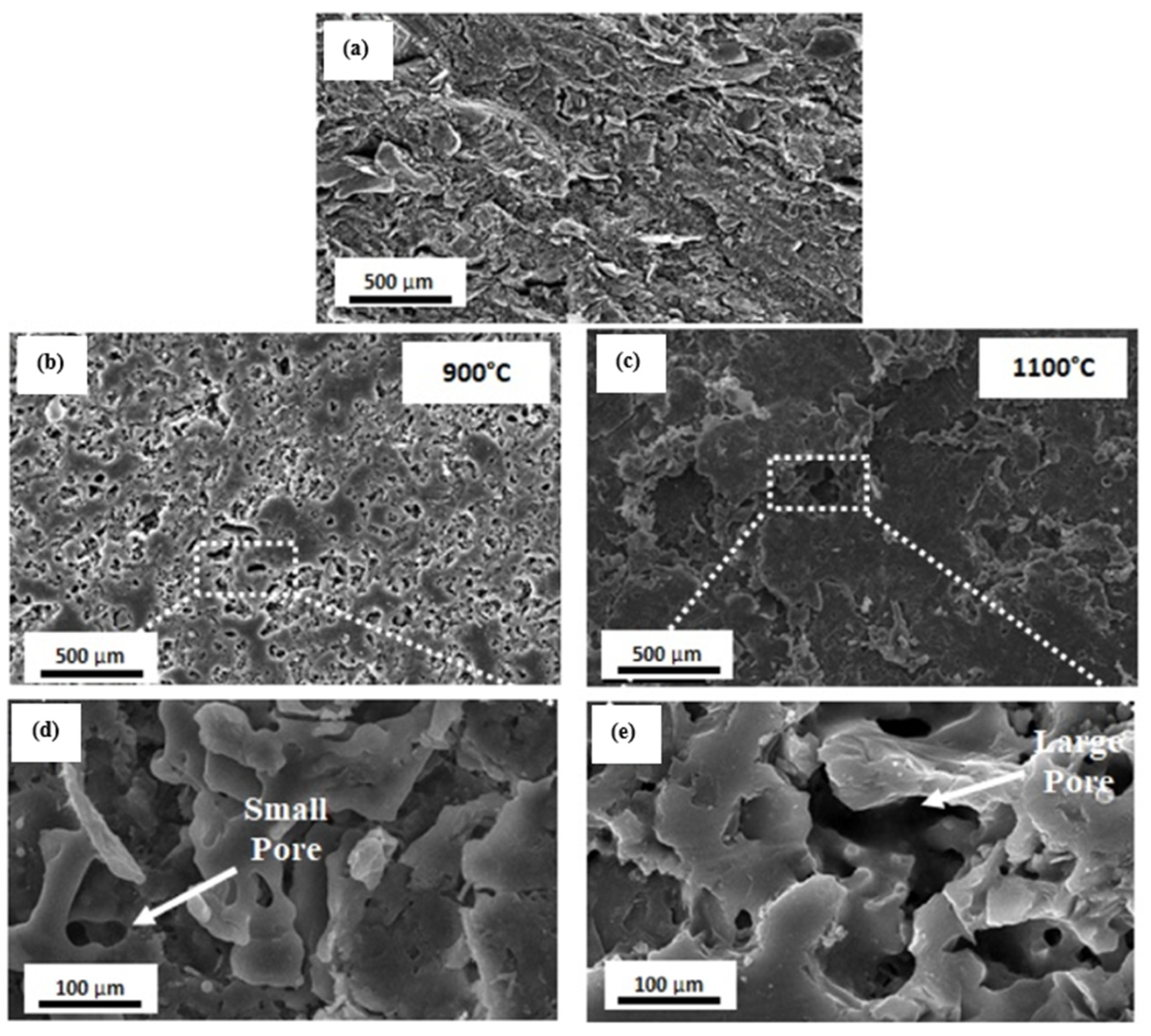
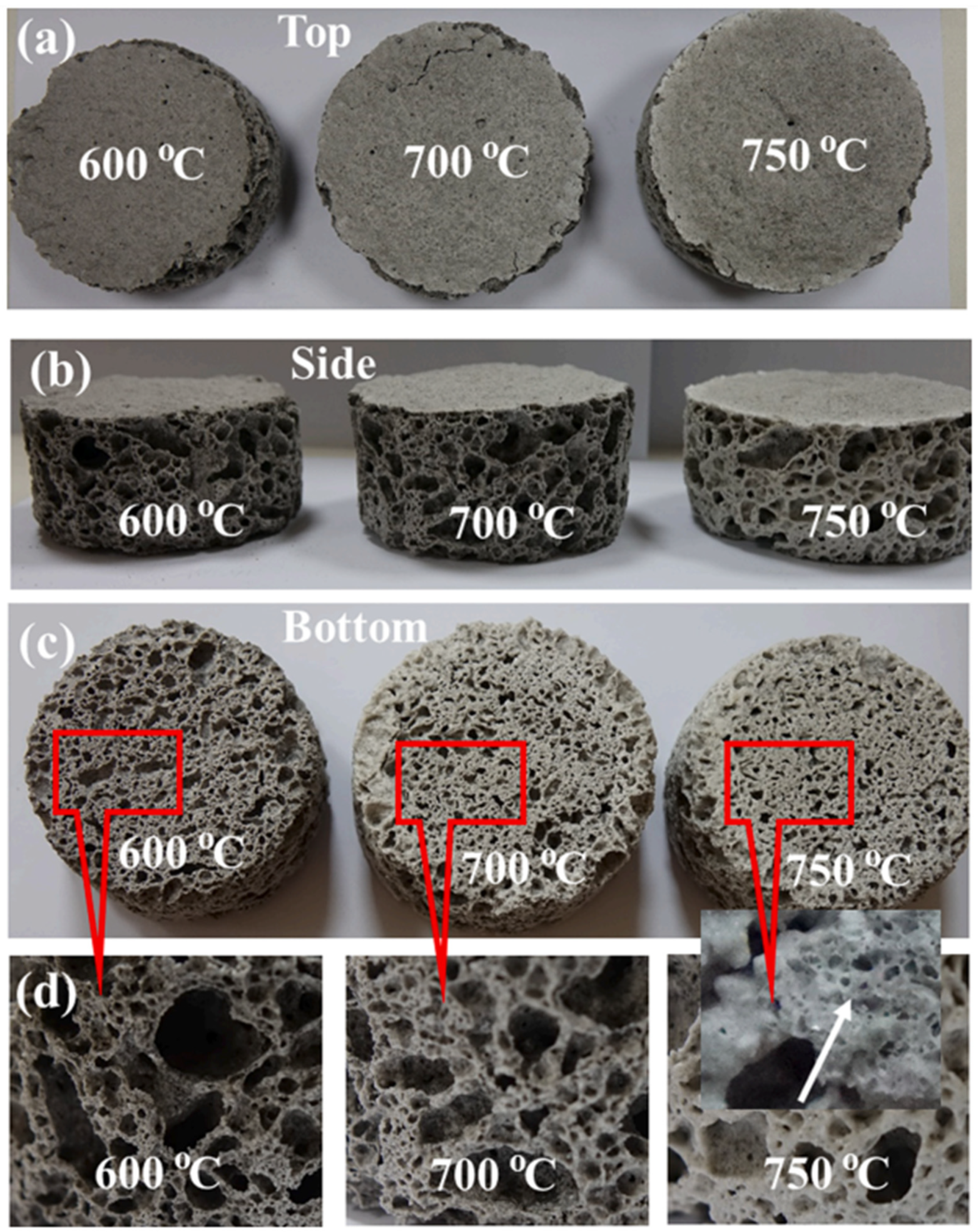
3.2. Properties of Geopolymer Ceramics
4. Sintering Mechanism of Ceramic Materials
4.1. Method of Sintering Kinetics
4.2. Model of Sintering
4.3. Effects of Sintering and Properties of Common Materials
5. Summary and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otitoju, T.A.; Okoye, P.U.; Chen, G.; Li, Y.; Okoye, M.O.; Li, S. Advanced ceramic components: Materials, fabrication, and applications. J. Ind. Eng. Chem. 2020, 85, 34–65. [Google Scholar] [CrossRef]
- He, P.; Jia, D.; Wang, M.; Zhou, Y. Thermal evolution and crystallization kinetics of potassium-based geopolymer. Ceram. Int. 2011, 37, 59–63. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, C.; Qiao, Y.; Wang, X.; Jia, D.; Li, H.; Colombo, P. Porous geopolymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2021, 150, 106629. [Google Scholar] [CrossRef]
- Almutairi, A.L.; Tayeh, B.A.; Adesina, A.; Isleem, H.F.; Zeyad, A.M. Potential applications of geopolymer concrete in construction: A review. Case Stud. Constr. Mater. 2021, 15, e00733. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Hussin, K. Correlation between pore structure, compressive strength and thermal conductivity of porous metakaolin geopolymer. Constr. Build. Mater. 2020, 247, 118641. [Google Scholar] [CrossRef]
- Hidayati, R.E.; Faradilla, F.S.; Dadang, D.; Harmelia, L.; Nurlina, N.; Prasetyoko, D.; Fansuri, H. Setting Time and Compressive Strength of Geopolymers Made of Three Indonesian Low Calcium Fly Ash with Variation of Sodium Silicate Addition. Arch. Metall. Mater. 2021, 66, 1115–1121. [Google Scholar]
- Ibrahim, M.; Ibrahim, W.M.W.; Abdullah, M.M.A.B.; Sauffi, A.S.; Vizureanu, P. Effect of Solid-To-Liquids and Na2SiO3-To-NaOH Ratio on Metakaolin Membrane Geopolymers. Arch. Metall. Mater. 2022, 67, 695–702. [Google Scholar]
- Ghanbari, M.; Hadian, A.; Nourbakhsh, A.; MacKenzie, K. Modeling and optimization of compressive strength and bulk density of metakaolin-based geopolymer using central composite design: A numerical and experimental study. Ceram. Int. 2017, 43, 324–335. [Google Scholar] [CrossRef]
- Nergis, D.D.B.; Vizureanu, P.; Lupescu, S.; Nergis, D.P.B.; Perju, M.C.; Sandu, A.V. Microstructural Analysis of Ambient Cured Phosphate Based-Geopolymers with Coal-Ash as Precursor. Arch. Metall. Mater. 2022, 67, 595–600. [Google Scholar]
- Azimi, E.A.; Salleh, M.M.; Abdullah, M.M.A.B.; Aziz, I.H.A.; Hussin, K.; Chaiprapa, J.; Vizureanu, P.; Yoriya, S.; Nabiałek, M.; Wyslocki, J.J. Effect of Naoh Molar Concentration on Microstructure and Compressive Strength of Dolomite/Fly Ash-Based Geopolymers. Arch. Met. Mater. 2022, 67, 993–998. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Aziz, I.H.A.; Zailani, W.W.A.; Rahim, S.Z.A.; Yong, H.C.; Sandu, A.V.; Peng, L.S. Silica Bonding Reaction on Fly Ash Based Geopolymer Repair Material System with Incorporation of Various Concrete Substrates. Arch. Met. Mater. 2022, 67, 1277–1281. [Google Scholar] [CrossRef]
- Tchadjie, L.N.; Ekolu, S.O. Enhancing the reactivity of aluminosilicate materials toward geopolymer synthesis. J. Mater. Sci. 2017, 53, 4709–4733. [Google Scholar] [CrossRef]
- Nagajothi, S.; Elavenil, S. Influence of Aluminosilicate for the Prediction of Mechanical Properties of Geopolymer Concrete–Artificial Neural Network. Silicon 2019, 12, 1011–1021. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X.; Davidovits, J.; Antenucci, D.; Nugteren, H.; Pereira, C.F. Coal fly ash-slag-based geopolymers: Microstructure and metal leaching. J. Hazard. Mater. 2009, 166, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Melinescu, A.; Eftimie, M.; Nicoară, A.; Tru, R.; Preda, M. Synthesis of The Ceramics with Nepheline from Geopolymeric Precursors Sinteza Ceramicilor Cu Nefelin Din Precursori Geopolimerici. Rev. Română De Mater. Rom. J. Mater. 2018, 48, 285–289. [Google Scholar]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Li, L.-Y.; Jaya, N.A.; Abdullah, M.M.A.B.; Tan, S.J.; Hussin, K. Formation of one-part-mixing geopolymers and geopolymer ceramics from geopolymer powder. Constr. Build. Mater. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Muñiz-Villarreal, M.S.; Manzano-Ramírez, A.; Sampieri-Bulbarela, S.; Gasca-Tirado, J.R.; Reyes-Araiza, J.L.; Rubio-Ávalos, J.C.; Pérez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigó, V. The effect of temperature on the geopolymerization process of a metakaolin-based geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Wang, Y.; Sun, R.; Rui, Y. Effects of the n(H2O:Na2Oeq) ratio on the geopolymerization process and microstructures of fly ash-based geopolymers. J. Non. Cryst. Solids 2019, 511, 19–28. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers-Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Ranjbar, N.; Zhang, M. Fiber-reinforced geopolymer composites: A review. Cem. Concr. Compos. 2020, 107, 103498. [Google Scholar] [CrossRef]
- Rouyer, J.; Poulesquen, A. Evidence of a Fractal Percolating Network During Geopolymerization. J. Am. Ceram. Soc. 2015, 98, 1580–1587. [Google Scholar] [CrossRef]
- Siyal, A.A.; Azizli, K.A.; Man, Z.; Ismail, L.; Khan, M.I. Geopolymerization kinetics of fly ash based geopolymers using JMAK model. Ceram. Int. 2016, 42, 15575–15584. [Google Scholar] [CrossRef]
- Yuan, J.; Li, L.; He, P.; Chen, Z.; Lao, C.; Jia, D.; Zhou, Y. Effects of kinds of alkali-activated ions on geopolymerization process of geopolymer cement pastes. Constr. Build. Mater. 2021, 293, 123536. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- De Azevedo, A.R.G.; Marvila, M.T.; Rocha, H.A.; Cruz, L.R.; Vieira, C.M.F. Use of glass polishing waste in the development of ecological ceramic roof tiles by the geopolymerization process. Int. J. Appl. Ceram. Technol. 2020, 17, 2649–2658. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Jindal, B.B. Investigations on the properties of geopolymer mortar and concrete with mineral admixtures: A review. Constr. Build. Mater. 2019, 227, 116644. [Google Scholar] [CrossRef]
- Ahsan, M.B.; Hossain, Z. Supplemental use of rice husk ash (RHA) as a cementitious material in concrete industry. Constr. Build. Mater. 2018, 178, 1–9. [Google Scholar] [CrossRef]
- Albidah, A.; Alghannam, M.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Characteristics of metakaolin-based geopolymer concrete for different mix design parameters. J. Mater. Res. Technol. 2020, 10, 84–98. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]
- Jindal, B.B.; Alomayri, T.; Hasan, A.; Kaze, C.R. Geopolymer concrete with metakaolin for sustainability: A comprehensive review on raw material’s properties, synthesis, performance, and potential application. Environ. Sci. Pollut. Res. 2022, 30, 25299–25324. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.C. Investigation of Mechanical and Microstructural Properties of Fiber-Reinforced Geopolymer Concrete with GGBFS and Metakaolin: Novel Raw Material for Geopolymerisation. Silicon 2021, 13, 4565–4573. [Google Scholar] [CrossRef]
- Moradikhou, A.B.; Esparham, A.; Avanaki, M.J. Physical & mechanical properties of fiber reinforced metakaolin-based geopolymer concrete. Constr. Build. Mater. 2020, 251, 118965. [Google Scholar] [CrossRef]
- Bong, S.H.; Nematollahi, B.; Xia, M.; Nazari, A.; Sanjayan, J. Properties of one-part geopolymer incorporating wollastonite as partial replacement of geopolymer precursor or sand. Mater. Lett. 2019, 263, 127236. [Google Scholar] [CrossRef]
- Ren, D.; Yan, C.; Duan, P.; Zhang, Z.; Li, L.; Yan, Z. Durability performances of wollastonite, tremolite and basalt fiber-reinforced metakaolin geopolymer composites under sulfate and chloride attack. Constr. Build. Mater. 2017, 134, 56–66. [Google Scholar] [CrossRef]
- Archez, J.; Texier-Mandoki, N.; Bourbon, X.; Caron, J.-F.; Rossignol, S. Influence of the wollastonite and glass fibers on geopolymer composites workability and mechanical properties. Constr. Build. Mater. 2020, 257, 119511. [Google Scholar] [CrossRef]
- Azad, N.M.; Samarakoon, S.S.M. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, J.; Kaur, M. Compressive strength of rice husk ash based geopolymer: The effect of alkaline activator. Constr. Build. Mater. 2018, 169, 188–192. [Google Scholar] [CrossRef]
- Krishna, R.; Mishra, J.; Zribi, M.; Adeniyi, F.; Saha, S.; Baklouti, S.; Shaikh, F.U.A.; Gökçe, H. A review on developments of environmentally friendly geopolymer technology. Materialia 2021, 20, 101212. [Google Scholar] [CrossRef]
- Suárez, M.; Fernández, A.; Díaz, L.; Sobrados, I.; Sanz, J.; Borrell, A.; Palomares, F.; Torrecillas, R.; Moya, J. Synthesis and sintering at low temperature of a new nanostructured beta-Eucryptite dense compact by spark plasma sintering. Ceram. Int. 2020, 46, 18469–18477. [Google Scholar] [CrossRef]
- Kljajević, L.M.; Nenadović, S.S.; Nenadović, M.T.; Bundaleski, N.K.; Todorović, B.; Pavlović, V.B.; Rakočević, Z.L. Structural and chemical properties of thermally treated geopolymer samples. Ceram. Int. 2017, 43, 6700–6708. [Google Scholar] [CrossRef]
- Longhi, M.A.; Walkley, B.; Rodríguez, E.D.; Kirchheim, A.P.; Zhang, Z.; Wang, H. New selective dissolution process to quantify reaction extent and product stability in metakaolin-based geopolymers. Compos. Part B Eng. 2019, 176, 107172. [Google Scholar] [CrossRef]
- Ji, Z.; Li, M.; Su, L.; Pei, Y. Porosity, mechanical strength and structure of waste-based geopolymer foams by different stabilizing agents. Constr. Build. Mater. 2020, 258, 119555. [Google Scholar] [CrossRef]
- Škvára, F.; Šcul, R.; Tišler, Z.; Skřičík, P.; Šmilauer, V.; Cílová, Z.Z. Preparation and properties of fly ash-based geopolymer foams. Ceram. Silik. 2014, 58, 188–197. [Google Scholar]
- Badanoiu, A.I.; Al Saadi, T.H.A.; Stoleriu, S.; Voicu, G. Preparation and characterization of foamed geopolymers from waste glass and red mud. Constr. Build. Mater. 2015, 84, 284–293. [Google Scholar] [CrossRef]
- Wattanasiriwech, D.; Yomthong, K.; Wattanasiriwech, S. Characterisation and properties of class C-fly ash based geopolymer foams: Effects of foaming agent content, aggregates, and surfactant. Constr. Build. Mater. 2021, 306, 124847. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Cao, L.; Wu, B. Development of metakaolin–fly ash based geopolymers for fire resistance applications. Constr. Build. Mater. 2014, 55, 38–45. [Google Scholar] [CrossRef]
- Zuda, L.; Rovnaník, P.; Bayer, P.; Černý, R. Effect of High Temperatures on the Properties of Alkali Activated Aluminosilicate with Electrical Porcelain Filler. Int. J. Thermophys. 2007, 29, 693–705. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.; Melo, U.C. Synthesis and thermal properties of inorganic polymers (geopolymers) for structural and refractory applications from volcanic ash. Ceram. Int. 2011, 37, 3011–3018. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; de Gutiérrez, R.M.; Gordillo, M.; Provis, J.L. Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486. [Google Scholar] [CrossRef]
- Zhao, R.; Sanjayan, J.G. Geopolymer and Portland cement concretes in simulated fire. Mag. Concr. Res. 2011, 63, 163–173. [Google Scholar] [CrossRef]
- Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.A.B.; Hussin, K. Thermal Resistance Variations of Fly Ash Geopolymers: Foaming Responses. Sci. Rep. 2017, 7, srep45355. [Google Scholar] [CrossRef]
- Ramli, M.I.I.; Salleh, M.A.A.M.; Abdullah, M.M.A.B.; Aziz, I.H.; Ying, T.C.; Shahedan, N.F.; Kockelmann, W.; Fedrigo, A.; Sandu, A.V.; Vizureanu, P.; et al. The Influence of Sintering Temperature on the Pore Structure of an Alkali-Activated Kaolin-Based Geopolymer Ceramic. Materials 2022, 15, 2667. [Google Scholar] [CrossRef]
- Polat, D.; Güden, M. Processing and characterization of geopolymer and sintered geopolymer foams of waste glass powders. Constr. Build. Mater. 2021, 300, 124259. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, H.-J.; Peng, T.-J.; Zheng, W.-M. The sintering kinetics and properties of sintered glass-ceramics from coal fly ash of different particle size. Results Phys. 2019, 15, 102774. [Google Scholar] [CrossRef]
- Lu, H.-X.; Liu, H.-Y.; He, M.; Yu, L.; Wan, F.; Chen, D.-L.; Wang, H.-L.; Xu, H.-L.; Zhang, R. Properties of Glass-Ceramics Synthesized from Blast Furnace Slag with Different Sizes. Steel Res. Int. 2011, 82, 741–745. [Google Scholar] [CrossRef]
- Erol, M.; Küçükbayrak, S.; Ersoy-Meriçboyu, A. Characterization of sintered coal fly ashes. Fuel 2007, 87, 1334–1340. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.; de Gutiérrez, R.M. Synthesis of ceramic materials from ecofriendly geopolymer precursors. Mater. Lett. 2018, 230, 300–304. [Google Scholar] [CrossRef]
- Jaya, N.A.; Abdullah, M.M.A.B.; Ghazali, C.M.R.; Binhussain, M.; Hussin, K.; Ahmad, R. Characterization and Microstructure of Kaolin-Based Ceramic Using Geopolymerization. Key Eng. Mater. 2016, 700, 3–11. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Factors affecting the performance of metakaolin geopolymers exposed to elevated temperatures. J. Mater. Sci. 2008, 43, 824–831. [Google Scholar] [CrossRef]
- Jaya, N.A.; Al Bakri Abdullah, M.M.; Ghazali, C.M.; Binhussain, M.; Hussin, K.; Ahmad, R.; Ekaputri, J.J. Correlation between Na2SiO3/NaOH and NaOH Molarity to Flexural Strength of Geopolymer Ceramic. Appl. Mech. Mater. 2015, 754–755, 152–156. [Google Scholar] [CrossRef]
- He, P.; Jia, D. Low-temperature sintered pollucite ceramic from geopolymer precursor using synthetic metakaolin. J. Mater. Sci. 2013, 48, 1812–1818. [Google Scholar] [CrossRef]
- Hussin, M.W.; Bhutta, M.A.R.; Azreen, M.; Ramadhansyah, P.J.; Mirza, J. Performance of blended ash geopolymer concrete at elevated temperatures. Mater. Struct. 2015, 48, 709–720. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Lee, W.-H.; Liao, P.-H. Using Geopolymer Technology on Synthesizing Leucite Ceramics. Polymers 2021, 13, 3621. [Google Scholar] [CrossRef] [PubMed]
- Zawrah, M.; Sawan, S.A.; Khattab, R.; Abdel-Shafi, A.A. Effect of nano sand on the properties of metakaolin-based geopolymer: Study on its low rate sintering. Constr. Build. Mater. 2020, 246, 118486. [Google Scholar] [CrossRef]
- He, P.; Jia, D.; Lin, T.; Wang, M.; Zhou, Y. Effects of high-temperature heat treatment on the mechanical properties of unidirectional carbon fiber reinforced geopolymer composites. Ceram Int. 2010, 36, 1447–1453. [Google Scholar] [CrossRef]
- Xie, N.; Bell, J.L.; Kriven, W.M. Fabrication of Structural Leucite Glass-Ceramics from Potassium-Based Geopolymer Precursors. J. Am. Ceram. Soc. 2010, 93, 2644–2649. [Google Scholar] [CrossRef]
- Kovářík, T.; Hájek, J.; Pola, M.; Rieger, D.; Svoboda, M.; Beneš, J.; Šutta, P.; Deshmukh, K.; Jandová, V. Cellular ceramic foam derived from potassium-based geopolymer composite: Thermal, mechanical and structural properties. Mater. Des. 2021, 198, 109355. [Google Scholar] [CrossRef]
- Motte, F.; Falcoz, Q.; Veron, E.; Py, X. Compatibility tests between Solar Salt and thermal storage ceramics from inorganic industrial wastes. Appl. Energy 2015, 155, 14–22. [Google Scholar] [CrossRef]
- Inokoshi, M.; Shimizu, H.; Nozaki, K.; Takagaki, T.; Yoshihara, K.; Nagaoka, N.; Zhang, F.; Vleugels, J.; Van Meerbeek, B.; Minakuchi, S. Crystallographic and morphological analysis of sandblasted highly translucent dental zirconia. Dent. Mater. 2018, 34, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.M.; Lai, A.; Gan, C.L.; Schuh, C.A. Crystal orientation dependence of the stress-induced martensitic transformation in zirconia-based shape memory ceramics. Acta Mater. 2016, 116, 124–135. [Google Scholar] [CrossRef]
- Shen, Z.; Nygren, M. Microstructural prototyping of ceramics by kinetic engineering: Applications of spark plasma sintering. Chem. Rec. 2005, 5, 173–184. [Google Scholar] [CrossRef]
- Biesuz, M.; Grasso, S.; Sglavo, V.M. What’s new in ceramics sintering? A short report on the latest trends and future prospects. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100868. [Google Scholar] [CrossRef]
- Wang, X.; Huan, Y.; Zhao, P.; Liu, X.; Wei, T.; Zhang, Q.; Wang, X. Optimizing the grain size and grain boundary morphology of (K,Na)NbO3-based ceramics: Paving the way for ultrahigh energy storage capacitors. J. Mater. 2021, 7, 780–789. [Google Scholar] [CrossRef]
- Zhang, Y.; Jung, J.I.; Luo, J. Thermal runaway, flash sintering and asymmetrical microstructural development of ZnO and ZnO–Bi2O3 under direct currents. Acta Mater. 2015, 94, 87–100. [Google Scholar] [CrossRef]
- Bordia, R.K.; Kang, S.J.L.; Olevsky, E.A. Current understanding and future research directions at the onset of the next century of sintering science and technology. J. Am. Ceram. Soc. 2017, 100, 2314–2352. [Google Scholar] [CrossRef]
- Erasmus, E.P.; Johnson, O.T.; Sigalas, I.; Massera, J. Effects of Sintering Temperature on Crystallization and Fabrication of Porous Bioactive Glass Scaffolds for Bone Regeneration. Sci. Rep. 2017, 7, 6046. [Google Scholar] [CrossRef]
- Gheiratmand, T.; Hosseini, H.R.M.; Davami, P.; Sarafidis, C. Fabrication of FINEMET bulk alloy from amorphous powders by spark plasma sintering. Powder Technol. 2015, 289, 163–168. [Google Scholar] [CrossRef]
- Kocjan, A.; Logar, M.; Shen, Z. The agglomeration, coalescence and sliding of nanoparticles, leading to the rapid sintering of zirconia nanoceramics. Sci. Rep. 2017, 7, 25. [Google Scholar] [CrossRef]
- Salama, H.; Kundin, J.; Shchyglo, O.; Mohles, V.; Marquardt, K.; Steinbach, I. Role of inclination dependence of grain boundary energy on the microstructure evolution during grain growth. Acta Mater. 2020, 188, 641–651. [Google Scholar] [CrossRef]
- Chaim, R.; Chevallier, G.; Weibel, A.; Estournès, C. Grain growth during spark plasma and flash sintering of ceramic nanoparticles: A review. J. Mater. Sci. 2017, 53, 3087–3105. [Google Scholar] [CrossRef]
- Presenda, Á.; Salvador, M.D.; Peñaranda-Foix, F.L.; Moreno, R.; Borrell, A. Effect of microwave sintering on microstructure and mechanical properties in Y-TZP materials used for dental applications. Ceram. Int. 2015, 41, 7125–7132. [Google Scholar] [CrossRef]
- Singh, L.K.; Bhadauria, A.; Jana, S.; Laha, T. Effect of Sintering Temperature and Heating Rate on Crystallite Size, Densification Behaviour and Mechanical Properties of Al-MWCNT Nanocomposite Consolidated via Spark Plasma Sintering. Acta Met. Sin. Engl. Lett. 2018, 31, 1019–1030. [Google Scholar] [CrossRef]
- Sutharsini, U.; Thanihaichelvan, M.; Ting, C.; Ramesh, S.; Tan, C.; Chandran, H.; Sarhan, A.A.; Urriés, I. Effect of two-step sintering on the hydrothermal ageing resistance of tetragonal zirconia polycrystals. Ceram. Int. 2017, 43, 7594–7599. [Google Scholar] [CrossRef]
- Huber, D.; Vogel, L.; Fischer, A. The effects of sintering temperature and hold time on densification, mechanical properties and microstructural characteristics of binder jet 3D printed 17-4 PH stainless steel. Addit. Manuf. 2021, 46, 102114. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, Y.; Jin, B.; Li, M.; Xing, B.; Zhao, Z. Effect of debinding and sintering profile on the optical properties of DLP-3D printed YAG transparent ceramic. Ceram. Int. 2022, 48, 21134–21140. [Google Scholar] [CrossRef]
- Frueh, T.; Ozer, I.; Poterala, S.; Lee, H.; Kupp, E.; Compson, C.; Atria, J.; Messing, G. A critique of master sintering curve analysis. J. Eur. Ceram. Soc. 2018, 38, 1030–1037. [Google Scholar] [CrossRef]
- Shao, W.; Chen, S.; Li, D.; Cao, H.; Zhang, Y.; Zhang, S. Prediction and control of microstructure evolution for sub-microscale α-Al2O3 during low-heating-rate sintering based on the master sintering curve theory. J. Eur. Ceram. Soc. 2009, 29, 201–204. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhang, X.; Shen, H.; Liu, J.; Zhang, S. A novel approach for preparing glass ceramic foams from MSWI fly ash: Foaming characteristics and hierarchical pore formation mechanism. J. Mater. Res. Technol. 2022, 18, 731–744. [Google Scholar] [CrossRef]
- Pei, F.; Zhu, G.; Li, P.; Guo, H.; Yang, P. Effects of CaF2 on the sintering and crystallisation of CaO–MgO–Al2O3–SiO2 glass-ceramics. Ceram Int. 2020, 46, 17825–17835. [Google Scholar] [CrossRef]
- Li, X.; Jiang, D.; Zhang, J.; Lin, Q.; Chen, Z.; Huang, Z. Densification behavior and related phenomena of spark plasma sintered boron carbide. Ceram. Int. 2014, 40, 4359–4366. [Google Scholar] [CrossRef]
- Moshtaghioun, B.M.; Cumbrera-Hernández, F.L.; Gómez-García, D.; de Bernardi-Martín, S.; Domínguez-Rodríguez, A.; Monshi, A.; Abbasi, M.H. Effect of spark plasma sintering parameters on microstructure and room-temperature hardness and toughness of fine-grained boron carbide (B4C). J. Eur. Ceram. Soc. 2013, 33, 361–369. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Liu, X.-Q.; Tao, S.-W.; Meng, G.-Y.; Sorensen, O.T. Kinetics of the reactive sintering of kaolinite-aluminum hydroxide extrudate. Ceram. Int. 2002, 28, 479–486. [Google Scholar] [CrossRef]
- Ptáček, P.; Křečková, M.; Šoukal, F.; Opravil, T.; Havlica, J.; Brandštetr, J. The kinetics and mechanism of kaolin powder sintering I. The dilatometric CRH study of sinter-crystallization of mullite and cristobalite. Powder Technol. 2012, 232, 24–30. [Google Scholar] [CrossRef]
- Fan, W.-D.; Yang, Q.-W.; Guo, B.; Liu, B.; Zhang, S.-G. Crystallization mechanism of glass-ceramics prepared from stainless steel slag. Rare Met. 2018, 37, 413–420. [Google Scholar] [CrossRef]
- Toussaint, A.; De Wilde, M. A comprehensive model of sintering and coalescence of unpigmented latexes. Prog. Org. Coat. 1997, 30, 113–126. [Google Scholar] [CrossRef]
- Prado, M.O.; Zanotto, E.D.; Müller, R. Model for sintering polydispersed glass particles. J. Non-Cryst. Solids 2001, 279, 169–178. [Google Scholar] [CrossRef]
- Prado, M.O.; Ferreira, E.B.; Zanotto, E.D. Sintering Kinetics of Crystallizing Glass Particles. A Review. Melt Chem. Relax. Solidif. Kinet. Glasses 2005, 170, 163–179. [Google Scholar] [CrossRef]
- Topuz, B.; Şimşek, D.; Çiftçioğlu, M. Preparation of monodisperse silica spheres and determination of their densification behaviour. Ceram Int. 2015, 41, 43–52. [Google Scholar] [CrossRef]
- Ban, C.W.; Choi, G.M. The effect of sintering on the grain boundary conductivity of lithium lanthanum titanates. Solid State Ion. 2001, 140, 285–292. [Google Scholar] [CrossRef]
- Gonzalez-Julian, J.; Neuhaus, K.; Bernemann, M.; da Silva, J.P.; Laptev, A.; Bram, M.; Guillon, O. Unveiling the mechanisms of cold sintering of ZnO at 250 °C by varying applied stress and characterizing grain boundaries by Kelvin Probe Force Microscopy. Acta Mater. 2018, 144, 116–128. [Google Scholar] [CrossRef]
- Xu, Q.; Tsai, C.-L.; Song, D.; Basak, S.; Kungl, H.; Tempel, H.; Hausen, F.; Yu, S.; Eichel, R.-A. Insights into the reactive sintering and separated specific grain/grain boundary conductivities of Li1.3Al0.3Ti1.7(PO4)3. J. Power Sources 2021, 492, 229631. [Google Scholar] [CrossRef]
- Yang, L.; Dai, Q.; Liu, L.; Shao, D.; Luo, K.; Jamil, S.; Liu, H.; Luo, Z.; Chang, B.; Wang, X. Rapid sintering method for highly conductive Li7La3Zr2O12 ceramic electrolyte. Ceram. Int. 2020, 46, 10917–10924. [Google Scholar] [CrossRef]
- Ayyappadas, C.; Muthuchamy, A.; Annamalai, A.R.; Agrawal, D.K. An investigation on the effect of sintering mode on various properties of copper-graphene metal matrix composite. Adv. Powder Technol. 2017, 28, 1760–1768. [Google Scholar] [CrossRef]
- Asadikiya, M.; Zhang, C.; Rudolf, C.; Boesl, B.; Agarwal, A.; Zhong, Y. The effect of sintering parameters on spark plasma sintering of B4C. Ceram. Int. 2017, 43, 11182–11188. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Liu, Y.; Zeng, Q.; Hu, K.; Lu, Z.; Liang, J. Effect of sintering temperature in argon atmosphere on microstructure and properties of 3D printed alumina ceramic cores. J. Adv. Ceram. 2020, 9, 220–231. [Google Scholar] [CrossRef]
- Liu, S.-S.; Li, M.; Wu, J.-M.; Chen, A.-N.; Shi, Y.-S.; Li, C.-H. Preparation of high-porosity Al2O3 ceramic foams via selective laser sintering of Al2O3 poly-hollow microspheres. Ceram. Int. 2019, 46, 4240–4247. [Google Scholar] [CrossRef]

| Author | Raw Materials | Properties |
|---|---|---|
| Bong et al. [38] | Wollastonite | Improve the flexural strength Increase the compressive strength |
| Ren et al. [39] | Metakaolin with reinforcement of wollastonite, tremolite, short basalt | Increase the compressive strength Enhance the resistance to sulfate and chloride attack |
| Archez et al. [40] | Wollastonite | Improve the viscosity and mechanical properties |
| Author | Exposed Temperature | Findings |
|---|---|---|
| Zhang et al. [56] | Up to 800 °C | The strength of fly-ash-based geopolymer increased as the temperature increased to 800 °C |
| Bernal et al. [57] | Up to 1000 °C | The strength of metakaolin-based geopolymer increased when further heated up to 1000 °C |
| Zhao and Sanjayan [58] | Up to 800 °C | Sintering of fly ash geopolymer increased the strength at 800 °C |
| Author | Sintering Profile | Findings |
|---|---|---|
| Shen et al. [94] | Sintering temperature: 1800 °C Heating rates: 5 and 10 °C/min Holding time: 5 h, 10 h | The transmittance was high when the heating rate of sintering was low |
| Frueh et al. [95] | Heating rates: 35–150 °C/min | High heating rate attributed to the lower Q value More particle coarsening when heated during slow heating process |
| Shao et al. [96] | Heating rates: 0.5 and 5 °C/min | Heating rate influences the densification; slow heating rate is favorable |
| Researcher | Type of Model Studied | Founding |
|---|---|---|
| Liu et al. (2002) [101] | Nucleation growth by Avrami Johnson and Mehl model Bimodal Johnson–Mehl–Avrami (JMA) model | The shrinkage process is divided into three stages: First stage (950–1100 °C) Second stage (1200–1300 °C) Third stage (1300–1450 °C) |
| Ptáček et al. (2012) [102] | Kissinger’s and Eyring’s laws | Heating rate affecting the sintering behavior, kinetics and mechanism of the process Increasing the heating rate produced ceramic with low content of cristobalite |
| Fan et al. (2018) [103] | Modified Kissinger’s equation | Activation energy increased with the decrease in the liquid–solid ratio The mechanism of crystallization was affected by the basicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapa, N.B.; Ahmad, R.; Ibrahim, W.M.W.; Abdullah, M.M.A.B.; Wattanasakulpong, N.; Nemeș, O.; Sandu, A.V.; Vizureanu, P.; Sandu, I.G.; Kartikowati, C.W.; et al. Effect of Sintering Mechanism towards Crystallization of Geopolymer Ceramic—A Review. Materials 2023, 16, 4103. https://doi.org/10.3390/ma16114103
Mustapa NB, Ahmad R, Ibrahim WMW, Abdullah MMAB, Wattanasakulpong N, Nemeș O, Sandu AV, Vizureanu P, Sandu IG, Kartikowati CW, et al. Effect of Sintering Mechanism towards Crystallization of Geopolymer Ceramic—A Review. Materials. 2023; 16(11):4103. https://doi.org/10.3390/ma16114103
Chicago/Turabian StyleMustapa, Nur Bahijah, Romisuhani Ahmad, Wan Mastura Wan Ibrahim, Mohd Mustafa Al Bakri Abdullah, Nuttawit Wattanasakulpong, Ovidiu Nemeș, Andrei Victor Sandu, Petrica Vizureanu, Ioan Gabriel Sandu, Christina W. Kartikowati, and et al. 2023. "Effect of Sintering Mechanism towards Crystallization of Geopolymer Ceramic—A Review" Materials 16, no. 11: 4103. https://doi.org/10.3390/ma16114103
APA StyleMustapa, N. B., Ahmad, R., Ibrahim, W. M. W., Abdullah, M. M. A. B., Wattanasakulpong, N., Nemeș, O., Sandu, A. V., Vizureanu, P., Sandu, I. G., Kartikowati, C. W., & Risdanareni, P. (2023). Effect of Sintering Mechanism towards Crystallization of Geopolymer Ceramic—A Review. Materials, 16(11), 4103. https://doi.org/10.3390/ma16114103











