Use of a Waste-Derived Linde Type-A Immobilized in Agarose for the Remediation of Water Impacted by Coal Acid Mine Drainage at Pilot Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Analytical Methods
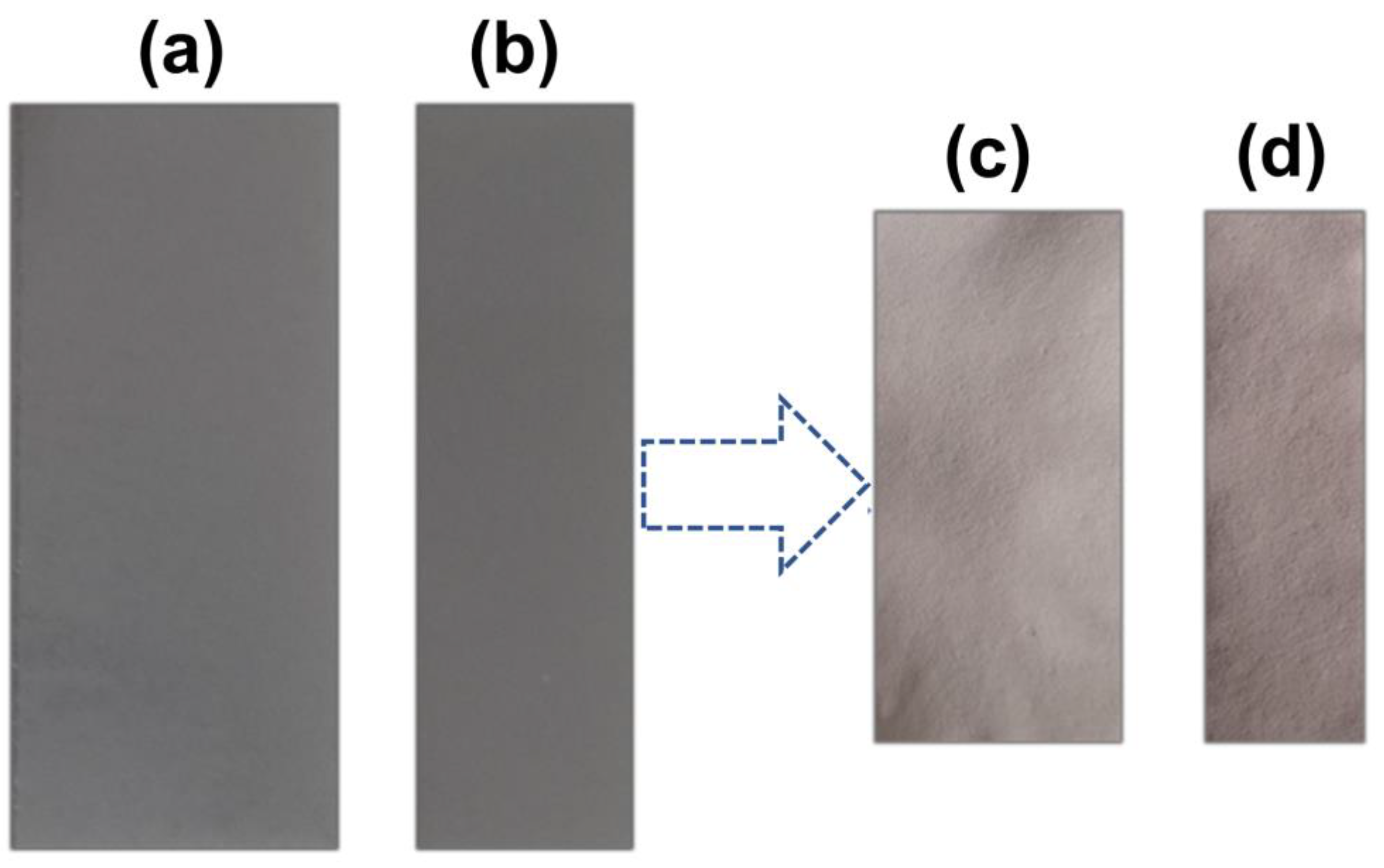
2.2. Preparing the Agarose-Immobilized LTA Zeolite
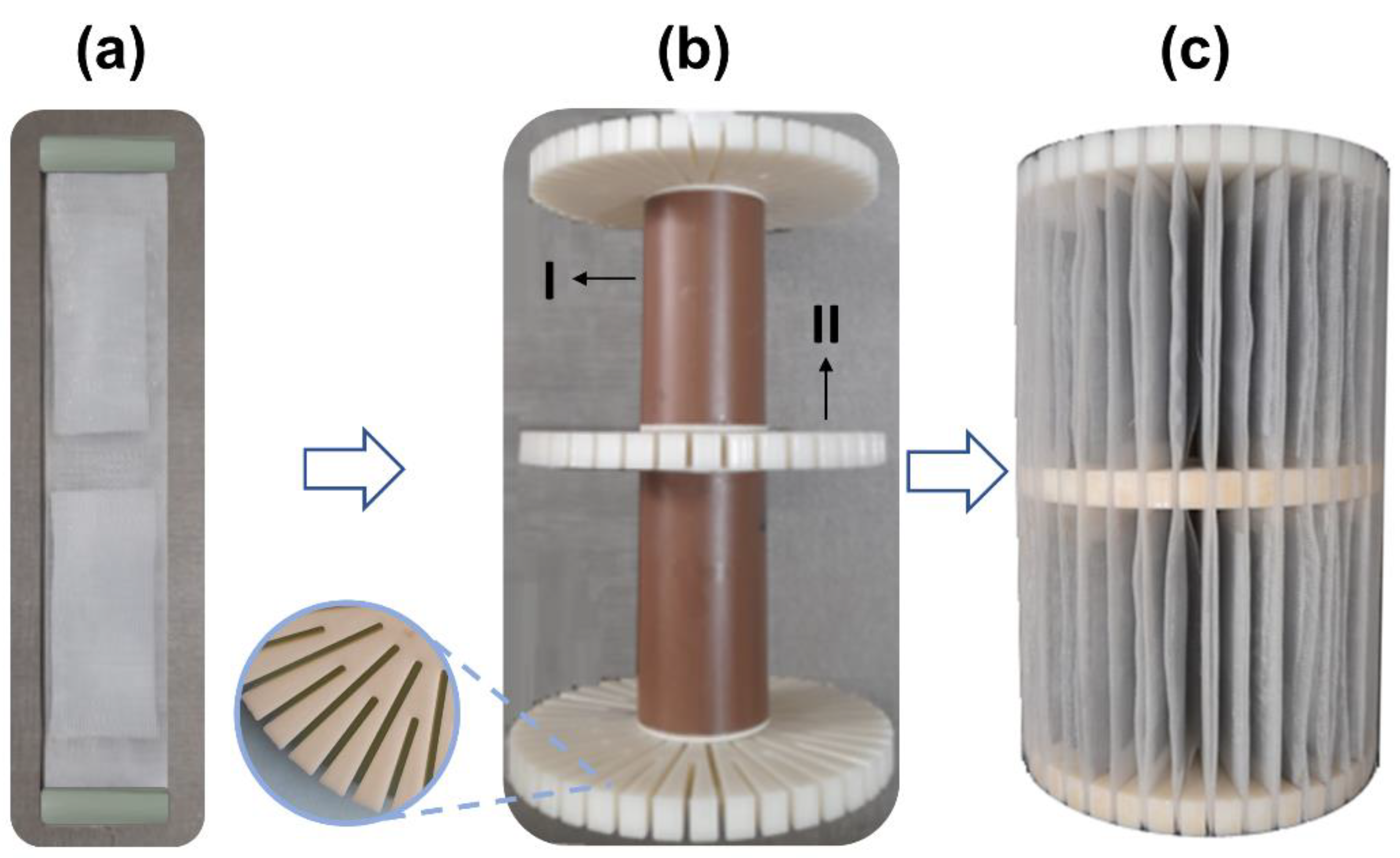
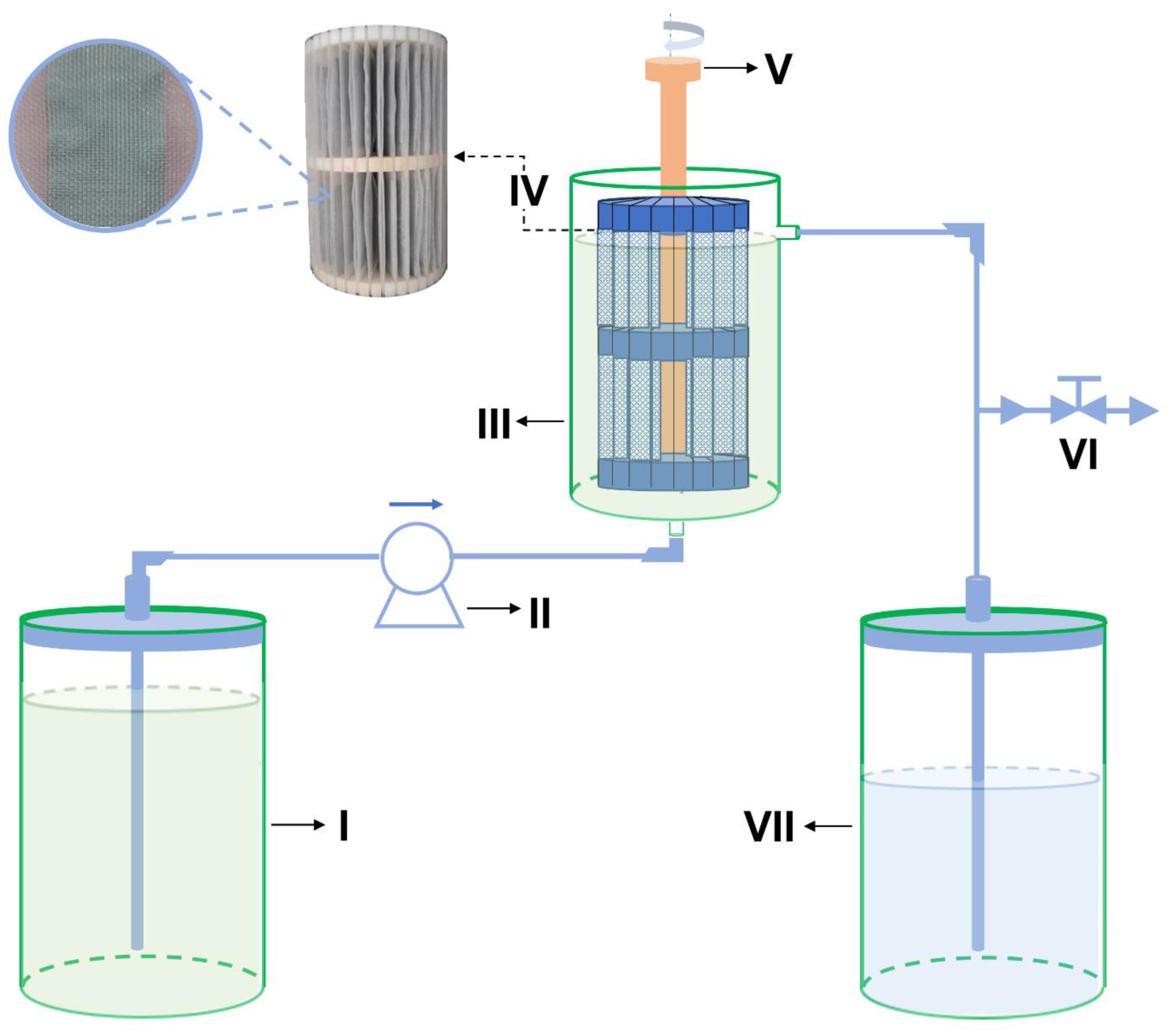
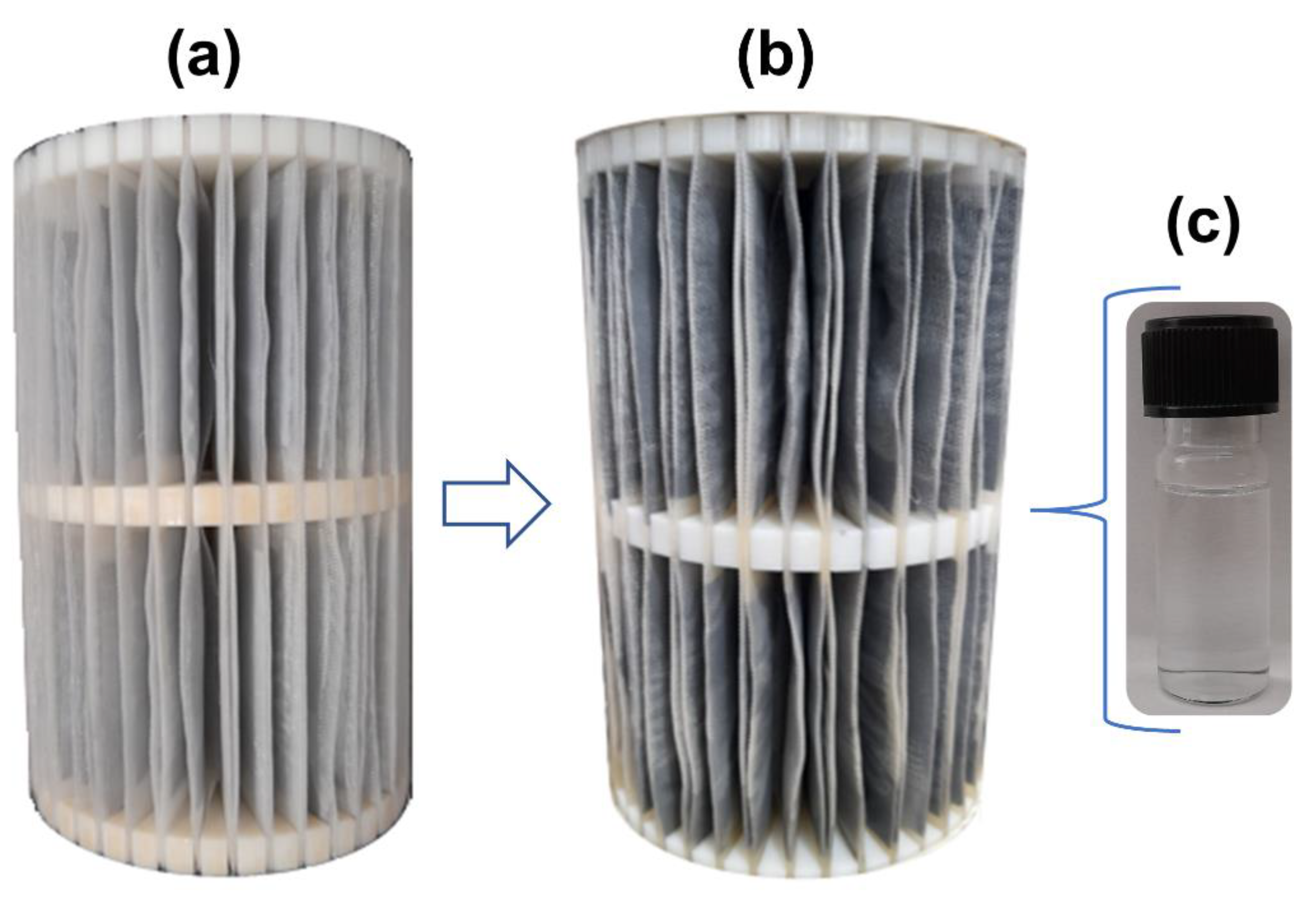
2.3. Construction and Experimental Design of the Pilot-Scale System
2.4. Operating Conditions for Adsorbent Application
2.5. Breakthrough Curves for the Pilot-Scale System
2.6. Mathematical Models for Breakthrough Curves
3. Results and Discussion
3.1. Characterization of the AMD-Impacted Water
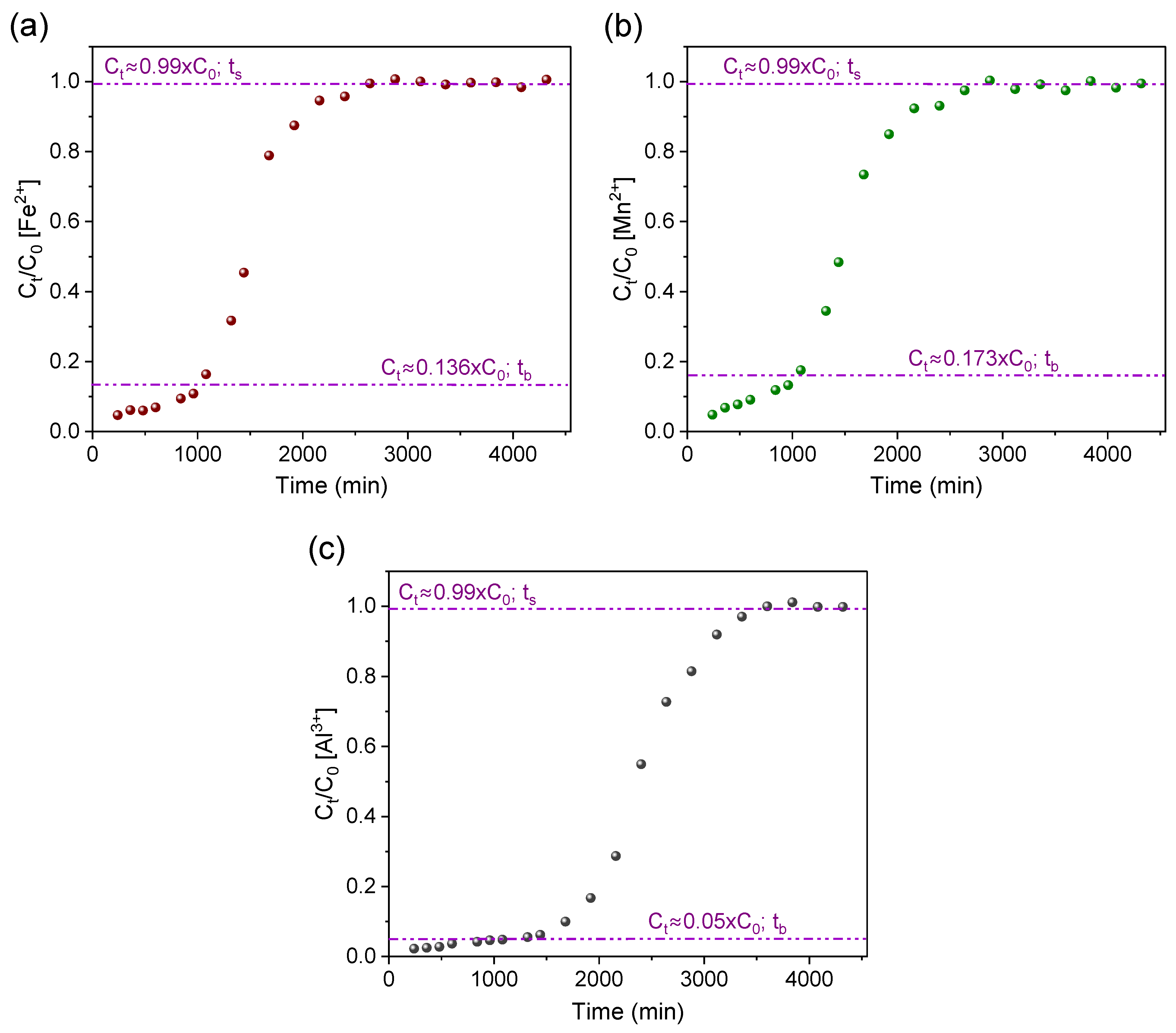
3.2. Operationalization and Breakthrough Curves
3.3. pH Variation during Operation
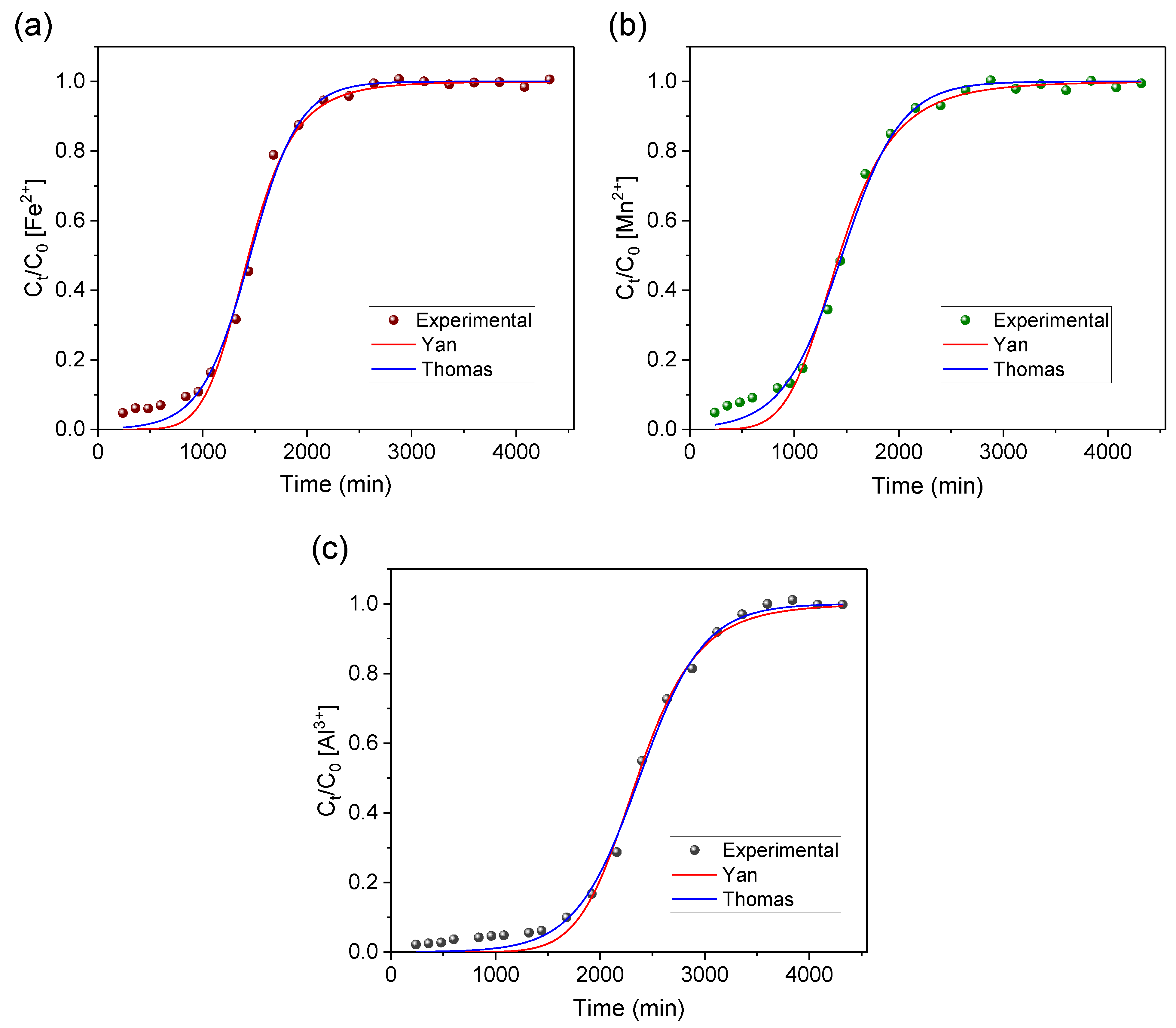
3.4. Applied Mathematical Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A Review of Treatment Technologies for the Mitigation of the Toxic Environmental Effects of Acid Mine Drainage (AMD). Process Saf. Environ. Prot. 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Rodrigues, C.; Lapolli, F.R.; Lobo-Recio, M.Á. Adsorption of Heavy Metals from Coal Acid Mine Drainage by Shrimp Shell Waste: Isotherm and Continuous-Flow Studies. J. Environ. Chem. Eng. 2019, 7, 102787. [Google Scholar] [CrossRef]
- Jeremias, T.C.; Pineda-Vásquez, T.; Lapolli, F.R.; Lobo-Recio, M.Á. Use of Eggshell as a Low-Cost Biomaterial for Coal Mine-Impacted Water (MIW) Remediation: Characterization and Statistical Determination of the Treatment Conditions. Water Air Soil Pollut. 2020, 231, 1–17. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable Resolutions for Environmental Threat of the Acid Mine Drainage. Sci. Total Environ. 2020, 717, 137211. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, C.; Su, P.; Tang, Y.; Huang, Z.; Ma, T. A Review of Acid Mine Drainage: Formation Mechanism, Treatment Technology, Typical Engineering Cases and Resource Utilization. Process Saf. Environ. Prot. 2023, 170, 1240–1260. [Google Scholar] [CrossRef]
- Daraz, U.; Li, Y.; Ahmad, I.; Iqbal, R.; Ditta, A. Remediation Technologies for Acid Mine Drainage: Recent Trends and Future Perspectives. Chemosphere 2023, 311, 137089. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.M.; Sarmiento, A.M.; Olías, M.; Canovas, C.R.; Riba, I.; Kalman, J.; Delvalls, T.A. Acid Mine Drainage Pollution in the Tinto and Odiel Rivers (Iberian Pyrite Belt, SW Spain) and Bioavailability of the Transported Metals to the Huelva Estuary. Environ. Int. 2007, 33, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Olías, M.; Cánovas, C.R.; Nieto, J.M.; Sarmiento, A.M. Evaluation of the Dissolved Contaminant Load Transported by the Tinto and Odiel Rivers (South West Spain). Appl. Geochem. 2006, 21, 1733–1749. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Alves, A.A.D.A.; Lapolli, F.R.; Lobo-Recio, M.A. Aplication of the Statistical Experimental Design to Optimize Mine-Impacted Water (MIW) Remediation Using Shrimp-Shell. Chemosphere 2017, 167, 322–329. [Google Scholar] [CrossRef]
- Rodrigues, C.; Núñez-Gómez, D.; Silveira, D.D.; Lapolli, F.R.; Lobo-Recio, M.A. Chitin as a Substrate for the Biostimulation of Sulfate-Reducing Bacteria in the Treatment of Mine-Impacted Water (MIW). J. Hazard. Mater. 2019, 375, 330–338. [Google Scholar] [CrossRef]
- Rodrigues, C.; Núñez-Gómez, D.; Follmann, H.V.D.M.; Silveira, D.D.; Nagel-Hassemer, M.E.; Lapolli, F.R.; Lobo-Recio, M.Á. Biostimulation of Sulfate-Reducing Bacteria and Metallic Ions Removal from Coal Mine-Impacted Water (MIW) Using Shrimp Shell as Treatment Agent. J. Hazard. Mater. 2020, 398, 122893. [Google Scholar] [CrossRef] [PubMed]
- Wesler, S.; de Brida, I.C.; Geremias, R.; de Menezes, C.T.B.; Pineda-Vasquez, T. Removal of Iron Ions from Water Contaminated with Acid Mine Drainage by Geopolymer Derived from Rice Husk Ash and Ceramic Residue. Eng. Sanit. E Ambient. 2021, 26, 1123–1133. [Google Scholar] [CrossRef]
- Brazil, National Council of the Environment (CONAMA). Resolution N° 357, from 17 March 2005. Available online: https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Resolucao/2005/res_conama_357_2005_classificacao_corpos_agua_rtfcda_altrd_res_393_2007_397_2008_410_2009_430_2011.pdf (accessed on 20 February 2023).
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1985; Volume 29, ISBN 92-5-102263-1. [Google Scholar]
- USEPA. Guidelines for Water Reuse; USEPA: Washington, DC, USA, 2012. [Google Scholar]
- Brazil, Ministry of Health (MS). Ordinance N° 518, from 25 March 2004. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/portaria_518_2004.pdf (accessed on 20 February 2023).
- Hernroth, B.; Tassidis, H.; Baden, S.P. Immunosuppression of Aquatic Organisms Exposed to Elevated Levels of Manganese: From Global to Molecular Perspective. Dev. Comp. Immunol. 2019, 104, 103536. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.; Borin, L.; Elli, E.; Latagliata, R.; Martino, B.; Palumbo, G.; Pilo, F.; Loscocco, F.; Visani, G.; Cianciulli, P. Iron Toxicity—Its Effect on the Bone Marrow. Blood Rev. 2018, 32, 473–479. [Google Scholar] [CrossRef]
- Klein, G.L. Aluminum Toxicity to Bone: A Multisystem Effect? Osteoporos Sarcopenia 2019, 5, 2–5. [Google Scholar] [CrossRef]
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and Avenues for Acid Mine Drainage Treatment, Beneficiation, and Valorisation in Circular Economy: A Review. Ecol. Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Chen, J.; Deng, S.; Jia, W.; Li, X.; Chang, J. Removal of Multiple Heavy Metals from Mining-Impacted Water by Biochar-Filled Constructed Wetlands: Adsorption and Biotic Removal Routes. Bioresour. Technol. 2021, 331, 125061. [Google Scholar] [CrossRef]
- Núñez-Gómez, D.; Nagel-Hassemer, M.E.; Lapolli, F.R.; Lobo-Recio, M.Á. Potencial Dos Resíduos Do Processamento de Camarão Para Remediação de Águas Contaminadas Com Drenagem Ácida Mineral. Polimeros 2016, 26, 1–7. [Google Scholar] [CrossRef]
- Falagán, C.; Yusta, I.; Sánchez-España, J.; Johnson, D.B. Biologically-Induced Precipitation of Aluminium in Synthetic Acid Mine Water. Miner. Eng. 2017, 106, 79–85. [Google Scholar] [CrossRef]
- Lobo-Recio, M.Á.; Rodrigues, C.; Custódio Jeremias, T.; Lapolli, F.R.; Padilla, I.; López-Delgado, A. Highly Efficient Removal of Aluminum, Iron, and Manganese Ions Using Linde Type-A Zeolite Obtained from Hazardous Waste. Chemosphere 2021, 267, 128919. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Huang, X.; Chen, L.; Hu, P.; Gao, J.; Zhen, Q.; Bashir, S.; et al. Facile Preparation of Zeolite-Activated Carbon Composite from Coal Gangue with Enhanced Adsorption Performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy Metal Adsorption with Zeolites: The Role of Hierarchical Pore Architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.; Padilla, I.; López-Andrés, S.; López-Delgado, A. Single and Competitive Adsorptive Removal of Lead, Cadmium, and Mercury Using Zeolite Adsorbent Prepared from Industrial Aluminum Waste. Desalination Water Treat 2018, 126, 181–195. [Google Scholar] [CrossRef]
- Banerjee, S.; Barman, S.; Halder, G. Sorptive Elucidation of Rice Husk Ash Derived Synthetic Zeolite towards Deionization of Coalmine Waste Water: A Comparative Study. Groundw. Sustain. Dev. 2017, 5, 137–151. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Song, Q.; Wang, S.; Cui, X.; Liu, F.; Liu, X. Efficient Recovery of Cu(II) by LTA-Zeolites with Hierarchical Pores and Their Resource Utilization in Electrochemical Denitrification: Environmentally Friendly Design and Reutilization of Waste in Water. J. Hazard. Mater. 2020, 394, 122554. [Google Scholar] [CrossRef]
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A Critical Review of Waste Resources, Synthesis, and Applications for Zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- López-Delgado, A.; Robla, J.I.; Padilla, I.; López-Andrés, S.; Romero, M. Zero-Waste Process for the Transformation of a Hazardous Aluminum Waste into a Raw Material to Obtain Zeolites. J. Clean. Prod. 2020, 255, 120178. [Google Scholar] [CrossRef]
- Li, W.; Jin, H.; Xie, H.; Wang, D.; Lei, E. Utilization of Electrolytic Manganese Residue to Synthesize Zeolite A and Zeolite X for Mn Ions Adsorption. J. Ind. Eng. Chem. 2022, 120, 147–158. [Google Scholar] [CrossRef]
- Chostak, C.L.; López-Delgado, A.; Padilla, I.; Lapolli, F.R.; Lobo-Recio, M.Á. Agarose-Immobilized LTA Zeolite: A Novel Material to Use in an Improved Treatment Process of Mine Impacted Water. Water Air Soil Pollut. 2023, 234, 365. [Google Scholar] [CrossRef]
- Chostak, C.L.; López-Delgado, A.; Padilla, I.; Lapolli, F.R.; Lobo-Recio, M.Á. Remoção de Íons de Alumínio, Ferro e Manganês de Águas Impactadas Pela Drenagem Ácida de Mina Com Zeólita Linde Type-A Imobilizada Em Gel de Agarose: Estudo Cinético e Isotérmico. Quim. Nova, 2023; submitted. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: New York, NY, USA, 2017. [Google Scholar]
- USEPA. Approved for Reporting Wastewater Analyses; USEPA: Washington, DC, USA, 1979. [Google Scholar]
- Chostak, C.L.; De Campos, M.S.; Da Silva, S.B.; Abate, G.; Grassi, M.T. Dispositivos DGT Modificados Com Materiais Alternativos Para Uso Na Especiação de Elementos Traço. Quim. Nova 2015, 38, 356–363. [Google Scholar] [CrossRef]
- Tafurt-Cardona, M.; Eismann, C.E.; Suárez, C.A.; Menegário, A.A.; Silva Luko, K.; Sargentini Junior, É. In Situ Selective Determination of Methylmercury in River Water by Diffusive Gradient in Thin Films Technique (DGT) Using Baker’s Yeast (Saccharomyces Cerevisiae) Immobilized in Agarose Gel as Binding Phase. Anal. Chim. Acta 2015, 887, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kumari, U.; Mishra, A.; Siddiqi, H.; Meikap, B.C. Effective Defluoridation of Industrial Wastewater by Using Acid Modified Alumina in Fixed-Bed Adsorption Column: Experimental and Breakthrough Curves Analysis. J. Clean. Prod. 2021, 279, 123645. [Google Scholar] [CrossRef]
- de Franco, M.A.E.; de Carvalho, C.B.; Bonetto, M.M.; Soares, R.D.P.; Féris, L.A. Removal of Amoxicillin from Water by Adsorption onto Activated Carbon in Batch Process and Fixed Bed Column: Kinetics, Isotherms, Experimental Design and Breakthrough Curves Modelling. J. Clean. Prod. 2017, 161, 947–956. [Google Scholar] [CrossRef]
- Amador, I.C.B.; Nunes, K.G.P.; de Franco, M.A.E.; Viegas, B.M.; Macêdo, E.N.; Féris, L.A.; Estumano, D.C. Application of Approximate Bayesian Computational Technique to Characterize the Breakthrough of Paracetamol Adsorption in Fixed Bed Column. Int. Commun. Heat Mass Transf. 2022, 132, 105917. [Google Scholar] [CrossRef]
- Maheshwari, U.; Gupta, S. Removal of Cr(VI) from Wastewater Using Activated Neem Bark in a Fixed-Bed Column: Interference of Other Ions and Kinetic Modelling Studies. Desalination Water Treat. 2016, 57, 8514–8525. [Google Scholar] [CrossRef]
- Chittoo, B.S.; Sutherland, C. Column Breakthrough Studies for the Removal and Recovery of Phosphate by Lime-Iron Sludge: Modeling and Optimization Using Artificial Neural Network and Adaptive Neuro-Fuzzy Inference System. Chin. J. Chem. Eng. 2020, 28, 1847–1859. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Apiratikul, R.; Chu, K.H. Improved Fixed Bed Models for Correlating Asymmetric Adsorption Breakthrough Curves. J. Water Process Eng. 2021, 40, 101810. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A New Model for Heavy Metal Removal in a Biosorption Column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Lee, C.G.; Kim, J.H.; Kang, J.K.; Kim, S.B.; Park, S.J.; Lee, S.H.; Choi, J.W. Comparative Analysis of Fixed-Bed Sorption Models Using Phosphate Breakthrough Curves in Slag Filter Media. Desalination Water Treat. 2015, 55, 1795–1805. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Cai, J.; Bao, W. Experimental Study on the Treatment of Acid Mine Drainage Containing Heavymetals with Domestic Waste Pyrolysis Ash. Water Sci. Technol. 2022, 85, 3225–3239. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of Solvation of Ions Part 5. Gibbs Free Energy of Hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Shavandi, M.A.; Haddadian, Z.; Ismail, M.H.S.; Abdullah, N.; Abidin, Z.Z. Removal of Fe(III), Mn(II) and Zn(II) from Palm Oil Mill Effluent (POME) by Natural Zeolite. J. Taiwan Inst. Chem. Eng. 2012, 43, 750–759. [Google Scholar] [CrossRef]
- Motsi, T.; Rowson, N.A.; Simmons, M.J.H. Adsorption of Heavy Metals from Acid Mine Drainage by Natural Zeolite. Int. J. Miner. Process 2009, 92, 42–48. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Assefi, M.; Maroufi, S.; Sahajwalla, V. Selective Isolation of Heavy Metals from Spent Electronic Waste Solution by Macroporous Ion-Exchange Resins. J. Hazard. Mater. 2019, 371, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Ebrahimi, A.; Mahvi, A.H.; Fatehizadeh, A.; Karakani, F.; Azarpira, H. Experimental Data for Aluminum Removal from Aqueous Solution by Raw and Iron-Modified Granular Activated Carbon. Data Brief 2018, 17, 731–738. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; El-Naas, M.H.; Abdallah, S. Removal of Aluminum from Aqueous Solutions by Adsorption on Date-Pit and BDH Activated Carbons. J. Hazard. Mater. 2008, 158, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Samanta, N.S.; Banerjee, S.; Mondal, P.; Anweshan; Bora, U.; Purkait, M.K. Preparation and Characterization of Zeolite from Waste Linz-Donawitz (LD) Process Slag of Steel Industry for Removal of Fe3+ from Drinking Water. Adv. Powder Technol. 2021, 32, 3372–3387. [Google Scholar] [CrossRef]
- Irdemez, Ş.; Demircioǧlu, N.; Yildiz, Y.Ş. The Effects of PH on Phosphate Removal from Wastewater by Electrocoagulation with Iron Plate Electrodes. J. Hazard. Mater. 2006, 137, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Fuerstenau, M.C.; Palmer, B.R. Anionic Flotation of Oxides and Silicates. In Flotation A.M. Gaudin Memorial; A.I.M.E.: New York, NY, USA, 1976; Volume 1, pp. 148–196. [Google Scholar]
- Tchobanoglus, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill Companies, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Mohan, D.; Chander, S. Single Component and Multi-Component Adsorption of Metal Ions by Activated Carbons. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 183–196. [Google Scholar] [CrossRef]

| Metal | C0 (mg/L) | pH | Conductivity (µS/cm) | Sulfate (mg/L) |
|---|---|---|---|---|
| Fe | 36.51 ± 0.54 | 3.5 ± 0.1 | 858 ± 5 | 416 ± 4 |
| Al | 19.55 ± 0.32 | |||
| Mn | 2.88 ± 0.17 |
| Metal | Breakthrough Point | Saturation Point | |||||||
|---|---|---|---|---|---|---|---|---|---|
| tb (min) | mtotal (mg) | Mtotal (mg) | R (%) | ts (min) | mtotal (mg) | Mtotal (mg) | R (%) | qmax (mg/g) | |
| Fe | 1027 | 364.41 | 389.96 | 93.45 | 2880 | 543.19 | 1093.55 | 49.67 | 17.42 |
| Mn | 1080 | 1529.64 | 32.35 | 91.62 | 2880 | 43.13 | 86.26 | 50.00 | 1.38 |
| Al | 1320 | 259.15 | 268.38 | 96.56 | 3600 | 474.10 | 731.95 | 64.77 | 15.20 |
| Adsorbent | Adsorbate | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|
| Shrimp shell | Fe2+ | 17.43 | [2] |
| Domestic waste Ash | Fe2+ | 18.519 | [49] |
| Mn2+ | 0.498 | [49] | |
| Natural zeolite | Mn2+ | 0.076 | [50,51] |
| Natural zeolite | Mn2+ | 0.52 | [50,51] |
| Modified activated carbon | Al3+ | 4.37 | [52] |
| Activated carbon date palm waste | Al3+ | 5.831 | [53] |
| Powdered LTA zeolite | Al3+ | 13.93 | [24] |
| AG-LTA | Fe2+ | 17.42 | This study |
| Mn2+ | 1.38 | ||
| Al3+ | 15.20 |
| Metal | Thomas’ Model | Yan’s Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KTH (mL/mg∙min) | qTH (mg/g) | R2 | χ2 | SSE | Ky (mL/mg∙min) | qy (mg/g) | R2 | χ2 | SSE | |
| Fe | 0.1169 | 17.75 | 0.9952 | 0.0009 | 0.0173 | 1.8596 | 17.54 | 0.9918 | 0.0015 | 0.0293 |
| Mn | 1.2292 | 1.40 | 0.9953 | 0.0008 | 0.0158 | 19.4993 | 1.37 | 0.9908 | 0.0016 | 0.0305 |
| Al | 0.1673 | 15.48 | 0.9966 | 0.0006 | 0.0125 | 4.3623 | 15.35 | 0.9945 | 0.0011 | 0.0201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chostak, C.L.; López-Delgado, A.; Padilla, I.; Lapolli, F.R.; Lobo-Recio, M.Á. Use of a Waste-Derived Linde Type-A Immobilized in Agarose for the Remediation of Water Impacted by Coal Acid Mine Drainage at Pilot Scale. Materials 2023, 16, 4038. https://doi.org/10.3390/ma16114038
Chostak CL, López-Delgado A, Padilla I, Lapolli FR, Lobo-Recio MÁ. Use of a Waste-Derived Linde Type-A Immobilized in Agarose for the Remediation of Water Impacted by Coal Acid Mine Drainage at Pilot Scale. Materials. 2023; 16(11):4038. https://doi.org/10.3390/ma16114038
Chicago/Turabian StyleChostak, Cristiano Luiz, Aurora López-Delgado, Isabel Padilla, Flávio Rubens Lapolli, and María Ángeles Lobo-Recio. 2023. "Use of a Waste-Derived Linde Type-A Immobilized in Agarose for the Remediation of Water Impacted by Coal Acid Mine Drainage at Pilot Scale" Materials 16, no. 11: 4038. https://doi.org/10.3390/ma16114038
APA StyleChostak, C. L., López-Delgado, A., Padilla, I., Lapolli, F. R., & Lobo-Recio, M. Á. (2023). Use of a Waste-Derived Linde Type-A Immobilized in Agarose for the Remediation of Water Impacted by Coal Acid Mine Drainage at Pilot Scale. Materials, 16(11), 4038. https://doi.org/10.3390/ma16114038








