A Rational Design of a CoS2-CoSe2 Heterostructure for the Catalytic Conversion of Polysulfides in Lithium-Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
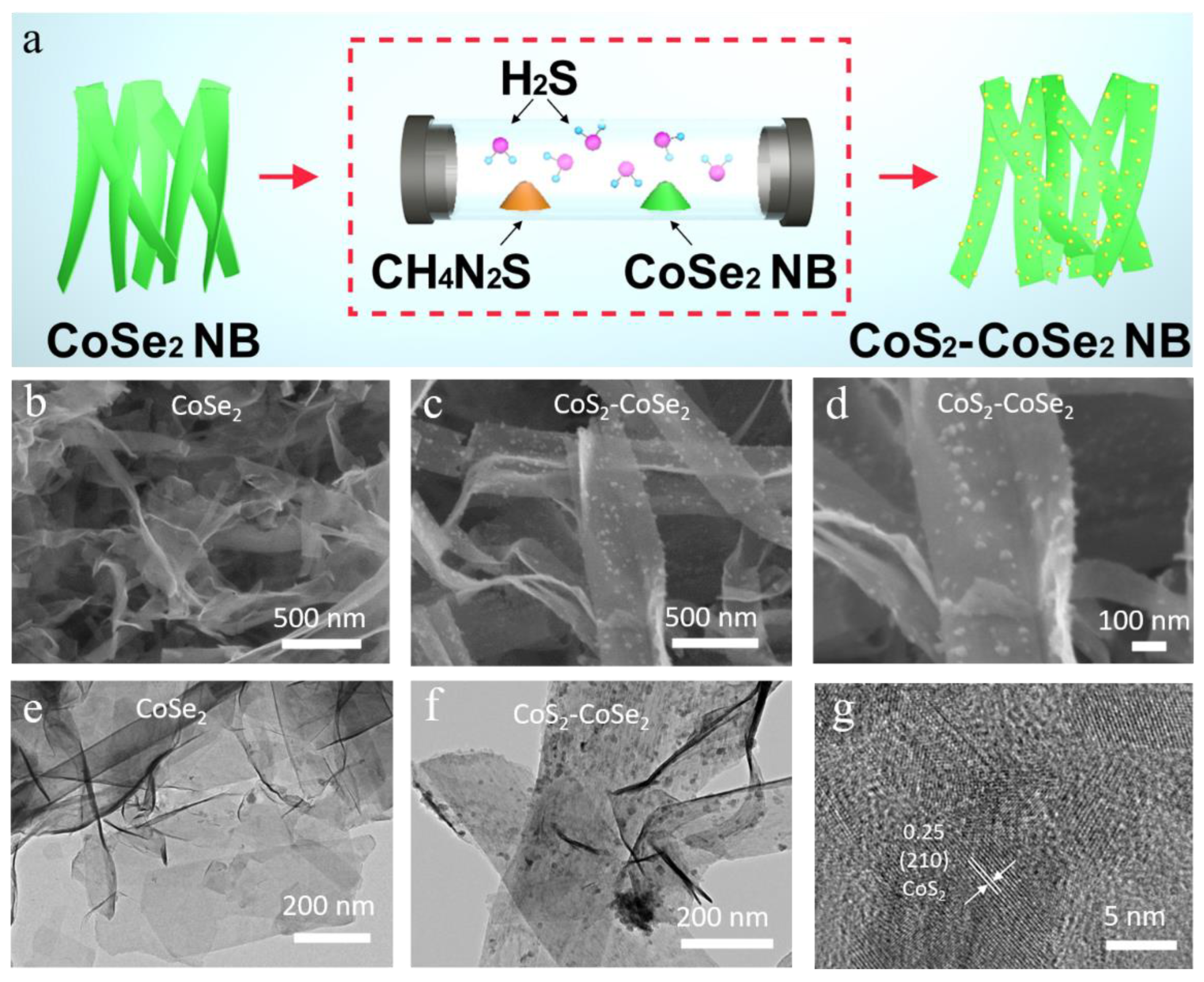
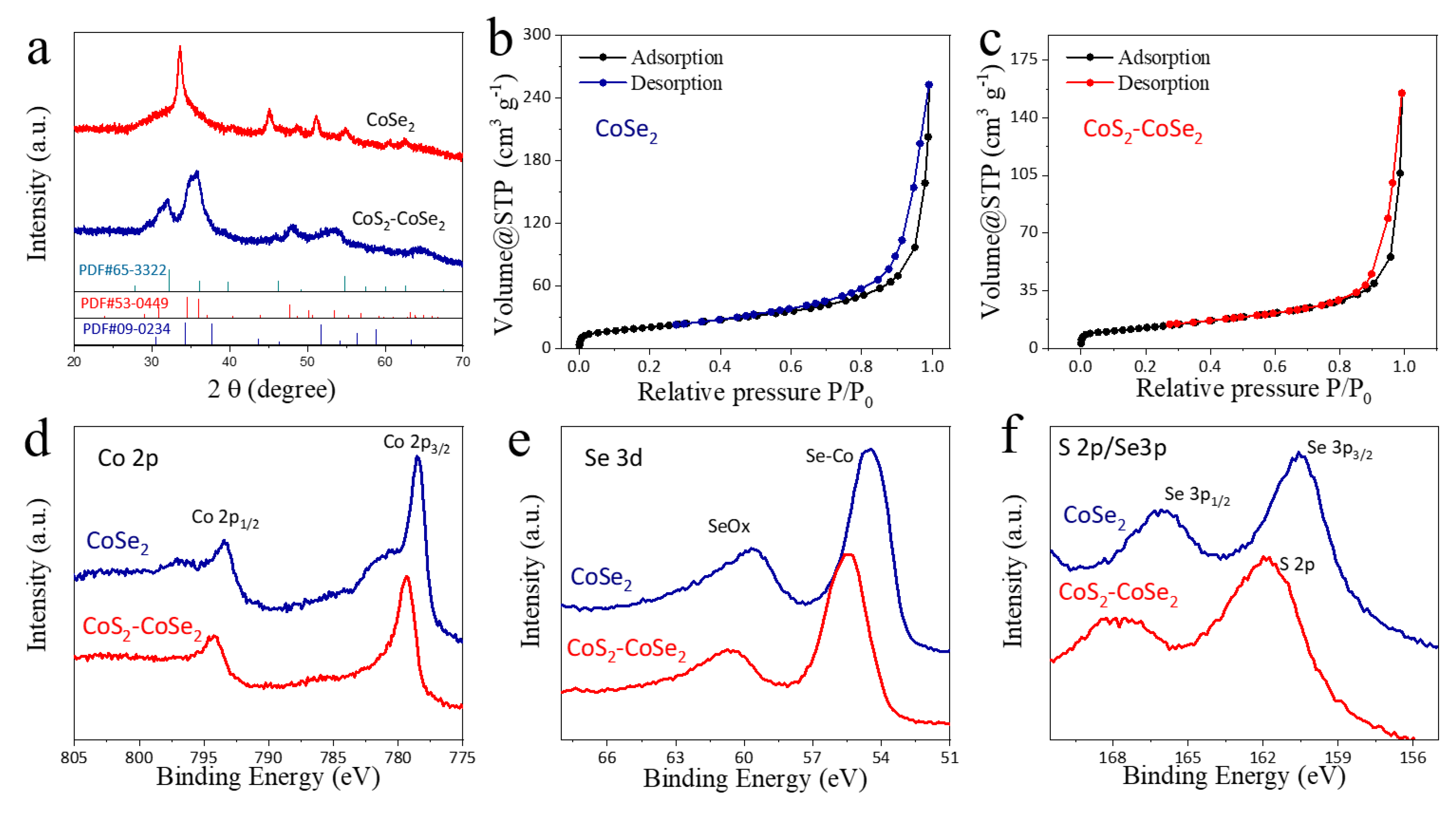
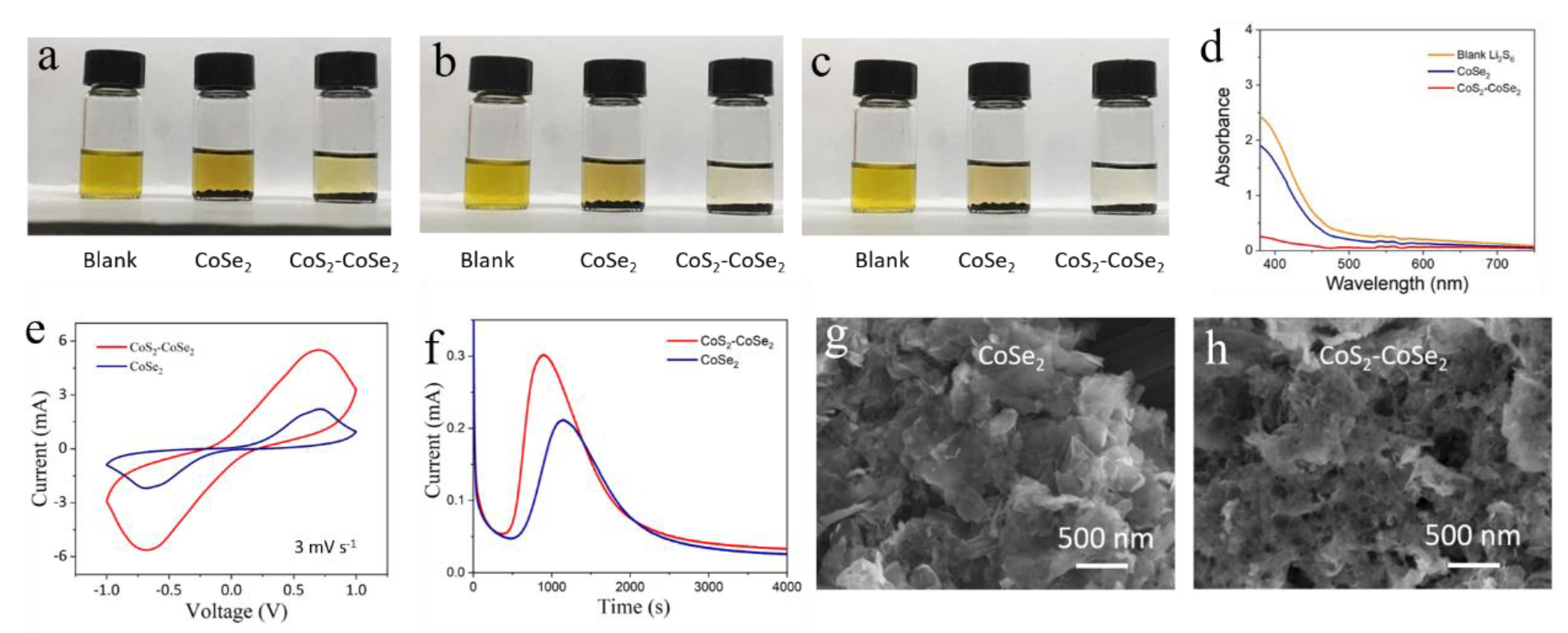
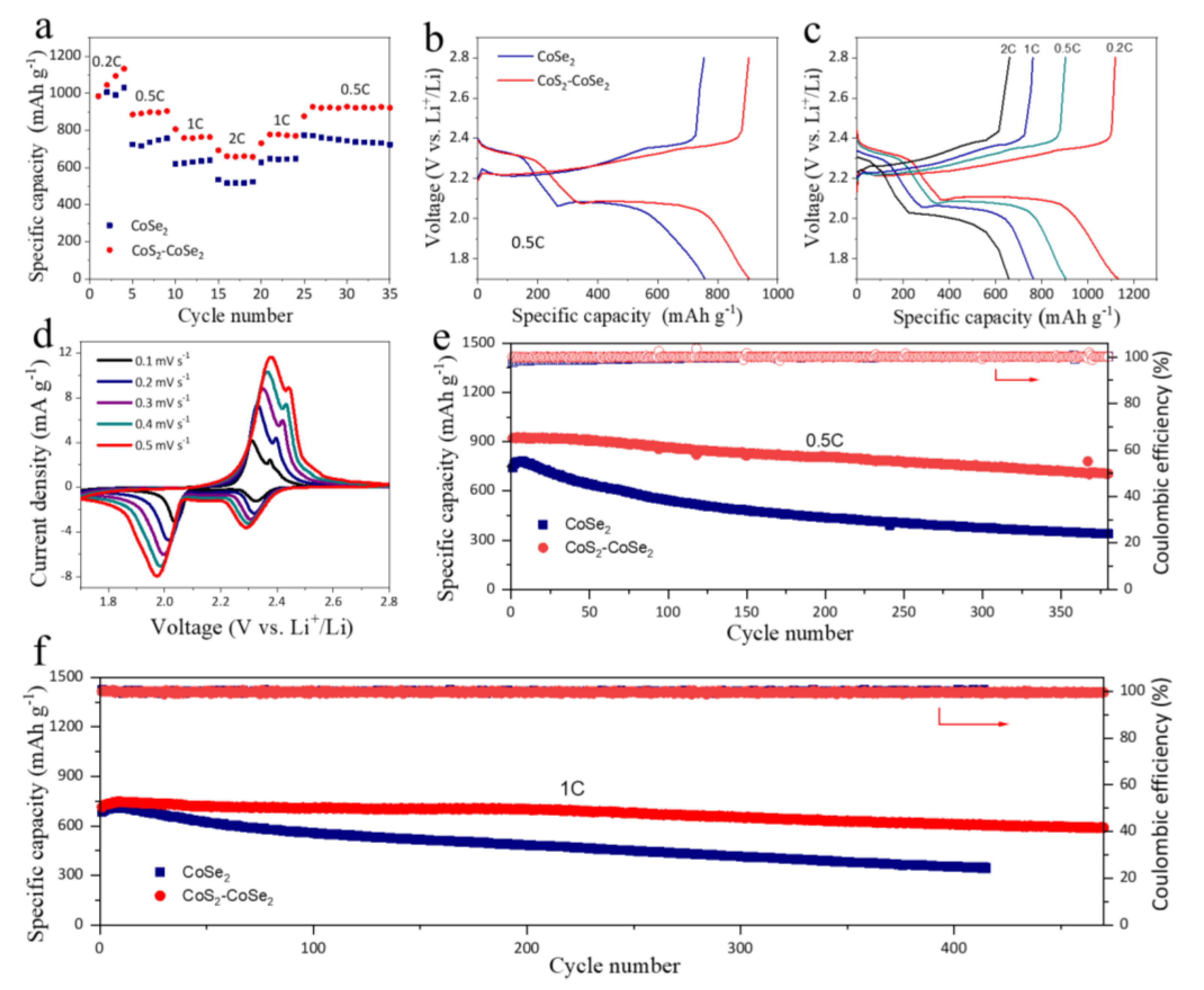
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing High-Energy Lithium-Sulfur Batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Al-Thabaiti, S.A.; Mostafa, M.M.M.; Ahmed, A.I.; Salama, R.S. Synthesis of Copper/Chromium Metal Organic Frameworks-Derivatives as an Advanced Electrode Material for High-Performance Supercapacitors. Ceram. Int. 2023, 49, 5119–5129. [Google Scholar] [CrossRef]
- Mostafa, M.M.M.; Alshehri, A.A.; Salama, R.S. High Performance of Supercapacitor Based on Alumina Nanoparticles Derived from Coca-Cola Cans. J. Energy Storage 2023, 64, 107168. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Zhang, Y.; Li, M.; Chen, Z.; Lu, J. Revisiting the Role of Polysulfides in Lithium-Sulfur Batteries. Adv. Mater. 2018, 30, 1705590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of Long Stability of Sulfur Cathode by Encapsulating Sulfur into Micropores of Carbon Spheres. Energy Environ. 2010, 3, 1531–1537. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Von Lim, Y.; Wang, Y.; Li, Y.; Zhang, D.; Yang, H.Y. Recent Advances in Heterostructure Engineering for Lithium-Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2003689. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A Highly Ordered Nanostructured Carbon-Sulphur Cathode for Lithium-Sulphur Batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Jung, D.S.; Hwang, T.H.; Lee, J.H.; Koo, H.Y.; Shakoor, R.A.; Kahraman, R.; Jo, Y.N.; Park, M.-S.; Choi, J.W. Hierarchical Porous Carbon by Ultrasonic Spray Pyrolysis Yields Stable Cycling in Lithium-Sulfur Battery. Nano Lett. 2014, 14, 4418–4425. [Google Scholar] [CrossRef]
- Peng, Z.; Fang, W.; Zhao, H.; Fang, J.; Cheng, H.; The Nam Long, D.; Xu, J.; Chen, P. Graphene-Based Ultrathin Microporous Carbon with Smaller Sulfur Molecules for Excellent Rate Performance of Lithium-Sulfur Cathode. J. Power Sources 2015, 282, 70–78. [Google Scholar] [CrossRef]
- Hai, C.; Peng, G.; Zhou, Y.; Shen, Y.; Li, X.; Sun, Y.; Zhang, G.; Zeng, J.; Dong, S. Gradually Anchoring Sulfur into in-Situ Doped Carbon Substrate as Cathode for Li-S Batteries with Excellent Wide-Temperature Performance. Ceram. Int. 2022, 48, 1622–1632. [Google Scholar] [CrossRef]
- Song, J.; Yu, Z.; Gordin, M.L.; Wang, D. Advanced Sulfur Cathode Enabled by Highly Crumpled Nitrogen-Doped Graphene Sheets for High-Energy-Density Lithium-Sulfur Batteries. Nano Lett. 2016, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Fan, T. Synergetic Pore Structure Optimization and Nitrogen Doping of 3d Porous Graphene for High Performance Lithium Sulfur Battery. J. Am. Chem. Soc. 2018, 256, 869–877. [Google Scholar] [CrossRef]
- Zhen, M.; Jiang, K.; Guo, S.-Q.; Shen, B.; Liu, H. Suitable Lithium Polysulfides Diffusion and Adsorption on Cnts@TiO2-Bronze Nanosheets Surface for High-Performance Lithium-Sulfur Batteries. Nano Res. 2022, 15, 933–941. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, S.; Zhang, X.; Zhang, Y.; Shi, H.; Liu, Y.; Hou, L.; Yuan, C. Construction of Co@N-Cnts Grown on N-MOxC Nanosheets for Separator Modification to Enhance Adsorption and Catalytic Conversion of Polysulfides in Li-S Batteries. J. Mater. Chem. A 2023, 11, 1856–1865. [Google Scholar] [CrossRef]
- Jiang, H.; Guo, J.; Tao, J.; Li, X.; Zheng, W.; He, G.; Dai, Y. Low-Cost Biomass-Gel-Induced Conductive Polymer Networks for High-Efficiency Polysulfide Immobilization and Catalytic Conversion in Li-S Batteries. ACS Appl. Energy Mater. 2022, 5, 2308–2317. [Google Scholar] [CrossRef]
- Wei, J.; Jiang, C.; Chen, B.; Li, X.; Zhang, H. Hollow C/Co9S8 Hybrid Polyhedra-Modified Carbon Nanofibers as Sulfur Hosts for Promising Li-S Batteries. Ceram. Int. 2021, 47, 25387–25397. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, H.-J.; Hou, T.-Z.; Huang, J.-Q.; Chen, C.-M.; Wang, D.-W.; Cheng, X.-B.; Wei, F.; Zhang, Q. Powering Lithium-Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, C.; Deng, Y.; Huang, Z.; Zhou, G.; Lv, W.; He, Y.-B.; Wan, Y.; Kang, F.; Yang, Q.-H. Optimized Catalytic WS2-WO3 Heterostructure Design for Accelerated Polysulfide Conversion in Lithium-Sulfur Batteries. Adv. Energy Mater. 2020, 10, 2000091. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, G.-L.; Yu, Z.; Zhang, L.; Hwang, I.; Mo, Y.-X.; Ren, Y.; Cheng, L.; Sun, C.-J.; Ren, Y.; et al. A High-Energy and Long-Cycling Lithium-Sulfur Pouch Cell Via a Macroporous Catalytic Cathode with Double-End Binding Sites . Nat. Nanotechnol. 2021, 16, 224. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.-H. Catalytic Effects in Lithium-Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2018, 5, 1700270. [Google Scholar] [CrossRef]
- Wang, R.; Luo, C.; Wang, T.; Zhou, G.; Deng, Y.; He, Y.; Zhang, Q.; Kang, F.; Lv, W.; Yang, Q.-H. Bidirectional Catalysts for Liquid-Solid Redox Conversion in Lithium-Sulfur Batteries. Adv. Mater. 2020, 32, 2000315. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zu, L.; Yang, S.; Chen, L.; Liao, K.; Meng, S.; Zhang, C.; Yang, J. Ultrahigh-Content Co-P Cluster as a Dual-Atom-Site Electrocatalyst for Accelerating Polysulfides Conversion in Li-S Batteries. Adv. Funct. Mater. 2022, 32, 2207579. [Google Scholar] [CrossRef]
- Theibault, M.J.; Chandler, C.; Dabo, I.; Abruna, H.D. Transition Metal Dichalcogenides as Effective Catalysts for High-Rate Lithium-Sulfur Batteries. ACS Catal. 2023, 13, 3684–3691. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Zhou, J.; Zhao, H.; Lin, N.; Zhu, L.; Zhu, Y.; Wang, G.; Qian, Y. Fully Integrated Hierarchical Double-Shelled Co9S8@CNT Nanostructures with Unprecedented Performance for Li-S Batteries. Nanoscale Horiz. 2019, 4, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-F.; Tian, D.-X.; Li, W.-C.; He, B.; Zhang, Y.; Chen, Z.-Y.; Lu, A.-H. One-Pot Synthesis of Highly Conductive Nickel-Rich Phosphide/CNTs Hybrid as a Polar Sulfur Host for High-Rate and Long-Cycle Li-S Battery. Nano Res. 2019, 12, 1193–1197. [Google Scholar] [CrossRef]
- Gueon, D.; Ju, M.Y.; Moon, J.H. Complete Encapsulation of Sulfur through Interfacial Energy Control of Sulfur Solutions for High-Performance Li-S Batteries. Proc. Natl. Acad. Sci. USA 2020, 117, 12686–12692. [Google Scholar] [CrossRef]
- Ai, W.; Li, J.; Du, Z.; Zou, C.; Du, H.; Xu, X.; Chen, Y.; Zhang, H.; Zhao, J.; Li, C.; et al. Dual Confinement of Polysulfides in Boron-Doped Porous Carbon Sphere/Graphene Hybrid for Advanced Li-S Batteries. Nano Res. 2018, 11, 4562–4573. [Google Scholar] [CrossRef]
- Rehman, S.; Guo, S.; Hou, Y. Rational Design of Si/SiO2@Hierarchical Porous Carbon Spheres as Efficient Polysulfide Reservoirs for High-Performance Li-S Battery. Adv. Mater. 2016, 28, 3167–3172. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Zhang, C.; Liu, D.; Luo, J.; Fan, W.; Xu, Y.; Li, Y.; You, C.; Pan, Z.-Z.; et al. Dense Graphene Monolith for High Volumetric Energy Density Li-S Batteries. Adv. Energy Mater. 2018, 8, 1703438. [Google Scholar] [CrossRef]
- Peng, L.; Wei, Z.; Wan, C.; Li, J.; Chen, Z.; Zhu, D.; Baumann, D.; Liu, H.; Allen, C.S.; Xu, X.; et al. A Fundamental Look at Electrocatalytic Sulfur Reduction Reaction. Nat. Catal. 2020, 3, 762–770. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.; Liu, L.; Koch, J.; Rosebrock, M.; Li, T.; Bettels, F.; He, T.; Pfnuer, H.; Bigall, N.C.; et al. Nitrogen Doping Improves the Immobilization and Catalytic Effects of Co9S8 in Li-S Batteries. Adv. Funct. Mater. 2020, 30, 2002462. [Google Scholar] [CrossRef]
- Wang, M.; Fan, L.; Sun, X.; Guan, B.; Jiang, B.; Wu, X.; Tian, D.; Sun, K.; Qiu, Y.; Yin, X.; et al. Nitrogen-Doped CoSe2 as a Bifunctional Catalyst for High Areal Capacity and Lean Electrolyte of Li-S Battery. ACS Energy Lett. 2020, 5, 3041–3050. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Cao, G.; Sari, H.M.K.; Duan, R.; Wang, J.; Xie, C.; Li, W.; Li, X. Bifunctional Catalytic Effect of CoSe2 for Lithium-Sulfur Batteries: Single Doping Versus Dual Doping. Adv. Funct. Mater. 2022, 32, 2107838. [Google Scholar] [CrossRef]
- Cai, D.; Liu, B.; Zhu, D.; Chen, D.; Lu, M.; Cao, J.; Wang, Y.; Huang, W.; Shao, Y.; Tu, H.; et al. Ultrafine Co3se4 Nanoparticles in Nitrogen-Doped 3D Carbon Matrix for High-Stable and Long-Cycle-Life Lithium Sulfur Batteries. Adv. Energy Mater. 2020, 10, 1904273. [Google Scholar] [CrossRef]
- Zhong, Y.; Yin, L.; He, P.; Liu, W.; Wu, Z.; Wang, H. Surface Chemistry in Cobalt Phosphide-Stabilized Lithium-Sulfur Batteries. J. Am. Chem. Soc. 2018, 256, 1455–1459. [Google Scholar] [CrossRef]
- Gouda, M.S.; Shehab, M.; Helmy, S.; Soliman, M.; Salama, R.S. Nickel and Cobalt Oxides Supported on Activated Carbon Derived from Willow Catkin for Efficient Supercapacitor Electrode. J. Energy Storage 2023, 61, 106806. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, W.; Kong, L.; Zhang, L.; Zhu, X.; Shao, Y.; Ding, F.; Zhang, Q.; Sun, J.; Liu, Z. Synchronous Immobilization and Conversion of Polysulfides on a VO2-VN Binary Host Targeting High Sulfur Load Li-S Batteries. Energy Environ. Sci. 2018, 11, 2620–2630. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, C.; Geng, C.; Wu, S.; Li, H.; Lv, W.; Tao, Y.; Chen, Z.; Zhou, G.; Li, J.; et al. Capture and Catalytic Conversion of Polysulfides by in Situ Built TiO2-MXene Heterostructures for Lithium-Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1900219. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, C.; Wang, E. An Efficient Cos2/CoSe2 Hybrid Catalyst for Electrocatalytic Hydrogen Evolution. J. Mater. Chem. A 2017, 5, 2504–2507. [Google Scholar] [CrossRef]
- Huang, S.; Wang, H.; Wang, S.; Hu, Z.; Zhou, L.; Chen, Z.; Jiang, Y.; Qian, X. Encapsulating CoS2-CoSe2 Heterostructured Nanocrystals in N-Doped Carbon Nanocubes as Highly Efficient Counter Electrodes for Dye-Sensitized Solar Cells. Dalton Trans. 2018, 47, 5236–5244. [Google Scholar] [CrossRef]
- Gao, M.-R.; Yao, W.-T.; Yao, H.-B.; Yu, S.-H. Synthesis of Unique Ultrathin Lamellar Mesostructured CoSe2-Amine (Protonated) Nanobelts in a Binary Solution. J. Am. Chem. Soc. 2009, 131, 7486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-R.; Wu, P.; Gao, M.-R.; Zhang, X.-L.; Gao, F.-Y.; Ju, H.-X.; Wu, R.; Gao, Q.; You, R.; Huang, W.-X.; et al. Doping-Induced Structural Phase Transition in Cobalt Diselenide Enables Enhanced Hydrogen Evolution Catalysis. Nat. Commun. 2018, 9, 2533. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Peng, H.-J.; Li, B.-Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J.-Q.; Zhang, Q. Conductive and Catalytic Triple-Phase Interfaces Enabling Uniform Nucleation in High-Rate Lithium-Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1802768. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. A High-Efficiency Cose Electrocatalyst with Hierarchical Porous Polyhedron Nanoarchitecture for Accelerating Polysulfides Conversion in Li-S Batteries. Adv. Mater. 2020, 32, 2002168. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Fan, X.-X.; Liu, T.; Xu, P.; Hou, Q.; Li, K.; Yuan, R.-M.; Zheng, M.-S.; Dong, Q.-F.; Chen, J.-J. Single-Dispersed Polyoxometalate Clusters Embedded on Multilayer Graphene as a Bifunctional Electrocatalyst for Efficient Li-S Batteries. Nat. Commun. 2022, 13, 202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Ma, J.; Cui, M.; Zhao, Y.; Wei, S. A Rational Design of a CoS2-CoSe2 Heterostructure for the Catalytic Conversion of Polysulfides in Lithium-Sulfur Batteries. Materials 2023, 16, 3992. https://doi.org/10.3390/ma16113992
Zhang B, Ma J, Cui M, Zhao Y, Wei S. A Rational Design of a CoS2-CoSe2 Heterostructure for the Catalytic Conversion of Polysulfides in Lithium-Sulfur Batteries. Materials. 2023; 16(11):3992. https://doi.org/10.3390/ma16113992
Chicago/Turabian StyleZhang, Bin, Jiping Ma, Manman Cui, Yang Zhao, and Shizhong Wei. 2023. "A Rational Design of a CoS2-CoSe2 Heterostructure for the Catalytic Conversion of Polysulfides in Lithium-Sulfur Batteries" Materials 16, no. 11: 3992. https://doi.org/10.3390/ma16113992
APA StyleZhang, B., Ma, J., Cui, M., Zhao, Y., & Wei, S. (2023). A Rational Design of a CoS2-CoSe2 Heterostructure for the Catalytic Conversion of Polysulfides in Lithium-Sulfur Batteries. Materials, 16(11), 3992. https://doi.org/10.3390/ma16113992





