Recent Advances in Processing of Titanium and Titanium Alloys through Metal Injection Molding for Biomedical Applications: 2013–2022
Abstract
1. Introduction
2. Biomedical Applications of Titanium and Titanium Alloys
3. MIM Process for Titanium and Titanium Alloys
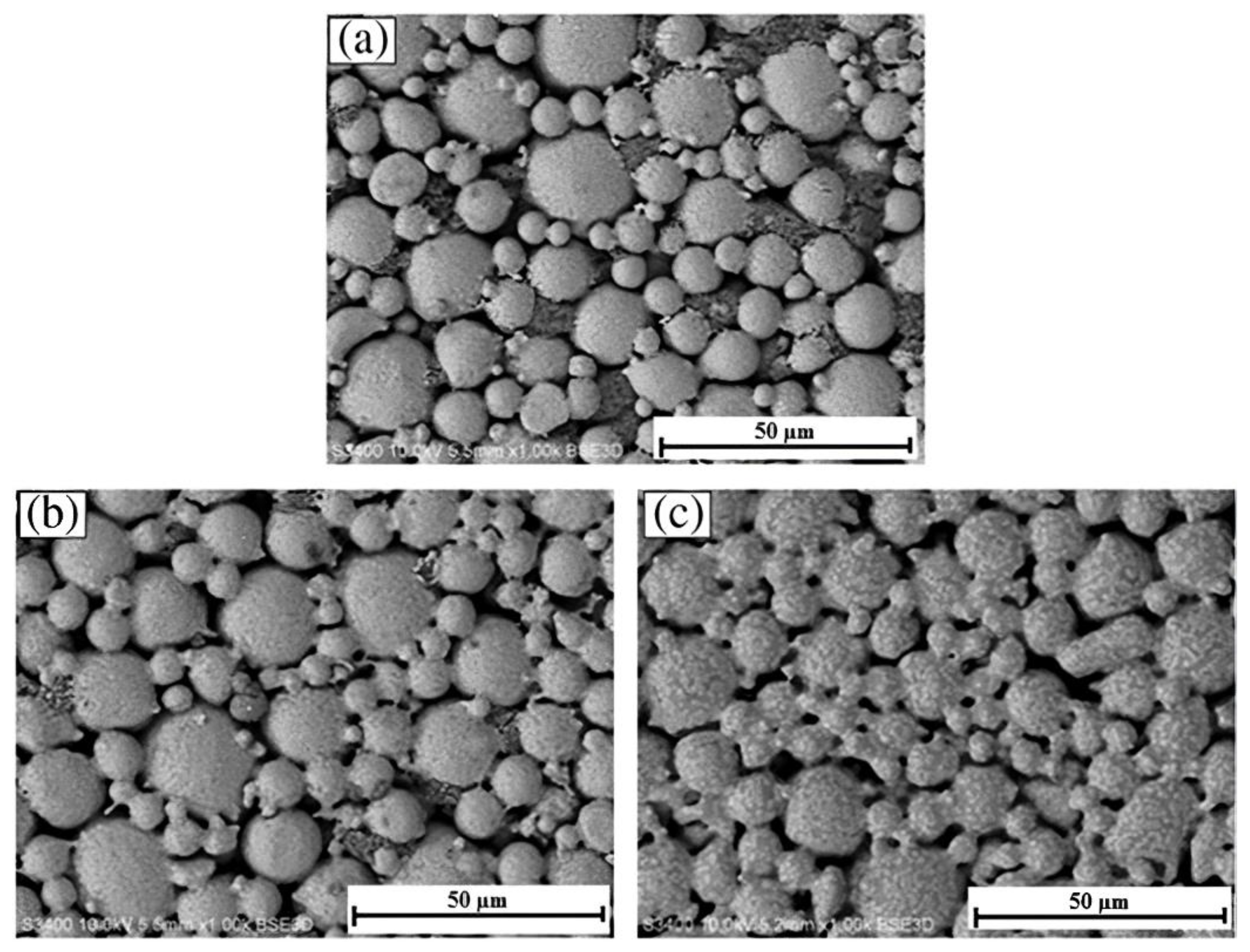
3.1. Metallic Powder Selection
- Spherical-shaped powder particles are preferred, as irregular-shaped particles cause imbalanced powder loading and more shrinkage;
- A higher packing density is required to maximally load the powder;
- Adequate interparticle friction is required to retain the shape throughout the debinding stage. Distortions are more likely to occur when larger powder particles are used, as the intraparticle contact per unit volume decreases;
- The powder particles must be non-agglomerated to produce defect-free sintered components;
- The particles should not react with the multicomponent binder system;
- The powder particles should be void-free to ensure that the sintered parts have excellent density.
3.2. Binder Selection
- Outstanding adhesion to Ti powder;
- Low likelihood of powder-binder separation;
- Superior wetting capabilities and flow properties;
- A viscosity that only varies slightly during MIM;
- Produce very little residual C post-burnout;
- Does not cause chemical reactions when interacting with Ti powder particles;
- Toxicity-free.
3.3. Preparation of Feedstock
3.4. Rheological Properties
3.5. Metal Injection Molding Process
3.6. Debinding Process
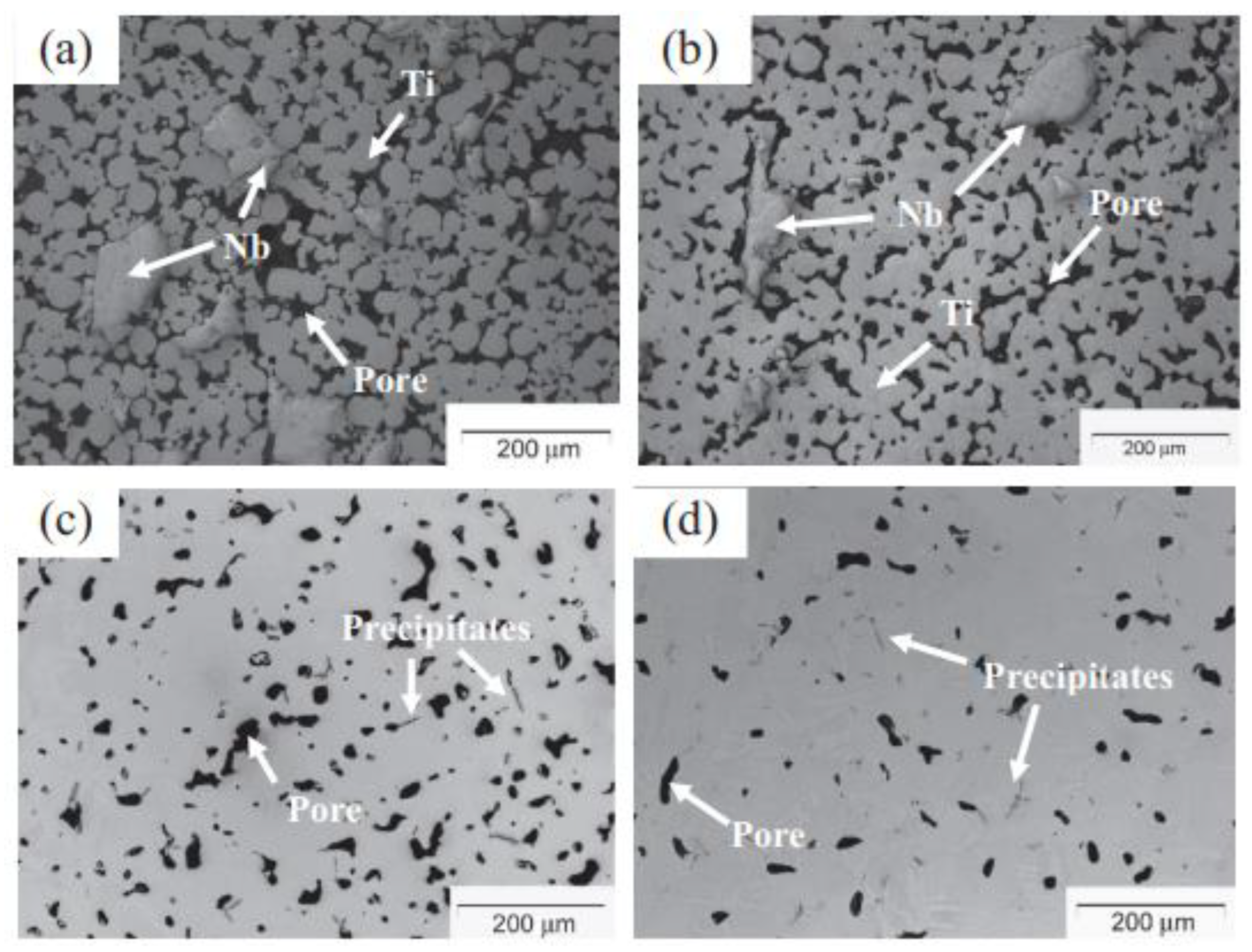
3.7. Sintering Process
4. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todros, S.; Todesco, M.; Bangno, A. Biomaterials and their biomedical applications: From replacement to regeneration. Processes 2021, 9, 1949. [Google Scholar] [CrossRef]
- Hamidi, M.F.F.A.; Harun, W.S.W.; Samykano, M.; Ghani, S.A.C.; Ghazalli, Z.; Ahmad, F.; Sulong, A.B. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Malekani, J.; Schmutz, B.; Gu, Y.; Schuetz, M.; Yarlagadda, P. Biomaterials in orthopedic bone plates: A review. In Proceedings of the Annual International Conference on Materials Science, Metal and Manufacturing, Singapore, 12–13 December 2011. [Google Scholar]
- Vallet-Regí, M. Evolution of biomaterials. Front. Mater. 2022, 9, 864016. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Antoniac, I.-V.; Lupescu, S.-C. Current research studies Mg-Ca-Zn biodegradable alloys used as orthopedic implant—Review. Crystals 2022, 12, 1468. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.-H.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Seemann, A.; Akbaba, S.; Buchholz, J.; Türkkan, S.; Tezcaner, A.; Woche, S.K.; Guggenberger, G.; Kirschning, A.; Dräger, G. RGD-modified titanium as an improved osteoinductive biomaterial for use in dental and orthopedic implants. Bioconjugate Chem. 2022, 33, 294–300. [Google Scholar] [CrossRef]
- Roman, A.-M.; Voiculescu, I.; Cimpoesu, R.; Istrate, B.; Chelariu, R.; Cimpoesu, N.; Zegan, G.; Panaghie, C.; Lohan, N.M.; Axinte, M.; et al. Microstructure, shape memory effect, chemical composition and corrosion resistance performance of biodegradable FeMnSi-Al alloy. Crystals 2023, 13, 109. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Z.; Li, Z.; Gu, L. A two-phase and long-lasting multi-antibacterial coating enables titanium biomaterials to prevent implants-related infections. Mater. Today Bio 2022, 15, 100330. [Google Scholar] [CrossRef]
- Soykan, C. A theoretical approach to the structural, elastic and electronic properties of Ti8-xV4-yMox+y+zAl4-z lightweight shape memory alloys for biomaterial implant applications. Phys. B Condens. Matter 2020, 598, 412416. [Google Scholar] [CrossRef]
- Trincă, L.C.; Mareci, D.; Solcan, C.; Fântânariu, M.; Burtan, L.; Hriţcu, L.; Chiruţă, C.; Fernández-Mérida, L.; Rodríguez-Raposo, R.; Santana, J.J.; et al. New Ti-6Al-2Nb-2Ta-1Mo alloy as implant biomaterial: In vitro corrosion and in vivo osseointegration evaluations. Mater. Chem. Phys. 2020, 240, 122229. [Google Scholar] [CrossRef]
- Gepreel, M.A.-H.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.H.; Gepreel, M.A.-H.; Gouda, M.K.; Hefnawy, A.M.; Kandil, S.H. Biocompatibility of new Ti-Nb-Ta base alloys. Mater. Sci. Eng. C 2016, 61, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.A.; Metz, C.M.; Callaghan, J.J.; Hennessy, D.W.; Liu, S.S. Durability of second-generation extensively porous-coated stems in patients age 50 and younger. Clin. Orthop. Relat. Res. 2010, 468, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Lenthe, G.H.V.; de Waal Malefijt, M.C.; Huiskes, R. Stress shielding after total knee replacement may cause bone resorption in the distal femur. J. Bone Jt. Surg. 1997, 79, 117–122. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Urtekin, L.; Bozkurt, F.; Çanli, M. Analysis of biomedical titanium implant green parts by X-Ray tomography. J. Mater. Res. Technol. 2021, 14, 277–286. [Google Scholar] [CrossRef]
- Shanmuganantha, L.; Khan, M.U.A.; Sulong, A.B.; Ramli, M.I.; Baharudin, A.; Ariffin, H.M.; Razak, S.I.A.; Ng, M.H. Characterization of titanium ceramic composite for bone implants applications. Ceram. Int. 2022, 48, 22808–22819. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A.; Punset, M.; Calero, J.A.; Gil, F.J.; Ruperez, E.; Manero, J.M. Powder metallurgy with space holder for porous titanium implants: A review. J. Mater. Sci. Technol. 2021, 76, 129–149. [Google Scholar] [CrossRef]
- Milone, D.; Fiorillo, L.; Alberti, F.; Cervino, G.; Filardi, V.; Pistone, A.; Cicciù, M.; Risitano, G. Stress distribution and failure analysis comparison between zirconia and titanium dental implants. Procedia Struct. Integr. 2022, 41, 680–691. [Google Scholar] [CrossRef]
- Stricker, A.; Bergfeldt, T.; Fretwurst, T.; Addison, O.; Schmelzeisen, R.; Rothweiler, R.; Nelson, K.; Gross, C. Impurities in commercial titanium dental implants—A mass and optical emission spectrometry elemental analysis. Dent. Mater. 2022, 38, 1395–1403. [Google Scholar] [CrossRef]
- Mühl, A.; Szabó, P.; Krafcsik, O.; Aigner, Z.; Kopniczky, J.; Nagy, Á.; Marada, G.; Turzó, K. Comparison of surface aspects of turned and anodized titanium dental implant, or abutment material for an optimal soft tissue integration. Heliyon 2022, 8, e10263. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Y.; Hou, Y.; Luo, Z.; Li, M.; Wei, Y.; Chen, M.; Tan, L.; Cai, K.; Hu, Y. Constructions of ROS-responsive titanium-hydroxyapatite implant for mesenchymal stem cell recruitment in peri-implant space and bone formation in osteoporosis microenvironment. Bioact. Mater. 2022, 18, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Kheder, W.; Kawas, S.A.; Khalaf, K.; Samsudin, A.R. Impact of tribocorrosion and titanium particles release on dental implant complications—A narrative review. Jpn. Dent. Sci. Rev. 2021, 57, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Alizade, C.; Jafarov, A.; Berchenko, G.; Bicer, O.S.; Alizada, F. Investigation of the process intergrowth of bone tissue into the hole in titanium implants (Experimental research). Injury 2022, 53, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.; Meng, Y.; Fan, Y.; Liu, J.; Shi, C.; Ren, F.; Wu, L.; Wang, J.; Sun, H. Osteoclast differentiation and formation induced by titanium implantation through complement C3a. Mater. Sci. Eng. C 2021, 122, 111932. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Y.; Wang, H.; Ren, L.; Yang, K. Study on mechanical behavior of Cu-bearing antibacterial titanium alloy implant. J. Mech. Behav. Biomed. Mater. 2022, 125, 104926. [Google Scholar] [CrossRef]
- Yan, B.; Tan, J.; Wang, D.; Qiu, J.; Liu, X. Surface alloyed Ti–Zr layer constructed on titanium by Zr ion implantation for improving physicochemical and osteogenic properties. Prog. Nat. Sci. Mater. Int. 2020, 30, 635–641. [Google Scholar] [CrossRef]
- Dewidar, M. Influence of processing parameters and sintering atmosphere on the mechanical properties and microstructure of porous 316L stainless steel for possible hard-tissue applications. Int. J. Mech. Mechatron. Eng. 2012, 12, 10–24. [Google Scholar]
- Fonseca-García, A.; Pérez-Alvarez, J.; Barrera, C.C.; Medina, J.C.; Almaguer-Flores, A.; Sánchez, R.B.; Rodil, S.E. The effect of simulated inflammatory conditions on the surface properties of titanium and stainless steel and their importance as biomaterials. Mater. Sci. Eng. C 2016, 66, 119–129. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Kuo, T.Y.; Yusof, F.; Hamdi, M. Characterization and hardness enhancement of amorphous Fe-based metallic glass laser cladded on nickel-free stainless steel for biomedical implant application. Mater. Chem. Phys. 2019, 235, 121745. [Google Scholar] [CrossRef]
- Muley, S.V.; Vidvans, A.N.; Chaudhari, G.P.; Udainiya, S. An assessment of ultra fine grained 316L stainless steel for implant applications. Acta Biomater. 2016, 30, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Marcos, C.; Baltatu, M.S.; Florido-Suárez, N.R.; Socorro-Perdomo, P.P.; Vizureanu, P.; Mirza-Rosca, J.C. Mechanical properties and corrosion resistance of two new titanium alloys for orthopaedics applications. Mater. Today Proc. 2023, 72, 544–549. [Google Scholar] [CrossRef]
- Abdulah, N.; Omar, M.A.; Jamaludin, S.B.; Zainon, N.M.; Roslani, N.; Meh, B.; Jalil, M.N.A.; Hijazi, M.B.M.; Omar, A.Z. Innovative metal injection molding (MIM) method for producing CoCrMo alloy metallic prosthesis for orthopedic applications. Adv. Mater. Res. 2014, 879, 102–106. [Google Scholar] [CrossRef]
- Yemisci, I.; Mutlu, O.; Gulsoy, N.; Kunal, K.; Atre, S.; Gulsoy, H.O. Experimentation and analysis of powder injection molded Ti10Nb10Zr alloy: A promising candidate for electrochemical and biomedical application. J. Mater. Res. Technol. 2019, 8, 5233–5245. [Google Scholar] [CrossRef]
- Raabe, D.; Sander, B.; Friák, M.; Ma, D.; Neugebauer, J. Theory-guided bottom-up design of β-titanium alloys as biomaterials based on first principles calculations: Theory and experiments. Acta Mater. 2007, 55, 4475–4487. [Google Scholar] [CrossRef]
- Basir, A.; Sulong, A.B.; Jamadon, N.H.; Muhamad, N.; Emeka, U.B. Process parameters used in macro/micro powder injection molding: An overview. Met. Mater. Int. 2021, 27, 2023–2045. [Google Scholar] [CrossRef]
- Ferreira, T.J.; -Fernandes, C.P.; Piedade, A.P.; Tondela, J.; Vieira, M.T. Mechanical behaviour of dental implants manufactured from metallic powders by µMIM. Cienc. Tecnol. Mater. 2014, 26, 89–95. [Google Scholar]
- Zhou, C.; Jiang, H.; Liu, C.; Yan, B.; Yan, P. Microstructure features of high performance soft magnetic alloy Fe-3 wt.% Si prepared by metal injection molding. Mater. Chem. Phys. 2021, 273, 125068. [Google Scholar] [CrossRef]
- Langlais, D.; Demers, V.; Brailovski, V. Rheology of dry powders and metal injection molding feedstocks formulated on their base. Powder Technol. 2022, 396, 13–26. [Google Scholar] [CrossRef]
- Subaşı, M. The investigation of sintering temperature and Ni interlayer effects on diffusion bonding in inserted metal injection molding. J. Manuf. Process. 2020, 58, 706–711. [Google Scholar] [CrossRef]
- Askari, A.; Momeni, V. Rheological investigation and injection optimization of Fe-2Ni-2Cu feedstock for metal injection molding process. Mater. Chem. Phys. 2021, 271, 124926. [Google Scholar] [CrossRef]
- Mukund, B.N.; Hausnerova, B. Variation in particle size fraction to optimize metal injection molding of water atomized 17–4PH stainless steel feedstocks. Powder Technol. 2020, 368, 130–136. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; StJohn, D.; Dargusch, M.; Chen, Y.; Sun, J.F.; Qian, M. Metal injection moulding of non-spherical titanium powders: Processing, microstructure and mechanical properties. J. Manuf. Process. 2018, 31, 416–423. [Google Scholar] [CrossRef]
- Thavanayagam, G.; Swan, J.E. Aqueous debinding of polyvinyl butyral based binder system for titanium metal injection moulding. Powder Technol. 2018, 326, 402–410. [Google Scholar] [CrossRef]
- Basir, A.; Sulong, A.B.; Jamadon, N.H.; Muhamad, N. Feedstock properties and debinding mechanism of yttria-stabilized zirconia/stainless steel 17-4PH micro-components fabricated via two-component micro-powder injection molding process. Ceram. Int. 2021, 47, 20476–20485. [Google Scholar] [CrossRef]
- Torres, Y.; Lascano, S.; Bris, J.; Pavón, J.; Rodriguez, J.A. Development of porous titanium for biomedical applications: A comparison between loose sintering and space-holder techniques. Mater. Sci. Eng. C 2014, 37, 148–155. [Google Scholar] [CrossRef]
- Gemelli, E.; de Jesus, J.; Camargo, N.H.A.; de Almeida Soares, G.D.; Henriques, V.A.R.; Nery, F. Microstructural study of a titanium-based biocomposite produced by the powder metallurgy process with TiH2 and nanometric β-TCP powders. Mater. Sci. Eng. C 2012, 32, 1011–1015. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Luo, T.; Song, M.; Wu, H.; Xiao, J.; Tan, Y.; Cheng, M.; Chen, B.; Niu, X.; et al. Powder metallurgical low-modulus Ti–Mg alloys for biomedical applications. Mater. Sci. Eng. C 2015, 56, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, D.P.; Paim, T.C.; Bertaco, I.; Zanatelli, C.; Naasani, L.I.S.; Slaviero, M.; Driemeier, D.; Tavares, A.C.; Martins, V.; Escobar, C.F.; et al. Mechanical properties, in vitro and in vivo biocompatibility analysis of pure iron porous implant produced by metal injection molding: A new eco-friendly feedstock from natural rubber (Hevea brasiliensis). Mater. Sci. Eng. C 2021, 131, 112532. [Google Scholar] [CrossRef]
- Melli, V.; Juszczyk, M.; Sandrini, E.; Bolelli, G.; Bonferroni, B.; Lusvarghi, L.; Cigada, A.; Manfredini, T.; De Nardo, L. Tribological and mechanical performance evaluation of metal prosthesis components manufactured via metal injection molding. J. Mater. Sci.: Mater. Med. 2015, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Pyczak, F.; Yan, M.; Kong, F.; Ebel, T. Impacts of yttrium on microstructure and tensile properties of biomedical β Ti-Nb-Zr fabricated by metal injection molding. Mater. Sci. Eng. A 2020, 792, 139816. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Alabort, E.; Tang, Y.T.; Panwisawas, C.; Reed, R.C.; Manonukul, A. A novel low-modulus titanium alloy for biomedical applications: A comparison between selective laser melting and metal injection moulding. Mater. Sci. Eng. A 2021, 812, 141081. [Google Scholar] [CrossRef]
- Aust, E.; Limberg, W.; Gerling, R.; Oger, B.; Ebel, T. Advanced TiAl6Nb7 bone screw implant fabricated by metal injection moulding. Adv. Eng. Mater. 2006, 8, 365–370. [Google Scholar] [CrossRef]
- Barbosa, A.P.C.; Bram, M.; Stöver, D.; Buchkremer, H.P. Realization of a titanium spinal implant with a gradient in porosity by 2-component-metal injection moulding. Adv. Eng. Mater. 2013, 15, 510–521. [Google Scholar] [CrossRef]
- Barbosa, A.P.C. Development of the 2-Component-Injection Moulding for Metal Powders; Forschungszentrum Jülich GmbH: Jülich, Germany, 2011. [Google Scholar]
- Shu, C.; He, H.; Fan, B.; Li, J.; Wang, T.; Li, D.; Li, Y.; He, H. Biocompatibility of vascular stents manufactured using metal inection molding in animal experiments. Trans. Nonferrous Met. Soc. China 2022, 32, 569–580. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Yu, P.; Dargusch, M.; StJohn, D.; Qian, M. Metal injection moulding of surgical tools, biomaterials and medical devices: A review. Powder Technol. 2020, 364, 189–204. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants—A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Ezugwu, E.O.; Da Silva, R.B.; Sales, W.F.; Machado, A.R. Overview of the machining of titanium alloys. In Encyclopedia of Sustainable Technologies; Abraham, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 487–506. [Google Scholar]
- Ronoh, K.; Mwema, F.; Dabees, S.; Sobola, D. Advances in sustainable grinding of different types of the titanium biomaterials for medical applications: A review. Biomed. Eng. Adv. 2022, 4, 100047. [Google Scholar] [CrossRef]
- Pede, D.; Li, M.; Virovac, L.; Poleske, T.; Balle, F.; Müller, C.; Mozaffari-Jovein, H. Microstructure and corrosion resistance of novel β-type titanium alloys manufactured by selective laser melting. J. Mater. Res. Technol. 2022, 19, 4598–4612. [Google Scholar] [CrossRef]
- Huang, Z.; Xiao, H.; Yu, J.; Zhang, H.; Huang, H.; Yu, K.; Zhou, R. Effects of different annealing cooling methods on the microstructure and properties of TA10 titanium alloys. J. Mater. Res. Technol. 2022, 18, 4859–4870. [Google Scholar] [CrossRef]
- Chen, K.; Fan, Q.; Yang, L.; Yao, J.; Xu, S.; Lei, W.; Gao, Y. Deciphering the microstructural evolution and adiabatic shearing behavior of the titanium alloy with stress-induced ω phase transformation during dynamic compression. Mater. Des. 2022, 221, 110939. [Google Scholar] [CrossRef]
- Yasin, M.S.; Soltani-Tehrani, A.; Shao, S.; Haghshenas, M.; Shamsaei, N. A comparative study on fatigue performance of various additively manufactured titanium alloys. Procedia Struct. Integr. 2022, 38, 519–525. [Google Scholar] [CrossRef]
- Moghadam, M.S.; Fayyaz, A.; Ardestani, M. Fabrication of titanium components by low-pressure powder injection moulding using hydride-dehydride titanium powder. Powder Technol. 2021, 377, 70–79. [Google Scholar] [CrossRef]
- Kim, S.; Jung, H.; Rim, H.J.; Lee, H.-S.; Lee, W. Fabrication of reinforced α+β titanium alloys by infiltration of Al into porous Ti-V compacts. J. Alloys Compd. 2018, 768, 775–781. [Google Scholar] [CrossRef]
- Dang, K.; Wang, K.; Chen, W.; Liu, G. Study on fast gas forming with in-die quenching for titanium alloys and the strengthening mechanisms of the components. J. Mater. Res. Technol. 2022, 18, 3916–3932. [Google Scholar] [CrossRef]
- Gupta, M.K.; Niesłony, P.; Sarikaya, M.; Korkmaz, M.E.; Kuntoğlu, M.; Królczyk, G.M.; Jamil, M. Tool wear patterns and their promoting mechanisms in hybrid cooling assisted machining of titanium Ti-3Al-2.5V/grade 9 alloy. Tribol. Int. 2022, 174, 107773. [Google Scholar] [CrossRef]
- Thavanayagam, G.; Swan, J.E. Optimizing hydride-dehydride Ti-6Al-4V feedstock composition for titanium powder injection moulding. Powder Technol. 2019, 355, 688–699. [Google Scholar] [CrossRef]
- De Freitas Daudt, N.; Bram, M.; Barbosa, A.P.C.; Laptev, A.M.; Alves, C. Manufacturing of highly porous titanium by metal injection molding in combination with plasma treatment. J. Mater. Process. Technol. 2017, 239, 202–209. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Ismail, M.H.; Husain, H.; Saedon, J.B.; Newishy, M. Sintered strength of Ti-6Al-4V by metal injection molding (MIM) using palm stearin binder. J. Mech. Eng. 2019, 16, 135–147. [Google Scholar]
- Shbeh, M.M.; Goodall, R. Open pore titanium foams via metal injection molding of metal powder with a space holder. Met. Powder Rep. 2016, 71, 450–455. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Molaei, M.; Nouri, M.; Babaei, K. Review of the role of graphene and its derivatives in enhancing the performance of plasma electrolytic oxidation coatings on titanium and its alloys. Appl. Surf. Sci. Adv. 2021, 6, 100140. [Google Scholar] [CrossRef]
- Alves, S.A.; Bayon, R.; de Viteri, V.S.; Garcia, M.P.; Igartua, A.; Fernandes, M.H.; Rocha, L.A. Tribocorrosion behavior of calcium- and phosphorous-enriched titanium oxide films and study of osteoblast interactions for dental implants. J. Bio Tribo Corros. 2015, 1, 23. [Google Scholar] [CrossRef]
- Jiang, H. Enhancement of titanium alloy corrosion resistance via anodic oxidation treatment. Int. J. Electrochem. Sci. 2018, 13, 3888–3896. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.R.; Shamanian, M. Improvement of corrosion and tribocorrosion behavior of pure titanium by subzero anodic spark oxidation. ACS Appl. Mater. Interfaces 2018, 10, 15281–15287. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rameshbabu, N.; Sreekanth, D.; Bose, A.C.; Muthupandi, V.; Babu, N.K.; Subramanian, S. Role of electrolyte additives on in-vitro electrochemical behavior of micro arc oxidized titania films on Cp Ti. Appl. Surf. Sci. 2012, 258, 6853–6863. [Google Scholar]
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A. Biomaterial properties of titanium in dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef]
- Lautenschlager, E.P.; Monaghan, P. Titanium and titanium alloys as dental materials. Int. Dent. J. 1993, 43, 245–253. [Google Scholar]
- Zakaria, M.Y.; Sulong, A.B.; Muhamad, N.; Raza, M.R.; Ramli, M.I. Incorporation of wollastonite bioactive ceramic with titanium for medical applications: An overview. Mater. Sci. Eng. C 2019, 97, 884–895. [Google Scholar] [CrossRef]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Cabrera, N.; Mott, N.F. Theory of the oxidation of metals. Rep. Prog. Phys. 1949, 12, 163–184. [Google Scholar] [CrossRef]
- Chunxiang, C.; BaoMin, H.; Lichen, Z.; Shuangjin, L. Titanium alloy production technology, market prospects and industry development. Mater. Des. 2011, 32, 1684–1691. [Google Scholar]
- Ivasyshyn, O.M.; Aleksandrov, A.V. Status of the titanium production, research, and applications in the CIS. Mater. Sci. 2008, 44, 311–327. [Google Scholar] [CrossRef]
- Kearns, M. Titanium: Alive, well, and booming! Adv. Mater. Process. 2005, 163, 63–64. [Google Scholar]
- Brånemark, R.; Brånemark, P.-I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation. J. Rehabil. Res. Dev. 2001, 38, 175–181. [Google Scholar] [PubMed]
- Alipal, J.; Lee, T.C.; Koshy, P.; Abdullah, H.Z.; Idris, M.I. Evolution of anodised titanium for implant applications. Heliyon 2021, 7, e07408. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, M.R.; Schreckenbach, J.P.; Graf, H.-L. Titanium dental implant surfaces obtained by anodic spark deposition—From the past to the future. Mater. Sci. Eng. C 2016, 69, 1429–1441. [Google Scholar] [CrossRef]
- Mohammadi, H.; Muhamad, N.; Sulong, A.B.; Ahmadipour, M. Recent advances on biofunctionalization of metallic substrate using ceramic coating: How far are we from clinically stable implant? J. Taiwan Inst. Chem. Eng. 2021, 118, 254–270. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: Toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty: A review. J. Int. Med. Res. 2018, 46, 2104–2119. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Lucaciu, G.D.O.; Crisan, B.; Crisan, L.; Bacuit, M.; Onisor, F.; Baciut, G.; Câmpian, R.S.; Bran, S. Systemic drugs that influence titanium implant osseointegration. Drug Metab. Rev. 2017, 49, 92–104. [Google Scholar] [CrossRef]
- John, A.A.; Jaganathan, S.K.; Supriyanto, E.; Manikandan, A. Surface modification of titanium and its alloys for the enhancement of osseointegration in orthopaedics. Curr. Sci. 2016, 111, 1003–1015. [Google Scholar] [CrossRef]
- Lewallen, E.A.; Riester, S.M.; Bonin, C.A.; Kremers, H.M.; Dudakovic, A.; Kakar, S.; Cohen, R.C.; Westendorf, J.J.; Lewallen, D.G.; Wijnen, A.J.V. Biological strategies for improved osseointegration and osteoinduction of porous metal orthopedic implants. Tissue Eng. Part B Rev. 2015, 21, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early osseointegration driven by the surface chemistry and wettability of dental implants. J. Appl. Oral Sci. 2015, 23, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants: Corrosion and its prevention—A review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Paschalis, E.I.; Chodosh, J.; Spurr-Michaud, S.; Cruzat, A.; Tauber, A.; Behlau, I.; Gipson, I.; Dohlman, C.H. In vitro and in vivo assessment of titanium surface modification for coloring the backplate of the Boston keratoprosthesis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3863–3873. [Google Scholar] [CrossRef]
- Kärrholm, J.; Razaznejad, R. Fixation and bone remodeling around a low stiffness stem in revision surgery. Clin. Orthop. Relat. Res. 2008, 466, 380–388. [Google Scholar] [CrossRef]
- Niinimaki, T.; Jalovaara, P. Bone loss from the proximal femur after arthroplasty with an isoelastic femoral stem: BMD measurements in 25 patients after 9 years. Acta Orthop. Scand. 1995, 66, 347–351. [Google Scholar] [CrossRef]
- Spoerke, E.D.; Murray, N.G.; Li, H.; Brinson, L.C.; Dunand, D.C.; Stupp, S.I. A bioactive titanium foam scaffold for bone repair. Acta Biomater. 2005, 1, 523–533. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Xu, G.; Liu, G.; Kou, H.; Zhou, L. Fabrication, pore structure and compressive behavior of anisotropic porous titanium for human trabecular bone implant applications. J. Mech. Behav. Biomed. Mater. 2015, 46, 104–114. [Google Scholar] [CrossRef]
- Johari, N.; Fathi, M.H.; Golozar, M.A. Fabrication, characterization and evaluation of the mechanical properties of poly (ε-caprolactone)/nano-fluoridated hydroxyapatite scaffold for bone tissue engineering. Compos. Part B 2012, 43, 1671–1675. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Wu, X.; Ren, S.; San, X.; Zhu, X. Ti/SiO2 composite fabricated by powder metallurgy for orthopedic implant. Mater. Des. 2013, 49, 76–80. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Katti, K.S. Biomaterials in total joint replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; John Wiley & Sons: Weinheim, Germany, 2006. [Google Scholar]
- Lampman, S. Titanium and its alloys for biomedical implants. In ASM Handbook Materials for Medical Devices; Narayan, R., Ed.; ASM International: Russell Township, OH, USA, 2012; pp. 225–238. [Google Scholar]
- Kafkas, F.; Ebel, T. Metallurgical and mechanical properties of Ti-24Nb-4Zr-8Sn alloy fabricated by metal injection molding. J. Alloys Compd. 2014, 617, 359–366. [Google Scholar] [CrossRef]
- Vishnu, J.; Sankar, M.; Rack, H.J.; Rao, N.; Singh, A.K.; Manivasagam, G. Effect of phase transformations during aging on tensile strength and ductility of metastable beta titanium alloy Ti-35Nb-7Zr-5Ta-0.35O for orthopedic applications. Mater. Sci. Eng. A 2020, 779, 139127. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Hynowska, A.; Pellicer, E.; Fornell, J.; González, S.; van Steenberge, N.; Suriñach, S.; Gebert, A.; Calin, M.; Eckert, J.; Baró, M.D.; et al. Nanostructured β-phase Ti-31.0Fe-9.0Sn and sub-μm structured Ti-39.3Nb-13.3Zr-10.7Ta alloys for biomedical applications: Microstructure benefits on the mechanical and corrosion performances. Mater. Sci. Eng. C 2012, 32, 2418–2425. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y.F. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, Z.; Yu, M.; Ji, Z.; Wang, T.; Cai, Y.; Liu, C.; Liu, Z. Osteogenic and antibacterial ability of micro-nano structures coated with ZnO on Ti-6Al-4V implant fabricated by two-step laser processing. J. Mater. Sci. Technol. 2022, 131, 240–252. [Google Scholar] [CrossRef]
- Lantang, Y.S.F.; Kobayashi, E.; Sato, T. Fabrication of spark plasma sintered porous Ti-6Al-4V using PCA-added space holder method for bone implant materials. Mater. Today Proc. 2022, 66, 2693–2701. [Google Scholar] [CrossRef]
- Dewidar, M.M.; Lim, J.K. Properties of solid core and porous surface Ti-6Al-4V implants manufactured by powder metallurgy. J. Alloys Compd. 2008, 454, 442–446. [Google Scholar] [CrossRef]
- Vazirgiantzikis, I.; George, S.L.; Pichon, L. Surface characterisation and silver release from Ti-6Al-4V and anodic TiO2 after surface modification by ion implantation. Surf. Coat. Technol. 2022, 433, 128115. [Google Scholar] [CrossRef]
- Lim, B.-S.; Cho, H.-R.; Choe, H.-C. Corrosion behaviors of macro/micro/nano-scale surface modification on Ti-6Al-4V alloy for bio-implant. Thin Solid Films 2022, 754, 139314. [Google Scholar] [CrossRef]
- Rahmouni, K.; Besnard, A.; Oulmi, K.; Nouveau, C.; Hidoussi, A.; Aissani, L.; Zaabat, M. In vitro corrosion response of CoCrMo and Ti-6Al-4V orthopedic implants with Zr columnar thin films. Surf. Coat. Technol. 2022, 436, 128310. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Qiao, N.; Wang, C.; Hu, M. Corrosion resistance characteristics of a Ti-6Al-4V alloy scaffold that is fabricated by electron beam melting and selective laser melting for implantation in vivo. Mater. Sci. Eng. C 2017, 70, 832–841. [Google Scholar] [CrossRef]
- Nagentrau, M.; Tobi, A.L.M.; Jamian, S.; Otsuka, Y.; Hussin, R. Delamination-fretting wear failure evaluation at HAp-Ti-6Al–4V interface of uncemented artificial hip implant. J. Mech. Behav. Biomed. Mater. 2021, 122, 104657. [Google Scholar] [CrossRef]
- Huang, H.-H.; Shiau, D.-K.; Chen, C.-S.; Chang, J.-H.; Wang, S.; Pan, H.; Wu, M.-F. Nitrogen plasma immersion ion implantation treatment to enhance corrosion resistance, bone cell growth, and antibacterial adhesion of Ti-6Al-4V alloy in dental applications. Sur. Coat. Technol. 2019, 365, 179–188. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C 2021, 118, 111505. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Xiao, S.; Liu, J.; Lv, S.; Xu, L.; Kong, F.; Chen, Y. Hot deformation behavior of Ti-3.5Al-5Mo-6V-3Cr-2Sn-0.5Fe Alloy in α + β field. Metals 2015, 5, 216–227. [Google Scholar] [CrossRef]
- Akahori, T.; Niinomi, M.; Fukui, H.; Ogawa, M.; Toda, H. Improvement in fatigue characteristics of newly developed beta type titanium alloy for biomedical applications by thermo-mechanical treatments. Mater. Sci. Eng. C 2005, 25, 248–254. [Google Scholar] [CrossRef]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Liu, H.; Niinomi, M.; Nakai, M.; Obara, S.; Fujii, H. Improved fatigue properties with maintaining low young’s modulus achieved in biomedical beta-type titanium alloy by oxygen addition. Mater. Sci. Eng. A 2017, 704, 10–17. [Google Scholar] [CrossRef]
- Afzali, P.; Ghomashchi, R.; Oskouei, R.H. On the corrosion behaviour of low modulus titanium alloys for medical implant applications: A review. Metals 2019, 9, 878. [Google Scholar] [CrossRef]
- Yilmazer, H.; Niinom, M.; Nakai, M.; Hieda, J.; Todaka, Y.; Akahori, T.; Miyazaki, T. Heterogeneous structure and mechanical hardness of biomedical β-type Ti-29Nb-13Ta-4.6Zr subjected to high-pressure torsion. J. Mech. Behav. Biomed. Mater. 2012, 10, 235–245. [Google Scholar] [CrossRef]
- Takematsu, E.; Cho, K.; Hieda, J.; Nakai, M.; Katsumata, K.; Okada, K.; Niinomi, M.; Matsushita, N. Adhesive strength of bioactive oxide layers fabricated on TNTZ alloy by three different alkali-solution treatments. J. Mech. Behav. Biomed. Mater. 2016, 61, 174–181. [Google Scholar] [CrossRef]
- Raducanu, D.; Vasilescu, E.; Cojocaru, V.D.; Cinca, I.; Drob, P.; Vasilescu, C.; Drob, S.I. Mechanical and corrosion resistance of a new nanostructured Ti-Zr-Ta-Nb alloy. J. Mech. Behav. Biomed. Mater. 2011, 4, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of low rigidity β-type titanium alloy for biomedical applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue performance and cyto-toxicity of low rigidity titanium alloy, Ti-29Nb-13Ta-4.6Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Milošev, I.; Žerjav, G.; Moreno, J.M.C.; Popa, M. Electrochemical properties, chemical composition and thickness of passive film formed on novel Ti-20Nb-10Zr-5Ta alloy. Electrochim. Acta 2013, 99, 176–189. [Google Scholar] [CrossRef]
- German, R.M. Powder Injection Moulding; Metal Powder Industries Federation: Princeton, NJ, USA, 1990. [Google Scholar]
- Emeka, U.B.; Sulong, A.B.; Muhamad, N.; Sajuri, Z.; Salleh, F. Two component injection molding of bi-material of stainless steel and yttria stabilized zirconia—Green Part. J. Kejuruter. 2017, 29, 49–55. [Google Scholar] [CrossRef]
- Fayyaz, A.; Muhamad, N.; Sulong, A.B. Microstructure and physical and mechanical properties of micro cemented carbide injection moulded components. Powder Technol. 2018, 326, 151–158. [Google Scholar] [CrossRef]
- Jabir, S.M.; Noorsyakirah, A.; Afian, O.M.; Nurazilah, M.Z.; Aswad, M.A.; Afiq, N.H.M.; Mazlan, M. Analysis of the rheological behavior of copper metal injection molding (MIM) feedstock. Procedia Chem. 2016, 19, 148–152. [Google Scholar] [CrossRef]
- Antusch, S.; Armstrong, D.E.J.; Britton, T.B.; Commin, L.; Gibson, J.S.K.-L.; Greuner, H.; Hoffmann, J.; Knabl, W.; Pintsuk, G.; Rieth, M.; et al. Mechanical and microstructural investigations of tungsten and doped tungsten materials produced via powder injection molding. Nucl. Mater. Energy 2015, 3–4, 22–31. [Google Scholar] [CrossRef]
- Thavanayagam, G.; Pickering, K.L.; Swan, J.E.; Cao, P. Analysis of rheological behaviour of titanium feedstocks formulated with a water-soluble binder system for powder injection moulding. Powder Technol. 2015, 269, 227–232. [Google Scholar] [CrossRef]
- You, W.-K.; Choi, J.-P.; Yoon, S.-M.; Lee, J.-S. Low temperature powder injection molding of iron micro-nano powder mixture. Powder Technol. 2012, 228, 199–205. [Google Scholar] [CrossRef]
- Mukund, B.N.; Hausnerova, B.; Shivashankar, T.S. Development of 17-4PH stainless steel bimodal powder injection molding feedstock with the help of interparticle spacing/lubricating liquid concept. Powder Technol. 2015, 283, 24–31. [Google Scholar] [CrossRef]
- Wang, J.; Edirisinghe, M.J. Ceramic injection molding. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar]
- Heaney, D.F. Handbook of Metal Injection Molding; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Piotter, V.; Bauer, W.; Knitter, R.; Mueller, M.; Mueller, T.; Plewa, K. Powder injection moulding of metallic and ceramic micro parts. Microsyst. Technol. 2011, 17, 251–263. [Google Scholar] [CrossRef]
- Tuncer, N.; Bram, M.; Laptev, A.; Beck, T.; Moser, A.; Buchkremer, H.P. Study of metal injection molding of highly porous titanium by physical modeling and direct experiments. J. Mater. Process. Technol. 2014, 214, 1352–1360. [Google Scholar] [CrossRef]
- Shbeh, M.M.; Yerokhin, A.; Goodall, R. Microporous titanium through metal injection moulding of coarse powder and surface modification by plasma oxidation. Appl. Sci. 2017, 7, 105. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE 2017, 12, e0172043. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Chen, Y.; Shi, Z.; Bermingham, M.; StJohn, D.; Dargusch, M.; Qian, M. Porous titanium scaffolds fabricated by metal injection moulding for biomedical applications. Materials 2018, 11, 1573. [Google Scholar] [CrossRef]
- Hayat, M.D.; Goswami, A.; Matthews, S.; Li, T.; Yuan, X.; Cao, P. Modification of PEG/PMMA binder by PVP for titanium metal injection moulding. Powder Technol. 2017, 315, 243–249. [Google Scholar] [CrossRef]
- Hayat, M.D.; Jadhav, P.P.; Zhang, H.; Ray, S.; Cao, P. Improving titanium injection moulding feedstock based on PEG/PPC based binder system. Powder Technol. 2018, 330, 304–309. [Google Scholar] [CrossRef]
- Hayat, M.D.; Wen, G.; Zulkifli, M.F.; Cao, P. Effect of PEG molecular weight on rheological properties of Ti-MIM feedstocks and water debinding behaviour. Powder Technol. 2015, 270, 296–301. [Google Scholar] [CrossRef]
- Bootchai, S.; Taweejun, N.; Manonukul, A.; Kanchanomai, C. Metal injection molded titanium: Mechanical properties of debinded powder and sintered metal. J. Mater. Eng. Perform. 2020, 29, 4559–4568. [Google Scholar] [CrossRef]
- Zheng, J.-p.; Chen, L.-j.; Chen, D.-y.; Shao, C.-s.; Yi, M.-f.; Zhang, B. Effects of pore size and porosity of surface-modified porous titanium implants on bone tissue ingrowth. Trans. Nonferrous Met. Soc. China 2019, 29, 2534–2545. [Google Scholar] [CrossRef]
- Chen, G.; Cao, P.; Wen, G.; Edmonds, N.; Li, Y. Using an agar-based binder to produce porous NiTi alloys by metal injection moulding. Intermetallics 2013, 37, 92–99. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Wang, L.N.; Shi, Z.M.; Lv, S.M.; Qian, M.; Qu, X.H. Mechanical properties, in vitro corrosion resistance and biocompatibility of metal injection molded Ti-12Mo alloy for dental applications. J. Mech. Behav. Biomed. Mater. 2018, 88, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Niinomi, M.; Nakai, M.; Liu, H.; Santos, P.F.; Itoh, Y.; Ikeda, M.; Abdel-Hady Gepreel, M.; Narushima, T. Improvement in mechanical strength of low-cost β-type Ti-Mn alloys fabricated by metal injection molding through cold rolling. J. Alloys Compd. 2016, 664, 272–283. [Google Scholar] [CrossRef]
- Santos, P.F.; Niinomi, M.; Liu, H.; Cho, K.; Nakai, M.; Itoh, Y.; Narushima, T.; Ikeda, M. Fabrication of low-cost beta-type Ti-Mn alloys for biomedical applications by metal injection molding process and their mechanical properties. J. Mech. Behav. Biomed. Mater. 2016, 59, 497–507. [Google Scholar] [CrossRef]
- Zhao, D.; Chang, K.; Ebel, T.; Qian, M.; Willumeit, R.; Yan, M.; Pyczak, F. Microstructure and mechanical behavior of metal injection molded Ti-Nb binary alloys as biomedical material. J. Mech. Behav. Biomed. Mater. 2013, 28, 171–182. [Google Scholar] [CrossRef]
- Zhao, D.; Chang, K.; Ebel, T.; Nie, H.; Willumeit, R.; Pyczak, F. Sintering behavior and mechanical properties of a metal injection molded Ti-Nb binary alloy as biomaterial. J. Alloys Compd. 2015, 640, 393–400. [Google Scholar] [CrossRef]
- Yılmaz, E.; Gökçe, A.; Findik, F.; Gulsoy, H. Metallurgical properties and biomimetic HA deposition performance of Ti-Nb PIM alloys. J. Alloys Compd. 2018, 746, 301–313. [Google Scholar] [CrossRef]
- Bidaux, J.-E.; Pasquier, R.; Rodriguez-Arbaizar, M.; Girard, H.; Carren~o-Morelli, E. Low elastic modulus Ti-17Nb processed by powder injection moulding and postsintering heat treatments. Powder Metall. 2014, 57, 320–323. [Google Scholar] [CrossRef]
- Zhao, D.-p.; Chen, Y.-k.; Chang, K.-k.; Ebel, T.; Luthrigner-Feyerabend, B.J.C.; Willumeit-Römer, R.; Pyczak, F. Surface topography and cytocompatibility of metal injection molded Ti-22Nb alloy as biomaterial. Trans. Nonferrous Met. Soc. China 2018, 28, 1342–1350. [Google Scholar] [CrossRef]
- Holm, M.; Ebel, T.; Dahms, M. Investigations on Ti-6Al-4V with gadolinium addition fabricated by metal injection moulding. Mater. Des. 2013, 51, 943–948. [Google Scholar] [CrossRef]
- Bian, T.; Ding, C.; Yao, X.; Wang, J.; Mo, W.; Wang, Z.; Yu, P.; Ye, S. Mechanisms of heat treatment and ductility improvement of high-oxygen Ti-6Al-4V alloy fabricated by metal injection molding. Mater. Sci. Eng. A 2022, 840, 142924. [Google Scholar] [CrossRef]
- Lin, D.; Sanetrnik, D.; Cho, H.; Chung, S.T.; Kwon, Y.S.; Kate, K.H.; Hausnerova, B.; Atre, S.V.; Park, S.J. Rheological and thermal debinding properties of blended elemental Ti-6Al-4V powder injection molding feedstock. Powder Technol. 2017, 311, 357–363. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Muhamad, N.; Ihsan, A.K.A.M.; Jamaludin, K.R. Sintering parameter optimization of Ti-6Al-4V metal injection molding for highest strength using palm stearin binder. Procedia Eng. 2013, 68, 359–364. [Google Scholar] [CrossRef]
- Zakaria, M.Y.; Ramli, M.I.; Sulong, A.B.; Muhamad, N.; Ismail, M.H. Application of sodium chloride as space holder for powder injection molding of alloy titanium-hydroxyapatite composites. J. Mater. Res. Technol. 2021, 12, 478–486. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Powder injection molding of HA/Ti6Al4V composite using palm stearin as based binder for implant material. Mater. Des. 2015, 65, 1028–1034. [Google Scholar] [CrossRef]
- Ramli, M.I.; Sulong, A.B.; Muhamad, N.; Muchtar, A.; Zakaria, M.Y. Effect of sintering on the microstructure and mechanical properties of alloy titanium-wollastonite composite fabricated by powder injection moulding process. Ceram. Int. 2019, 45, 11648–11653. [Google Scholar] [CrossRef]
- Yılmaz, E.; Gökçe, A.; Findik, F.; Gülsoy, H.Ö. Characterization of biomedical Ti-16Nb-(0-4)Sn alloys produced by powder injection molding. Vacuum 2017, 142, 164–174. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Bermingham, M.J.; Dargusch, M.S.; StJohn, D.H.; Qian, M. Metal injection moulding of titanium and titanium alloys: Challenges and recent development. Powder Technol. 2017, 319, 289–301. [Google Scholar] [CrossRef]
- Virdhian, S.; Osada, T.; Kang, H.G.; Tsumori, F.; Miura, H. Evaluation and analysis of distortion of complex shaped Ti-6Al-4V compacts by metal injection molding process. Key Eng. Mater. 2012, 520, 187–194. [Google Scholar] [CrossRef]
- Ferri, O.M.; Ebel, T.; Bormann, R. The influence of a small boron addition on the microstructure and mechanical properties of Ti-6Al-4V fabricated by metal injection moulding. Adv. Eng. Mater. 2011, 13, 436–447. [Google Scholar] [CrossRef]
- Ebel, T.; Blawert, C.; Willumeit, R.; Luthringer, B.J.C.; Ferri, O.M.; Feyerabend, F. Ti-6Al-4V–0.5B--a modified alloy for implants produced by metal injection molding. Adv. Eng. Mater. 2011, 13, B440–B453. [Google Scholar] [CrossRef]
- Uematsu, T.; Itoh, Y.; Sato, K.; Miura, H. Effects of substrate for sintering on the mechanical properties of injection molded Ti-6Al-4V alloy. J. Jpn. Soc. Powder Metall. 2006, 53, 755–759. [Google Scholar] [CrossRef]
- Tingskong, T.; Larouche, F.; Lefebvre, L.-P. New titanium alloy feedstock for high performance metal injection moulding parts. Key Eng. Mater. 2016, 704, 118–121. [Google Scholar] [CrossRef]
- Pelletier, R.; Lefebvre, L.-P.; Baril, E. A MIM route for producing Ti6Al4V-TiC composites. Key Eng. Mater. 2016, 704, 139–147. [Google Scholar] [CrossRef]
- Obasi, G.C.; Ferri, O.M.; Ebel, T.; Bormann, R. Influence of processing parameters on mechanical properties of Ti-6Al-4V alloy fabricated by MIM. Mater. Sci. Eng. A 2010, 527, 3929–3935. [Google Scholar] [CrossRef]
- Attia, U.M.; Alcock, J.R. A review of micro-powder injection moulding as a microfabrication technique. J. Micromech. Microeng. 2011, 21, 043001. [Google Scholar] [CrossRef]
- Baek, E.-R.; Supriadi, S.; Choi, C.-J.; Lee, B.-T.; Lee, J.-W. Effect of particle size in feedstock properties in micro powder injection molding. Mater. Sci. Forum 2007, 534–536, 349–352. [Google Scholar] [CrossRef]
- Kusaka, K.; Kohno, T.; Kondo, T.; Horata, A. Tensile behavior of sintered titanium by MIM process. J. Jpn. Soc. Powder Metall. 1995, 42, 383–387. [Google Scholar] [CrossRef]
- Carreño-Morelli, E.; Rodríguez-Arbaizar, M.; Amherd, A.; Bidaux, J.-E. Porous titanium processed by powder injection moulding of titanium hydride and space holders. Powder Metall. 2014, 57, 93–96. [Google Scholar] [CrossRef]
- Carreño-Morelli, E.; Amherd, A.; Rodriguez-Arbaizar, M.; Zufferey, D.; Várez, A.; Bidaux, J.-E. Porous titanium by powder injection moulding of titanium hydride and PMMA space holders. Eur. Cells Mater. 2013, 26, 16. [Google Scholar]
- Carreño-Morelli, E.; Bidaux, J.-E.; Rodríguez-Arbaizar, M.; Girard, H.; Hamdan, H. Production of titanium grade 4 components by powder injection moulding of titanium hydride. Powder Metall. 2014, 57, 89–92. [Google Scholar] [CrossRef]
- German, R.M. Progress in titanium metal powder injection molding. Materials 2013, 6, 3641–3662. [Google Scholar] [CrossRef] [PubMed]
- Tosello, G. Micro Injection Molding; Hanser Verlag GmbH & Co. KG: Munich, Germany, 2018. [Google Scholar]
- Mahmud, N.N.; Azam, F.A.A.; Ramli, M.I.; Foudzi, F.M.; Ameyama, K.; Sulong, A.B. Rheological properties of irregular-shaped titanium-hydroxyapatite bimodal powder composite moulded by powder injection moulding. J. Mater. Res. Technol. 2021, 11, 2255–2264. [Google Scholar] [CrossRef]
- Iriany, M.N.; Sahari, J.; Arifin, A.K. Stability of metal injection molding feedstock containing palm oil. J. Inst. Mater. Malaysia 2001, 2, 71–83. [Google Scholar]
- Basir, A.; Sulong, A.B.; Jamadon, N.H.; Muhamad, N. Sintering behavior of bi-material micro-component of 17-4PH stainless steel and yttria-stabilized zirconia produced by two-component micro-powder injection molding process. Materials 2022, 15, 2059. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Gunawan; Mohruni, A.S.; Yani, I. Palm stearin for alternative binder for MIM: A review. J. Ocean. Mech. Aerosp. 2014, 7, 18–23. [Google Scholar]
- German, R.M.; Bose, A. Injection Molding of Metals and Ceramics; Metal Powder Industries Federation: Princeton, NJ, USA, 1997. [Google Scholar]
- Fayyaz, A.; Muhamad, N.; Sulong, A.B.; Yunn, H.S.; Amin, S.Y.M.; Rajabi, J. Micro-powder injection molding of cemented tungsten carbide: Feedstock preparation and properties. Ceram. Int. 2015, 41, 3605–3612. [Google Scholar] [CrossRef]
- Fayyaz, A.; Muhamad, N.; Sulong, A.B.; Rajabi, J.; Wong, Y.N. Fabrication of cemented tungsten carbide components by micro-powder injection moulding. J. Mater. Process. Technol. 2014, 214, 1436–1444. [Google Scholar] [CrossRef]
- Sotomayor, M.E.; Várez, A.; Levenfeld, B. Influence of powder particle size distribution on rheological properties of 316L powder injection moulding feedstocks. Powder Technol. 2010, 200, 30–36. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Khalil, K.A. Effect of powder loading on metal injection molding stainless steels. J. Mater. Process. Technol. 2007, 183, 432–439. [Google Scholar] [CrossRef]
- Zakaria, H.; Muhamad, N.; Sulong, A.B.; Ibrahim, M.H.I.; Foudzi, F. Moldability characteristics of 3 mol% yttria stabilized zirconia feedstock for micro-powder injection molding process. Sains Malays. 2014, 43, 129–136. [Google Scholar]
- Aggarwal, G.; Park, S.J.; Smid, I. Development of niobium powder injection molding: Part I. Feedstock and injection molding. Int. J. Refract. Met. Hard Mater. 2006, 24, 253–262. [Google Scholar] [CrossRef]
- Kong, X.; Barriere, T.; Gelin, J.C. Determination of critical and optimal powder loadings for 316L fine stainless steel feedstocks for micro-powder injection molding. J. Mater. Process. Technol. 2012, 212, 2173–2182. [Google Scholar] [CrossRef]
- Mutsuddy, B.C.; Ford, R.G. Ceramic Injection Molding; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Reddy, J.J.; Vijayakumar, M.; Mohan, T.R.R.; Ramakrishnan, P. Loading of solids in a liquid medium: Determination of CBVC by torque rheometry. J. Eur. Ceram. Soc. 1996, 16, 567–574. [Google Scholar] [CrossRef]
- Zakaria, M.Y.; Sulong, A.B.; Muhamad, N.; Ramli, M.I. Rheological properties of titanium-hydroxyapatite with powder space holder composite feedstock for powder injection moulding. Int. J. Adv. Manuf. Technol. 2019, 102, 2591–2599. [Google Scholar] [CrossRef]
- Huang, B.; Liang, S.; Qu, X. The rheology of metal injection molding. J. Mater. Process. Technol. 2003, 137, 132–137. [Google Scholar] [CrossRef]
- Agote, I.; Odriozola, A.; Gutierrez, M.; Santamaría, A.; Quintanilla, J.; Coupelle, P.; Soares, J. Rheological study of waste porcelain feedstocks for injection moulding. J. Eur. Ceram. Soc. 2001, 21, 2843–2853. [Google Scholar] [CrossRef]
- Basir, A.; Sulong, A.B.; Jamadon, N.H.; Muhamad, N. Bi-material micro-part of stainless steel and zirconia by two-component micro-powder injection molding: Rheological properties and solvent debinding behavior. Metals 2020, 10, 595. [Google Scholar] [CrossRef]
- Yang, W.-W.; Yang, K.-Y.; Wang, M.-C.; Hon, M.-H. Solvent debinding mechanism for alumina injection molded compacts with water-soluble binders. Ceram. Int. 2003, 29, 745–756. [Google Scholar] [CrossRef]
- Lin, S.T.; German, R.M. Extraction debinding of injection-molded parts by condensed solvent. Int. J. Powder Metall. 1989, 21, 19–24. [Google Scholar]
- Aggarwal, G.; Smid, I.; Park, S.J.; German, R.M. Development of niobium powder injection molding. Part II: Debinding and sintering. Int. J. Refract. Met. Hard Mater. 2007, 25, 226–236. [Google Scholar] [CrossRef]
- Enneti, R.K.; Park, S.J.; German, R.M.; Atre, S.V. Review: Thermal debinding process in particulate materials processing. Mater. Manuf. Process. 2012, 27, 103–118. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, F.; Yusoff, P.S.M.B.M.; Altaf, K.; Omar, M.A.; German, R.M. Powder injection molding of biocompatible stainless steel biodevices. Powder Technol. 2016, 295, 84–95. [Google Scholar] [CrossRef]
- Lame, O.; Bellet, D.; Michiel, M.D.; Bouvard, D. In situ microtomography investigation of metal powder compacts during sintering. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 200, 287–294. [Google Scholar] [CrossRef]
- Li, K. Ceramic Membranes for Separation and Reaction; John Wiley & Sons: Weinheim, Germany, 2007. [Google Scholar]
- Luo, S.D.; Song, T.; Lu, S.L.; Liu, B.; Tian, J.; Qian, M. High oxygen-content titanium and titanium alloys made from powder. J. Alloys Compd. 2020, 836, 155526. [Google Scholar] [CrossRef]
- Zhao, D.; Ebel, T.; Yan, M.; Qian, M. Trace carbon in biomedical beta-titanium alloys: Recent progress. JOM 2015, 67, 2236–2243. [Google Scholar] [CrossRef]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef]
- Itälä, A.I.; Ylänen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 µm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.Y.; Hong, H. Characterization of sintered titanium/hydroxyapatite biocomposite using FTIR spectroscopy. J. Mater. Sci. Mater. Med. 2009, 20, 843–850. [Google Scholar] [CrossRef]
- Chu, C.; Lin, P.; Dong, Y.; Xue, X.; Zhu, J.; Yin, Z. Fabrication and characterization of hydroxyapatite reinforced with 20 vol% Ti particles for use as hard tissue replacement. J. Mater. Sci. Mater. Med. 2002, 13, 985–992. [Google Scholar] [CrossRef] [PubMed]


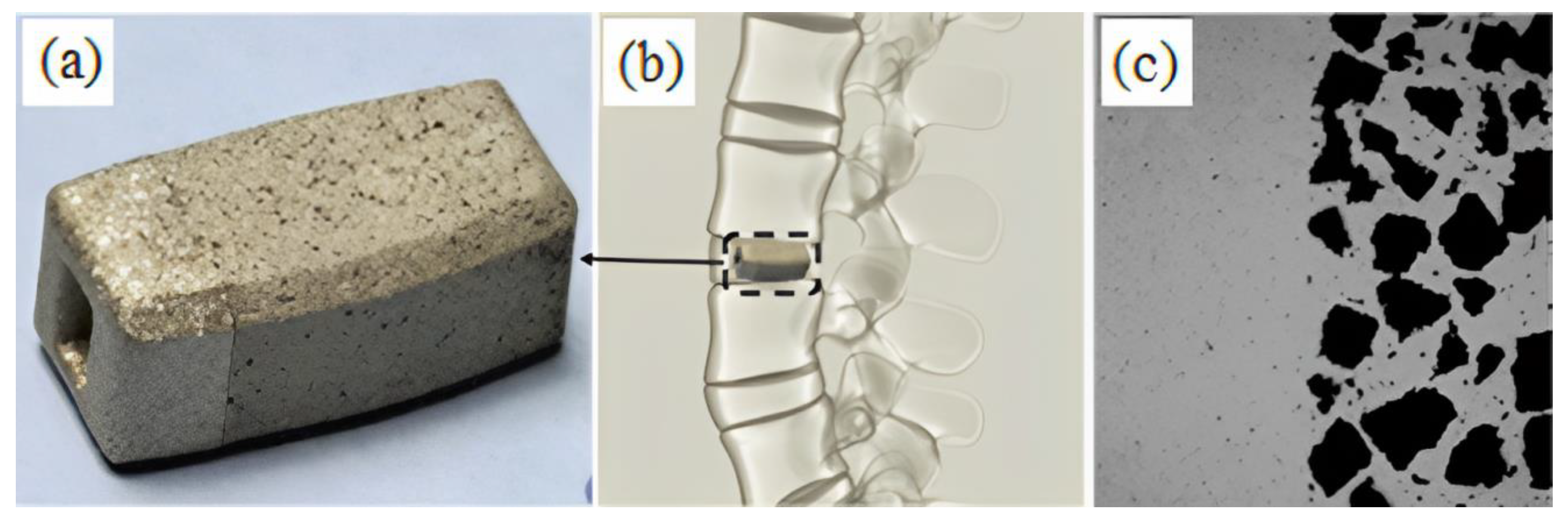



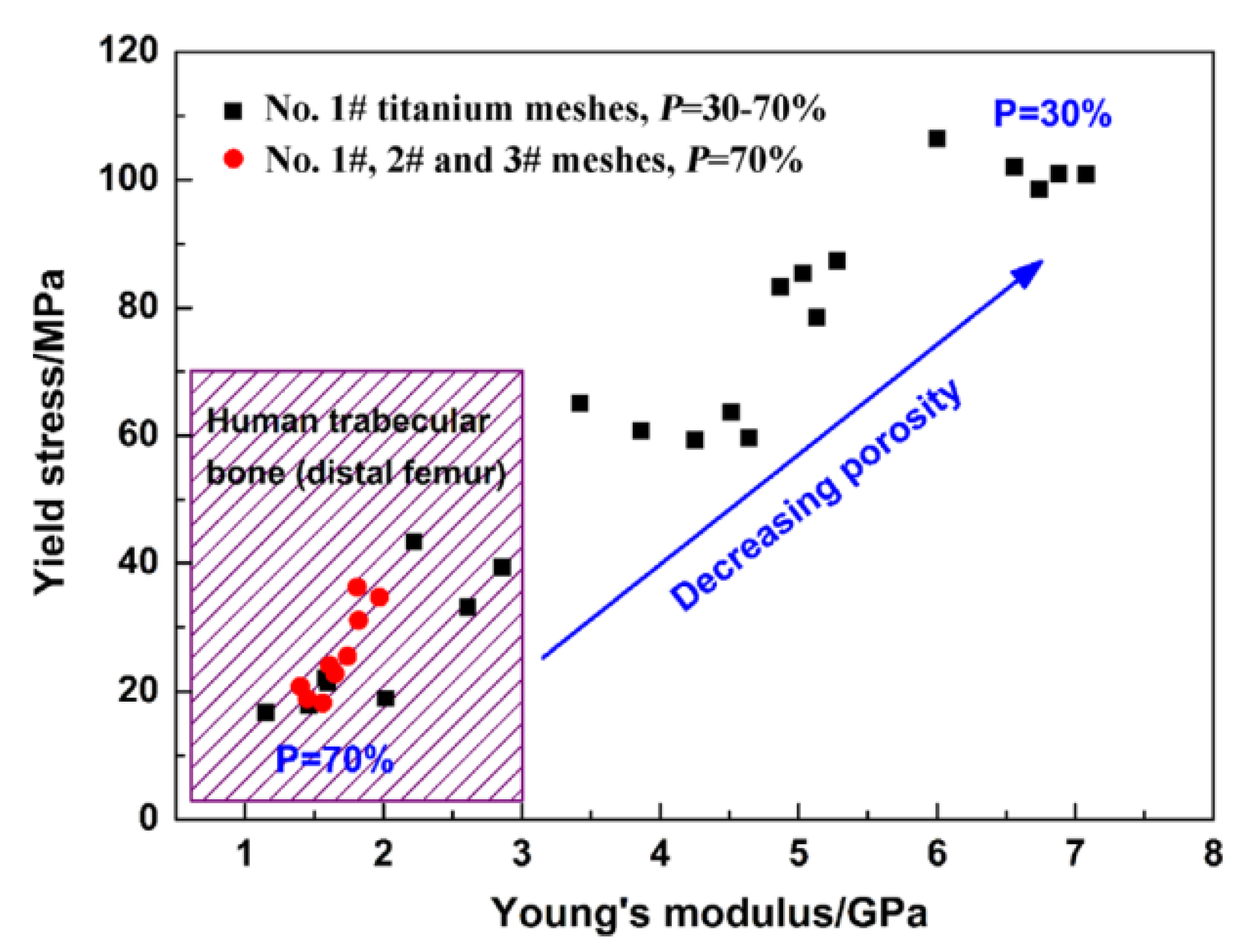
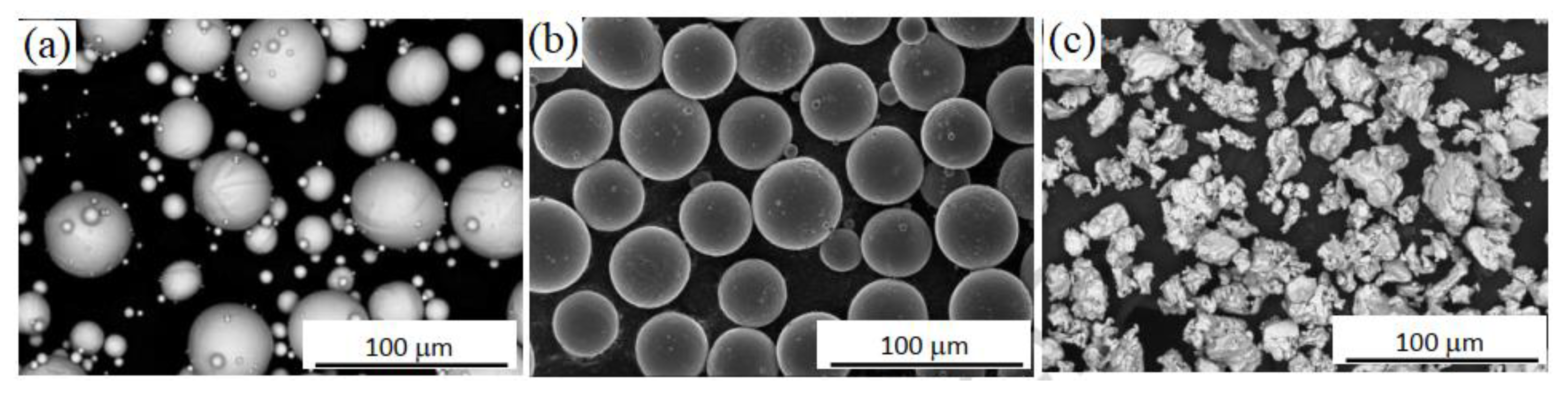
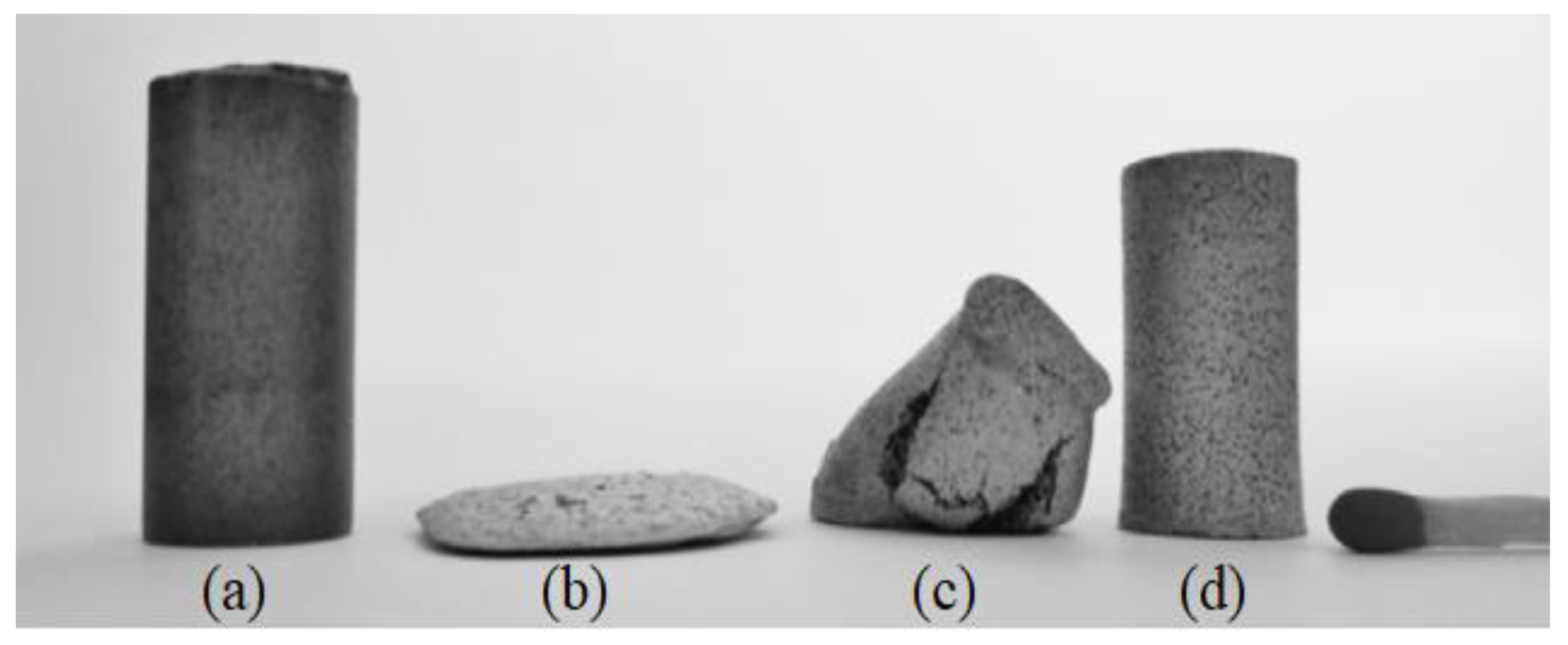

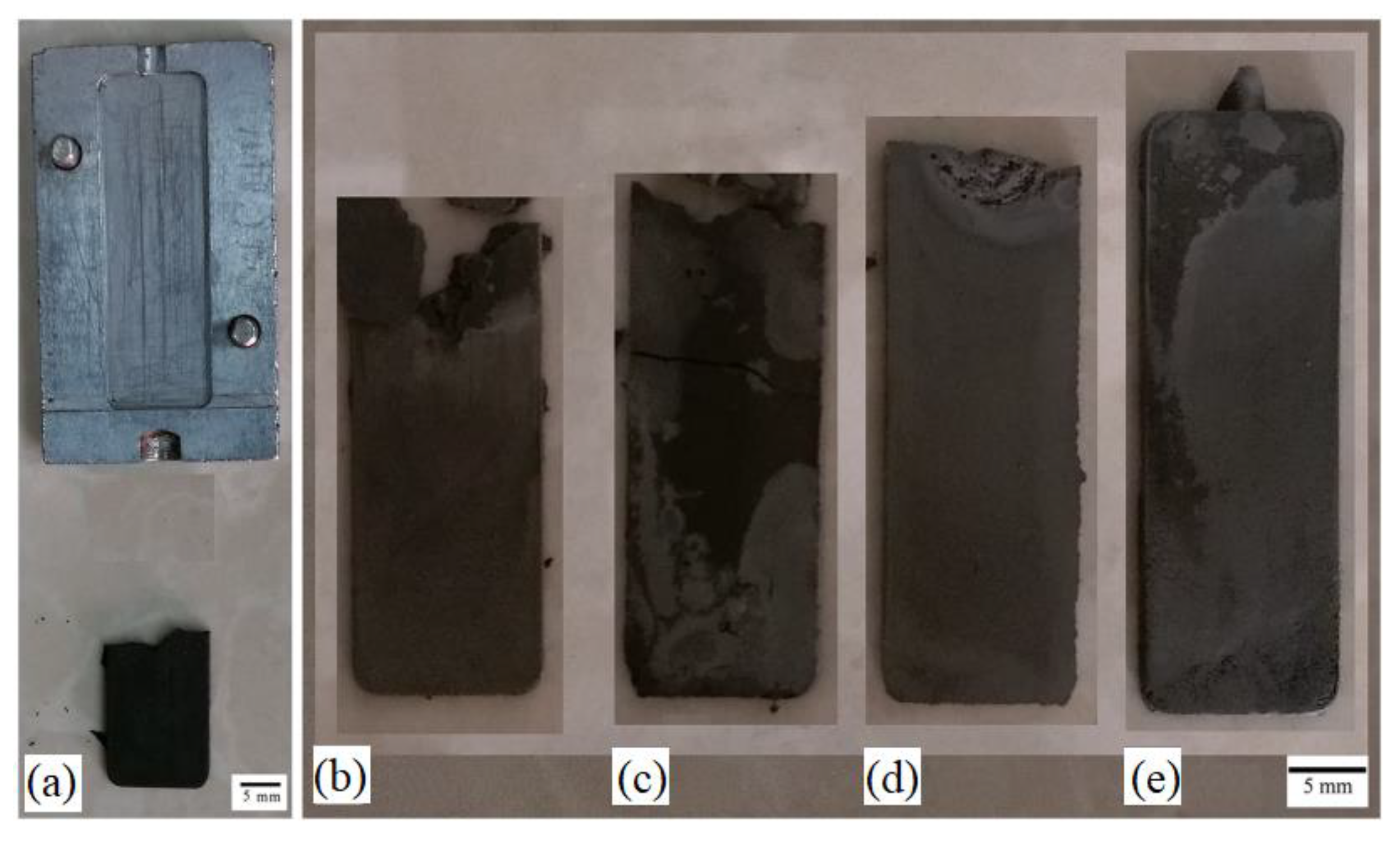
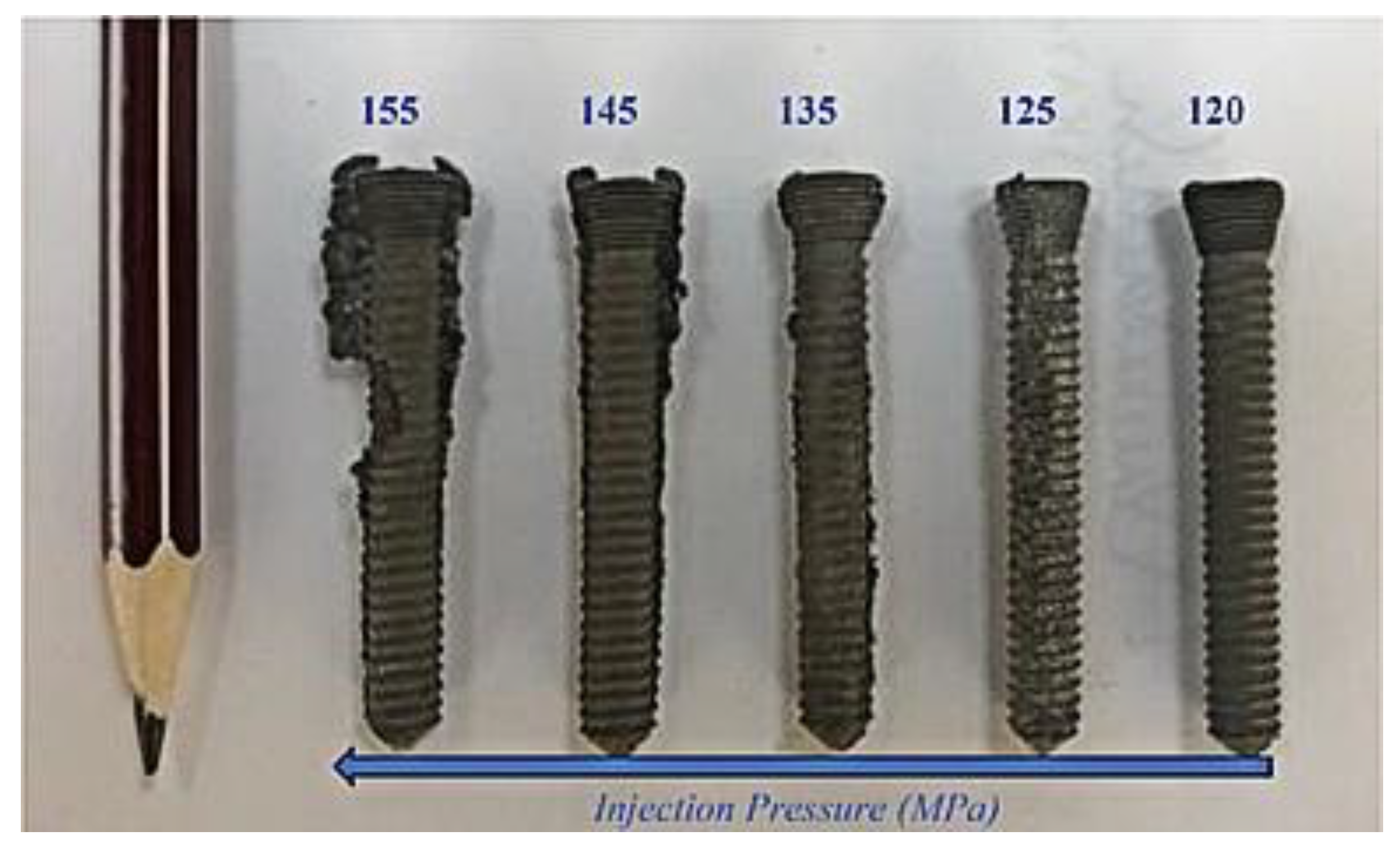

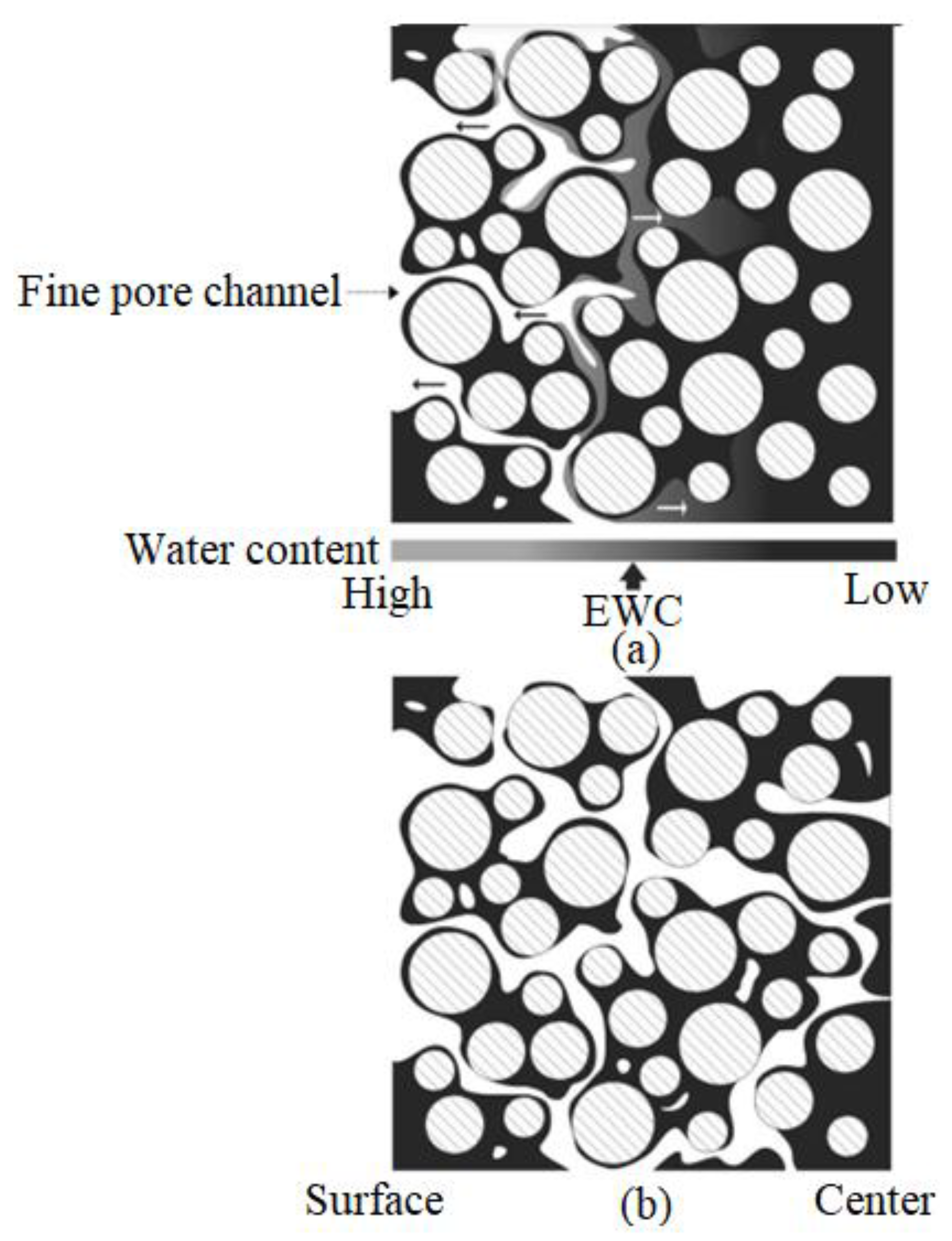
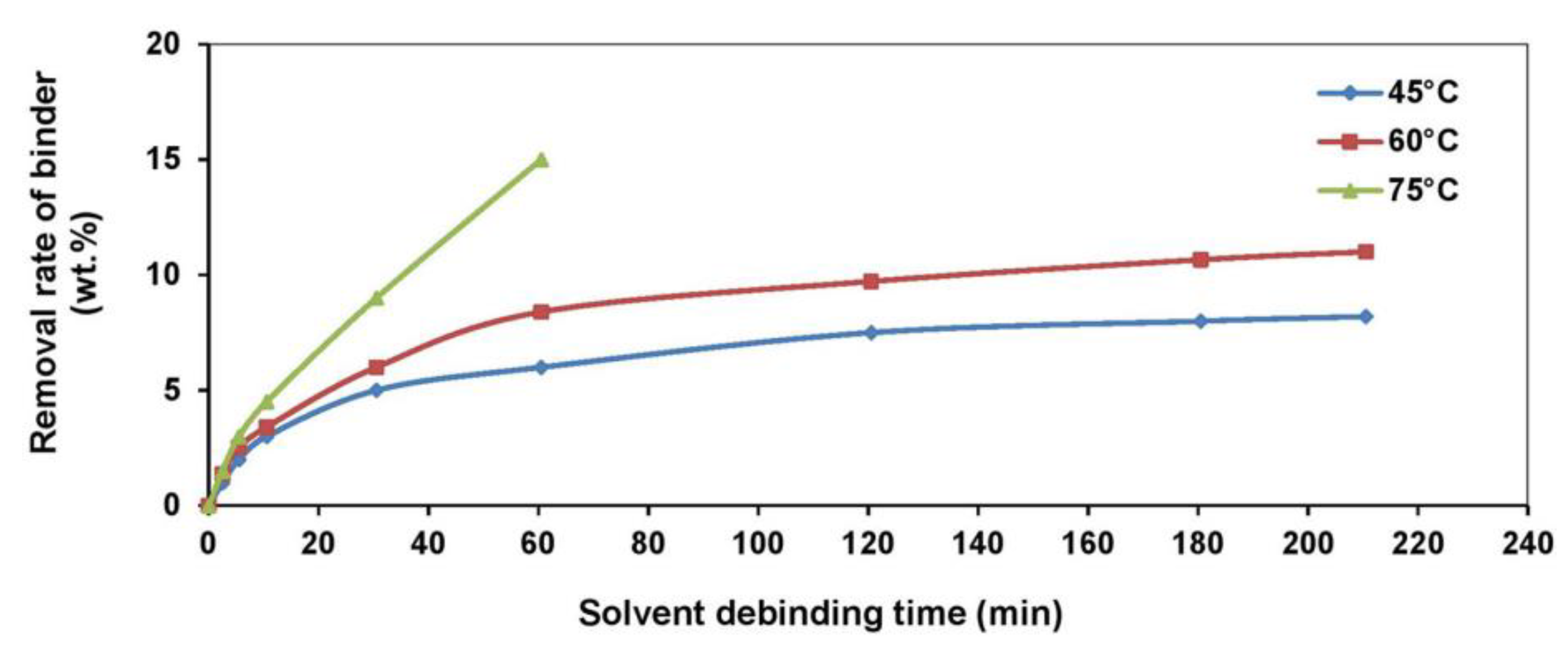
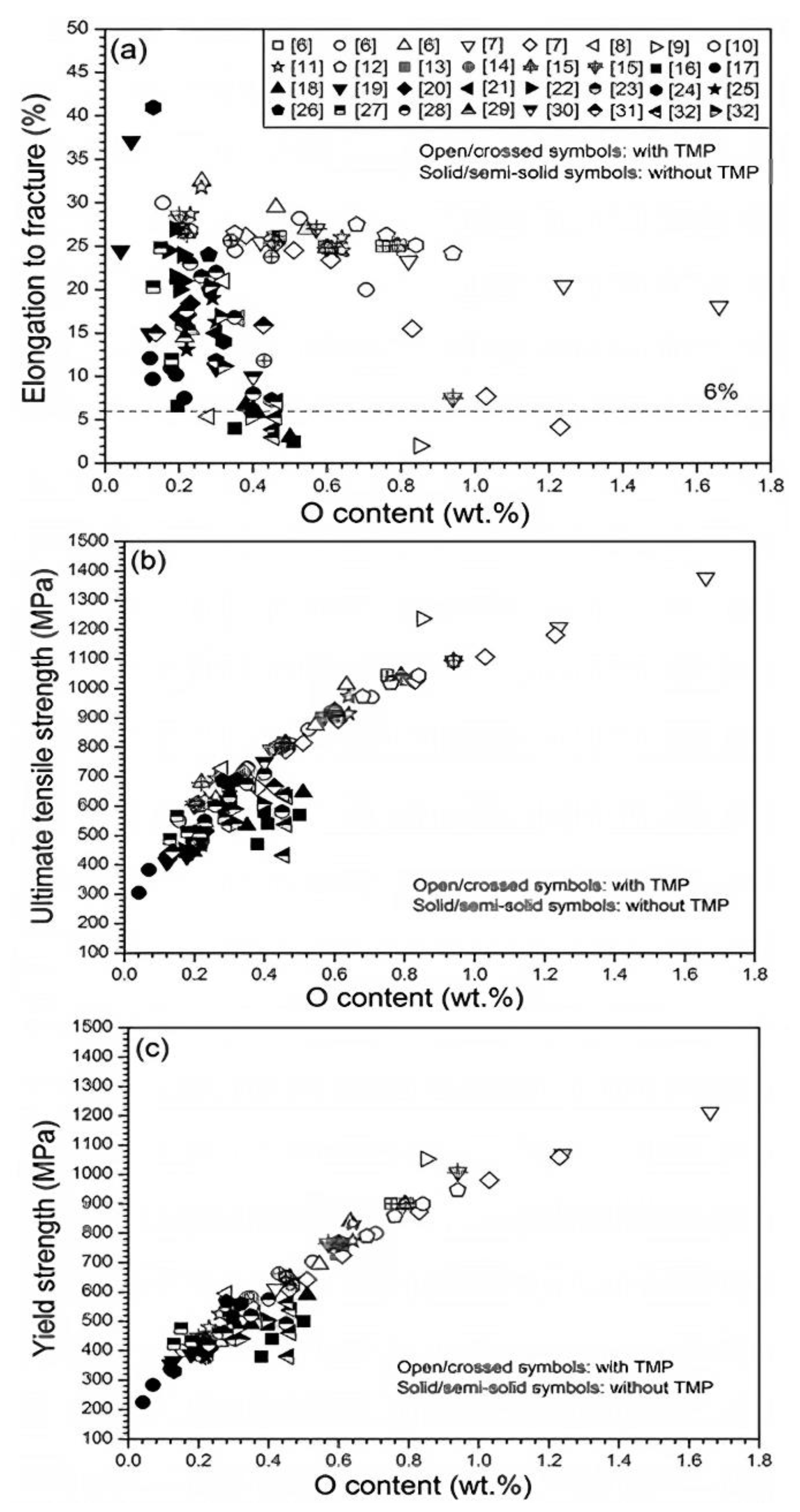

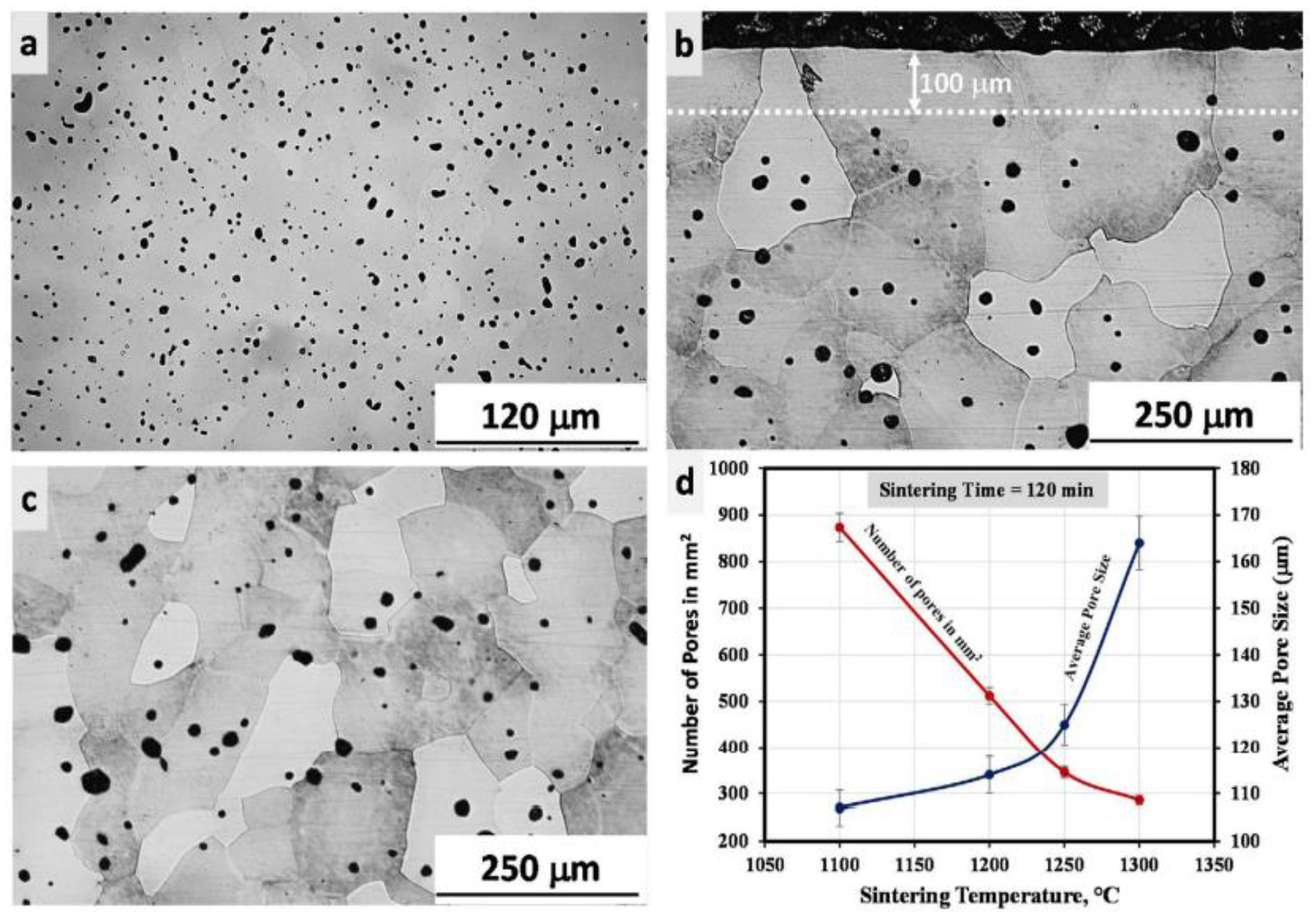
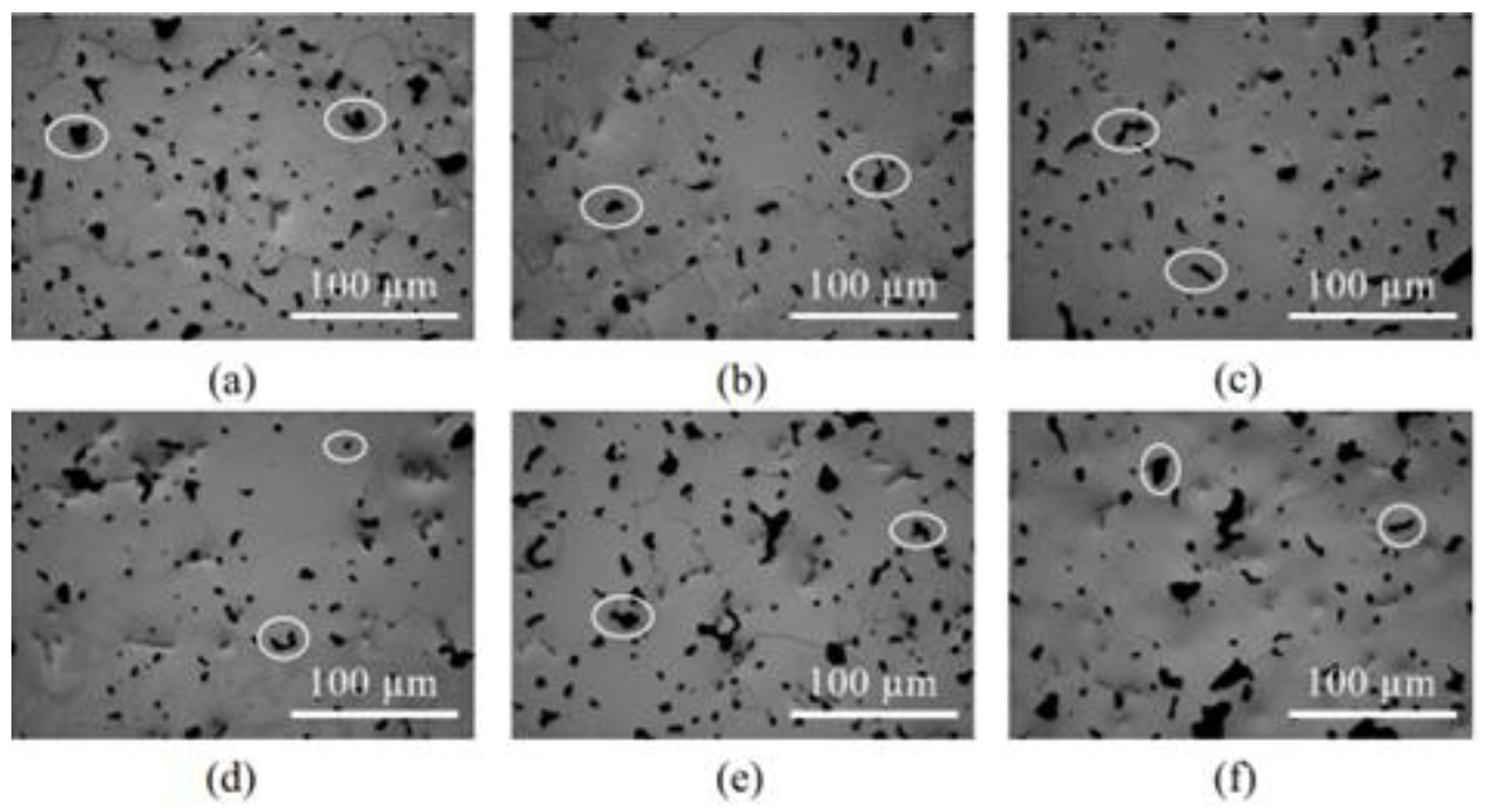
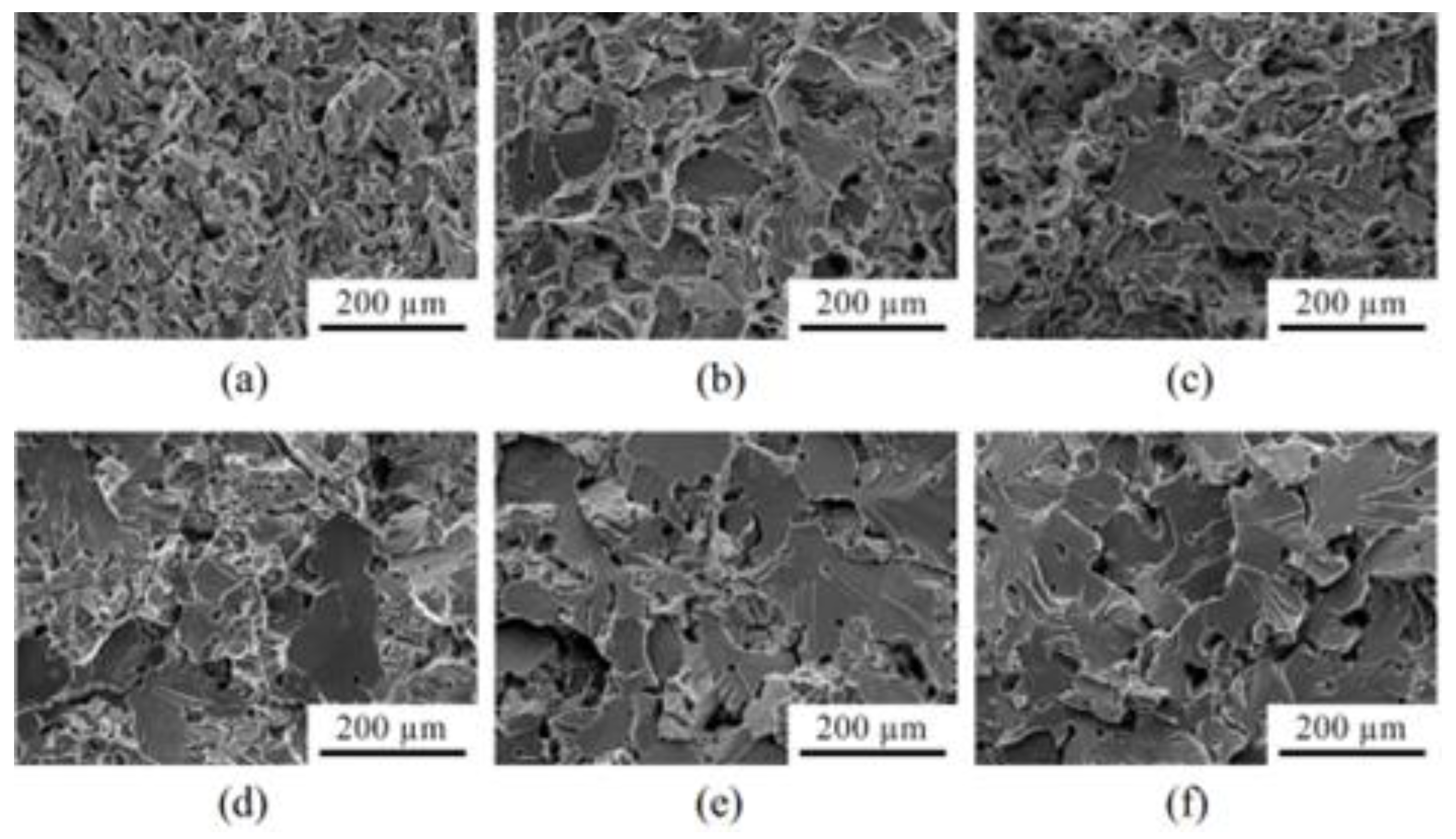
| Material | Young’s Modulus (GPa) | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) | References |
|---|---|---|---|---|---|
| Stainless steels and Co-based alloys | |||||
| 316L stainless steel | 200 | 500–1350 | 200–700 | 10–40 | [2,18,60,83,109,110,111,112,113,114,115,116,117,118,119] |
| Co-Cr | 200 | – | 500 | 8 | |
| Co-Ni-Cr | 220 | – | 850 | 20 | |
| Type of alloy: α | |||||
| Pure Ti grade 1 | 103 | 240 | 170 | 24 | |
| Pure Ti grade 2 | 103 | 345 | 275 | 20 | |
| Pure Ti grade 3 | 103 | 450 | 380 | 18 | |
| Pure Ti grade 4 | 104 | 550 | 485 | 15 | |
| Type of alloy: α + β | |||||
| Ti-6Al-4V | 110–114 | 895–930 | 860 | 6–10 | |
| Ti-6Al-7Nb | 114 | 900–1050 | 880–950 | 8.1–15 | |
| Ti–5Al-2.5Fe | 110 | 1020 | 780 | 6 | |
| Ti-3Al-2.5V | 100 | 585 | 690 | 15 | |
| Type of alloy: β | |||||
| Ti-13Nb-13Zr | 79–84 | 973–1037 | 836–908 | 10–16 | |
| Ti-35Nb-7Zr-5Ta | 55 | 590 | 800 | 20 | |
| Ti-35Nb-7Zr-5Ta-0.35O | 41–46 | 876–1015 | 864–966 | 2.2–15.6 | |
| Ti-29Nb-13Ta-4.6Zr | 80 | 911 | 864 | 13.2 | |
| Ti-35.3Nb-5.1Ta-7.1Zr | 55 | 597 | 547 | 19 | |
| Ti-24Nb-4Zr-8Sn | 54–62 | 655–720 | 627–685 | 2–9 | |
| Ti-12Mo-6Zr-2Fe | 74–85 | 1060–1100 | 100–1060 | 18–22 | |
| Ti-15Mo-5Zr-3Al | 80 | 852–1100 | 838–1060 | 18–25 |
| Materials | Particle Size (µm) | References |
|---|---|---|
| Ti | 19.1 | [73] |
| <20 | [74] | |
| 74.9 | [75] | |
| 33.2 | [153] | |
| 75 | [154] | |
| 5 | [155] | |
| <45 | [156,157,158] | |
| 45 | [159] | |
| 26.5 | [160] | |
| Hydride-dehydride (HDH) Ti | 45 | [46] |
| 29 | [68] | |
| <77 | [161] | |
| 35.43 | [162] | |
| Ti-12Mo | HDH Ti: ≤45, hydrogen reduced Mo: ≤25 | [163] |
| Ti-Mn | <45 | [164,165] |
| Ti-Nb | Ti: <45, Nb: <110 | [166] |
| Ti: 21, Nb: <75 | [167] | |
| Ti: 32.95, Nb: 30.15 | [168] | |
| Ti: <14, Nb: <36 | [169] | |
| Ti: 21, HDH Nb: 75 | [170] | |
| Ti-6Al-4V | 13.4 | [19] |
| <45 | [171] | |
| 15 | [172] | |
| 25.5 | [173] | |
| 18 | [174] | |
| HDH Ti-6Al-4V | 51.8 | [47] |
| 70 | [147] | |
| Ti-6Al-4V/ Hydroxyapatite (HA) | Ti-6Al-4V: 19.54, HA: 61.95 | [175] |
| Ti-6Al-4V: 19.6, HA: 5 | [176] | |
| Ti-6Al-4V/Wollastonite (WA) | Ti-6Al-4V: 19.54, WA: 10.10 | [177] |
| Ti-16Nb-(0-4) Sn | Ti: 23.81, Nb: 14.98, Sn: 20.19 | [178] |
| Ti-24Nb-4Zr-8Sn | <45 | [113] |
| Ti-27.5Nb-8.5Ta-3.5Mo-2.5Zr-5Sn | 6.08 | [55] |
| Materials | Binders | References |
|---|---|---|
| Ti | Paraffin wax, polyethylene, stearic acid | [73] |
| Polyethylene glycol 1500, poly methyl methacrylate, stearic acid | [75,154] | |
| Paraffin wax, high-density polyethylene, stearic acid | [153] | |
| Polyvinyl butyral, benzyl butyl phthalate, solsperse 20,000 | [155] | |
| Paraffin wax, high-density polyethylene, stearic acid | [156] | |
| Polyethylene glycol, poly methyl methacrylate, polyvinylpyrrolidone | [157] | |
| Polyethylene glycol, polypropylene carbonate, poly methyl methacrylate | [158] | |
| Polyethylene glycol, poly methyl methacrylate, stearic acid | [159] | |
| Polyacetal-based thermoplastic binder | [160] | |
| HDH Ti | Paraffin wax, high-density polyethylene, stearic acid | [46] |
| Paraffin wax, low-density polyethylene, stearic acid | [68] | |
| Wax-based binders | [161] | |
| Agar, sucrose | [162] | |
| Ti-12Mo | Liquid paraffin wax, stearic acid, low-density polyethylene, polypropylene, Polyethylene glycol, naphthalene, solid paraffin | [163] |
| Ti-Nb | Paraffin wax, polyethylene vinyl acetate, stearic acid | [167,170] |
| Paraffin wax, carnauba wax, polypropylene, stearic acid | [168] | |
| Paraffin wax, low-density polyethylene, stearic acid | [169] | |
| Ti-6Al-4V | Polyethylene glycol, polypropylene, stearic acid | [19] |
| Paraffin wax, polyethylene vinyl acetate, stearic acid | [171] | |
| Polyoxymethylene, stearic acid, paraffin wax, ethylene vinyl acetate, polyethylene | [172] | |
| Paraffin wax, polypropylene, polyethylene, stearic acid | [173] | |
| Palm stearin, polyethylene | [174] | |
| HDH Ti-6Al-4V | Polyethylene glycol, polyvinyl butyral, stearic acid | [47,147] |
| Ti-6Al-4V/HA | Palm stearin, low-density polyethylene | [175,195] |
| Palm stearin, polyethylene | [176] | |
| Ti-6Al-4V/WA | Palm stearin, polyethylene | [177] |
| Ti-16Nb-(0-4) Sn | Paraffin wax, carnauba wax, polypropylene, stearic acid | [178] |
| Ti-24Nb-4Zr-8Sn | Paraffin wax, polyethylene-vinyl acetate co-polymer, stearic acid | [113] |
| Ti-27.5Nb-8.5Ta-3.5Mo-2.5Zr-5Sn | Polyacetal-based binder | [55] |
| Materials | Particle Size (µm) | Powder Loading (vol.%) | References |
|---|---|---|---|
| Ti | 19.1 | 72, 75, 80 | [73] |
| 75 | 58 | [154] | |
| <45 | 69 | [156] | |
| <45 | 67 | [157,158] | |
| 45 | 60 | [159] | |
| 26.5 | 65.6 | [160] | |
| 28.4 | 67 | [173] | |
| HDH Ti | 45 | 61 | [46] |
| 29 | 53 | [68] | |
| <77 | 55 | [161] | |
| Ti-12Mo | HDH Ti: ≤45, hydrogen reduced Mo: ≤25 | 65 | [163] |
| Ti-Nb | Ti: 32.95, Nb: 30.15 | 50 | [168] |
| Ti: <14, Nb: <36 | 60 | [169] | |
| Ti-6Al-4V | 13.4 | 60 | [19] |
| 25.5 | 64 | [173] | |
| 18 | 65 | [174] | |
| HDH Ti-6Al-4V | 51.8 | 55, 60 | [47] |
| 70 | 55, 60 | [147] | |
| Ti-6Al-4V/HA | Ti-6Al-4V: 19.61, HA: 20 | 68, 69, 70 | [209] |
| Ti-6Al-4V: 19.54, HA: 61.95 | 68 | [175] | |
| Ti-6Al-4V: 19.6, HA: 5 | 78.21 | [176] | |
| Ti-6Al-4V/WA | Ti-6Al-4V: 19.54, WA: 10.10 | 67 | [177] |
| Ti-16Nb-(0-4) Sn | Ti: 23.81, Nb: 14.98, Sn: 20.19 | 50 | [178] |
| Ti-24Nb-4Zr-8Sn | <45 | 65 | [113] |
| Ti-27.5Nb-8.5Ta-3.5Mo-2.5Zr-5Sn | 6.08 | 65 | [55] |
| Materials | Solvent | Temperature (°C) | Time (h) | References |
|---|---|---|---|---|
| Ti | Hexane | 50 | 24 | [73,153] |
| Water | 50 | 35 | [154] | |
| Hexane | 50 | 20 | [156] | |
| Water | 40 | 28 | [157] | |
| Water | 50 | – | [159] | |
| HDH Ti | Hexane | 50 | 20 | [46] |
| Heptane | 45, 60, 75 | 6.5 | [68] | |
| Ti-12Mo | Heptane | 50 | 15 | [163] |
| Ti-Nb | Hexane | 40 | 20 | [166,167] |
| Heptane | 60 | 8 | [168] | |
| Heptane | 50 | 20 | [169] | |
| Hexane | 40 | 20 | [170] | |
| Ti-6Al-4V | Water | 60 | 24 | [19] |
| – | 40 | 15 | [171] | |
| Heptane | 60 | 6 | [174] | |
| Ti-6Al-4V/HA | Heptane | 60 | 6 | [175] |
| Ti-6Al-4V/WA | Heptane | 60 | 6 | [177] |
| Ti-16Nb-(0-4) Sn | Heptane | 60 | 8 | [178] |
| Ti-24Nb-4Zr-8Sn | Hexane | 40 | 20 | [113] |
| Materials | Thermal Debinding Schedule | References |
|---|---|---|
| Ti | 500 °C for 2 h | [73,153] |
| 450 °C for 1 h | [75,160] | |
| 550 °C for 1 h | [156] | |
| 570 °C for 2 h | [158] | |
| HDH Ti | 550 °C | [46] |
| 350 and 550 °C at 3 °C/min | [68] | |
| Ti-12Mo | 700 °C for 2 h | [163] |
| Ti-Nb | 200–400–600 °C at 2 °C/min for 2 h | [168] |
| 250 °C for 2 h and 450 °C for 2 h | [169] | |
| Ti-6Al-4V | 700 °C at 2, 5, and 10 °C/min | [173] |
| 550 °C for 4 h | [174] | |
| Ti-6Al-4V/ HA | 500 °C | [175] |
| 320 °C at 3 °C/min for 1 h and 500 °C at 5 °C/min for 1 h | [176] | |
| Ti-6Al-4V/WA | 500 °C at 5 °C/min for 1 h | [177] |
| Ti-24Nb-4Zr-8Sn | 500 °C | [113] |
| Ti-27.5Nb-8.5Ta-3.5Mo-2.5Zr-5Sn | 500 °C for 2 h | [55] |
| Materials | Sintering Temperature (°C) | Time (h) | Heating Rate (°C/min) | Sintering Atmosphere | References |
|---|---|---|---|---|---|
| Ti | 1200 | 3 | – | Vacuum | [73,153] |
| 1250 | 2 | – | Argon | [75] | |
| 1320 | 2 | – | Argon | [154] | |
| 1250 | 2 | 8 | Argon | [155] | |
| 1150, 1250, 1300 | – | – | – | [156] | |
| 1300 | 2 | – | – | [157] | |
| 1150 | 2 | – | Vacuum | [160] | |
| HDH Ti | 1100–1300 | 2 | – | Vacuum | [46] |
| 1350 | 2 | 6 | Argon | [68] | |
| 1150 | 2 | – | Vacuum | [161] | |
| Ti-Mn | 1100 | 8 | – | Vacuum | [164,165] |
| Ti-Nb | 1500 | 4 | – | Vacuum | [166] |
| 900–1500 | 2 | 5 | Vacuum | [167] | |
| 1500 | 4 | 10, 5 | Vacuum | [168] | |
| 1400 | 4 | – | Argon | [169] | |
| 1500 | 2 | – | – | [170] | |
| Ti-6Al-4V | 1350 | 2 | – | Vacuum | [171] |
| 1200, 1250, 1300 | 1, 2, 3 | 3, 4, 5 | Vacuum | [174] | |
| Ti-6Al-4V/HA | 1350 | 3 | – | Vacuum | [175] |
| 1100, 1200, 1300 | 2 | – | Vacuum | [176] | |
| Ti-6Al-4V/WA | 1100, 1200, 1300 | 5 | 3 | Vacuum | [177] |
| Ti-16Nb-(0-4) Sn | 1250, 1400, 1550 | 2 | 10, 5 | Vacuum | [178] |
| Ti-24Nb-4Zr-8Sn | 1400, 1500 | 2, 4 | – | Vacuum | [113] |
| Ti-27.5Nb-8.5Ta-3.5Mo-2.5Zr-5Sn | 1000, 1100, 1200, 1300, 1400 | 8 | – | Vacuum | [55] |
| Materials | Sintering Temperature (°C) | Young’s Modulus (GPa) | Tensile Strength (Mpa) | Elongation (%) | References |
|---|---|---|---|---|---|
| Ti | 1250, 1300 | 7.80, 22 | – | – | [156] |
| 1300 | – | 617 | – | [157] | |
| 1150 | 99 | 542 | – | [160] | |
| HDH Ti | 1250 | – | 395 | 12.5 | [46] |
| Ti-8Mn | 1100 | 87 | – | – | [165] |
| Ti-9Mn | 1100 | 89 | 1046 | 4.7 | [165] |
| Ti-12Mn | 1100 | 96 | – | – | [165] |
| Ti-13Mn | 1100 | 99 | – | – | [165] |
| Ti-15Mn | 1100 | 98 | – | – | [165] |
| Ti-17Mn | 1100 | 103 | – | – | [165] |
| Ti-16Nb | 1500 | 80 | 667 | – | [167] |
| Ti-Nb | 1500 | 100 | – | – | [168] |
| Ti-17Nb | 1400 | 76 | – | – | [169] |
| Ti-6Al-4V | 1350 | – | 824 | – | [171] |
| 1200 | – | 934.33 | – | [174] | |
| Ti-6Al-4V + Gd | 1350 | – | 749 | – | [171] |
| Ti-6Al-4V/HA | 1300 | 44.26 | – | – | [176] |
| Ti6Al4V/WA | 1100–1300 | 14.57–18.10 | – | – | [177] |
| Ti-24Nb-4Zr-8Sn | 1400 | 54 | 656 | – | [113] |
| Ti-27.5Nb-8.5Ta-3.5Mo-2.5Zr-5Sn | 1100 | 98 | 1154 | – | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basir, A.; Muhamad, N.; Sulong, A.B.; Jamadon, N.H.; Foudzi, F.M. Recent Advances in Processing of Titanium and Titanium Alloys through Metal Injection Molding for Biomedical Applications: 2013–2022. Materials 2023, 16, 3991. https://doi.org/10.3390/ma16113991
Basir A, Muhamad N, Sulong AB, Jamadon NH, Foudzi FM. Recent Advances in Processing of Titanium and Titanium Alloys through Metal Injection Molding for Biomedical Applications: 2013–2022. Materials. 2023; 16(11):3991. https://doi.org/10.3390/ma16113991
Chicago/Turabian StyleBasir, Al, Norhamidi Muhamad, Abu Bakar Sulong, Nashrah Hani Jamadon, and Farhana Mohd Foudzi. 2023. "Recent Advances in Processing of Titanium and Titanium Alloys through Metal Injection Molding for Biomedical Applications: 2013–2022" Materials 16, no. 11: 3991. https://doi.org/10.3390/ma16113991
APA StyleBasir, A., Muhamad, N., Sulong, A. B., Jamadon, N. H., & Foudzi, F. M. (2023). Recent Advances in Processing of Titanium and Titanium Alloys through Metal Injection Molding for Biomedical Applications: 2013–2022. Materials, 16(11), 3991. https://doi.org/10.3390/ma16113991









