Alloy Disordering Effects on the Thermal Conductivity and Energy Gap Temperature Dependence of Cd1−xZnxSe Ternary Crystals Grown by the Bridgman Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
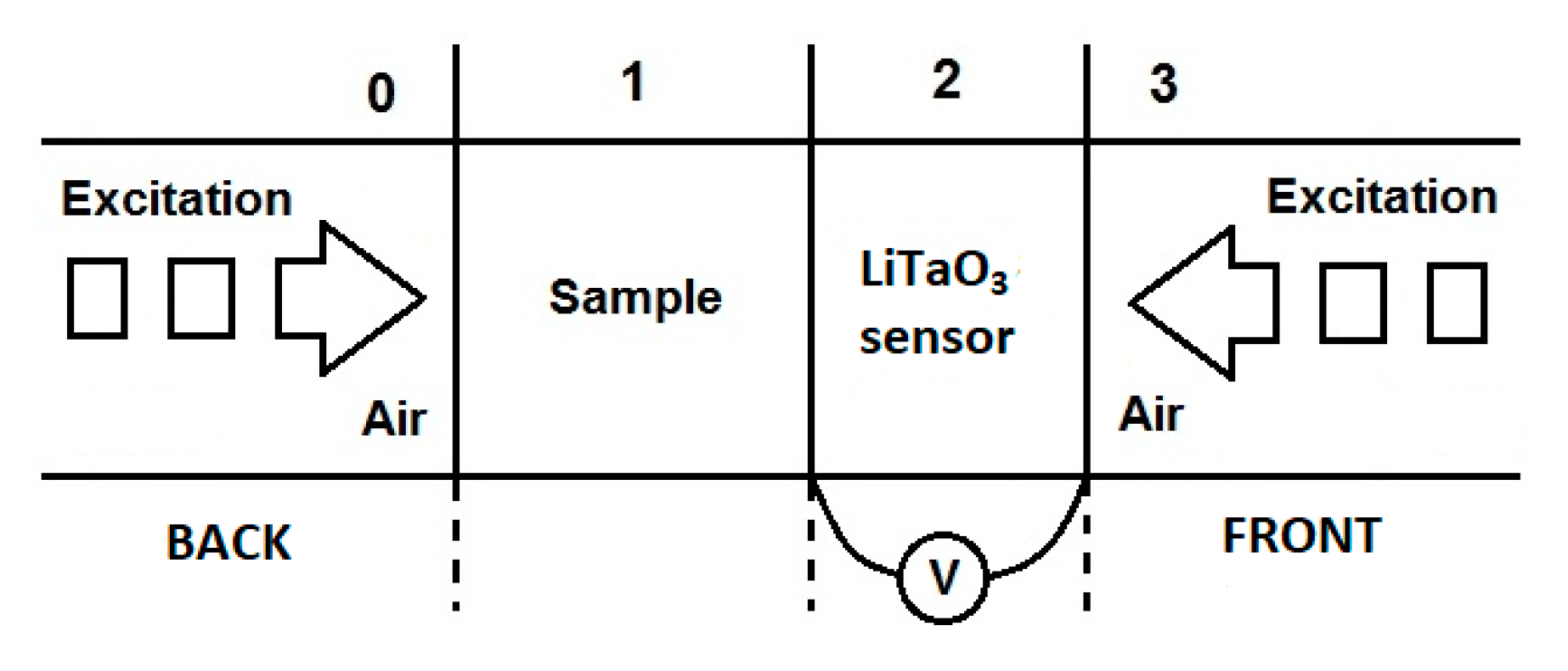
2.2. Experimental Systems
2.3. Basic Theory of PPE
3. Experimental Results and Discussion
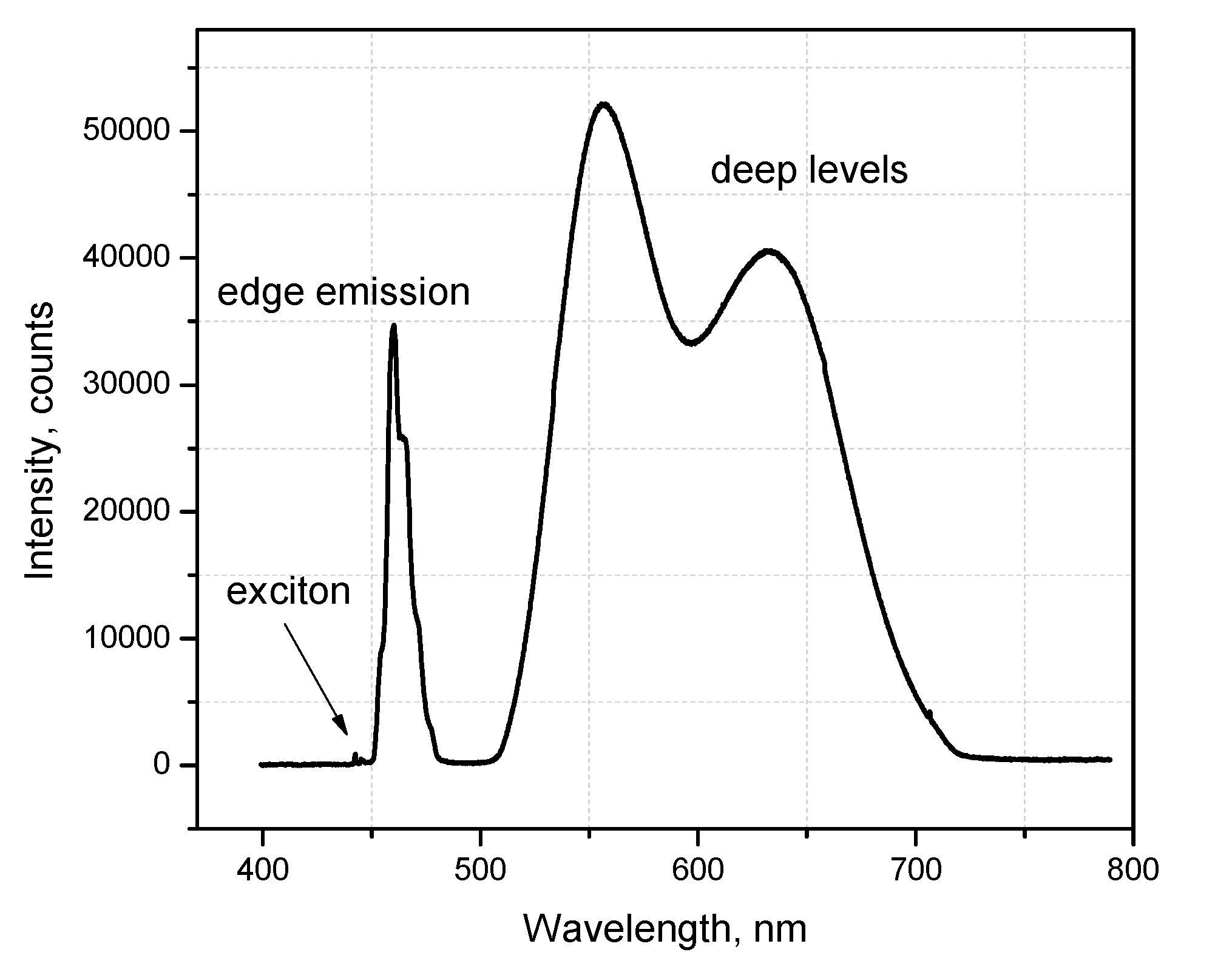
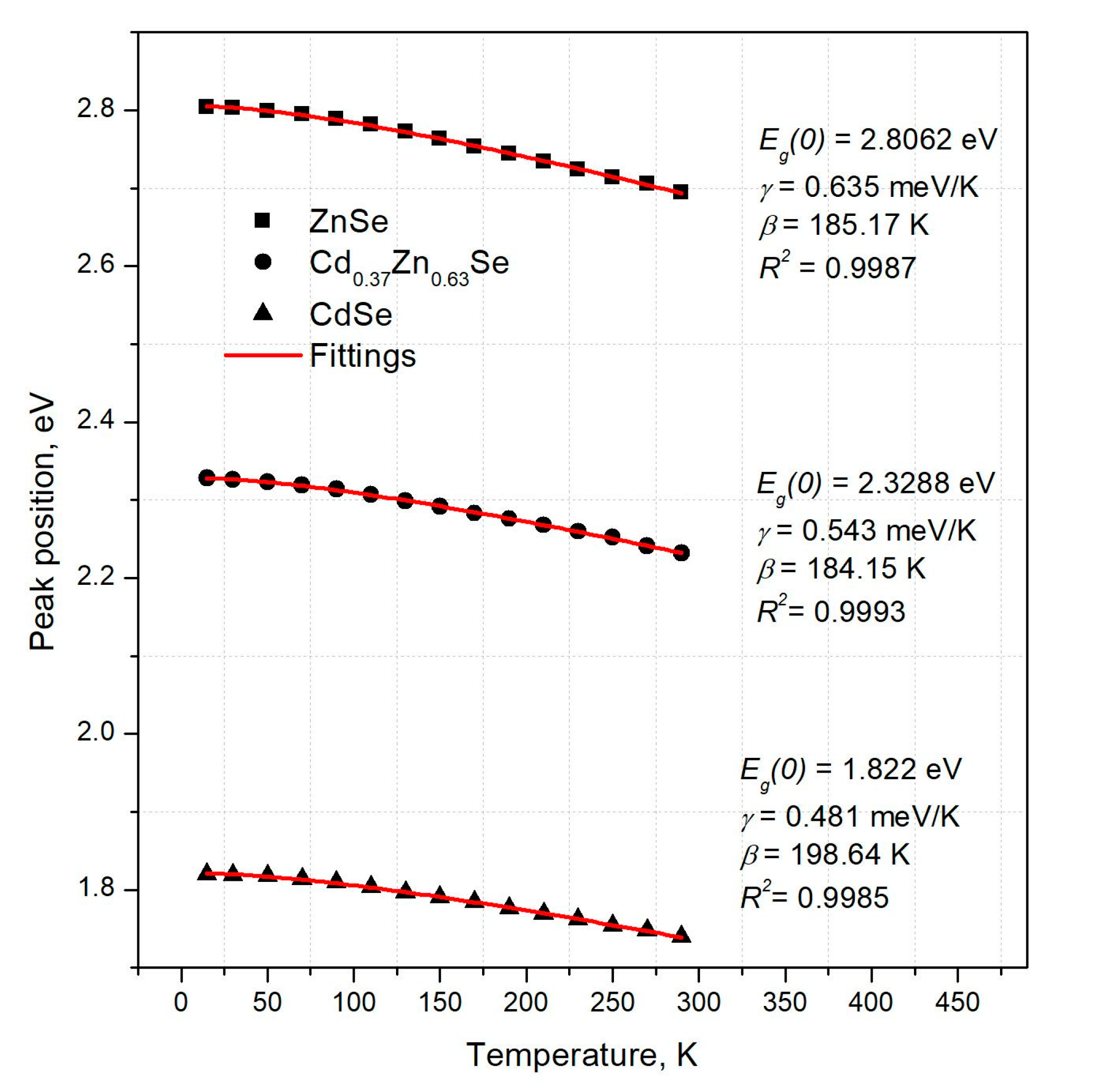
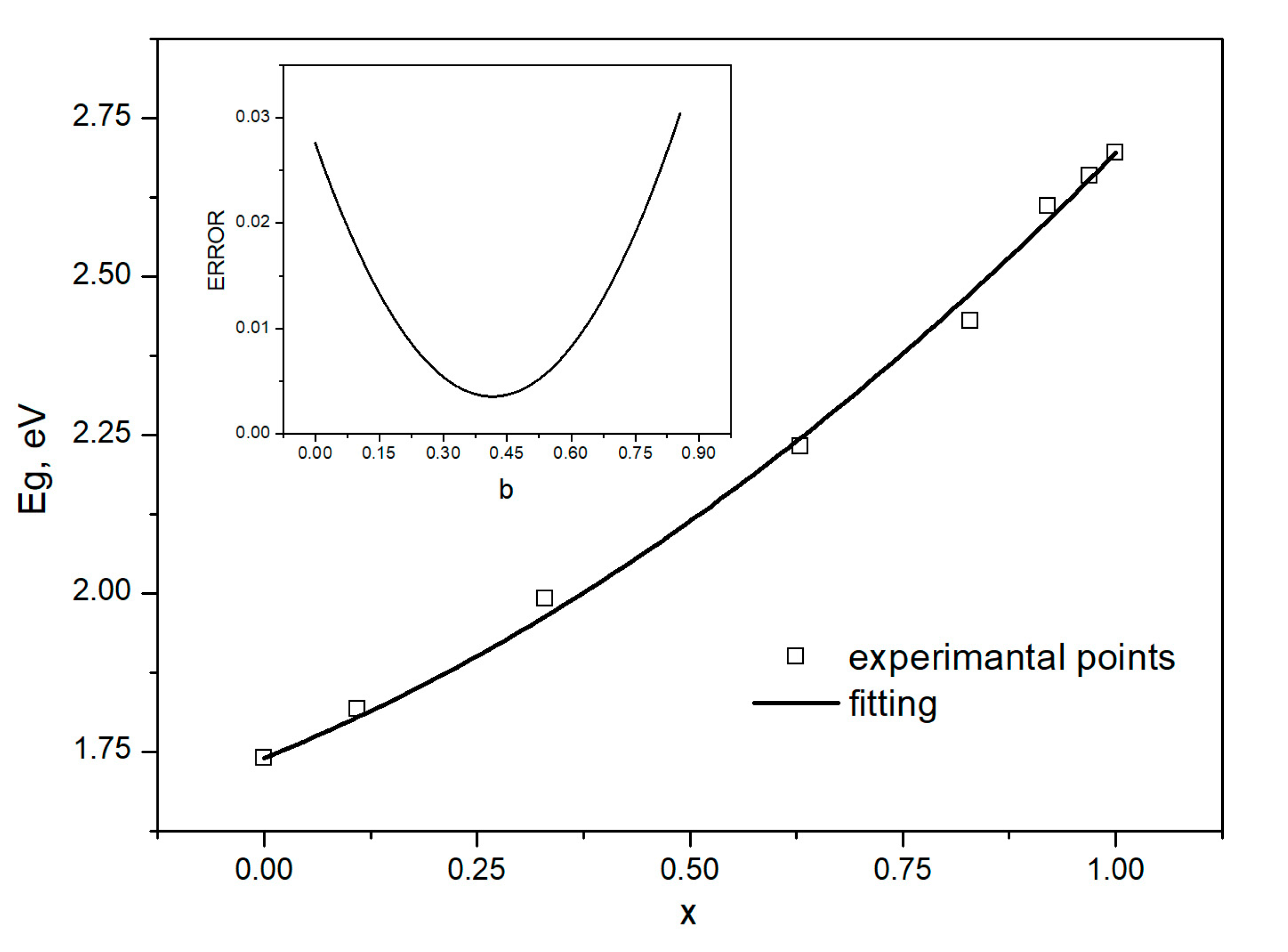
3.1. Photoluminescence Characterization
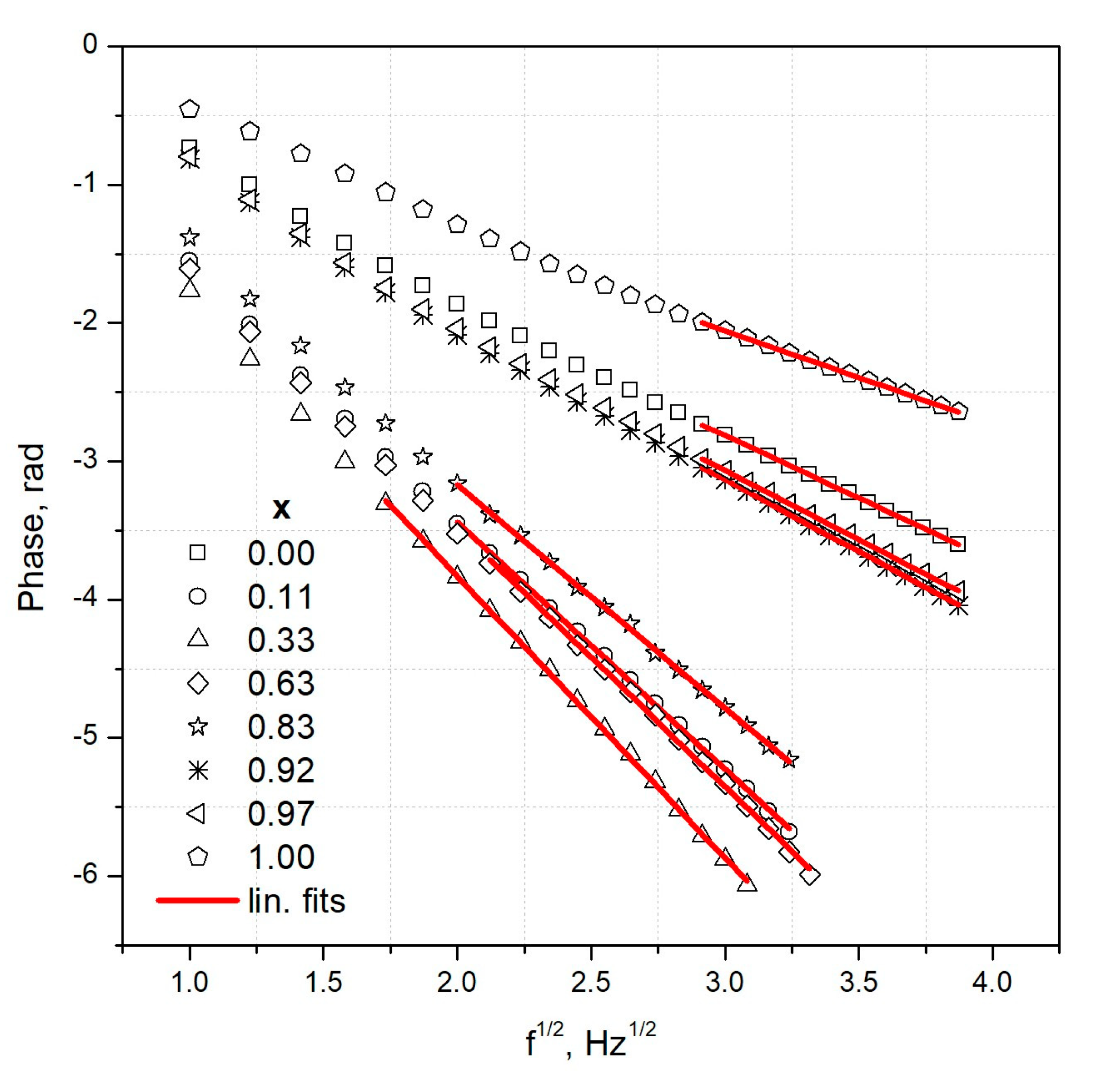
3.2. Thermal Results
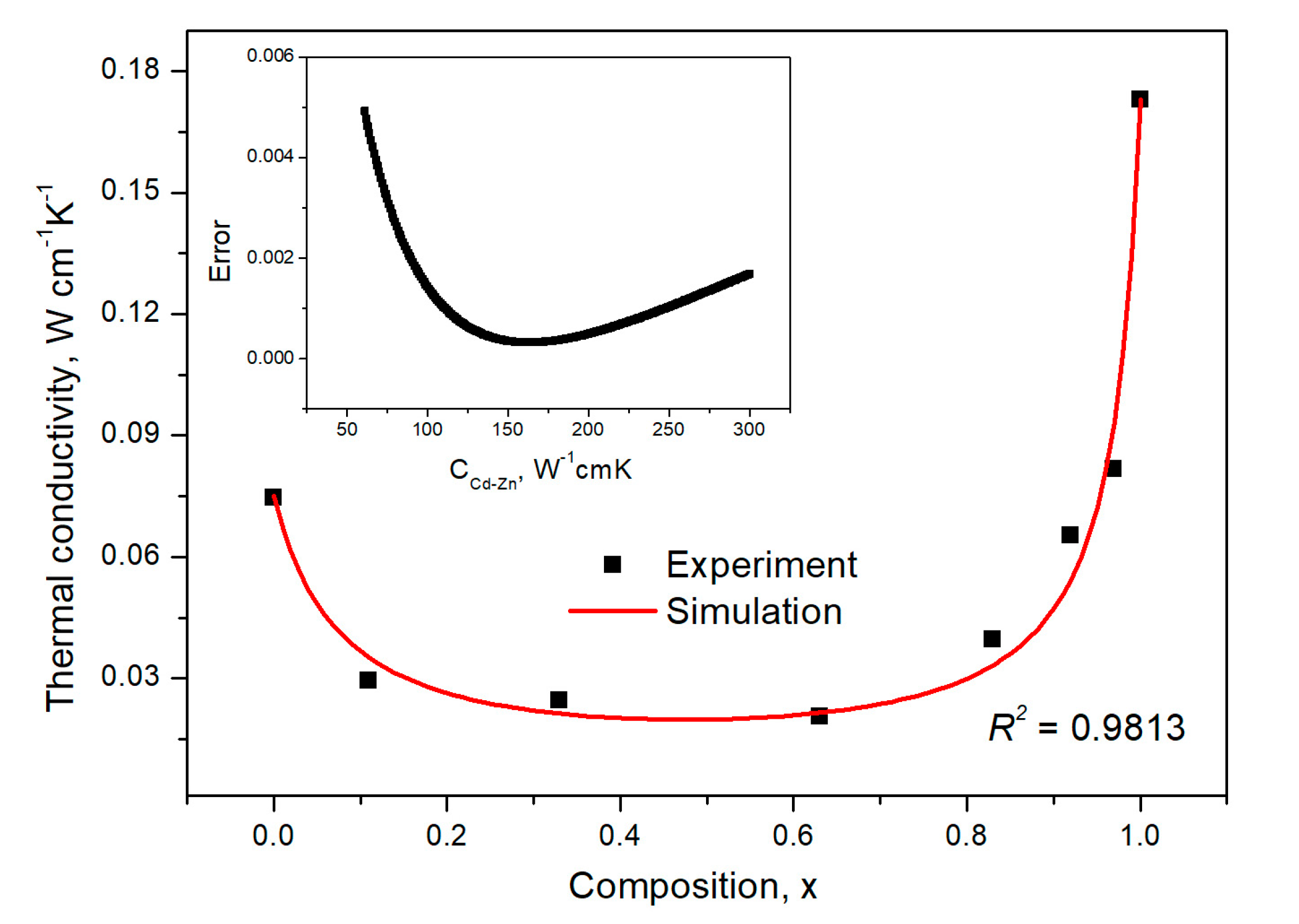
3.3. Lattice Thermal Resistivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kniproth, R.; Rabe, J.P.; McLesky, J.T., Jr.; Wang, D.Y.; Kirstein, S. Hybrid photovoltaic cells with I–VI quantum dot sensitizers fabricated by layer-by-layer deposition of water soluble components. Thin Solid Film. 2009, 518, 295–298. [Google Scholar] [CrossRef]
- Gur, N.A.; Fromer, A.P. Alivisatos Controlled assembly of hybrid bulk-heterojunction solar cells by sequential deposition. J. Phys. Chem. B 2006, 110, 25543–25546. [Google Scholar] [CrossRef][Green Version]
- Van Calster, A.; Vervaet, A.; De Rycke, I.; De Baets, J.; Vanfleteren, J. Polycrystalline CdSe films for thin film transistors. J. Cryst. Growth 1989, 86, 924–928. [Google Scholar] [CrossRef]
- Gruszecki, T.; Holmstrom, B. Preparation of thin films of polycrystalline CdSe for solar energy conversion I. A literature survey. Sol. Energy Mater. Sol. Cells 1993, 31, 227–234. [Google Scholar] [CrossRef]
- Deshmukh, L.P.; Rotti, C.B.; Garadkar, K.M. Cd1−xZnxS thin film electrode for photoelectrochemical (PEC) applications. Mater. Chem. Phys. 1997, 50, 45–49. [Google Scholar] [CrossRef]
- Edmund, J.W.; Dale, E.M.; Donald, R.N.; Arthur, B.E.; John, F.G.; Kucch, T.F. Detection of ammonia, phosphine, and arene gases by reversible modulation of cadmium selenide photoluminescence intensity. J. Cryst. Growth 1995, 148, 63–69. [Google Scholar]
- Wang, C.I.; Yang, Z.; Periasamy, A.P.; Chang, H.T. High-efficiency photochemical water splitting of CdZnS/CdZnSe nanostructures. J. Mater. 2013, 2013, 703985. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Jakobson, H.P.; Kou, R.; Tang, J.; Fineman, R.Z.; Yu, D.; Lu, Y. Metal and Semiconductor Nanowire Network Thin Films with Hierarchical Pore Structures. Chem. Mater. 2006, 18, 4231–4237. [Google Scholar] [CrossRef]
- Banfi, G.P.; Degiorgio, V.; Tan, H.M. Optical non-linearity of semiconductor-doped glasses at frequencies below the band gap: The role of free carriers. J. Opt. Soc. Am. B 1995, 12, 621–628. [Google Scholar] [CrossRef]
- Subba Ramaiah, K.; Su, Y.K.; Chang, S.J.; Juang, F.S.; Ohdaira, K.; Shiraki, Y.; Liu, H.P.; Chen, I.J.; Bhatnagar, A.K. Characterization of Cu-doped CdSe thin films grown by vacuum evaporation. J. Cryst. Growth 2001, 224, 74–82. [Google Scholar] [CrossRef]
- Hankare, P.P.; Bhuse, V.M.; Garadkar, K.M.; Delekar, S.D.; Bhagat, P.R. HgSe thin films, preparation, characterization and optoelectronic studies. Semicond. Sci. Tech. 2004, 19, 277–284. [Google Scholar] [CrossRef]
- Mariappan, R.; Ponnuswamy, V.; Ragavendar, M. Characterization of CdS1−xSex thin films by chemical bath deposition technique. Optik 2012, 123, 1196–1200. [Google Scholar] [CrossRef]
- Mahalingam, T.; Dhanasekaran, V.; Rajendran, S.; Chandramohan, R.; Ixtlilco, L.; Sebastian, P.J. Electrosynthesis and Studies on CdZnSe Thin Films. J. New Mater. Electrochem. Syst. 2012, 15, 37–42. [Google Scholar] [CrossRef]
- Murali, K.R.; Balasubramanian, M. Characteristics of pulse plated CdxZn1−xSe films. Curr. Appl. Phys. 2010, 10, 734. [Google Scholar] [CrossRef]
- Schoemaker, G.H.; Bakkers, E.P.A.M.; Kelly, J.J. Electroless Etching of ZnSe in Aqueous Ferricyanide Solutions: An Electrochemical Study. J. Electrochem. Soc. 1997, 144, 2329. [Google Scholar] [CrossRef]
- Singh, K.; Pathak, R.K. Redox and solution chemistry of the SeSO32−–Zn–EDTA2− system and electrodeposition behavior of ZnSe from alkaline solutions. Electrochim. Acta 1994, 39, 2693. [Google Scholar] [CrossRef]
- Kishore, V.; Saxena, N.S.; Saraswat, V.K.; Sharma, R.; Sharma, K.; Sharma, T.P. Temperature dependence of effective thermal conductivity and effective thermal diffusivity of CdZnSe composite. J. Phys. D Appl. Phys. 2006, 39, 2592–2595. [Google Scholar] [CrossRef]
- Yang, S.; Lin, C.; Chen, K.; Fan, H.; Chen, J.; Fang, S.; Ye, N.; Luo, M. Trade-Off for Better Balanced Nonlinear Optical Performance with Disordered Si in ZnGeP2. Chem. Mater. 2022, 34, 11007–11013. [Google Scholar] [CrossRef]
- Sung, Y.-M.; Lee, Y.-J.; Park, K.-S. Kinetic Analysis for Formation of Cd1-xZnxTeSe Solid-Solution Nanocrystals. J. Am. Chem. Soc. 2006, 128, 9002–9003. [Google Scholar] [CrossRef]
- Chrobak, Ł.; Maliński, M. On Investigations of the Optical Absorption Coefficient of Gold and Germanium Implanted Silicon with the Use of the Non-destructive Contactless Photo Thermal Infrared Radiometry. J. Electron. Mater. 2019, 48, 5273–5278. [Google Scholar] [CrossRef][Green Version]
- Dorywalski, K.; Chrobak, Ł.; Maliński, M. Comparative Studies of the optical absorption coefficient spectra in the implanted layers in silicon with the use of nondestructive spectroscopic techniques. Metrol. Meas. Syst. 2020, 27, 323–337. [Google Scholar]
- Kaźmierczak-Bałata, A.; Juszczyk, J.; Trefon-Radziejewska, D.; Bodzenta, J. Influence of probe-sample temperature difference on thermal mapping contrast in scanning thermal microscopy imaging. J. Appl. Phys. 2017, 121, 114502. [Google Scholar] [CrossRef]
- Adachi, S. GaAs, AlAs, and AlxGa1−xAs: Material parameters for use in research and device applications. J. Appl. Phys. 1983, 54, 1844. [Google Scholar] [CrossRef]
- Firszt, F.; Łegowski, S.; Meczynska, H.; Szatkowski, J.; Paszkowicz, W.; Godwod, K. Growth and characterisation of Zn1−xBexSe mixed crystals. J. Cryst. Growth 1998, 184/185, 1335. [Google Scholar] [CrossRef]
- Strzałkowski, K. Effect of lattice disorder on the thermal conductivity of ZnBeSe, ZnMgSe and ZnBeMgSe crystals. Mater. Chem. Phys. 2015, 163, 453–459. [Google Scholar] [CrossRef]
- Dadarlat, D. Photopyroelectric calorimetry of liquids; recent development and applications. Laser Phys. 2009, 54, 1330. [Google Scholar] [CrossRef]
- Strzałkowski, K.; Zakrzewski, J.; Maliński, M. Determination of the Exciton Binding Energy Using Photothermal and Photoluminescence Spectroscopy. Int. J. Thermophys. 2013, 34, 691. [Google Scholar] [CrossRef][Green Version]
- Varshni, Y.P. Temperature Dependence of the Energy Gap in Semi-Conductor. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Hernández-Calderón, I. II-VI Semiconductor Materials and their Applications; Tamargo, M.C., Ed.; Taylor and Francis: New York, NY, USA, 2002; pp. 113–170. [Google Scholar]
- Mourad, N.D.; Czycholl, G.; Kruse, C.; Klembt, S.; Retzlaff, R.; Hommel, D.; Gartner, M.; Anastasescu, M. Compound semiconductor alloys: From atomic-scale structure to bandgap bowing. Phys. Rev. B 2010, 82, 165204. [Google Scholar]
- Venugopal, R.; Lin, P.; Chen, Y. Photoluminescence and Raman Scattering from Catalytically Grown ZnxCd1-xSe Alloy Nanowires. J. Phys. Chem. B 2006, 110, 11691. [Google Scholar] [CrossRef]
- Strzałkowski, K.; Dadarlat, D.; Streza, M.; Firszt, F. On the optimization of experimental parameters in photopyroelectric investigation of thermal diffusivity of solids. Thermochim. Acta 2015, 614, 232–238. [Google Scholar] [CrossRef]
- Strzałkowski, K. The composition effect on the thermal and optical properties across CdZnTe crystals. J. Phys. D Appl. Phys. 2016, 49, 435106. [Google Scholar] [CrossRef]
- Slack, G. Thermal Conductivity of II-VI Compounds and Phonon Scattering by Fe2+ Impurities. Phys. Rev. B 1972, 6, 3791. [Google Scholar] [CrossRef]
- Abeles, B. Lattice Thermal Conductivity of Disordered Semiconductor Alloys at High Temperatures. Phys. Rev. 1963, 131, 1906. [Google Scholar] [CrossRef]
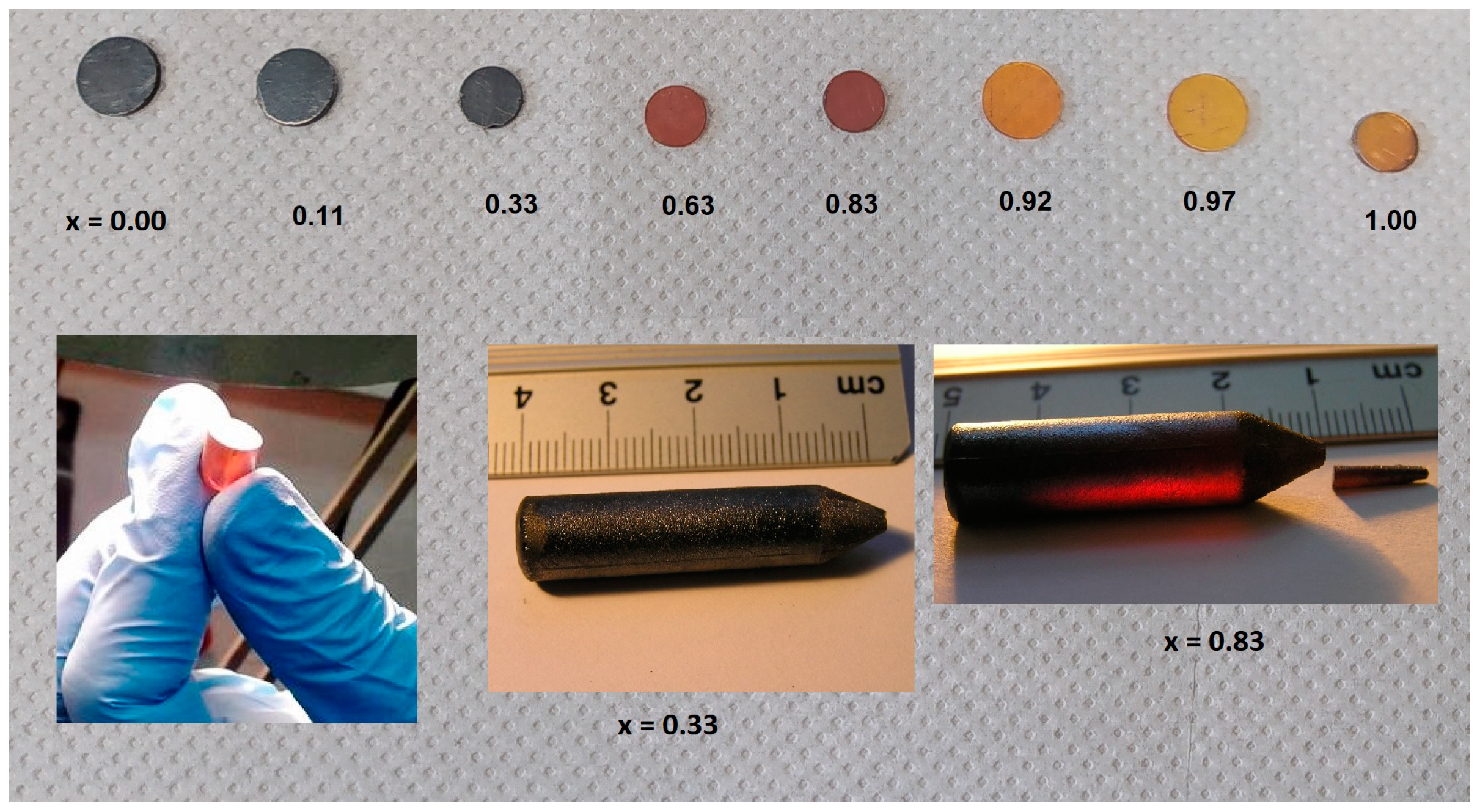
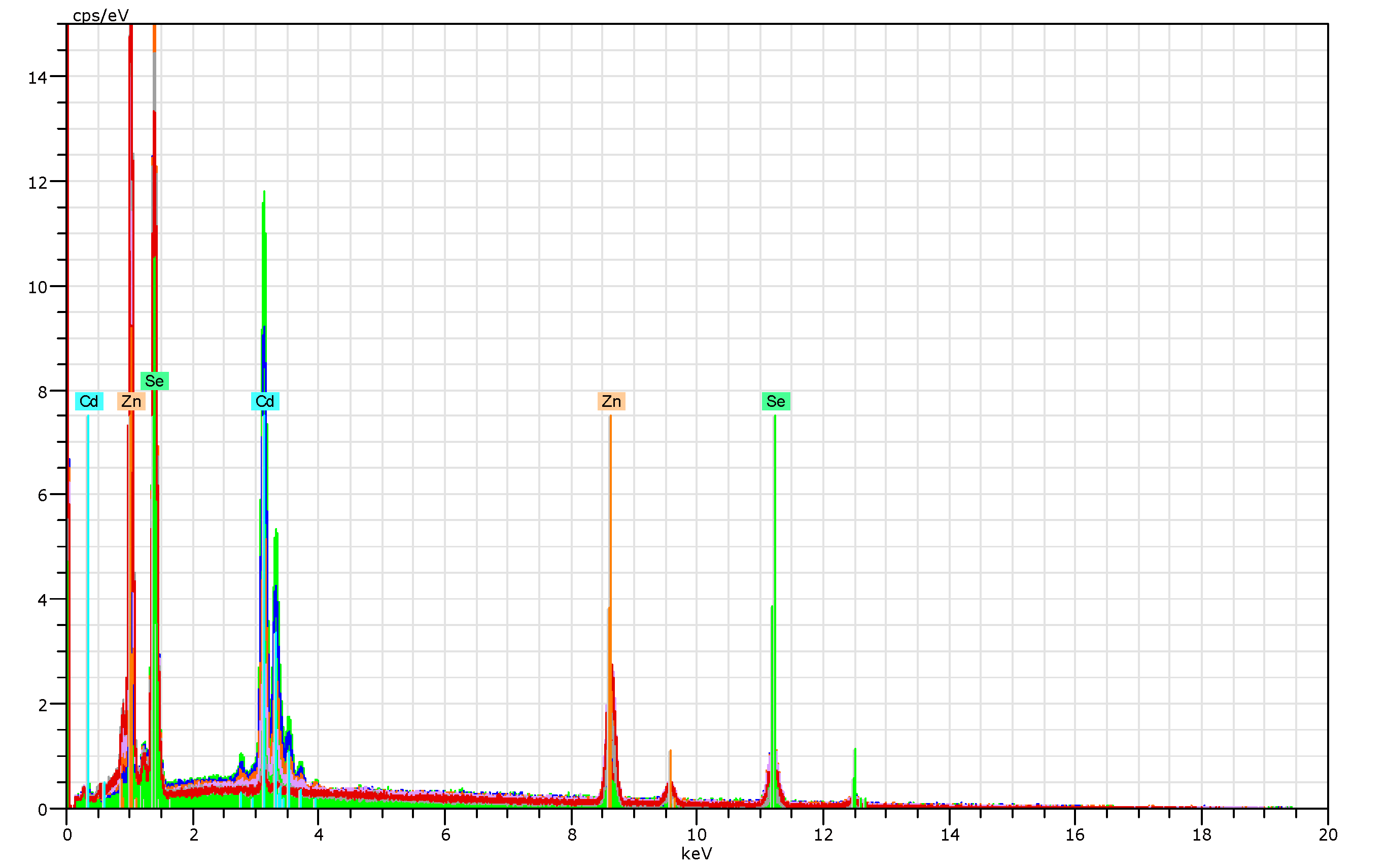
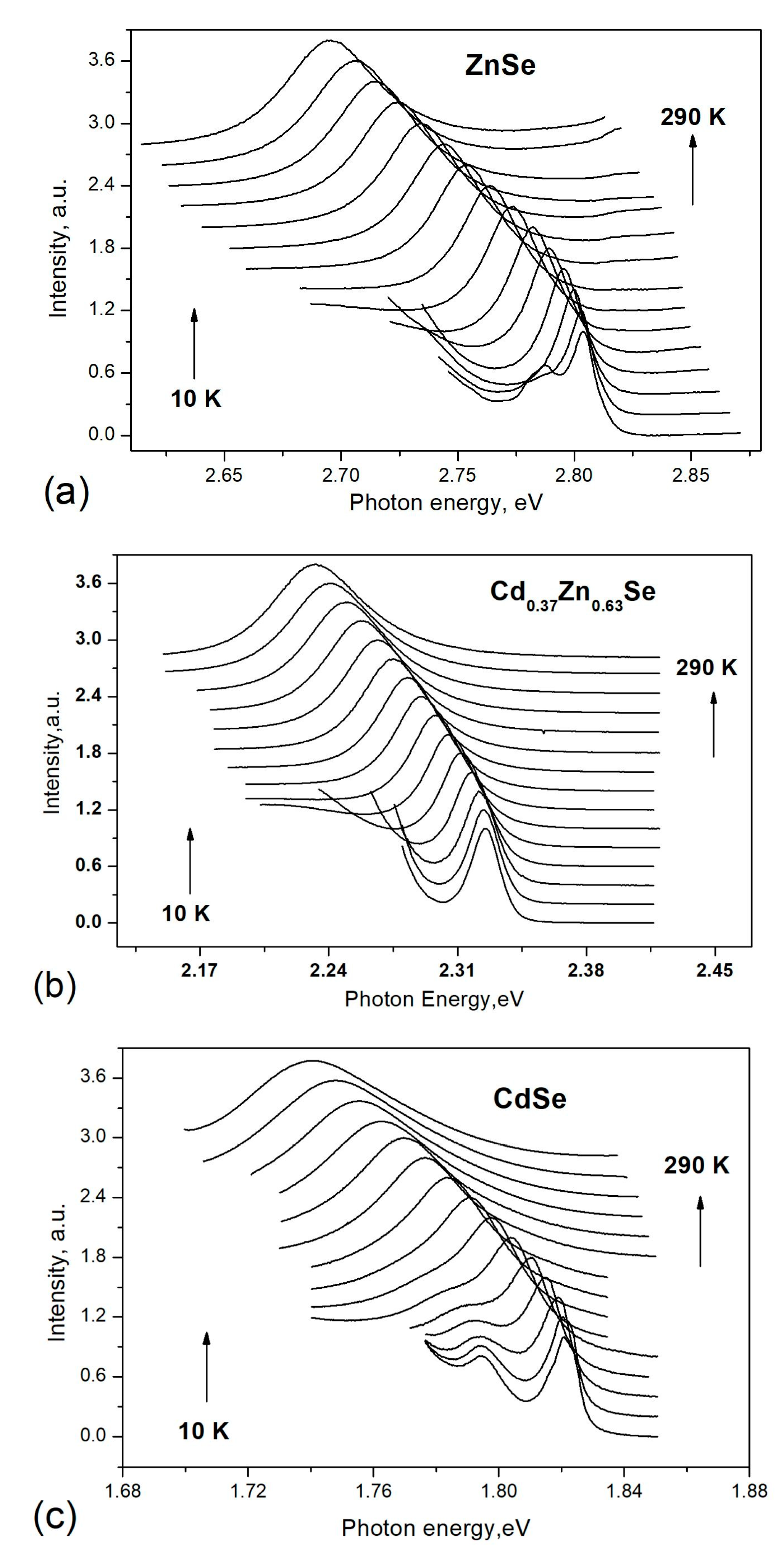

| Starting x, % | Measured x, % | Cd Atomic% | Zn Atomic% | Se Atomic% | Thickness mm |
|---|---|---|---|---|---|
| 0 | - | - | - | - | 1.15 ± 0.01 |
| 10 | 11 ± 0.5 | 42.37 ± 0.19 | 5.49 ± 0.27 | 52.14 ± 0.36 | 1.38 ± 0.09 |
| 30 | 33 ± 0.2 | 32.24 ± 0.72 | 16.35 ± 0.52 | 51.41 ± 1.26 | 1.33 ± 0.02 |
| 50 | 63 ± 0.5 | 17.72 ± 0.41 | 31.01 ± 0.95 | 52.27 ± 1.21 | 1.13 ± 0.03 |
| 70 | 83 ± 0.4 | 8.24 ± 0.28 | 41.32 ± 0.88 | 51.02 ± 1.01 | 1.35 ± 0.02 |
| 90 | 92 ± 0.4 | 4.38 ± 0.18 | 45.89 ± 1.05 | 50.54 ± 2.29 | 1.12 ± 0.02 |
| 95 | 97 ± 0.3 | 1.74 ± 0.22 | 48.64 ± 0.88 | 51.24 ± 1.09 | 1.24 ± 0.08 |
| 100 | - | 1.09 ± 0.01 |
| x | Eg at RT eV | γ meV·K−1 | γ K | R2 - |
|---|---|---|---|---|
| 0 | 1.740 ± 0.003 | 0.504 ± 0.025 | 220.13 ± 25.32 | 0.99850 |
| 11 | 1.817 ± 0.003 | 0.360 ± 0.003 | 60.49 ± 3.42 | 0.99988 |
| 33 | 1.991 ± 0.003 | 0.375 ± 0.013 | 75.03 ± 8.71 | 0.99941 |
| 63 | 2.232 ± 0.003 | 0.551 ± 0.013 | 190.23 ± 11.78 | 0.99930 |
| 83 | 2.430 ± 0.003 | 0.549 ± 0.001 | 95.34 ± 13.31 | 0.99975 |
| 92 | 2.611 ± 0.003 | 0.525 ± 0.018 | 160.17 ± 24.46 | 0.99844 |
| 97 | 2.658 ± 0.003 | 0.583 ± 0.021 | 199.69 ± 17.41 | 0.99956 |
| 100 | 2.695 ± 0.003 | 0.635 ± 0.037 | 185.17 ± 27.71 | 0.99870 |
| x | Thermal Diffusivity (m2·s−1) × 10−6 | Thermal Effusivity (W·s1/2·m−2·K−1) | Thermal Conductivity (W·cm−1·K−1) × 102 |
|---|---|---|---|
| 0 | 4.981 ± 0.098 | 3359.7 ± 15.9 | 7.498 ± 0.109 |
| 0.11 | 1.847 ± 0.050 | 2163.3 ± 2.0 | 2.939 ± 0.043 |
| 0.33 | 1.420 ± 0.035 | 2055.0 ± 13.2 | 2.449 ± 0.046 |
| 0.63 | 1.148 ± 0.031 | 1923.3 ± 83.3 | 2.060 ± 0.117 |
| 0.83 | 2.237 ± 0.045 | 2638.3 ± 28.4 | 3.946 ± 0.082 |
| 0.92 | 3.623 ± 0.045 | 3423.3 ± 23.1 | 6.516 ± 0.085 |
| 0.97 | 4.835 ± 0.007 | 3714.0 ± 68.5 | 8.067 ± 0.157 |
| 1 | 9.853 ± 0.084 | 5515.0 ± 136.1 | 17.312 ± 0.501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzałkowski, K.; Abouais, A.; Alaoui-Belghiti, A.; Singh, D.; Hajjaji, A. Alloy Disordering Effects on the Thermal Conductivity and Energy Gap Temperature Dependence of Cd1−xZnxSe Ternary Crystals Grown by the Bridgman Method. Materials 2023, 16, 3945. https://doi.org/10.3390/ma16113945
Strzałkowski K, Abouais A, Alaoui-Belghiti A, Singh D, Hajjaji A. Alloy Disordering Effects on the Thermal Conductivity and Energy Gap Temperature Dependence of Cd1−xZnxSe Ternary Crystals Grown by the Bridgman Method. Materials. 2023; 16(11):3945. https://doi.org/10.3390/ma16113945
Chicago/Turabian StyleStrzałkowski, Karol, Ali Abouais, Amine Alaoui-Belghiti, Diksha Singh, and Abdelowahed Hajjaji. 2023. "Alloy Disordering Effects on the Thermal Conductivity and Energy Gap Temperature Dependence of Cd1−xZnxSe Ternary Crystals Grown by the Bridgman Method" Materials 16, no. 11: 3945. https://doi.org/10.3390/ma16113945
APA StyleStrzałkowski, K., Abouais, A., Alaoui-Belghiti, A., Singh, D., & Hajjaji, A. (2023). Alloy Disordering Effects on the Thermal Conductivity and Energy Gap Temperature Dependence of Cd1−xZnxSe Ternary Crystals Grown by the Bridgman Method. Materials, 16(11), 3945. https://doi.org/10.3390/ma16113945









