A Selective Review of Ceramic, Glass and Glass–Ceramic Protective Coatings: General Properties and Specific Characteristics for Solar Cell Applications
Abstract
1. Introduction
2. Literature Review
2.1. General Properties and Classification
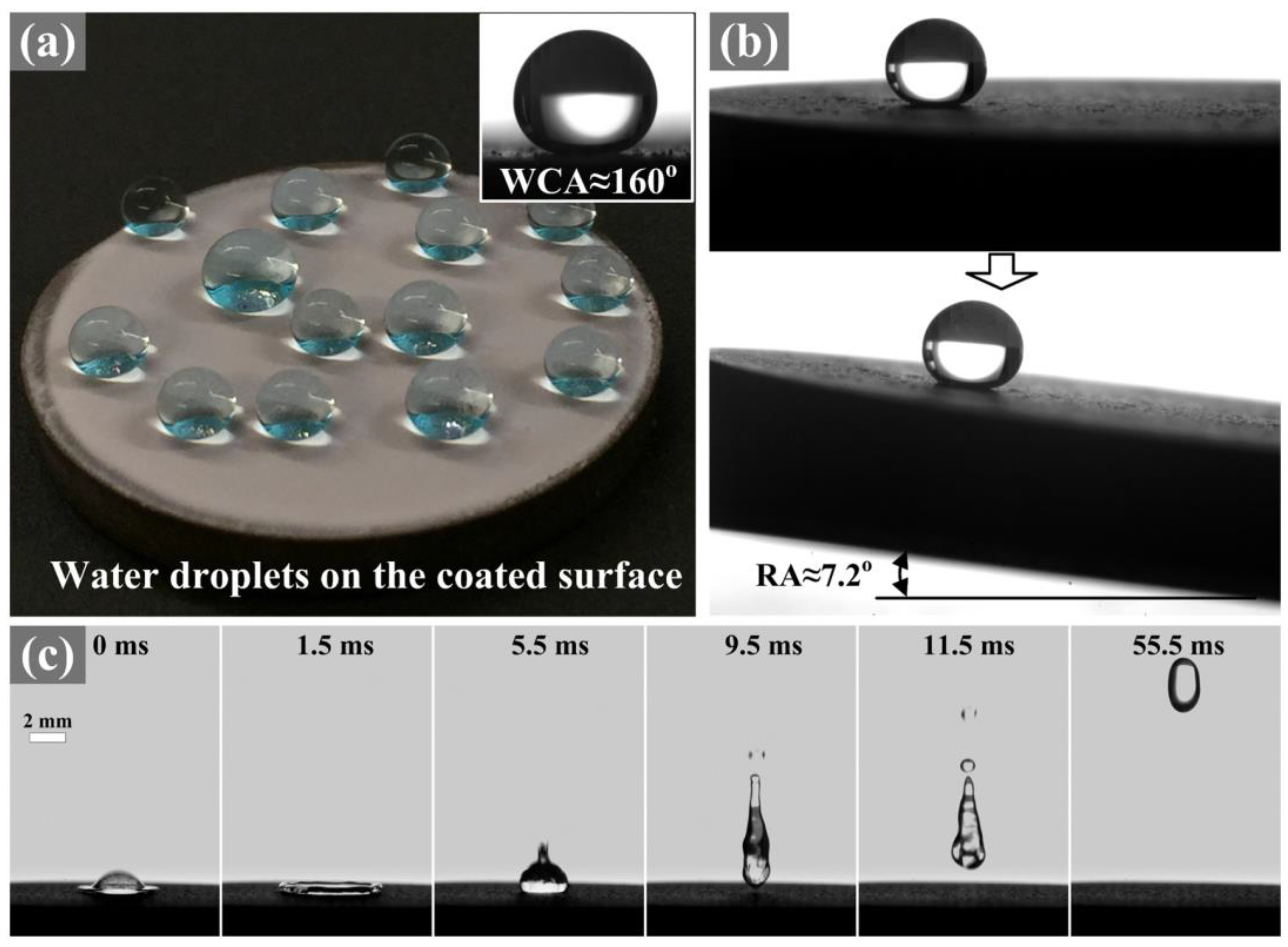
2.1.1. Mechanical Properties
2.1.2. Optical Properties
2.1.3. Classification
2.1.4. Types of Deposition
2.2. Ceramic, Glass and Glass–Ceramic Applications as Protective Coatings
2.2.1. Ceramics
2.2.2. Glasses
2.2.3. Glass–Ceramics
3. Discussion
4. Conclusions
- High transparency;
- Low water permeation;
- Low oxygen permeation;
- Hydrophobic or superhydrophobic;
- Self-cleaning/anti-fouling activity;
- Anti-reflective/low reflectivity of useful incident light;
- Light downshift;
- Corrosion resistance;
- Chemical resistance;
- Wear resistance;
- Scratch resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APS | Atmospheric Plasma Spraying |
| AYZ | Al2O3-Y2O3-ZrO2 |
| CBS | CaO-B2O3-SiO2 |
| CGDS | Cold Gas Dynamic Spraying |
| CMAS | Calcium Magnesium Aluminosilicate |
| CTE | Coefficient of Thermal Expansion |
| CVD | Chemical Vapour Deposition |
| DDS | Dimethyldichlorosilane |
| DSSC | Dye Sensitised Solar Cell |
| EIS | Electrochemical Impedance Spectroscopy |
| HMDS | Hexamethyldisilazane |
| LSAO | Lanthanide-doped Strontium Aluminium Oxide |
| MB | Methylene Blue |
| MCVD | Modified Chemical Vapour Deposition |
| PCE | Power Conversion Efficiency |
| PDC | Polymer Derived Ceramic |
| PDDA | Poly(diallyldimethylammonium) |
| PDMS | Polydimethylsiloxane |
| PECVD | Plasma Enhanced Chemical Vapour Deposition |
| PFOTES | 1H,1H,2H,2H-perfluorooctyltriethoxysilane |
| PHPS | Perhydropolysilazane |
| PS | Polystyrene |
| PSC | Perovskite Solar Cell |
| PVD | Physical Vapour Deposition |
| RH | Relative Humidity |
| RTIC | Room-Temperature Impact Consolidation |
| Sialon | Silicon/Aluminium/Oxygen/Nitrogen mix |
| SOPS | Solid Omniphobic Slippery |
| YSZ | Yttria-Stabilised Zirconia |
References
- Atkins, P.W.; Jones, L.L. Chemical Principles: The Quest for Insight; W. H. Freeman and Company: New York, NY, USA, 2000; pp. 692–693. [Google Scholar]
- Gülen, S.M.; Çöpoğlu, N.; Yılmaz, Y.B.; Karaahmet, O.; Cengiz, T.; Gökdemir, H.; Çiçek, B. TiO2-based glass-ceramic coatings: An innovative approach to architectural panel applications. Case Stud. Constr. Mater. 2022, 16, e00805. [Google Scholar] [CrossRef]
- Deubener, J.; Allix, M.; Davis, M.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Singh, S.P.; Sontakke, A.D. Transparent Glass Ceramics. Crystals 2021, 11, 156. [Google Scholar] [CrossRef]
- The American Ceramic Society. Structure and Properties of Ceramics. Available online: https://ceramics.org/about/what-are-engineered-ceramics-and-glass/structure-and-properties-of-ceramics (accessed on 8 January 2023).
- Karmaker, B. Functional Glasses and Glass-Ceramics Processing, Properties, and Applications; Butterworth-Heinemann: Oxford, UK, 2017; pp. 4–6. [Google Scholar] [CrossRef]
- Honma, T.; Maeda, K.; Nakane, S.; Shinozaki, K. Unique properties and potential of glass-ceramics. J. Ceram. Soc. Jpn. 2022, 130, 545–551. [Google Scholar] [CrossRef]
- Maalej, O.; Merigeon, J.; Boulard, B.; Girtan, M. Visible to near-infrared down-shifting in Tm3+ doped fluoride glasses for solar cells efficiency enhancement. Opt. Mater. 2016, 60, 235–239. [Google Scholar] [CrossRef]
- Merigeon, J.; Maalej, O.; Boulard, B.; Stanculescu, A.; Leontie, L.; Mardare, D.; Girtan, M. Studies on Pr3+–Yb3+ codoped ZBLA as rare earth down convertor glasses for solar cells encapsulation. Opt. Mater. 2015, 48, 243–246. [Google Scholar] [CrossRef]
- Pietrzyk, B.; Miszczak, S. Functional Ceramic Coatings; MDPI: Basel, Switzerland, 2021; Volume 11, p. 130. [Google Scholar] [CrossRef]
- Wu, Y.; Du, J.; Liu, G.; Ma, D.; Jia, F.; Klemeš, J.J.; Wang, J. A review of self-cleaning technology to reduce dust and ice accumulation in photovoltaic power generation using superhydrophobic coating. Renew. Energy 2022, 185, 1034–1061. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.K.; Moon, C.S.; Jeon, N.M.; Correa-Baena, J.P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Mann, S.A.; de Wild-Scholten, M.J.; Fthenakis, V.M.; van Sark, W.G.; Sinke, W.C. The energy payback time of advanced crystalline silicon PV modules in 2020: A prospective study. Prog. Photovolt. Res. Appl. 2014, 22, 1180–1194. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, Z.; Meng, X.; Yue, Y.; Ahmad, M.A.; Zhang, W.; Zhang, S.; Zhang, Y.; Liu, Z.; Chen, W. A Review on Encapsulation Technology from Organic Light Emitting Diodes to Organic and Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2100151. [Google Scholar] [CrossRef]
- Salamon, D. Advanced Ceramics. In Advanced Ceramics for Dentistry; Shen, J.Z., Kosmač, T., Eds.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 103–122. [Google Scholar] [CrossRef]
- Tran, T.N.L.; Szczurek, A.; Carlotto, A.; Varas, S.; Righini, G.C.; Ferrari, M.; Krzak, J.; Lukowiak, A.; Chiasera, A. Sol-gel-derived transparent glass-ceramics for photonics. Opt. Mater. 2022, 130, 112577. [Google Scholar] [CrossRef]
- Zanurin, A.; Johari, N.A.; Alias, J.; Ayu, H.M.; Redzuan, N.; Izman, S. Research progress of sol-gel ceramic coating: A review. Mater. Today Proc. 2022, 48, 1849–1954. [Google Scholar] [CrossRef]
- Winnicki, M. Advanced Functional Metal-Ceramic and Ceramic Coatings Deposited by Low-Pressure Cold Spraying: A Review. Coatings 2021, 11, 1044. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Ma, K.; Pan, X.D.; Li, C.X.; Yang, G.J.; Li, C.J. Microstructure and Transparent Super Hydrophobic Performance of Vacuum Cold-Sprayed Al2O3 and SiO2 Aerogel Composite Coating. J. Therm. Spray Technol. 2018, 27, 471–482. [Google Scholar] [CrossRef]
- Kaya, C.; Kaya, F.; Su, B.; Thomas, B.; Boccaccini, A.R. Structural and functional thick ceramic coatings by electrophoretic deposition. Surf. Coat. Technol. 2005, 191, 303–310. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Y.; Liang, L.; Wang, Y.; Song, J.; Long, H.; Liu, Y. Microstructure Observation and Nanoindentation Size Effect Characterization for Micron-/Nano-Grain TBCs. Coatings 2020, 10, 345. [Google Scholar] [CrossRef]
- Grimm, M.; Conze, S.; Berger, L.M.; Paczkowski, G.; Lindner, T.; Lampke, T. Microstructure and Sliding Wear Resistance of Plasma Sprayed Al2O3-Cr2O3-TiO2 Ternary Coatings from Blends of Single Oxides. Coatings 2020, 10, 42. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wang, H.; Fu, Y.; Chen, G.; Xi, Z. Long-life ablation resistance ZrB2-SiC-TiSi2 ceramic coating for SiC coated C/C composites under oxidizing environments up to 2200 K. J. Alloys Compd. 2020, 824, 153934. [Google Scholar] [CrossRef]
- Zavareh, M.A.; Sarhan, A.A.D.M.; Zadeh, R.K.; Singh, R.S.A. Analysis of corrosion protection behavior of Al2O3-TiO2 oxide ceramic coating on carbon steel pipes for petroleum industry. Ceram. Int. 2018, 44, 5967–5975. [Google Scholar] [CrossRef]
- Shao, T.; Ma, H.; Feng, M.; Wang, J.; Yan, M.; Wang, J.; Zhao, S.; Qu, S. A thin dielectric ceramic coating with good absorbing properties composed by tungsten carbide and alumina. J. Alloys Compd. 2020, 818, 152851. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, X.; Ai, Y.; Zhuang, Y.; Sheng, J.; Ni, J.; Yang, K. In Situ Deposition of Amorphous Al2O3-GAP Ceramic Coating with Excellent Microstructure Stability and Uniformity via Atmospheric Plasma Spraying. Coatings 2022, 12, 119. [Google Scholar] [CrossRef]
- Xu, P.; Coyle, T.W.; Pershin, L.; Mostaghimi, J. Fabrication of micro-/nano-structured superhydrophobic ceramic coating with reversible wettability via a novel solution precursor vacuum plasma spray process. Mater. Des. 2018, 160, 974–984. [Google Scholar] [CrossRef]
- Zhou, L.; Li, F.; Liu, J.X.; Hu, Q.; Bao, W.; Wu, Y.; Cao, X.; Xu, F.; Zhang, G.J. High-entropy thermal barrier coating of rare-earth zirconate: A case study on (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 prepared by atmospheric plasma spraying. J. Eur. Ceram. Soc. 2020, 40, 5731–5739. [Google Scholar] [CrossRef]
- Li, Q.; Xie, J.; Hu, J.; Wang, X.; Yu, C.; Jiang, S.; Liu, P.; Sun, C.; Li, E.; Deng, L. Influence of Ca/Al ratio on physical and dielectric properties of Al2O3/CaO-B2O3-SiO2 glass-ceramics composite coatings prepared by high enthalpy atmospheric plasma spraying. J. Eur. Ceram. Soc. 2022, 42, 1501–1509. [Google Scholar] [CrossRef]
- Li, Q.; Hu, J.; Xie, J.; Wang, X.; Yu, C.; Jiang, S.; Liu, Z.; Jiang, X.; Sun, C.; Li, E.; et al. Effect of spray process on dielectric properties of APS-deposited CaO–B2O3–SiO2 glass-ceramic coatings. J. Eur. Ceram. Soc. 2020, 40, 4527–4535. [Google Scholar] [CrossRef]
- Akedo, J. Room temperature impact consolidation and application to ceramic coatings: Aerosol deposition method. J. Ceram. Soc. Jpn. 2020, 128, 101–116. [Google Scholar] [CrossRef]
- Soraru, G.D. Silicon oxycarbide glasses from gels. J. Sol Gel Sci. Technol. 1994, 2, 843–848. [Google Scholar] [CrossRef]
- Ak, A.; Çiçek, B. Synthesis and characterization of hydrophobic glass-ceramic thin film derived from colloidal silica, zirconium(IV) propoxide and methyltrimethoxysilane via sol–gel method. J. Sol-Gel Sci. Technol. 2021, 98, 252–263. [Google Scholar] [CrossRef]
- Gorni, G.; Velázquez, J.J.; Mosa, J.; Mather, G.C.; Serrano, A.; Vila, M.; Castro, G.R.; Bravo, D.; Balda, R.; Fernández, J.; et al. Transparent Sol-Gel Oxyfluoride Glass-Ceramics with High Crystalline Fraction and Study of RE Incorporation. Nanomaterials 2019, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.E.; Durán, A.; Balda, R.; Fernández, J.; Mather, G.C.; Castro, Y. A new sol–gel route towards Nd3+-doped SiO2–LaF3 glass-ceramics for photonic applications. Mater. Adv. 2020, 1, 3589–3596. [Google Scholar] [CrossRef]
- Cruz, M.E.; Fernández, J.; Durán, A.; Balda, R.; Castro, Y. Optically active nano-glass-ceramic coatings of Nd3+ doped-80SiO2-20LaF3 prepared by the pre-crystallized nanoparticles sol-gel route. J. Non Cryst. Solids 2023, 601, 122050. [Google Scholar] [CrossRef]
- Van Zele, M.; Watté, J.; Hasselmeyer, J.; Rijckaert, H.; Vercammen, Y.; Verstuyft, S.; Deduytsche, D.; Debecker, D.P.; Poleunis, C.; Van Driessche, I.; et al. Thickness Characterization Toolbox for Transparent Protective Coatings on Polymer Substrates. Materials 2018, 11, 1101. [Google Scholar] [CrossRef]
- Adak, D.; Ghosh, S.; Chakraborty, P.; Srivatsa, K.M.; Mondal, A.; Saha, H.; Mukherjee, R.; Bhattacharyya, R. Non lithographic block copolymer directed self-assembled and plasma treated self-cleaning transparent coating for photovoltaic modules and other solar energy devices. Sol. Energy Mater. Sol. Cells 2018, 188, 127–139. [Google Scholar] [CrossRef]
- Bukhari, S.; Imran, M.; Bashir, M.; Riaz, S.; Naseem, S. Room temperature stabilized TiO2 doped ZrO2 thin films for teeth coatings—A sol-gel approach. J. Alloys Compd. 2018, 767, 1238–1252. [Google Scholar] [CrossRef]
- Parchovianský, M.; Parchovianská, I.; Švancárek, P.; Motz, G.; Galusek, D. PDC Glass/Ceramic Coatings Applied to Differently Pretreated AISI441 Stainless Steel Substrates. Materials 2020, 13, 629. [Google Scholar] [CrossRef]
- Çöpoğlu, N.; Çiçek, B. Abrasion resistant glass-ceramic coatings reinforced with WC-nanoparticles. Surf. Coat. Technol. 2021, 419, 127275. [Google Scholar] [CrossRef]
- Ito, A. Chemical vapor deposition of highly oriented ceramic coatings in a high-intensity laser irradiation. J. Ceram. Soc. Jpn. 2021, 129, 646–653. [Google Scholar] [CrossRef]
- Rashidi, M.; Tamizifar, M.; Boutorabi, S.M.A. Characteristics of TiAlCN ceramic coatings prepared via pulsed-DC PACVD, part I: Influence of precursors’ ratio. Ceram. Int. 2020, 46, 1269–1280. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Fu, T.; Wang, J.; Shen, F.; Cui, K.; Wang, H. Microstructure and oxidation resistance of Si-MoSi2 ceramic coating on TZM (Mo-0.5Ti-0.1Zr-0.02C) alloy at 1500 °C. Surf. Coat. Technol. 2002, 431, 128037. [Google Scholar] [CrossRef]
- Li, Z.; Wei, M.; Xiao, K.; Bai, Z.; Xue, W.; Dong, C.; Wei, D.; Li, X. Microhardness and wear resistance of Al2O3-TiB2-TiC ceramic coatings on carbon steel fabricated by laser cladding. Ceram. Int. 2019, 45, 115–121. [Google Scholar] [CrossRef]
- Girtan, M.; Hrostea, L.; Boclinca, M.; Negulescu, B. Study of oxide/metal/oxide thin films for transparent electronics and solar cells applications by spectroscopic ellipsometry. AIMS Mater. Sci. 2017, 4, 594–613. [Google Scholar] [CrossRef]
- Hrostea, L.; Lisnic, P.; Mallet, R.; Leontie, L.; Girtan, M. Studies on the Physical Properties of TiO2:Nb/Ag/TiO2:Nb and NiO/Ag/NiO Three-Layer Structures on Glass and Plastic Substrates as Transparent Conductive Electrodes for Solar Cells. Nanomaterials 2021, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Hrostea, L.; Boclinca, M.; Socol, M.; Leontie, L.; Stanculescu, A.; Girtan, M. Oxide/metal/oxide electrodes for solar cell applications. Sol. Energy 2017, 146, 464–469. [Google Scholar] [CrossRef]
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics: “All-Rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Ionescu, E.; Papendorf, B.; Kleebe, H.J.; Poli, F.; Müller, K.; Riedel, R. Polymer-derived silicon oxycarbide/hafnia ceramic nanocomposites. Part I: Phase and microstructure evolution during the ceramization process. J. Am. Ceram. Soc. 2010, 93, 1774–1782. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based polymer-derived ceramics: Synthesis properties and applications-a review. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Gorni, G.; Balda, R.; Fernández, J.; Velázquez, J.J.; Pascual, L.; Mosa, J.; Durán, A.; Castro, Y. 80SiO2-20LaF3 oxyfluoride glass ceramic coatings doped with Nd3+ for optical applications. Int. J. Appl. Glass Sci. 2018, 9, 208–217. [Google Scholar] [CrossRef]
- Ramesh, M.; Marimuthu, K.; Karuppuswamy, P.; Rajeshkumar, L. Microstructure and properties of YSZ-Al2O3 functional ceramic thermal barrier coatings for military applications. Bol. Soc. Esp. Ceram. V 2022, 61, 641–652. [Google Scholar] [CrossRef]
- Newkirk, J.M.; Nayshevsky, I.; Sinha, A.; Law, A.M.; Xu, Q.; To, B.; Ndione, P.F.; Schelhas, L.T.; Walls, J.M.; Lyons, A.M.; et al. Artificial linear brush abrasion of coatings for photovoltaic module first-surfaces. Sol. Energy Mater. Sol. Cells 2021, 219, 110757. [Google Scholar] [CrossRef]
- Mohamedkhair, A.K.; Hakeem, A.S.; Drmosh, Q.A.; Mohammed, A.S.; Baig, M.M.A.; Ul-Hamid, A.; Gondal, M.A.; Yamani, Z.H. Fabrication and Characterization of Transparent and Scratch-Proof Yttrium/Sialon Thin Films. Nanomaterials 2020, 10, 2283. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, D.F.; Villarroel, R.; Espinoza-González, R.; Carvajal, N.; Rosenkranz, A.; Montaño-Figueroa, A.G.; Arellano-Jiménez, M.J.; Quevedo-Lopez, M.; Valenzuela, P.; Gacitúa, W. Mechanical and microstructural properties of broadband anti-reflective TiO2/SiO2 coatings for photovoltaic applications fabricated by magnetron sputtering. Sol. Energy Mater. Sol. Cells 2021, 220, 110841. [Google Scholar] [CrossRef]
- Zambrano-Mera, D.F.; Espinoza-González, R.; Villarroel, R.; Rosenkranz, A.; Carvajal, N.; Pintor-Monroy, M.I.; Montaño-Figueroa, A.G.; Arellano-Jiménez, M.J.; Quevedo-López, M.; Valenzuela, P.; et al. Optical and mechanical properties of Zr-oxide doped TiO2/SiO2 anti-reflective coatings for PV glass covers. Sol. Energy Mater. Sol. Cells 2022, 243, 111784. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Xie, Q.; Chen, Z.; Ma, C.; Zhang, G. Transparent Polymer-Ceramic Hybrid Antifouling Coating with Superior Mechanical Properties. Adv. Funct. Mater. 2021, 31, 2011145. [Google Scholar] [CrossRef]
- Walshe, J.; Girtan, M.; McCormack, S.; Doran, J.; Amarandei, G. Combined Experimental and Modeling Analysis for the Development of Optical Materials Suitable to Enhance the Implementation of Plasmonic-Enhanced Luminescent Down-Shifting Solutions on Existing Silicon-Based Photovoltaic Devices. ACS Appl. Electron. Mater. 2021, 3, 2512–2525. [Google Scholar] [CrossRef]
- Walshe, J.; Girtan, M.; McCormack, S.; Doran, J.; Amarandei, G. Exploring the development of nanocomposite encapsulation solutions for enhancing the efficiency of PV systems using optical modelling. Optima Mater. 2021, 111, 110654. [Google Scholar] [CrossRef]
- Kim, T.S.; Kim, H.J.; Geum, D.M.; Han, J.H.; Kim, I.S.; Hong, N.; Ryu, G.H.; Kang, J.H.; Choi, W.J.; Yu, K.J. Ultra-Lightweight, Flexible InGaP/GaAs Tandem Solar Cells with a Dual-Function Encapsulation Layer. ACS Appl. Mater. Interfaces 2021, 13, 13248–13253. [Google Scholar] [CrossRef]
- Channa, I.A.; Shah, A.A.; Rizwan, M.; Makhdoom, M.A.; Chandio, A.D.; Shar, M.A.; Mahmood, A. Process Parameter Optimization of a Polymer Derived Ceramic Coatings for Producing Ultra-High Gas Barrier. Materials 2021, 14, 7000. [Google Scholar] [CrossRef]
- Ji, R.; Ma, M.; He, Y.; Liu, C.; Fang, T.; Zhang, Z.; Wang, Y.; He, Y.; Wu, J. Improved corrosion resistance of Al2O3 ceramic coatings on AZ31 magnesium alloy fabricated through cathode plasma electrolytic deposition combined with surface pore-sealing treatment. Ceram. Int. 2018, 44, 15192–15199. [Google Scholar] [CrossRef]
- Liu, Y.; Bian, D.; Zhao, Y.; Wang, Y. Anti-corrosion performance of chemically bonded phosphate ceramic coatings reinforced by nano-TiO2. J. Mech. Behav. Biomed. Mater. 2018, 86, 208–214. [Google Scholar] [CrossRef]
- Appleget, C.D.; Hodge, A.M. Synthesis and Characterization of Optically Transparent Ceramic Crystalline/Amorphous and Amorphous/Amorphous Multilayers. Scr. Mater. 2020, 187, 157–162. [Google Scholar] [CrossRef]
- du Merac, M.R.; Bram, M.; Malzbender, J.; Ziegner, M.; Rasinski, M.; Guillon, O. Increasing Fracture Toughness and Transmittance of Transparent Ceramics using Functional Low-Thermal Expansion Coatings. Sci. Rep. 2018, 8, 15644. [Google Scholar] [CrossRef]
- Evstropiev, S.K.; Soshnikov, I.P.; Kolobkova, E.V.; Evstropyev, K.S.; Nikonorov, N.V.; Khrebtov, A.I.; Dukelskii, K.V.; Kotlyar, K.P.; Oreshkina, K.V.; Nashekin, A.V. Polymer-salt synthesis and characterization of MgO-ZnO ceramic coatings with the high transparency in UV spectral range. Opt. Mater. 2018, 82, 81–87. [Google Scholar] [CrossRef]
- Watté, J.; Van Zele, M.; De Buysser, K.; Van Driessche, I. Recent Advances in Low-Temperature Deposition Methods of Transparent, Photocatalytic TiO2 Coatings on Polymers. Coatings 2018, 8, 131. [Google Scholar] [CrossRef]
- Fares, C.; Ehassani, R.; Partain, J.; Hsu, S.M.; Craciun, V.; Ren, F.; Esquivel-Upshaw, J.F. Annealing and N2 Plasma Treatment to Minimize Corrosion of SiC-Coated Glass-Ceramics. Materials 2020, 13, 2375. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Coyle, T.W.; Pershin, L.; Mostaghimi, J. Superhydrophobic ceramic coating: Fabrication by solution precursor plasma spray and investigation of wetting behavior. J. Colloid Interface Sci. 2018, 523, 35–44. [Google Scholar] [CrossRef]
- Niu, W.; Chen, G.Y.; Xu, H.; Liu, X.; Sun, J. Highly Transparent and Self-Healable Solar Thermal Anti-/Deicing Surfaces: When Ultrathin MXene Multilayers Marry a Solid Slippery Self-Cleaning Coating. Adv Mater. 2022, 34, 2108232. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Z. A mechanically robust transparent coating for anti-icing and self-cleaning applications. J. Mater. Chem. A 2018, 6, 16043–16052. [Google Scholar] [CrossRef]
- Zhong, W.; Liao, H.; Wu, M.; Xiong, B.; Zhan, W. Superhydrophobic surface based on the self-growing structure of BaAl2Si2O8 glass-ceramics. Ceram. Int. 2022, 48, 1990–1998. [Google Scholar] [CrossRef]
- Zhu, T.; Cheng, Y.; Huang, J.; Xiong, J.; Ge, M.; Mao, J.; Liu, Z.; Dong, X.; Chen, Z.; Lai, Y. A transparent superhydrophobic coating with mechanochemical robustness for anti-icing, photocatalysis and self-cleaning. Chem. Eng. J. 2020, 399, 125746. [Google Scholar] [CrossRef]
- Polizos, G.; Jang, G.G.; Smith, D.B.; List, F.A.; Lassiter, M.G.; Park, J.; Datskos, P.G. Transparent superhydrophobic surfaces using a spray coating process. Sol. Energy Mater. Sol. Cells 2018, 176, 405–410. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Li, C. Highly transparent and scratch resistant polysiloxane coatings containing silica nanoparticles. J. Colloid Interface Sci. 2020, 559, 273–281. [Google Scholar] [CrossRef]
- Johansson, W.; Peralta, A.; Jonson, B.; Anand, S.; Österlund, L.; Karlsson, S. Transparent TiO2 and ZnO Thin Films on Glass for UV Protection of PV Modules. Front. Mater. 2019, 6, 259. [Google Scholar] [CrossRef]
- Moysan, C.; Riedel, R.; Harshe, R.R.; Rouxel, T.; Augereau, F. Mechanical characterization of a polysiloxane-derived SiOC glass. J. Eur. Ceram. Soc. 2007, 27, 397–403. [Google Scholar] [CrossRef]
- Cordelair, J.; Greil, P. Electrical conductivity measurements as a microprobe for structure transitions in polysiloxane derived Si–O–C ceramics. J. Eur. Ceram. Soc. 2000, 20, 1947–1957. [Google Scholar] [CrossRef]
- Kim, K.J.; Eom, J.H.; Koh, T.Y.; Kim, Y.W.; Seo, W.S. Effects of carbon addition on the electrical properties of bulk silicon-oxycarbide ceramics. J. Eur. Ceram. Soc. 2016, 36, 2705–2711. [Google Scholar] [CrossRef]
- Sorarù, G.D.; Modena, S.; Guadagnino, E.; Colombo, P.; Egan, J.; Pantano, C. Chemical durability of silicon oxycarbide glasses. J. Am. Ceram. Soc. 2002, 85, 1529–1536. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, C.; Lee, S.; Kim, C.; Choi, W.; Park, Y.; Kim, J.H.; Joung, Y.-H. Characteristics of Functional Film Synthesized on the Cover Glass of Photovoltaic Modules. Energies 2021, 14, 6671. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Chen, S.; Mahmood, A.; Wang, J. Development of Durable, Fluorine-free, and Transparent Superhydrophobic Surfaces for Oil/Water Separation. ACS Omega 2019, 4, 6947–6954. [Google Scholar] [CrossRef]
- Wang, P.; Yan, X.; Zeng, J.; Luo, C.; Wang, C. Anti-Reflective superhydrophobic coatings with excellent durable and Self-cleaning properties for solar cells. Appl. Surf. Sci. 2022, 602, 154408. [Google Scholar] [CrossRef]
- Chi, F.; Liu, D.; Wu, H.; Lei, J. Mechanically robust and self-cleaning antireflection coatings from nanoscale binding of hydrophobic silica nanoparticles. Sol. Energy Mater. Sol. Cells 2019, 200, 109939. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Binyaseen, A.M.; Aljuhani, E.; Aljohani, M.; Alzahrani, H.K.; Shah, R.; El-Metwaly, N.M. Production of smart nanocomposite for glass coating toward photochromic and long-persistent photoluminescent smart windows. Ceram. Int. 2022, 48, 903–912. [Google Scholar] [CrossRef]
- Yan, G.; Wang, M.; Sun, T.; Li, X.; Wang, G.; Yin, W. Anti-Corrosion Property of Glass Flake Reinforced Chemically Bonded Phosphate Ceramic Coatings. Materials 2019, 12, 2082. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.; Cao, L.; Hao, W.; Wu, W. A novel design of oxidation protective β-Y2Si2O7 nanowire toughened Y2SiO5/Y2O3-Al2O-SiO2 glass ceramic coating for SiC coated carbon/carbon composites. Corros. Sci. 2018, 135, 233–242. [Google Scholar] [CrossRef]
- Chen, K.; Lin, J.; Li, W.; Zhu, W.; Li, K.; Yi, A.; Hu, S.; Chen, M.; Wang, F. Improved Oxidation and Hot Corrosion Resistance of 1Cr11Ni2W2MoV Stainless Steel at 650 °C by a Novel Glass-Ceramic Coating. Crystals 2021, 11, 1213. [Google Scholar] [CrossRef]
- Deng, J.; Lu, B.; Hu, K.; Li, H.; Wang, J.; Fan, S.; Zhang, L.; Cheng, L. Interaction between Y-Al-Si-O glass-ceramics for environmental barrier coating materials and Ca-Mg-Al-Si-O melts. Ceram. Int. 2020, 46, 18262–18273. [Google Scholar] [CrossRef]
- Feng, M.; Chen, M.; Yu, Z.; Chen, Z.; Chen, J.; Zhu, S.; Wang, F. Crystallization and wear behavior of SiO2-Al2O3-ZrO2-Ba(Sr, Ca)O glass-ceramics added with Cr2O3 by different methods. Ceram. Int. 2019, 45, 22617–22624. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, D.; Yang, Z.; Li, H.; Niu, B.; Duan, W.; Jia, D.; Zhou, Y. Fabrication and properties of dense α-cordierite glass-ceramic coating on porous BN/Si2N2O ceramic. J. Eur. Ceram. Soc. 2018, 38, 4749–4755. [Google Scholar] [CrossRef]
- Singh, V.P.; Kushwaha, H.S.; Vaish, R. Photocatalytic study on SrBi2B2O7 (SrO-Bi2O3-B2O3) transparent glass ceramics. Mater. Res. Bull. 2018, 99, 453–459. [Google Scholar] [CrossRef]
- Thakur, V.; Kushwaha, H.S.; Singh, A.; Vaish, R.; Punia, R.; Singh, L. A study on the structural and photocatalytic degradation of ciprofloxacine using (70B2O3–29Bi2O3–1Dy2O3)–x(BaO–TiO2) glass ceramics. J. Non Cryst. Solids 2015, 428, 197–203. [Google Scholar] [CrossRef]
- Ida, T.; Shinozaki, K.; Honma, T.; Komatsu, T. Synthesis and photocatalytic properties of α-ZnWO4 nanocrystals in tungsten zinc borate glasses. J. Asian Ceram. Soc. 2014, 2, 253–257. [Google Scholar] [CrossRef]
- Ismail, S.F.; Sahar, M.R.; Ghoshal, S.K. Effects of titanium nanoparticles on self-cleaning and structural features of zinc-magnesium-phosphate glass. Mater. Res. Bull. 2016, 74, 502–506. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nasu, H.; Kamiya, K. Ti3+-free multicomponent titanophosphate glasses as ecologically sustainable optical glasses. J. Am. Ceram. Soc. 2006, 89, 2521–2527. [Google Scholar] [CrossRef]
- Masai, H.; Fujiwara, T.; Mori, H. Effect of SnO addition on optical absorption of bismuth borate glass and photocatalytic property of the crystallized glass. Appl. Phys. Lett. 2008, 92, 141902. [Google Scholar] [CrossRef]
- D’Isanto, F.; Smeacetto, F.; Martin, H.P.; Sedlák, R.; Lisnichuk, M.; Chrysanthou, A.; Salvo, M. Development and characterisation of a Y2Ti2O7-based glass-ceramic as a potential oxidation protective coating for titanium suboxide (TiOx). Ceram. Int. 2021, 47, 19774–19783. [Google Scholar] [CrossRef]
- Gorokhovsky, A.V.; Yurkov, G.Y.; Burmistrov, I.N.; Villalpando-Reyna, A.F.; Kuznetsov, D.V.; Gusev, A.A.; Khaidarov, B.B.; Konyukhov, Y.V.; Zakharova, O.V.; Kiselev, N.V. Glass-Ceramic Protective Coatings Based on Metallurgical Slag. Coatings 2023, 13, 269. [Google Scholar] [CrossRef]
- Singh, D.; Saini, A.; Dhayal, V.; Agarwal, D.C. Oxime-Modified Aluminum(III)Isopropoxide: A Promising Sol-Gel Precursor for Corrosion Resistive Nano-Alumina Coating on an Aluminum Alloy. Prot. Met. Phys. Chem. 2019, 55, 682–688. [Google Scholar] [CrossRef]
- Sharifiyan, M.S.; Shanaghi, A.; Moradi, H.; Chu, P.K. Effects of high concentration of Benzotriazole on corrosion behavior of nanostructured titania-alumina composite coating deposited on Al 2024 by sol-gel method. Surf. Coat. Technol. 2017, 321, 36–44. [Google Scholar] [CrossRef]
- Winnicki, M.; Małachowska, A.; Baszczuk, A.; Rutkowska-Gorczyca, M.; Kukla, D.; Lachowicz, M.; Ambroziak, A. Corrosion protection and electrical conductivity of copper coatings deposited by low-pressure cold spraying. Surf. Coat. Technol. 2017, 318, 90–98. [Google Scholar] [CrossRef]
- Winnicki, M.; Baszczuk, A.; Jasiorski, M.; Małachowska, A. Corrosion resistance of copper coatings deposited by cold spraying. J. Therm. Spray Technol. 2017, 26, 1935–1946. [Google Scholar] [CrossRef]
- Szala, M.; Łatka, L.; Walczak, M.; Winnicki, M. Comparative study on the cavitation erosion and sliding wear of cold-sprayed Al/Al2O3 and Cu/Al2O3 coatings, and stainless steel, aluminium alloy, copper and brass. Metals 2020, 10, 856. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Coleman, A.; Murray, K.; Kearns, M.; Vuoristo, P. High pressure cold sprayed (HPCS) and low pressure cold sprayed (LPCS) coatings prepared from OFHC Cu feedstock: Overview from powder characteristics to coating properties. J. Therm. Spray Technol. 2012, 21, 1065–1075. [Google Scholar] [CrossRef]
- El-Aziz, K.A.; Saber, D.; Sallam, H.E.-D.M. Wear and corrosion behavior of Al–Si matrix composite reinforced with alumina. J. Bio. Tribo Corros. 2015, 1, 5. [Google Scholar] [CrossRef]
- Winnicki, M.; Kozerski, S.; Małachowska, A.; Pawłowski, L.; Rutkowska-Gorczyca, M. Optimization of ceramic content in nickel–alumina composite coatings obtained by low pressure cold spraying. Surf. Coat. Technol. 2021, 405, 126732. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Li, X.; Huang, G.; Li, C.; Li, Y. Microstructure and mechanical properties of Zn-Ni-Al2O3 composite coatings. Materials 2018, 11, 853. [Google Scholar] [CrossRef]
- Kılıçay, K. Development of Protective MMC Coating on TZM Alloy for high temperature oxidation resistance by LPCS. Surf. Coat. Technol. 2020, 393, 125777. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Z.; Shen, L.; Pu, J.; Liu, W.; Sun, X.; Li, Z. Performance evaluation of mercapto functional hybrid silica sol–gel coating and its synergistic effect with f-GNs for corrosion protection of copper surface. RSC Adv. 2018, 8, 7438–7449. [Google Scholar] [CrossRef]
- Dhayal, V.; Singh, D.; Saini, A.; Sonewane, S.; Agarwal, D.C. Vibration and Corrosion Analysis of Modified Alumina Coating over Aluminum Alloy. J. Fail. Anal. Prev. 2021, 21, 130–137. [Google Scholar] [CrossRef]
- Singh, D.; Dhayal, V.; Agarwal, D.C. Corrosion Performance of Nano-Alumina Coatings over Anodized Aluminum Alloy by Dip Coating Method. Surf. Eng. Appl. Electrochem. 2019, 55, 436–442. [Google Scholar] [CrossRef]
- Nofz, M.; Dörfel, I.; Sojref, R.; Wollschläger, N.; Mosquera-Feijoo, M.; Schulz, W.; Kranzmann, A. Thin Sol-Gel Alumina Coating as Protection of a 9% Cr Steel Against Flue Gas Corrosion at 650 C. Oxid. Met. 2018, 89, 453–470. [Google Scholar] [CrossRef]
- Koivuluoto, H.; Vuoristo, P. Effect of powder type and composition on structure and mechanical properties of Cu + Al2O3 coatings prepared by using low-pressure cold spray process. J. Therm. Spray Technol. 2010, 19, 1081–1092. [Google Scholar] [CrossRef]
- Winnicki, M.; Małachowska, A.; Piwowarczyk, T.; Rutkowska-Gorczyca, M.; Ambroziak, A. The bond strength of Al+Al2O3 cermet coatings deposited by low-pressure cold spraying. Arch. Civ. Mech. Eng. 2016, 16, 743–752. [Google Scholar] [CrossRef]
- Melendez, N.M.; McDonald, A.G. Development of WC-based metal matrix composite coatings using low-pressure cold gas dynamic spraying. Surf. Coat. Technol. 2013, 214, 101–109. [Google Scholar] [CrossRef]
- Melendez, N.M.; Narulkar, V.V.; Fisher, G.A.; McDonald, A.G. Effect of reinforcing particles on the wear rate of low-pressure cold-sprayed WC-based MMC coatings. Wear 2013, 306, 185–195. [Google Scholar] [CrossRef]
- Yuan, S.; Lin, N.; Zou, J.; Lin, X.; Liu, Z.; Yu, Y.; Wang, Z.; Zeng, Q.; Chen, W.; Tian, L.; et al. In-situ fabrication of gradient titanium oxide ceramic coating on laser surface textured Ti6Al4V alloy with improved mechanical property and wear performance. Vacuum 2020, 176, 109327. [Google Scholar] [CrossRef]
- Rother, B.; Mucha, A. Transparent glass–ceramic coatings: Property distribution on 3D parts. Surf. Coat. Technol. 2000, 124, 128–138. [Google Scholar] [CrossRef]
- Dey, T.; Naughton, D. Nano-porous sol-gel derived hydrophobic glass coating for increased light transmittance through greenhouse. Mater. Res. Bull. 2022, 116, 126–130. [Google Scholar] [CrossRef]
- Wojdat, T.; Winnicki, M.; Łamasz, S.; Zuk, A. Application of interlayers in the soldering process of graphite composite to aluminium alloy 6060. Arch. Civ. Mech. Eng. 2019, 19, 91–99. [Google Scholar] [CrossRef]
- Winnicki, M.; Małachowska, A.; Korzeniowski, M.; Jasiorski, M.; Baszczuk, A. Aluminium to steel resistance spot welding with cold sprayed interlayer. Surf. Eng. 2018, 34, 235–242. [Google Scholar] [CrossRef]
- Baszczuk, A.; Jasiorski, M.; Winnicki, M. Low-Temperature Transformation of Amorphous Sol–Gel TiO2 Powder to Anatase during Cold Spray Deposition. J. Therm. Spray Technol. 2018, 27, 1551–1562. [Google Scholar] [CrossRef]
- Seremak, W.; Baszczuk, A.; Jasiorski, M.; Gibas, A.; Winnicki, M. Photocatalytic Activity Enhancement of Low-pressure Cold-Sprayed TiO2 Coatings Induced by Long-term Water Vapor Exposure. J. Therm. Spray Techol. 2021, 30, 1827–1836. [Google Scholar] [CrossRef]
- Sirirerkratana, K.; Kemacheevakul, P.; Chuangchote, S. Color removal from wastewater by photocatalytic process using titanium dioxide-coated glass, ceramic tile, and stainless steel sheets. J. Clean. Prod. 2019, 215, 123–130. [Google Scholar] [CrossRef]
- Zhou, S.; Bernard, C.A.; Ravi, K.; Saito, H.; Ichikawa, Y.; Ogawa, K.; Yin, S. Development and Characterization of Photocatalytic GaN Coatings by Cold Spray Process. J. Therm. Spray Techol. 2021, 30, 1294–1309. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Cinca, N.; Dosta, S.; Cano, I.G.; Guilemany, J.M. Feasibility of using low pressure cold gas spray for the spraying of thick ceramic hydroxyapatite coatings. Int. J. Appl. Ceram. Technol. 2019, 16, 221–229. [Google Scholar] [CrossRef]
- Shane, M.; Mecartney, M.L. Sol-gel synthesis of zirconia barrier coatings. J. Mater. Sci. 1990, 25, 1537–1544. [Google Scholar] [CrossRef]
- Won, Y.; Schwartzenberg, K.; Gray, K.A. TiO2-based transparent coatings create self-cleaning surfaces. Chemosphere 2018, 208, 899–906. [Google Scholar] [CrossRef]
- Ippili, S.; Jella, V.; Lee, J.M.; Jung, J.S.; Lee, D.H.; Yang, T.Y.; Yoon, S.G. ZnO–PTFE-based antimicrobial, anti-reflective display coatings and high-sensitivity touch sensors. J. Mater. Chem. A 2022, 10, 22067–22079. [Google Scholar] [CrossRef]
- Chen, W.; Yu, Y.; Cheng, J.; Wang, S.; Zhu, S.; Liu, W.; Yang, J. Microstructure, mechanical properties and dry sliding wear behavior of Cu-Al2O3-graphite solid-lubricating coatings deposited by low-pressure cold spraying. J. Therm. Spray Technol. 2018, 27, 1652–1663. [Google Scholar] [CrossRef]
- Chen, W.; Yu, Y.; Sui, X.; Zhu, S.; Huang, G.; Yang, J. Tribological behavior of the low-pressure cold sprayed (Cu-5Sn)/Al2O3-Ag solid-lubricating coating in artificial seawater. Surf. Coat. Technol. 2020, 403, 126359. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Cui, J.; Wu, S.; Han, J.; Zou, Y.; Huang, C. Hydrothermal synthesized UV-resistance and transparent coating composited superoloephilic electrospun membrane for high efficiency oily wastewater treatment. J. Hazard. Mater. 2020, 383, 121152. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Yu, Y.H.; Lee, Y.C.; Hong, Y.P.; Ko, K.H. Thin film coatings of WO3 by cold gas dynamic spray: A technical note. J. Therm. Spray Technol. 2005, 14, 183–186. [Google Scholar] [CrossRef]
- Suharto, Y.; Lee, Y.; Yu, J.S.; Choi, W.; Kim, K.J. Microporous ceramic coated separators with superior wettability for enhancing the electrochemical performance of sodium-ion batteries. J. Power Sources 2018, 376, 184–190. [Google Scholar] [CrossRef]
- Liu, K.; Hu, Y.; Yin, B.; Liu, X.; Lian, W.; Tang, S. Hydrophobic anti-reflective silica hybrid film with tunable refractive index. Opt. Laser Technol. 2023, 159, 108998. [Google Scholar] [CrossRef]
- Joshi, D.N.; Atchuta, S.; Reddy, Y.L.; Kumar, A.N.; Sakthivel, S. Super-hydrophilic broadband anti-reflective coating with high weather stability for solar and optical applications. Sol. Energy Mater. Sol. Cells. 2019, 200, 110023. [Google Scholar] [CrossRef]
- Gibas, A.; Baszczuk, A.; Jasiorski, M.; Winniki, M. Prospects of Low-Pressure Cold Spray for Superhydrophobic Coatings. Coatings 2019, 9, 829. [Google Scholar] [CrossRef]
- Ristok, S.; Flad, P.; Giessen, H. Atomic layer deposition of conformal anti-reflective coatings on complex 3D printed micro-optical systems. Opt. Mater. Express 2022, 12, 2063–2071. [Google Scholar] [CrossRef]
- Iftimie, S.; Mallet, R.; Merigeon, J.; Ion, L.; Girtan, M.; Antohe, S. On the structural, morphological and optical properties of ITO, ZnO, ZnO:Al and NiO thin films obtained by thermal oxidation. Dig. J. Nanomater. Biostruct. 2015, 10, 221–229. [Google Scholar]
- Girtan, M.; Negulescu, B. A review on oxide/metal/oxide thin films on flexible substrates as electrodes for organic and perovskite solar cells. Opt. Mater. X 2022, 13, 100122. [Google Scholar] [CrossRef]
- Reuna, J.; Aho, A.; Isoaho, R.; Raappana, M.; Aho, T.; Anttola, E.; Hietalahti, A.; Tukiainen, A.; Guina, M. Use of nanostructured alumina thin films in multilayer anti-reflective coatings. Nanotechnology 2021, 32, 215602. [Google Scholar] [CrossRef] [PubMed]
- Saidi, W.; Hfayedh, N.; Megriche, A.; Girtan, M.; El Maaoui, M. Hydrophilic/hydrophobic and optical properties of B2O3 doped TiO2 sol-gel thin films: Effect of B2O3 content, film thickness and surface roughness. Mater. Chem. Phys. 2018, 215, 31–39. [Google Scholar] [CrossRef]
- Sertel, T.; Sonmez, N.A.; Cetin, S.S.; Ozcelik, S. Influences of annealing temperature on anti-reflective performance of amorphous Ta2O5 thin films. Ceram. Int. 2019, 45, 11–18. [Google Scholar] [CrossRef]
- Sertel, T.; Ozen, Y.; Baran, V.; Ozcelik, S. Effect of single-layer Ta2O5 and double-layer SiO2/Ta2O5 anti-reflective coatings on GaInP/GaAs/Ge triple-junction solar cell performance. J. Alloys Compd. 2019, 806, 439–450. [Google Scholar] [CrossRef]
- Wu, J.; Fei, F.; Wei, C.; Chen, X.; Nie, S.; Zhang, D.; Su, W.; Cui, Z. Efficient multi-barrier thin film encapsulation of OLED using alternating Al2O3 and polymer layers. RSC Adv. 2018, 8, 5721–5727. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, N.; Pugazhendhi, R.; Elavarasan, R.M.; Kasiviswanathan, P.; Das, N. Anti-Reflective Coating Materials: A Holistic Review from PV Perspective. Energies 2020, 13, 2631. [Google Scholar] [CrossRef]
- Kócs, L.; Jilavi, M.H.; Koch, M.; de Oliveira, P.W. An environmentally friendly approach to produce single-layer anti-reflective coatings on large surfaces using wet chemical method. Ceram. Int. 2020, 46, 25865–25872. [Google Scholar] [CrossRef]
- Kócs, L.; Jilavi, M.H.; Beckelmann, D.; Schäfer, B.; May, A.; Koch, M.; de Oliveira, P.W. Water-based silica coatings: An environmentally friendly process on an industrial scale of single-layer anti-reflective coatings for large substrates. Ceram. Int. 2022, 48, 4165–4171. [Google Scholar] [CrossRef]
- Spence, M.; Hammond, R.; Pockett, A.; Wei, Z.; Johnson, A.; Watson, T.; Carnie, M.J. A Comparison of Different Textured and Non-Textured Anti-Reflective Coatings for Planar Monolithic Silicon-Perovskite Tandem Solar Cells. ACS Appl. Energy Mater. 2022, 5, 5974–5982. [Google Scholar] [CrossRef]
- Minegishi, S.; Ueda, N.; Saito, M.; Lee, J.; Fujima, T. Highly Water-Repellent and Anti-Reflective Glass Based on a Hierarchical Nanoporous Layer. Coatings 2022, 12, 961. [Google Scholar] [CrossRef]
- Lee, H.; Yi, A.; Choi, J.; Ko, D.H.; Kim, H.J. Texturing of polydimethylsiloxane surface for anti-reflective films with super-hydrophobicity in solar cell application. Appl. Surf. Sci. 2022, 584, 15262. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, K.; Cui, P.; Qi, H.; Qin, H.; Gu, G.; Shang, W.; Wang, S.; Cheng, G.; Du, Z. Hybrid energy harvester with bi-functional nano-wrinkled anti-reflective PDMS film for enhancing energies conversion from sunlight and raindrops. Nano Energy 2019, 66, 104188. [Google Scholar] [CrossRef]
- Seo, D.; Sayar, M.; Ogawa, K. SiO2 and MoSi2 formation on inconel 625 surface via SiC coating deposited by cold spray. Surf. Coat. Technol. 2012, 206, 2851–2858. [Google Scholar] [CrossRef]
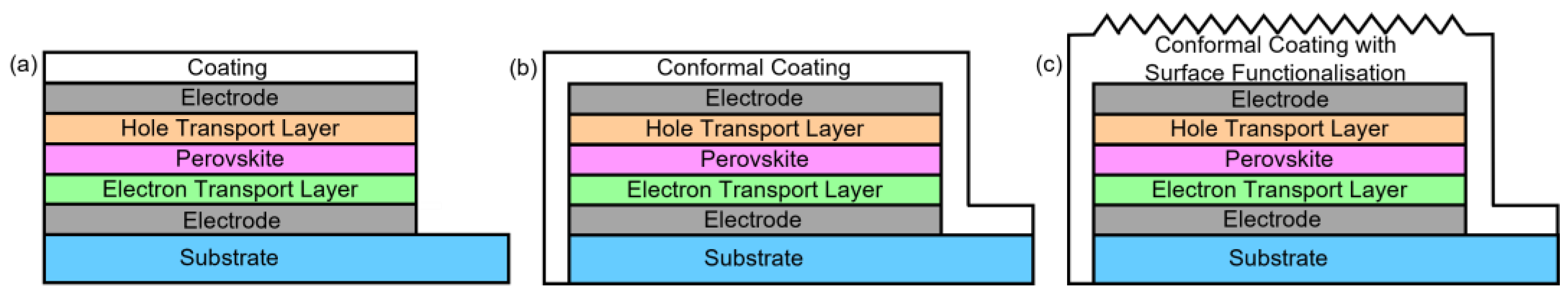

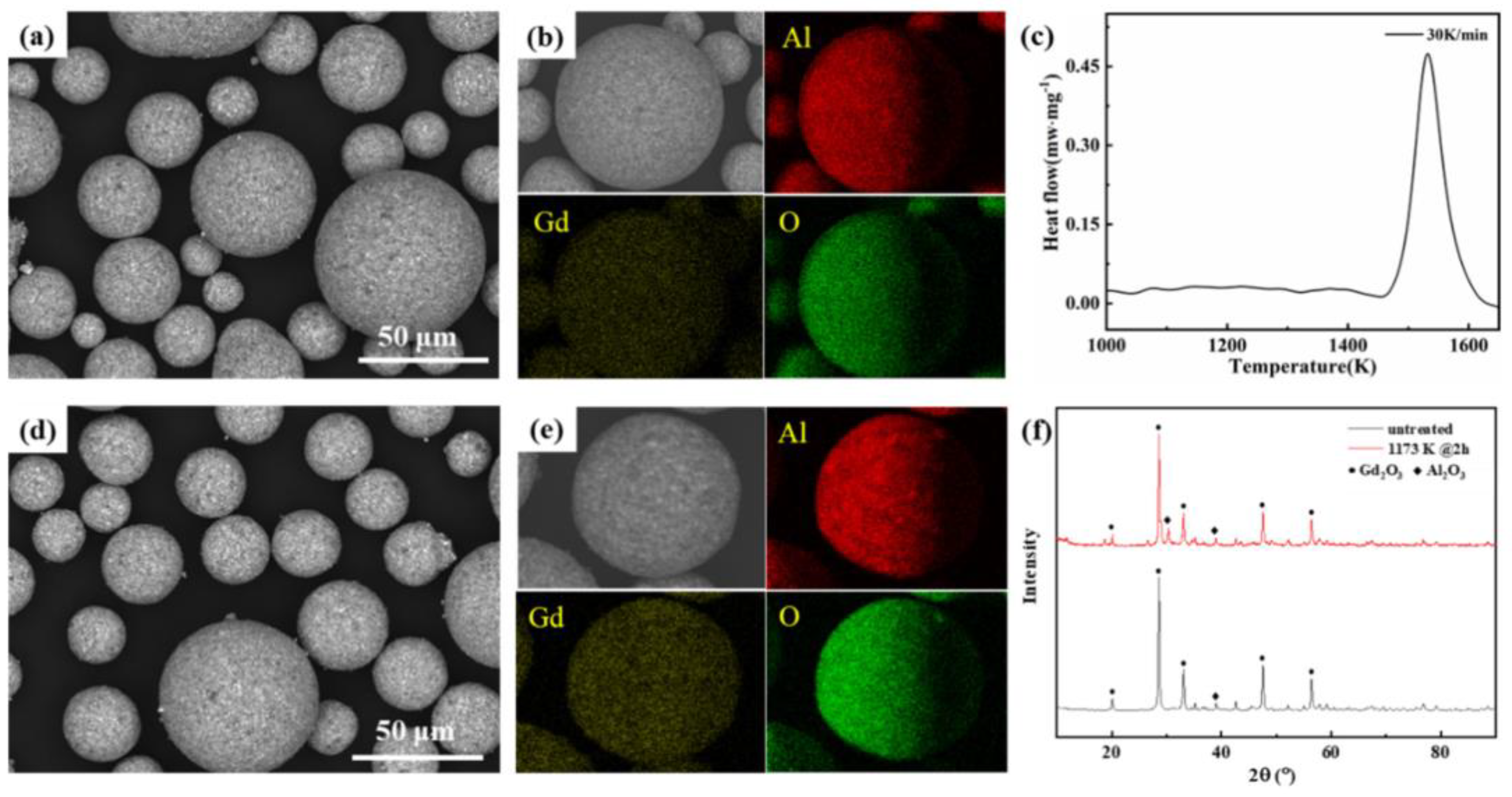
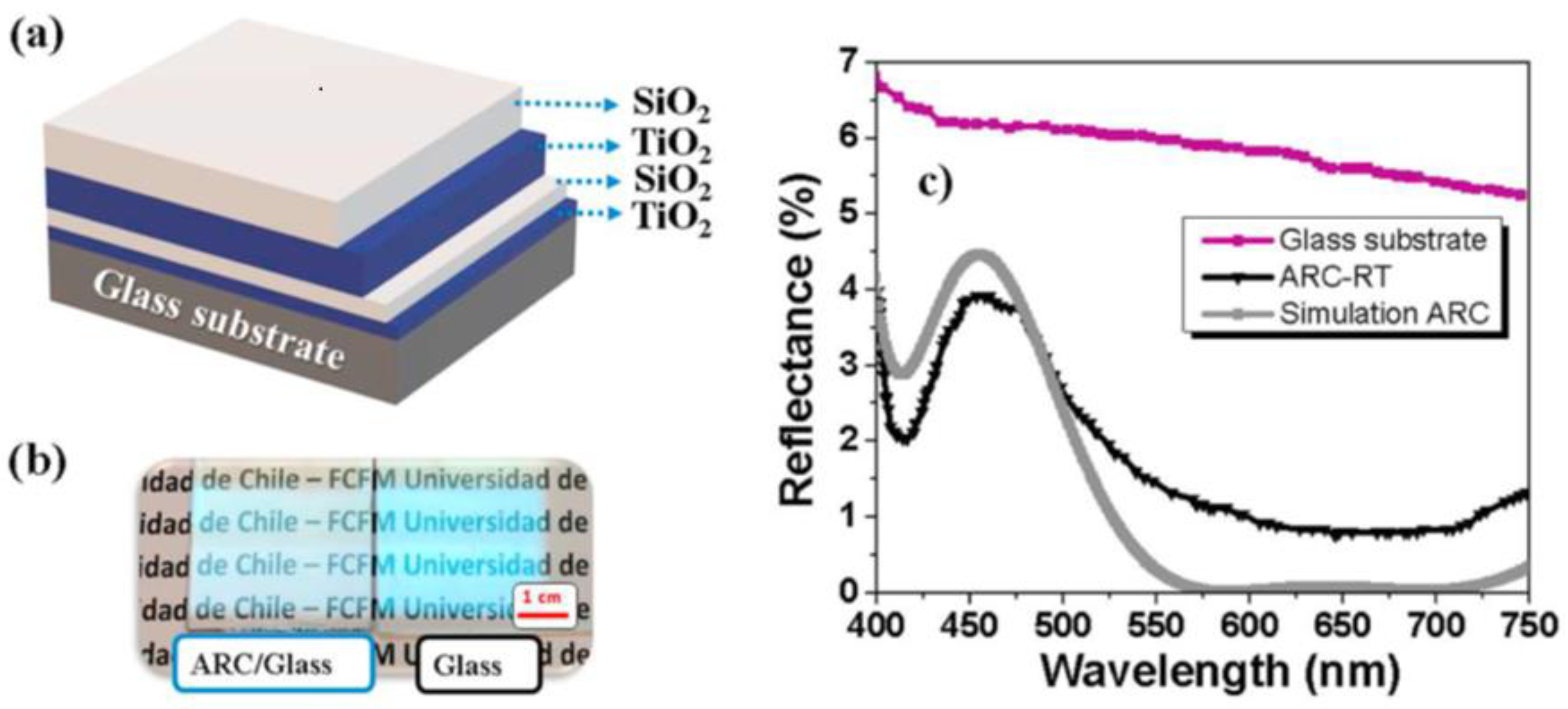
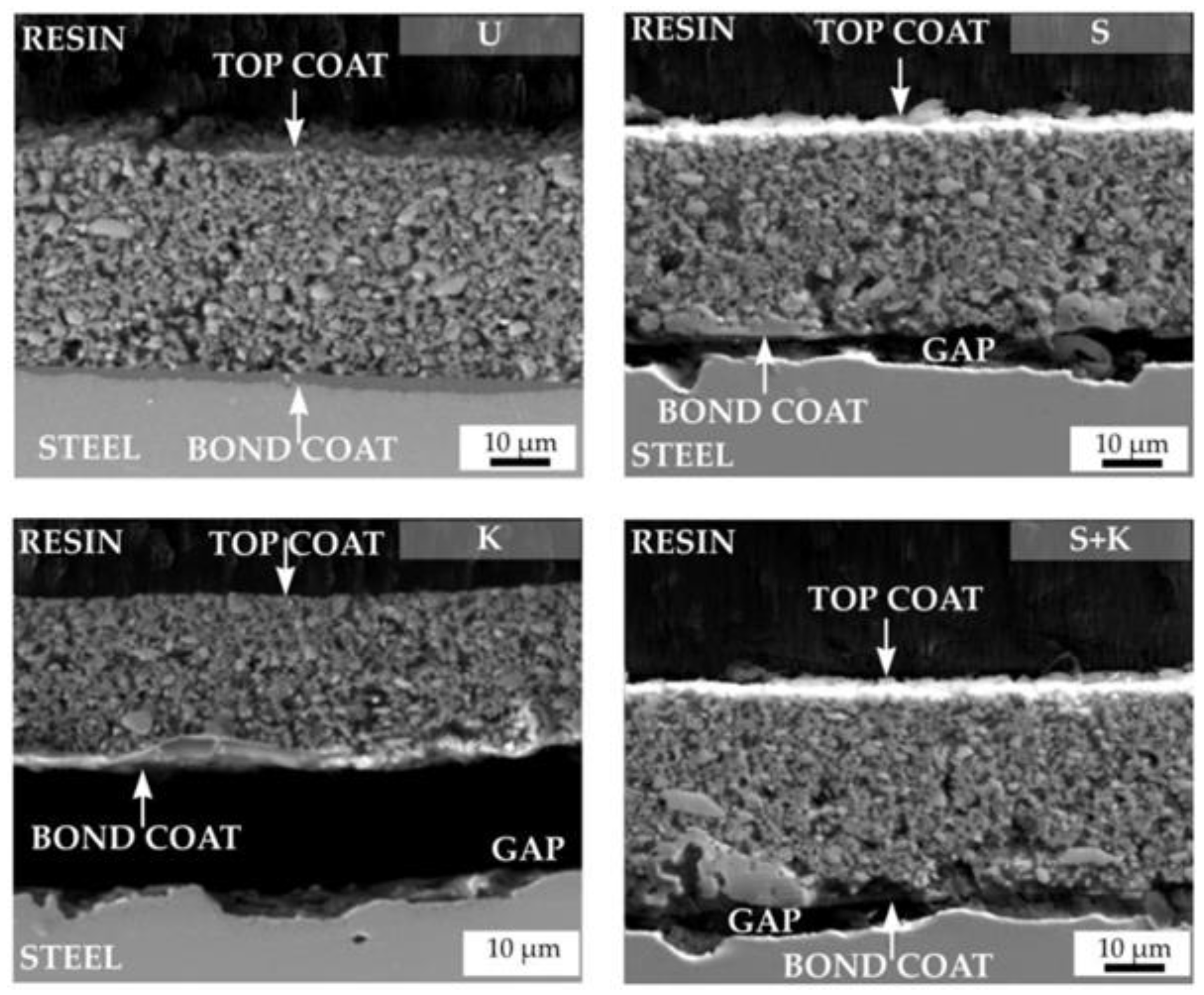
| Property | Ceramics | Glasses | Glass–Ceramics |
|---|---|---|---|
| Hardness | High | High | High |
| Elastic modulus | High | High | High |
| Ductility | Low | Low | Low |
| Wear resistance | High | High | High |
| Corrosion resistance | High | High | High |
| Chemical resistance | High | High | High |
| Weather resistance | High | High | High |
| Melting point | High | Medium–High | Medium–High |
| Working temperature | High | Medium–High | Medium–High |
| Thermal expansion | Low | Medium–Low | Medium–Low |
| Thermal conductivity | Medium–Low | Low | Medium–Low |
| Electrical insulation | High | High | High |
| Tensile strength | Low–Medium | Low | Low–Medium |
| Compressive strength | High | High | High |
| Transparency | Low | High | Low–High |
| Brittle | High | High | High |
| Impact strength | Low | Low | Low |
| Thermal shock resistance | Low | Low | Low |
| Method | Key Features | References |
|---|---|---|
| Cold gas dynamic spraying (CGDS) |
| [20,21] |
| Electrophoretic deposition |
| [22] |
| Plasma spraying |
| [23,24,25,26,27,28], [29,30,31,32] |
| Aerosol deposition |
| [33] |
| Sol–gel |
| [4,18,19,34,35,36,37,38,39,40,41] |
| Wet spray |
| [2,42,43] |
| Laser assisted CVD |
| [44] |
| Plasma assisted CVD |
| [45] |
| Hot dip plating |
| [46] |
| Laser cladding |
| [47] |
| Application | Coating Type | Deposition Method | Refs. |
|---|---|---|---|
| Electrical/corrosion protection | Al2O3-TiO2 | Plasma spray | [26,103,104] |
| WC-Al2O3 | Plasma spray | [27] | |
| CBS/Al2O3 | Plasma spray | [31] | |
| CaO–B2O3–SiO2 | Plasma spray | [32] | |
| Al2O3 Aluminium nitride | Aerosol | [33] | |
| Al2O3/SiO2 | ALD | [63] | |
| Polymer ceramic SiO2 | Blade coating | [64] | |
| γ-Al2O3 with epoxy resin topcoat | Electrolytic | [65] | |
| Al2O3/ZnO phosphate ceramic reinforced with TiO2 nanoparticles | Powder coating | [66] | |
| Glass flake nanoparticle-reinforced MgO phosphate ceramic coating | Brush coating | [89] | |
| SiOC Glass | Pyrolysis | [81] | |
| SiO2-Na2O-Al2O3-K2O-MgO-CaO-BaO | Melt quench/spray | [91] | |
| PRMMC Al2O3-Cu PRMMC Al2O3-Al PRMMC Al2O3-Ni PRMMC Al2O3-Ni-Zn | CGDS | [105,106,107,108,109] [110] [111,112] | |
| Mercapto-functionalised SiO2 | Sol–gel | [113] | |
| Al2O3 | Sol–gel | [114,115,116] | |
| Wear resistance/scratch resistance | Li2O-SiO2 | Crystallisation | [7] |
| Cr2O3 TiOx Cr2O3–TiOx–Al2O3 Ternary coating | Plasma spray | [24] | |
| ZrB2-SiC-TiSi2 | Plasma spray | [25] | |
| Al2O3-TiO2 | Plasma spray | [26] | |
| Al2O3-GdAlO3 | Plasma spray | [28] | |
| SiO2, ZnO, Al doped ZnO, or Al2O3 coating on TiO2/Ag/TiO2 stacks for IR reflectivity | Sol–gel | [39] | |
| TiO2-doped ZrO2 | Sol–gel | [41] | |
| SiO2-Na2O-B2O3-Al2O3-CaO with WC nanoparticles | Wet spray | [43] | |
| TiAlCN | Plasma-assisted CVD | [45] | |
| Al2O3-TiB2-TiC | Laser cladding | [47] | |
| Y/Sialon | Pulsed laser deposition | [57] | |
| Zirconium epoxy–ceramic | Droplet | [60] | |
| Polycrystalline Y2O3 on YSZ ceramic substrates | Electron-beam PVD | [68] | |
| PFOTES-functionalised SiO2 nanoparticles | Sol–gel | [74] | |
| TiO2 functionalised PDMS | Wet spray | [76] | |
| Epoxy modified SiO2 in polysiloxane | Brush coating | [78] | |
| DDS-functionalised SiO2 nanoparticles | Wet spray | [85] | |
| Hydrophobic and long-chain functionalised SiO2 on a polymer base layer | Dip coating | [86] | |
| HDMS-functionalised SiO2 nanoparticles in organosilica binder | Dip coating | [87] | |
| Y2O3-Al2O3-SiO2 | Melt quench | [92] | |
| Cr2O3-doped SiO2-Al2O3-ZrO2-Ba(Sr, Ca)O | Melt quench | [93] | |
| Y2Ti2O7-SiO2 | Melt casting | [101] | |
| SiO2-Al2O3-Fe2O3-CaO-MgO-R2O | Dip coating | [102] | |
| PRMMC Al2O3-Cu PRMMC Al2O3-Al PRMMC WC-Ni | CGDS | [107,117] [107,109,118] [119,120] | |
| Gradient TiOx-Al2O3 | Thermal oxidation | [121] | |
| Transparent TiO2/Al2O3 | Electron beam evaporation | [122] | |
| SiO2 nanoplates in cellulose | Sol–gel | [123] | |
| Electrical conductivity | BaAl11O17 YBa2Cu3O7−δ | Laser-assisted CVD | [44] |
| SiOC Glass | Pyrolysis | [81] | |
| PRMMC Al2O3-Cu | CGDS | [105] | |
| Intermediate compatibility layer | PRMMC Al2O3-Al PRMMC Al2O3-Ni PRMMC Al2O3-Ni-Zn | CGDS | [124,125] |
| Photocatalyst | Porous TiO2 | Aerosol | [33] |
| TiO2 with nitrogen plasma treatment | Sol–gel | [40] | |
| TiO2-functionalised PDMS | Spray | [76] | |
| Porous TiO2 | Sol–gel | [126] | |
| TiO2 | CGDS | [127,128] | |
| GaN | CGDS | [129] | |
| Biomedical | Rare earth-doped glass–ceramics | Sol–gel | [18] |
| Ceramic HA | CGDS | [130] | |
| Al2O3 | Sol–gel | [131] | |
| TiO2 | Dip coating | [132] | |
| ZnO-PTFE composites | Sputtering | [133] | |
| Solid lubricating coatings | TiAlCN | Plasma assisted CVD | [45] |
| Cr2O3 doped SiO2-Al2O3-ZrO2-Ba(Sr, Ca)O | Melt quench | [93] | |
| PRMMC Al2O3-Cu-graphite PRMMC Al2O3-(Cu-5Sn)-Ag | CGDS | [134] [135] | |
| Chemical/UV protection/IR protection | Glass ceramics composed of mainly TiO2, Na2O/K2O/Li2O, SiO2/ZrO2, and Fe2O3/Al2O3/B2O3 | Wet spray | [2] |
| SiO2, ZnO, Al doped ZnO, or Al2O3 coating on TiO2/Ag/TiO2 stacks for IR reflectivity | Sol–gel | [39] | |
| Zirconium epoxy–ceramic | Droplet coating | [60] | |
| TiO2 functionalised PDMS | Wet spray | [76] | |
| TiO2 functionalisation on glass ZnO functionalisation on glass | Spray pyrolysis | [79] | |
| SiOC glass | Spray pyrolysis | [83] | |
| PS-LSAO functionalised glass | Dip coating | [88] | |
| Hydrophobic and long-chain functionalised SiO2 on a polymer base layer | Dip coating | [86] | |
| α-cordierite | Powder sintering | [94] | |
| SiO2-Al2O3-Fe2O3-CaO-MgO-R2O | Dip coating | [102] | |
| ZnO-PDMS on polyimide | Dip coating/hydrothermal | [136] | |
| Fuel cell component coatings | WO3 Polyvinylidene fluoride– hexafluoropropylene/ZrO2NPs | CGDS | [137] [138] |
| Thermal barriers | Li2O-Al2O3-SiO2 | Crystallisation | [7] |
| SiC | Plasma spray | [23] | |
| ZrB2-SiC-TiSi2 | Plasma spray | [25] | |
| Al2O3-GdAlO3 | Plasma spray | [28] | |
| (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 | Plasma spray | [30] | |
| β-Al2TiO5 | Laser-assisted CVD | [44] | |
| YSZ-Al2O3 | Plasma spray | [55] | |
| α-cordierite | Powder sintering | [94] | |
| SiO2-Al2O3-Fe2O3-CaO-MgO-R2O | Dip coating | [102] | |
| Hydrophobic/anti-fouling surfaces | Crystalline Yb2O3 | Plasma spray | [29] |
| Zirconium(IV) Propoxide/Colloidal SiO2/methyltrimethoxysilane glass–ceramic | Sol–gel | [35] | |
| TiO2 with nitrogen plasma treatment | Sol–gel | [40] | |
| Zirconium epoxy-ceramic | Droplet coating | [60] | |
| Al2O3/ZnO phosphate ceramic reinforced with TiO2 nanoparticles | Powder | [66] | |
| Ti3C2Tx MXene nanosheet | Dip coating | [73] | |
| PFOTES-functionalised SiO2 nanoparticles | Sol–gel | [74] | |
| PFOTES-functionalised BaAl2Si2O8 | Wet spray | [75] | |
| TiO2-functionalised PDMS | Wet spray | [76] | |
| SiO2 nanoparticles/ceramic–polymer matrix | Wet spray | [77] | |
| PS-LSAO-functionalised glass | Dip coating | [88] | |
| SiO2 | Bar coating | [84] | |
| Hydrophobic and long-chain-functionalised SiO2 on a polymer base layer | Dip coating | [86] | |
| DDS functionalised SiO2 nanoparticles | Wet spray | [85] | |
| HDMS functionalised SiO2 nanoparticles in organosilica binder | Dip coating | [87] | |
| TiO2 | Dip coating | [132] | |
| ZnO-PTFE composites | Sputtering | [133] | |
| ZnO-PDMS on polyimide | Dip coating/hydrothermal | [136] | |
| Double layer SiO2 hybrid film | Dip coating | [139] | |
| Hydrophilic SiO2-MgF2 | Sol–gel | [140] | |
| SiO2 | CGDS | [141] | |
| Anti-reflective/transparent coating for solar cells | SiO2, ZnO, Al-doped ZnO, or Al2O3 coating on TiO2/Ag/TiO2 stacks for IR reflectivity | Sol–gel | [39] |
| TiO2 with nitrogen plasma treatment | Sol–gel | [40] | |
| Y/Sialon | Pulsed laser deposition | [57] | |
| TiO2/SiO2 multilayers | Sputtering, ALD | [58,142] | |
| Zr-oxide doped TiO2/SiO2 multilayers | Sputtering | [59] | |
| Al2O3/SiO2 | ALD | [63] | |
| Polymer ceramic SiO2 | Blade coating | [64] | |
| Polycrystalline Y2O3 on YSZ ceramic substrates | Electron beam PVD | [68] | |
| MgO-ZnO | Dip coating | [69] | |
| Ti3C2Tx MXene nanosheet | Dip coating | [73] | |
| PFOTES-functionalised SiO2 nanoparticles | Sol–gel | [74] | |
| TiO2-functionalised PDMS | Wet spray | [76] | |
| SiO2 nanoparticles in ceramic–polymer matrix | Wet spray | [77] | |
| Epoxy-modified SiO2 in polysiloxane | Brush coating | [78] | |
| ZnO | Sputtering | [79,143] | |
| TiO2 | Sputtering | [79,144] | |
| DDS-functionalised SiO2 nanoparticles | Wet spray | [85] | |
| Hydrophobic and long-chain-functionalised SiO2 on a polymer base layer | Dip coating | [86] | |
| HDMS-functionalised SiO2 nanoparticles in organosilica binder | Dip coating | [87] | |
| SrO-Bi2O3-B2O3 | Melt quenching | [95] | |
| SiO2 nanoplates in cellulose | Sol–gel | [123] | |
| TiO2 | Dip coating | [132] | |
| ZnO-PTFE composites | Sputtering | [133] | |
| Double-layer SiO2 hybrid film | Dip coating | [139] | |
| Hydrophilic SiO2-MgF2 | Sol–gel | [140] | |
| Al-ZnO | Sputtering | [143] | |
| Nanostructured Al2O3 multilayers | Electron beam evaporation/sputtering | [145] | |
| B2O5 doped TiO2 | Sol–gel | [146] | |
| a-Ta2O5 | Sputtering | [147,148] | |
| Ta2O5/SiO2 multilayers | Sputtering | [148] | |
| Al2O3/Parylene-C alternating layers | ALD/CVD | [149] | |
| SiO2 | Sol–gel | [150,151,152] | |
| Textured PDMS | CVD, spin coating, etching | [153,154,155,156] | |
| Photonics | SiO2 | Sol–gel | [18] |
| Doped oxyfluoride glass–ceramics | Sol–gel | [36,37,38,54] | |
| SiO2, ZnO, Al doped ZnO, or Al2O3 coating on TiO2/Ag/TiO2 stacks for IR reflectivity | Sol–gel | [39] | |
| TiO2 with nitrogen plasma treatment | Sol–gel | [40] | |
| Polycrystalline Y2O3 on YSZ ceramic substrates | Electron beam PVD | [68] | |
| MgO-ZnO | Dip coating | [69] | |
| TiO2-functionalised PDMS | Spray | [76] | |
| PS-LSAO-functionalised glass | Dip coating | [88] | |
| SiO2-based film | Bar coating | [84] | |
| Double-layer SiO2 hybrid film | Dip coating | [139] | |
| TiO2/SiO2 multilayers | ALD | [142] | |
| Oxidation barriers | Al2O3-TiO2 | Plasma spray | [26] |
| Al2O3-GdAlO3 | Plasma spray | [28] | |
| MoSi2 | Hot dip plating | [46] | |
| Al2O3/SiO2 | ALD | [63] | |
| Y2SiO5/Y2O3-Al2O3-SiO2 | Pulsed arc discharge/hot dipping | [90] | |
| SiO2-Na2O-Al2O3-K2O-MgO-CaO-BaO | Melt quench/spray | [91] | |
| Y2Ti2O7-SiO2 | Melt casting | [101] | |
| Gradient TiOx-Al2O3 | Thermal oxidation | [121] | |
| Al2O3/Parylene-C alternating layers | ALD/CVD | [149] | |
| MoSi2-SiO2-SiC | CGDS | [157] |
| Layer Material | Thickness/nm | Transparency/% | Reflectivity/% | Self- Cleaning | Contact Angle/° | Refs. | ||
|---|---|---|---|---|---|---|---|---|
| 400 nm | 600 nm | 400 nm | 600 nm | |||||
| SiO2 on TiO2/Ag/TiO2 | 2.7 × 102 | >60 | ~80 | ~10 | <10 | Not specified | Not specified | [39] |
| TiO2 + N2 Plasma | ~6.3 × 101–9.5 × 101 | ~90 | 92–95 | Not specified | MB degradation | Not specified | [40] * | |
| Y/Sialon | ~1.8 × 101–3.2 × 102 | ~50 | ~90 | Not specified | Not specified | Not specified | [57] | |
| TiO2/SiO2 | 2.6 × 102 | 80–92 | 92–97 | 3 | 1 | Not specified | Not specified | [58] * |
| ~2.0 × 102 | >90 at 525 nm | ~20 | <5 | Not specified | Not specified | [142] * | ||
| ZrO-doped TiO2/SiO2 | 2.5 × 102 | ~88–92 | 88–92 | <10 | <5 | Not specified | Not specified | [59] * |
| TiO2 | <1.0 × 102 | ~75–85 | ~75–85 | ~15–35 | ~10–30 | Not specified | Not specified | [79] |
| Particle size 2.5 × 101–1.0 × 102 | Not specified | Not specified | MB degradation | <30 | [132] | |||
| 2.8 × 101–3.0 × 101 | Not specified | Not specified | Not specified | 55–70 | [144] | |||
| ZnO | 3.6 × 101 | ~70 | ~90 | Not specified | Not specified | Not specified | [143] | |
| <1.0 × 102 | ~75 | ~75–85 | ~10–25 | ~10–20 | Not specified | Not specified | [79] | |
| Al2O3/SiO2 | 9.4 × 101 | Not specified | ~30 | <10 | Not specified | Not specified | [63] * | |
| SiO2 | 7.0 × 101–2.5 × 103 | ~85 | ~90 | Not specified | Not specified | 88 | [64] | |
| 7.5 × 101–1.4 × 102 | 89–95 | 96–99 | Not specified | Not specified | Not specified | [151] | ||
| ~7.7 × 101 | 93–95 | 94–96 | 5–6 | 2 | Not specified | Not specified | [152] * | |
| Y2O3 on YSZ | 5.0 × 102–1.5 × 103 | ~20–30 | 40–60 | Not specified | Not specified | Not specified | [68] | |
| (better than substrate) | ||||||||
| MgO-ZnO | Not specified | (absorbance <0.5 at 400 nm) | Not specified | Not specified | Not specified | [69] | ||
| Ti3C2Tx MXene | ~4.0 × 101 | 50–65 | 71–77 | Not specified | Contaminant removal | 25 (glycerol sliding angle) | [73] | |
| PFOTES/SiO2 NPs | 3.0 × 104 | >80 | >80 | Not specified | Contaminant removal | 105 | [74] | |
| (10 days stirring) | ||||||||
| TiO2/PDMS | ~5.0 × 101–5.0 × 102 | 65–80 | 80–85 | Not specified | MB degradation, Contaminant removal | >130–>160 (recoverable) | [76] | |
| SiO2 NPs in ceramic- polymer matrix | Not specified | ~90 | ~90 | Not specified | Not specified | 115–>140 | [77] | |
| Epoxy-modified SiO2 polysiloxane | 2.0 × 105 | ~90 | ~90 | Not specified | Not specified | Not specified | [78] | |
| DDS/SiO2 NPs | 1.8 × 105 | ~30–70 | ~35–80 | Not specified | Not specified | ~160 | [85] | |
| (thickness dependent) | ||||||||
| Hydrophobic SiO2 | Not specified | 91–95 | 92–95 | Not specified | Contaminant removal | >150 | [86] * | |
| HDMS/SiO2 NPs in Organosilica binder | 1.1 × 102 | 95–97 | 96–100 | Not specified | Contaminant removal | ~160 | [87] * | |
| SrO-Bi2O3-B2O3 glass–ceramic | Particle size 1.0 × 101–3.0 × 101 | 0 | ~70 | Not specified | Indicator ink and MB degradation | 63 | [95] | |
| SiO2 nanoplates in cellulose | Ave. plate 5.4 × 102 | ~75–80 | ~75–80 | Not specified | Not specified | 71–91 | [123] | |
| ZnO-PTFE | 1.5 × 102 | ~70–90 | ~85–90 | Not specified | Not specified | Up to 110 | [133] | |
| Double-layer SiO2 | ~2.2 × 102 | ~94–98 | ~95–98 | Not specified | Contaminant removal | 108–142 | [139] * | |
| SiO2-MgF2 | 6.9 × 101–1.3 × 102 | 87–94 | 92–98 | <4 | <3 | Not specified | <6 | [140] |
| Al-ZnO | 3.6 × 101–4.0 × 101 | ~80 | ~80 | Not specified | Not specified | 109 | [143,144] | |
| Al2O3 multilayers | <1.0 × 102 | 95–97 | 95–99 | <20 | <5 | Not specified | Not specified | [145] * |
| B2O5 doped TiO2 | 4.7–2.3 × 102 | ~75–100 | 80–100 | Not specified | Not specified | 16–75 | [146] | |
| a-Ta2O5 | 1.0 × 102–1.1 × 102 | ~72–88 | ~66–76 | ~35–48 | <2 | Not specified | Not specified | [147,148] |
| Ta2O5/SiO2 | 1.7 × 102 | ~88 | ~92 | 5 | <5 | Not specified | Not specified | [148] |
| Al2O3/Parylene-C | 1.6 × 103 | Visual only | Not specified | Not specified | Not specified | [149] | ||
| Textured PDMS | 1.0 × 104–7.0 × 104 | 89–91 | 90–92 | 9–10 | ~8 | Not specified | Not specified | [153] |
| Not specified | Not specified | <1 | <1 | Not specified | 140 | [154] | ||
| 2.5 × 105 | ~80–92 | ~80–92 | ~3–5 | ~3–5 | Not specified | ~126–155 | [155] | |
| Not specified | ~85–98 | 90 | Not specified | Not specified | 138 | [156] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraser, R.; Girtan, M. A Selective Review of Ceramic, Glass and Glass–Ceramic Protective Coatings: General Properties and Specific Characteristics for Solar Cell Applications. Materials 2023, 16, 3906. https://doi.org/10.3390/ma16113906
Fraser R, Girtan M. A Selective Review of Ceramic, Glass and Glass–Ceramic Protective Coatings: General Properties and Specific Characteristics for Solar Cell Applications. Materials. 2023; 16(11):3906. https://doi.org/10.3390/ma16113906
Chicago/Turabian StyleFraser, Rebekah, and Mihaela Girtan. 2023. "A Selective Review of Ceramic, Glass and Glass–Ceramic Protective Coatings: General Properties and Specific Characteristics for Solar Cell Applications" Materials 16, no. 11: 3906. https://doi.org/10.3390/ma16113906
APA StyleFraser, R., & Girtan, M. (2023). A Selective Review of Ceramic, Glass and Glass–Ceramic Protective Coatings: General Properties and Specific Characteristics for Solar Cell Applications. Materials, 16(11), 3906. https://doi.org/10.3390/ma16113906






