Properties of Alkali-Activated Slag Cement Activated by Weakly Alkaline Activator
Abstract
1. Introduction
2. Experimentation
2.1. Materials
2.2. Experimental Procedures
2.2.1. Experimental Designs
2.2.2. Experimental Methods
3. Results and Discussions
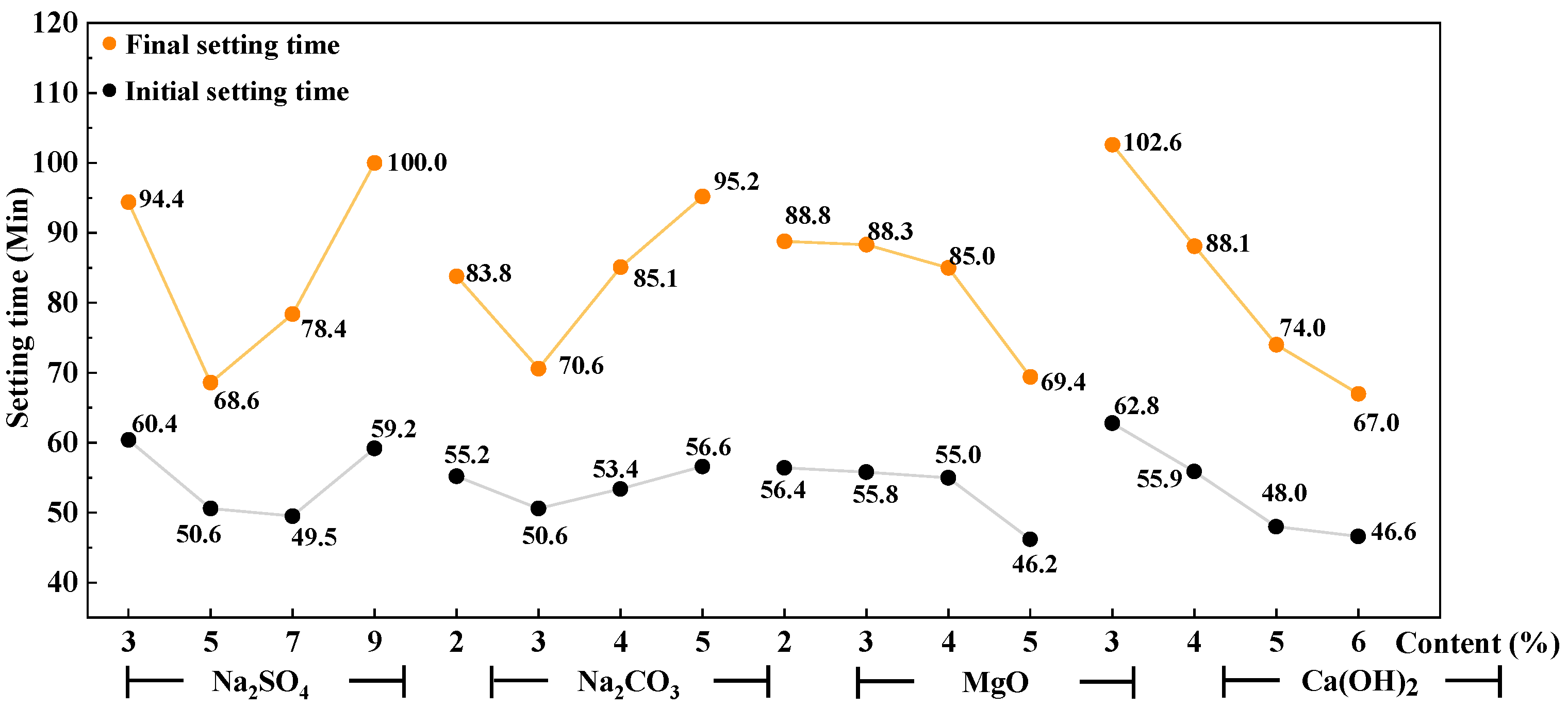
3.1. Setting Time of AAS Paste
| Factors | R′ of Initial Setting Time | R′ of Final Setting Time |
|---|---|---|
| A (Na2SO4) | 12.01 | 34.61 |
| B (Na2CO3) | 6.61 | 27.12 |
| C (Reactive MgO) | 11.24 | 21.38 |
| D(Ca(OH)2) | 17.86 | 39.24 |
| A × D (Na2SO4 × Ca(OH)2) | 3.22 | 14.49 |
| B × D (Na2CO3 × Ca(OH)2) | 12.16 | 24.33 |
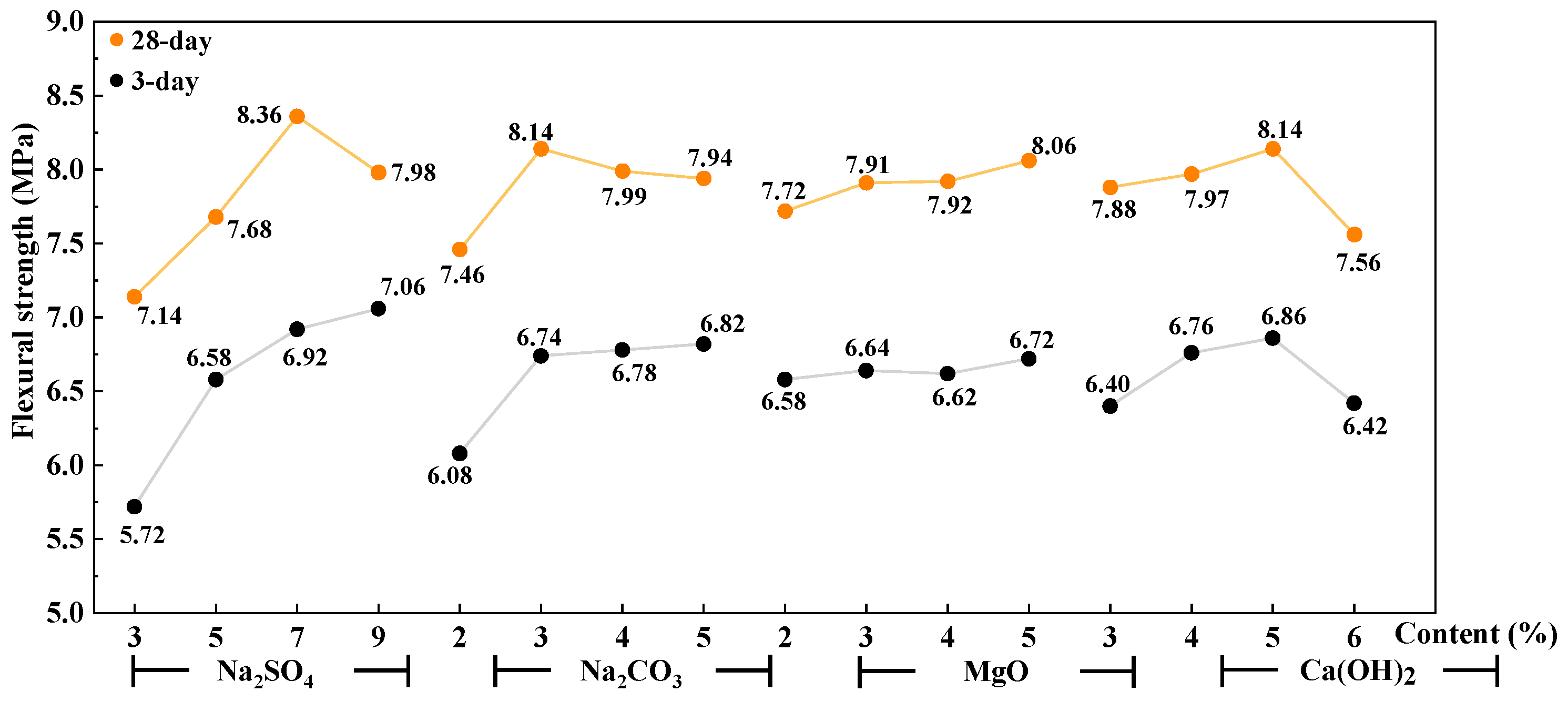
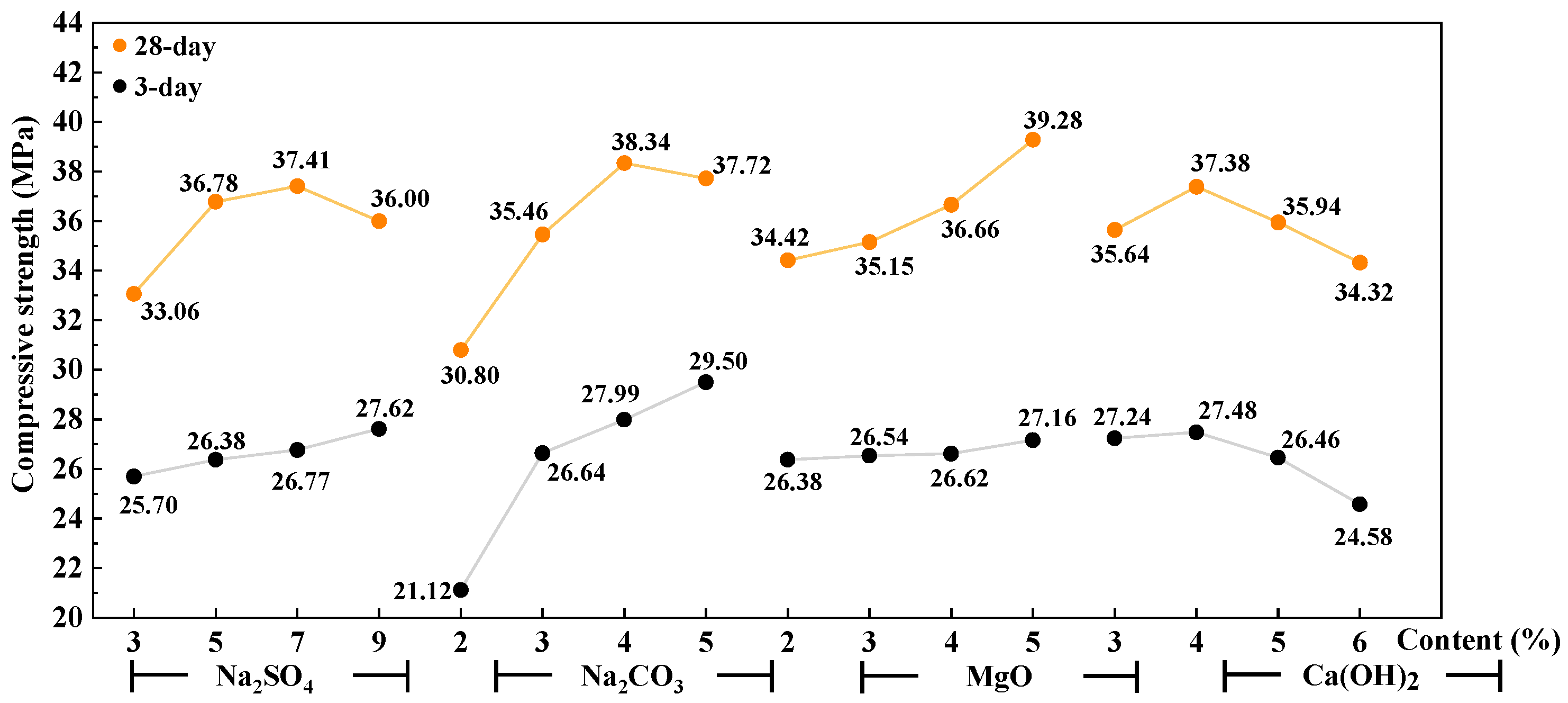
3.2. Mechanical Properties of AAS Mortar
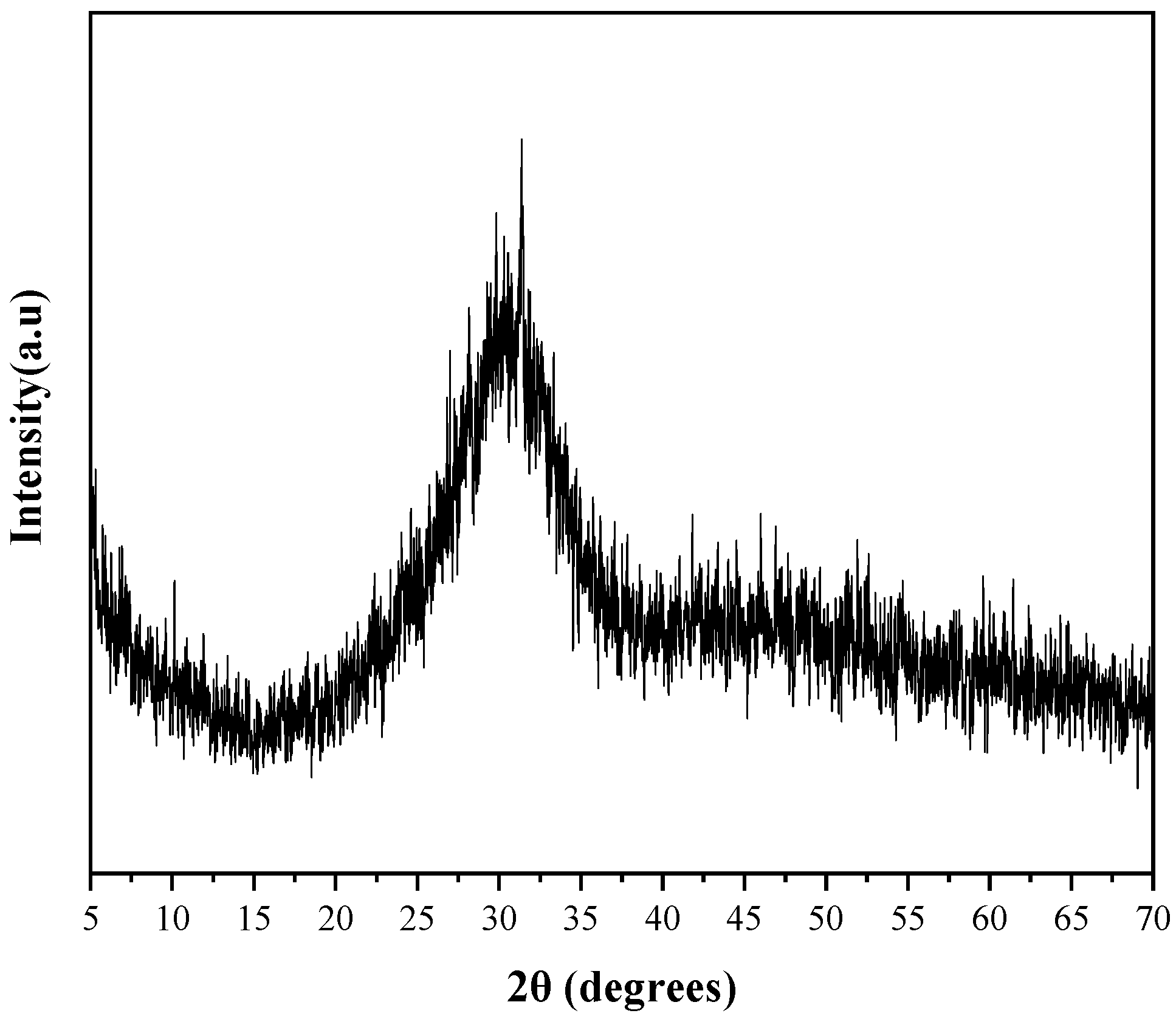
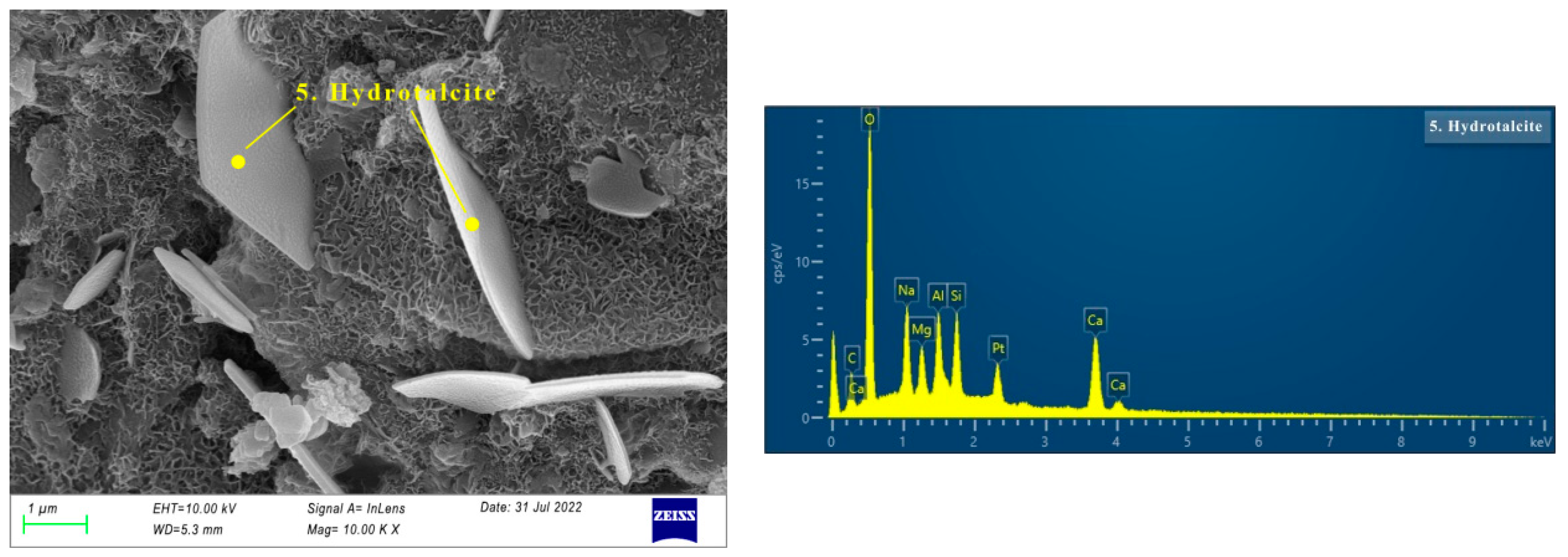
3.3. The Microstructure of AAS Paste
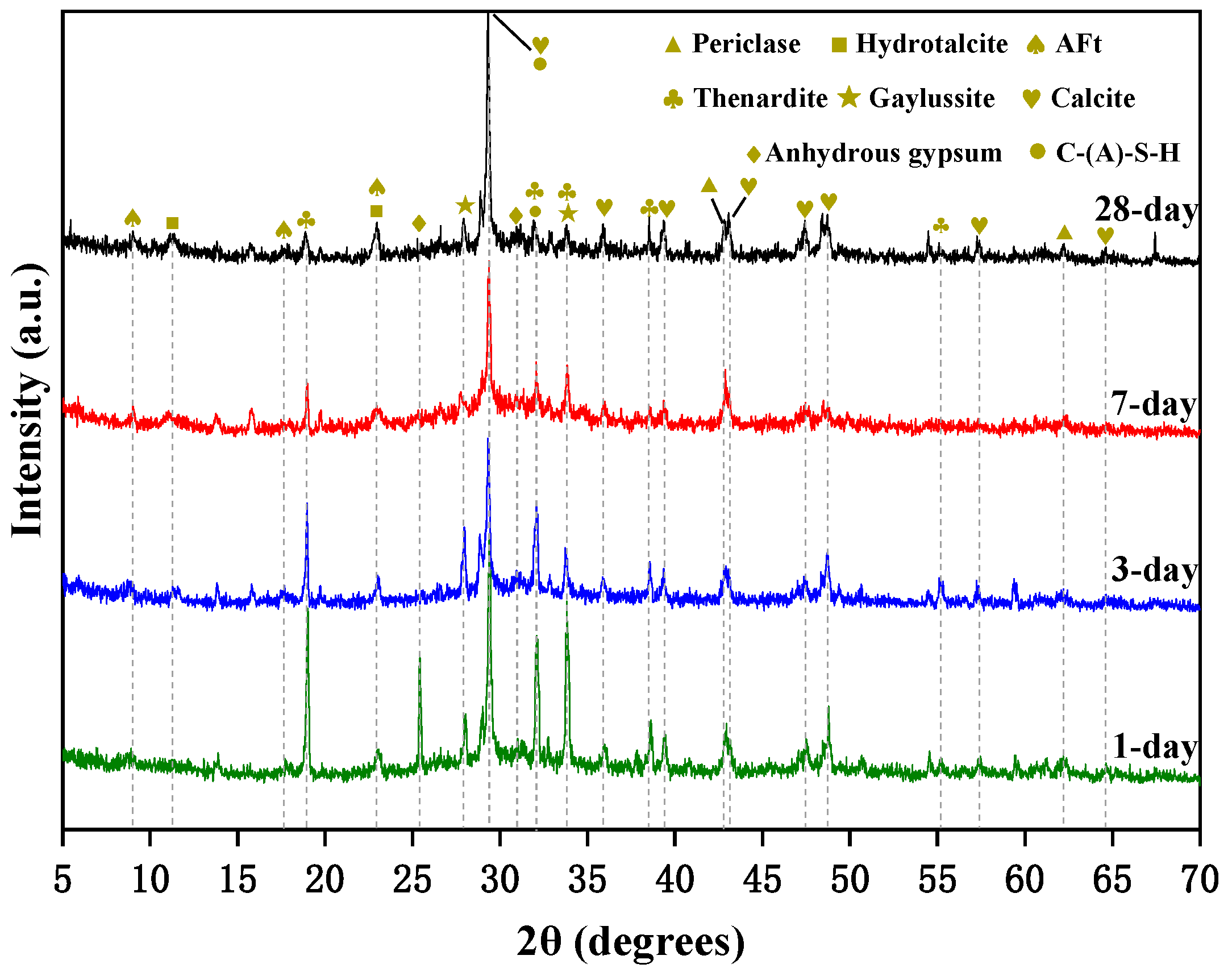
3.3.1. XRD of AAS Paste
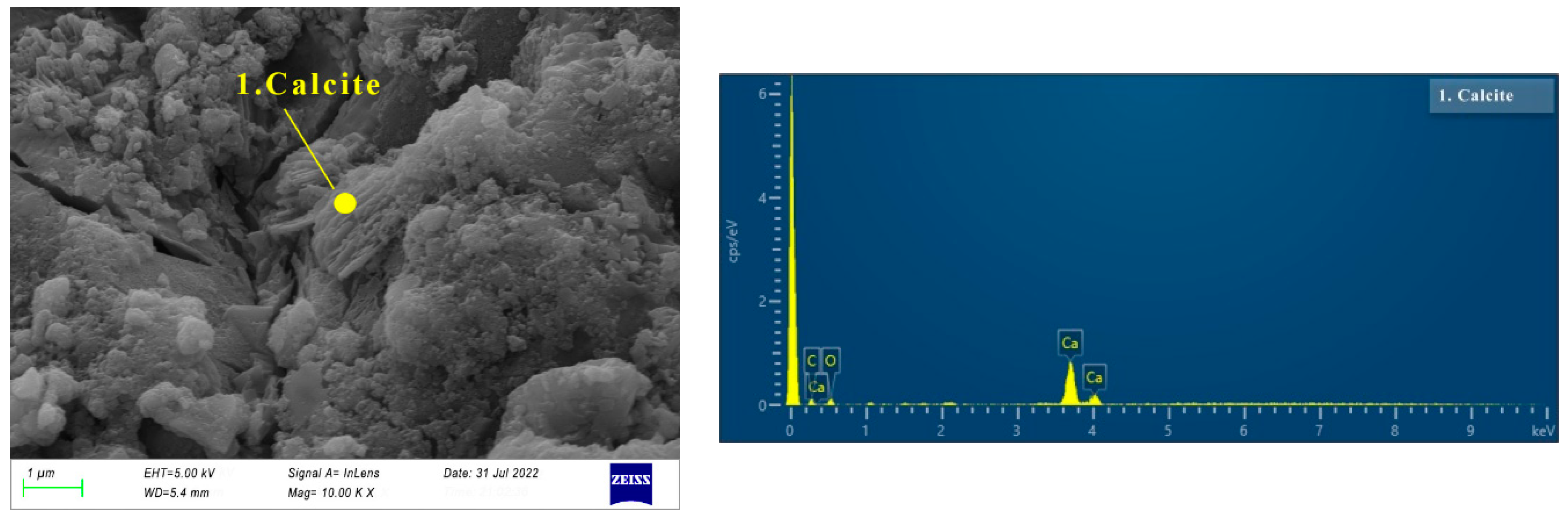
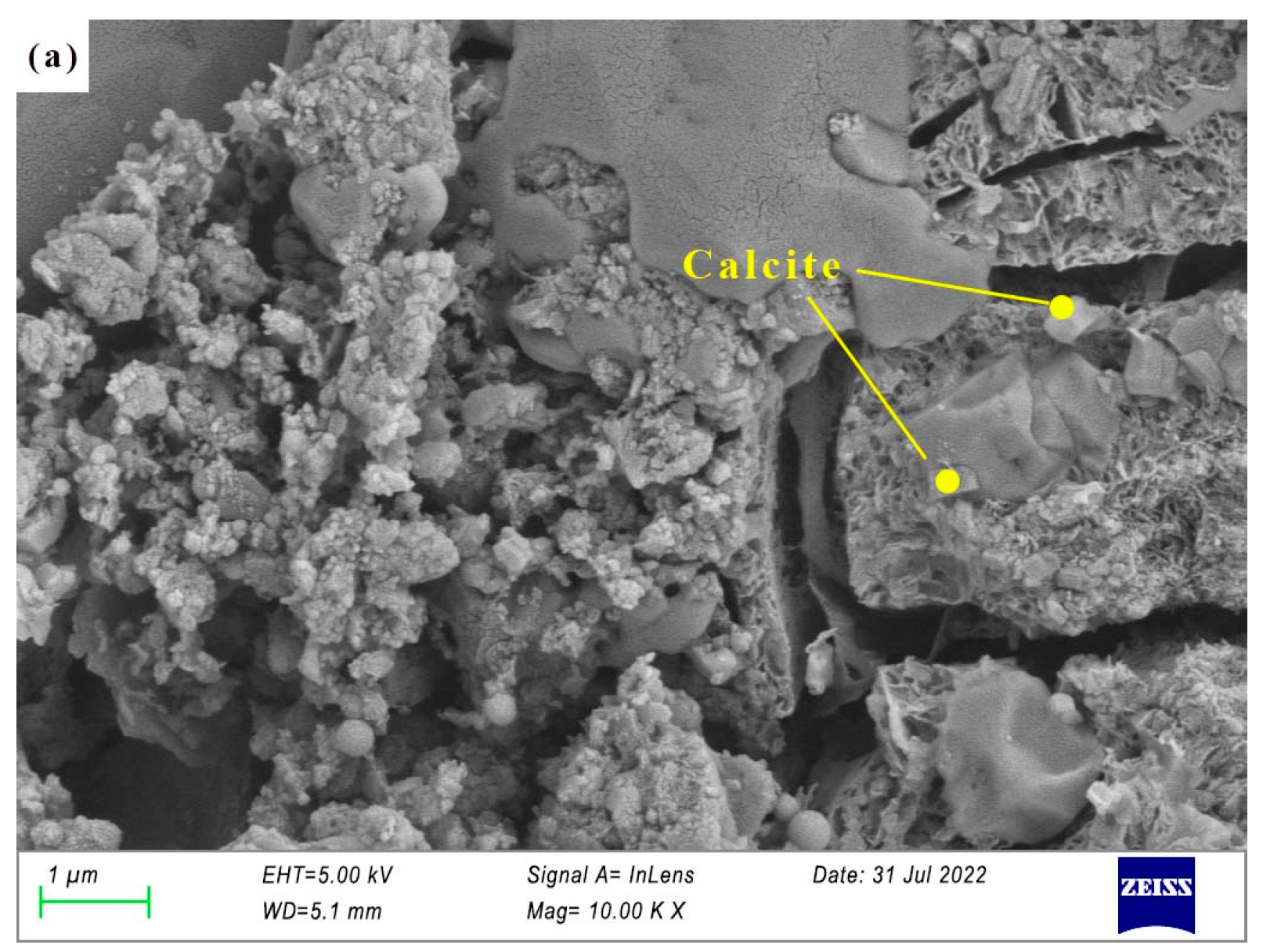
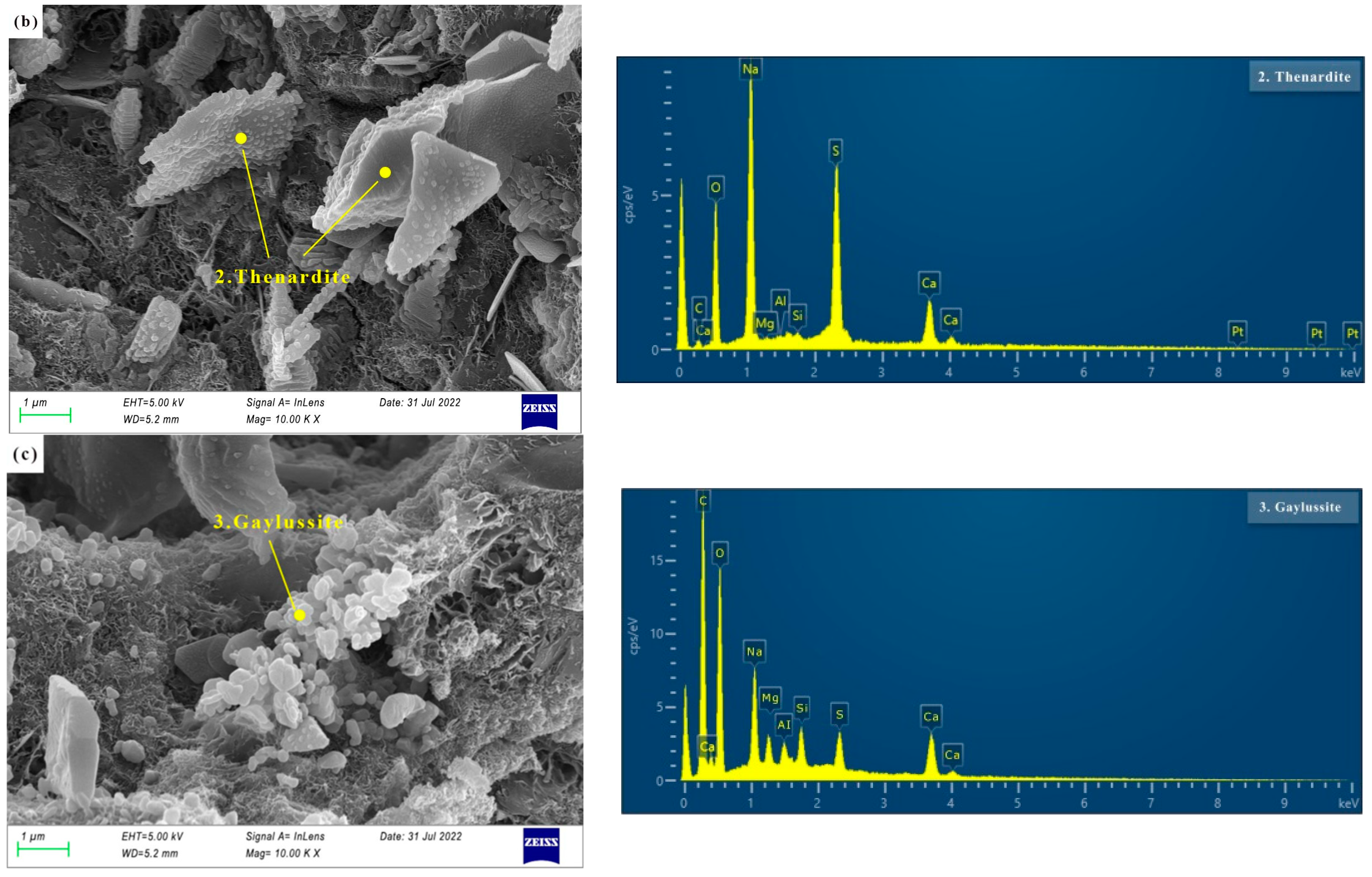
3.3.2. SEM of AAS Paste
3.4. Production Cost, CO2 Emission, and Energy Consumption
4. Conclusions
- (1)
- Ca(OH)2 was the significant factor affecting the setting time of AAS cement activated by a weakly alkaline activator. Na2SO4 and Na2CO3 are significant factors affecting the flexural strength and compressive strength of AAS mortar, respectively. The interaction between Ca(OH)2 and Na2CO3 has a greater effect on the initial setting time than on the final setting time. That interaction has a greater effect on the 3-day compressive strength than that on the 28-day compressive strength.
- (2)
- The reaction between the activators of Ca(OH)2 and Na2CO3 to form CaCO3 crystals is the main reason that affects the initial setting time and the early strength of AAS cement. The rapid reaction of Ca(OH)2 with Na2CO3 and Na2SO4 enhances the alkalinity of the AAS system and further promotes the hydration of GBFS. When an activator is composed of 7% Na2SO4, 4–5% Na2CO3, 3–5% Ca(OH)2, and 2–4% reactive MgO, the setting time of the AAS cement is reasonable and the mechanical property is good.
- (3)
- There are more crystal phases in hydration products, namely calcite, AFt, and hydrotalcite. Na2SO4 and reactive MgO contribute to the formation of AFt and hydrotalcite, respectively. The effect of the crystal phases on dry shrinkage needs to be further studied.
- (4)
- Compared with P·O 42.5 of OPC and AAS cement activated by NH and WG with the same alkali equivalent of 5.4%, the cost of producing 1 ton of cementitious material is reduced by 12.7%, 20.0%, and 41.2%, and energy consumption is reduced by 87.7%, 11.0%, and 61.1%. Compared with P·O 42.5, CO2 emission is reduced by 77.6%. The engineering application of AAS cement activated by weakly alkaline activators will bring good economic and environmental benefits.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Neelakanta Reddy, I.; Shim, J. Preparation and validation of sustainable metakaolin based geopolymer concrete for structural application. Constr. Build. Mater. 2023, 371, 130688. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, X.; Wang, F.; Wang, J. Mechanical Properties and Durability of Geopolymer Recycled Aggregate Concrete: A Review. Polymers 2023, 15, 615. [Google Scholar] [CrossRef] [PubMed]
- Marvila, M.T.; Garcez de Azevedo, A.R.; Tostes Linhares Júnior, J.A.; Fontes Vieira, C.M. Activated alkali cement based on blast furnace slag: Effect of curing type and concentration of Na2O. J. Mater. Res. Technol. 2023, 23, 4551–4565. [Google Scholar] [CrossRef]
- Fu, Q.; Bu, M.; Zhang, Z.; Xu, W.; Yuan, Q.; Niu, D. Hydration Characteristics and Microstructure of Alkali-Activated Slag Concrete: A Review. Engineering 2021, 20, 162–179. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment—A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Yang, K.-H.; Cho, A.-R.; Song, J.-K.; Nam, S.-H. Hydration products and strength development of calcium hydroxide-based alkali-activated slag mortars. Constr. Build. Mater. 2012, 29, 410–419. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Ou, Z. Effect of alkali dosage on alkali-silica reaction in sodium hydroxide activated slag mortars. Constr. Build. Mater. 2017, 143, 16–23. [Google Scholar] [CrossRef]
- Fang, S.; Lam, E.S.S.; Li, B.; Wu, B. Effect of alkali contents, moduli and curing time on engineering properties of alkali activated slag. Constr. Build. Mater. 2020, 249, 118799. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D.A. Alkali–silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Brice, D.G. Chemical Research and Climate Change as Drivers in the Commercial Adoption of Alkali Activated Materials. Waste Biomass Valorization 2010, 1, 145–155. [Google Scholar] [CrossRef]
- Duran Atiş, C.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Bernal, S.A. Advances in near-neutral salts activation of blast furnace slags. RILEM Tech. Lett. 2016, 1, 39–44. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, B.; Wang, L.; Long, G.; Xie, Y. Clean and low-alkalinity one-part geopolymeric cement: Effects of sodium sulfate on microstructure and properties. J. Clean. Prod. 2019, 252, 119279. [Google Scholar] [CrossRef]
- Kozhukhova, N.I.; Alfimova, N.I.; Kozhukhova, M.I.; Nikulin, I.S.; Glazkov, R.A.; Kolomytceva, A.I. The Effect of Recycled Citrogypsum as a Supplementary Mineral Additive on the Physical and Mechanical Performance of Granulated Blast Furnace Slag-Based Alkali-Activated Binders. Recycling 2023, 8, 22. [Google Scholar] [CrossRef]
- Kozhukhova, N.I.; Shurakov, I.M.; Kozhukhova, M.I.; Yu Elistratkin, M.; Alfimova, N.I. Understanding the relationship between composition and rheology in alkali-activated binders. J. Phys. Conf. Ser. 2021, 2124, 012004. [Google Scholar] [CrossRef]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Milestone, N.B.; Collier, N.C. Hydration and properties of sodium sulfate activated slag. Cem. Concr. Compos. 2013, 37, 20–29. [Google Scholar] [CrossRef]
- Chen, W.; Brouwers, H.J.H. The hydration of slag, part 1: Reaction models for alkali-activated slag. J. Mater. Sci. 2006, 42, 428–443. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y. Preliminary study on combined-alkali-slag paste materials. Constr. Build. Mater. 2000, 30, 963–966. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, J.G.; Puertas, F. Alkali-activated slag mortars Mechanical strength behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Huo, J.-H.; Yu, B.-S.; Peng, Z.-G.; Wu, Z.-S.; Zhang, L.-H. Thermal control effects and mechanism of slag and fly ash on heat development of cement slurry used in hydrate formation. J. Nat. Gas Sci. Eng. 2021, 91, 103967. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Z.; Zhu, H.; Zhang, W.; Gao, Y.; Zhang, X.; Wu, Q. Effects of calcined dolomite addition on reaction kinetics of one-part sodium carbonate-activated slag cements. Constr. Build. Mater. 2019, 211, 329–336. [Google Scholar] [CrossRef]
- He, J.; Bai, W.; Zheng, W.; He, J.; Sang, G. Influence of hydrated lime on mechanical and shrinkage properties of alkali-activated slag cement. Constr. Build. Mater. 2021, 289, 123201. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Yang, T.; Zhou, S.; Wei, H.; Zhang, Z. Calcium carbide residue as auxiliary activator for one-part sodium carbonate-activated slag cements: Compressive strength, phase assemblage and environmental benefits. Constr. Build. Mater. 2021, 308, 125015. [Google Scholar] [CrossRef]
- GB/T 18046-2017; Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete. SAC: Beijing, China, 2017.
- YB/T 4019-2006; Test Methods for Chemical Activity of Caustic Burned Magnesia. SAC: Beijing, China, 2006.
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. SAC: Beijing, China, 2011.
- GB/T 17671-2022; Method of Testing Cements-Determination of Strength. SAC: Beijing, China, 2021.
- Sujin Song, H.M.J. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar] [CrossRef]
- Jeong, Y.; Oh, J.E.; Jun, Y.; Park, J.; Ha, J.-H.; Sohn, S.G. Influence of four additional activators on hydrated-lime [Ca(OH)2] activated ground granulated blast-furnace slag. Cem. Concr. Compos. 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Moseson, A.J.; Moseson, D.E.; Barsoum, M.W. High volume limestone alkali-activated cement developed by design of experiment. Cem. Concr. Compos. 2012, 34, 328–336. [Google Scholar] [CrossRef]
- Hangxing, D.; Shiyu, Z.; Xiaolong, Z.; Zhaohao, Z.; Yingliang, Z. Low carbon cementitious composites: Calcined quarry dust modified lime/sodium sulfate-activated slag. Constr. Build. Mater. 2021, 282, 122521. [Google Scholar] [CrossRef]
- Sahu, P.; Upadhyay, S.C.; Bhatti, S.; Mahey, J.K.; Sanghavi, R.J.; Kumar, A. Sodium-sulphate production from sulphate-rich bittern: A parametric study and economic evaluation. J. Environ. Chem. Eng. 2021, 9, 105632. [Google Scholar] [CrossRef]
- Nguyen, L.; Moseson, A.J.; Farnam, Y.; Spatari, S. Effects of composition and transportation logistics on environmental, energy and cost metrics for the production of alternative cementitious binders. J. Clean. Prod. 2018, 185, 628–645. [Google Scholar] [CrossRef]
- Yang, K.-H.; Song, J.-K.; Song, K.-I. Assessment of CO2 reduction of alkali-activated concrete. J. Clean. Prod. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Fawer, M.; Concannon, M.; Rieber, W. Life cycle inventories for the production of sodium silicates. Int. J. Life Cycle Assess. 1999, 4, 207–212. [Google Scholar] [CrossRef]


| Composition | GBFS | Reactive MgO |
|---|---|---|
| CaO | 41.15 | 5.07 |
| MgO | 7.67 | 87.00 |
| Al2O3 | 14.41 | 0.58 |
| Fe2O3 | 0.49 | 0.99 |
| SiO2 | 30.91 | 5.21 |
| SO3 | 1.61 | 0.86 |
| Na2O | 0.75 | 0.02 |
| K2O | 0.57 | 0.06 |
| Loss of ignition | 2.44 | 0.21 |
| Level | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Na2SO4 (%) | Na2CO3 (%) | Reactive MgO (%) | Ca(OH)2 (%) | Na2SO4 ×Ca(OH)2 | Na2CO3 ×Ca(OH)2 | |
| 1 | 3 | 2 | 2 | 3 | 1 | 1 |
| 2 | 5 | 3 | 3 | 4 | 2 | 2 |
| 3 | 7 | 4 | 4 | 5 | 3 | 3 |
| 4 | 9 | 5 | 5 | 6 | 4 | 4 |
| 5 | 7 | 4 | 3 | 4 | 5 | 5 |
| Serial Number | Na2SO4 (%) | Na2CO3 (%) | Reactive MgO(%) | Ca(OH)2 (%) | Na2SO4 ×Ca(OH)2 | Na2CO3 ×Ca(OH)2 | nCa(OH)2 /nNa2CO3 | nCa(OH)2 /nNa2SO4 | nCa(OH)2 /(nNa2SO4 + nNa2CO3) |
|---|---|---|---|---|---|---|---|---|---|
| N1 | 3 | 2 | 2 | 3 | 1 | 1 | 2.1 | 1.9 | 1 |
| N2 | 3 | 3 | 3 | 4 | 2 | 2 | 1.9 | 2.6 | 1.1 |
| N3 | 3 | 4 | 4 | 5 | 3 | 3 | 1.8 | 3.2 | 1.1 |
| N4 | 3 | 5 | 5 | 6 | 4 | 4 | 1.7 | 3.8 | 1.2 |
| N5 | 3 | 4 | 3 | 4 | 5 | 5 | 1.4 | 2.6 | 0.9 |
| N6 | 5 | 2 | 3 | 5 | 4 | 5 | 3.6 | 1.9 | 1.2 |
| N7 | 5 | 3 | 4 | 6 | 5 | 1 | 2.9 | 2.3 | 1.3 |
| N8 | 5 | 4 | 5 | 4 | 1 | 2 | 1.4 | 1.5 | 0.7 |
| N9 | 5 | 5 | 3 | 3 | 2 | 3 | 0.9 | 1.2 | 0.5 |
| N10 | 5 | 4 | 2 | 4 | 3 | 4 | 1.4 | 1.5 | 0.7 |
| N11 | 7 | 2 | 4 | 4 | 2 | 4 | 2.9 | 1.1 | 0.8 |
| N12 | 7 | 3 | 5 | 3 | 3 | 5 | 1.4 | 0.8 | 0.5 |
| N13 | 7 | 4 | 3 | 4 | 4 | 1 | 1.4 | 1.1 | 0.6 |
| N14 | 7 | 5 | 2 | 5 | 5 | 2 | 1.4 | 1.4 | 0.7 |
| N15 | 7 | 4 | 3 | 6 | 1 | 3 | 2.1 | 1.6 | 0.9 |
| N16 | 9 | 2 | 5 | 4 | 5 | 3 | 2.9 | 0.9 | 0.7 |
| N17 | 9 | 3 | 3 | 5 | 1 | 4 | 2.4 | 1.1 | 0.7 |
| N18 | 9 | 4 | 2 | 6 | 2 | 5 | 2.1 | 1.3 | 0.8 |
| N19 | 9 | 5 | 3 | 4 | 3 | 1 | 1.1 | 0.9 | 0.5 |
| N20 | 9 | 4 | 4 | 3 | 4 | 2 | 1.1 | 0.6 | 0.4 |
| N21 | 7 | 2 | 3 | 6 | 3 | 2 | 4.3 | 1.6 | 1.2 |
| N22 | 7 | 3 | 2 | 4 | 4 | 3 | 1.9 | 1.1 | 0.7 |
| N23 | 7 | 4 | 3 | 3 | 5 | 4 | 1.1 | 0.8 | 0.5 |
| N24 | 7 | 5 | 4 | 4 | 1 | 5 | 1.1 | 1.1 | 0.6 |
| N25 | 7 | 4 | 5 | 5 | 2 | 1 | 1.8 | 1.4 | 0.8 |
| Serial Number | Initial Setting Time (min) | Final Setting Time (min) | 3-Day Flexural Strength (MPa) | 3-Day Compressive Strength (MPa) | 28-Day Flexural Strength (MPa) | 28-Day Compressive Strength (MPa) |
|---|---|---|---|---|---|---|
| N1 | 80 | 140 | 5.1 | 21.9 | 6.2 | 26.9 |
| N2 | 58 | 83 | 5.6 | 27.0 | 7.3 | 33.2 |
| N3 | 49 | 77 | 5.9 | 26.9 | 7.6 | 34.7 |
| N4 | 44 | 67 | 5.9 | 25.3 | 6.9 | 35.5 |
| N5 | 71 | 105 | 6.1 | 27.4 | 7.7 | 35.0 |
| N6 | 49 | 66 | 6.0 | 18.3 | 7.4 | 28.4 |
| N7 | 47 | 53 | 6.7 | 25.8 | 7.6 | 36.8 |
| N8 | 49 | 79 | 6.4 | 26.2 | 7.7 | 44.4 |
| N9 | 59 | 77 | 6.6 | 31.5 | 7.8 | 36.7 |
| N10 | 49 | 68 | 7.2 | 30.1 | 7.9 | 37.6 |
| N11 | 51 | 73 | 6.2 | 19.4 | 7.3 | 29.6 |
| N12 | 47 | 69 | 6.5 | 25.7 | 8.5 | 34.3 |
| N13 | 51 | 88 | 7.2 | 27.8 | 8.0 | 39.0 |
| N14 | 50 | 87 | 6.8 | 29.0 | 8.2 | 37.2 |
| N15 | 43 | 66 | 6.7 | 30.1 | 7.6 | 35.6 |
| N16 | 44 | 68 | 6.9 | 28.0 | 8.2 | 37.6 |
| N17 | 45 | 76 | 7.7 | 27.5 | 8.5 | 34.8 |
| N18 | 47 | 77 | 6.6 | 23.7 | 7.5 | 32.2 |
| N19 | 81 | 151 | 7.3 | 29.8 | 7.7 | 36.2 |
| N20 | 79 | 128 | 6.8 | 29.1 | 8.0 | 39.2 |
| N21 | 52 | 72 | 6.2 | 18.0 | 8.2 | 31.5 |
| N22 | 56 | 72 | 7.2 | 27.2 | 8.8 | 38.2 |
| N23 | 49 | 99 | 7.0 | 28.0 | 8.9 | 41.1 |
| N24 | 49 | 94 | 7.5 | 31.9 | 9.1 | 43.0 |
| N25 | 47 | 64 | 7.9 | 30.6 | 9.0 | 44.6 |
| D (Ca(OH)2) | D1 | D2 | D3 | D4 | |
|---|---|---|---|---|---|
| B (Na2CO3) | |||||
| B1 | 80 | 48 | 49 | 52 | |
| B2 | 47 | 57 | 45 | 47 | |
| B3 | 64 | 55 | 48 | 45 | |
| B4 | 59 | 65 | 50 | 44 | |
| Factors | R′ of 3-Day Flexural Strength | R′ of 3-Day Compressive Strength | R′ of 28-Day Flexural Strength | R′ of 28-Day Compressive Strength |
|---|---|---|---|---|
| A (Na2SO4) | 1.48 | 2.12 | 1.34 | 4.79 |
| B (Na2CO3) | 0.82 | 9.24 | 0.75 | 8.31 |
| C (Reactive MgO) | 0.15 | 0.86 | 0.37 | 5.36 |
| D (Ca(OH)2) | 0.51 | 3.20 | 0.64 | 3.37 |
| A × D (Na2SO4 ×Ca(OH)2) | 0.11 | 1.88 | 0.30 | 2.40 |
| B × D (Na2CO3 ×Ca(OH)2) | 0.43 | 2.99 | 0.30 | 2.25 |
| Serial Number | Initial Setting Time (min) | Final Setting Time (min) | 3-Day Flexural Strength (MPa) | 3-Day Compressive Strength (Mpa) | 28-Day Flexural Strength (Mpa) | 28-Day Compressive Strength (Mpa) |
|---|---|---|---|---|---|---|
| N13 | 51 | 88 | 7.2 | 27.8 | 8.0 | 39.0 |
| N23 | 49 | 99 | 7.0 | 28.0 | 8.9 | 41.1 |
| N24 | 49 | 94 | 7.5 | 31.9 | 9.1 | 43.0 |
| N25 | 47 | 64 | 7.9 | 30.6 | 9.0 | 44.6 |
| N8 | 49 | 79 | 6.4 | 26.2 | 7.7 | 44.4 |
| Cost (CNY/t) c | CO2 Emission (kg/t) d | Energy Consumption (GJ/t) d | Reference | |
|---|---|---|---|---|
| Na2CO3 | 1580 | 111 | 1.3 | [36] |
| Na2SO4 | 720 | 640 | 0.3 | [37,38] |
| NH | 3800 | 750 | 9.5 | [39] |
| Ca(OH)2 | 700 | 830 | 4 | [36] |
| Reactive MgO a | 450 | 1200 | 6.3 | |
| P·O 42.5 | 525 | 944 | 5.5 | [17,40] |
| GBFS | 330 | 70 | 0.1 | [39] |
| WG b | 1100 | 425 | 4.6 | [41] |
| Cementitious Material | GBFS | Na2SO4 | Na2CO3 | Ca(OH)2 | Reactive MgO | P·O 42.5 | NH | WG |
|---|---|---|---|---|---|---|---|---|
| P·O 42.5 | 100 | |||||||
| N25 | 79 | 7 | 4 | 5 | 5 | |||
| NH-AAS cement | 93 | 7 | ||||||
| WG AAS cement a | 73.6 | 9.1 | 17.3 |
| Cementitious Material | 28-Day Compressive Strength (MPa) | Cost/Strength (CNY/(MPa)) | CO2 Emission/Strength (kg/(MPa)) | Energy Consumption/Strength (GJ/(MPa)) |
|---|---|---|---|---|
| P·O 42.5 | 43.5 | 0.0054 | 9.77 | 0.057 |
| N25-AAS cement | 44.6 | 0.0046 | 2.14 | 0.007 |
| NH-AAS cement | 38.0 | 0.0068 | 1.39 | 0.009 |
| WG | 68.0 | 0.0052 | 1.28 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Yu, S.; Sang, G.; He, J.; Wang, J.; Chen, Z. Properties of Alkali-Activated Slag Cement Activated by Weakly Alkaline Activator. Materials 2023, 16, 3871. https://doi.org/10.3390/ma16103871
He J, Yu S, Sang G, He J, Wang J, Chen Z. Properties of Alkali-Activated Slag Cement Activated by Weakly Alkaline Activator. Materials. 2023; 16(10):3871. https://doi.org/10.3390/ma16103871
Chicago/Turabian StyleHe, Juan, Shuya Yu, Guochen Sang, Junhong He, Jie Wang, and Zheng Chen. 2023. "Properties of Alkali-Activated Slag Cement Activated by Weakly Alkaline Activator" Materials 16, no. 10: 3871. https://doi.org/10.3390/ma16103871
APA StyleHe, J., Yu, S., Sang, G., He, J., Wang, J., & Chen, Z. (2023). Properties of Alkali-Activated Slag Cement Activated by Weakly Alkaline Activator. Materials, 16(10), 3871. https://doi.org/10.3390/ma16103871





