Insight into the Desolvation of Quaternary Ammonium Cation with Acetonitrile as a Solvent in Hydroxyl-Flat Pores: A First-Principles Calculation
Abstract
1. Introduction
2. Calculation Method
3. Results and Discussion
3.1. Reaction Principles
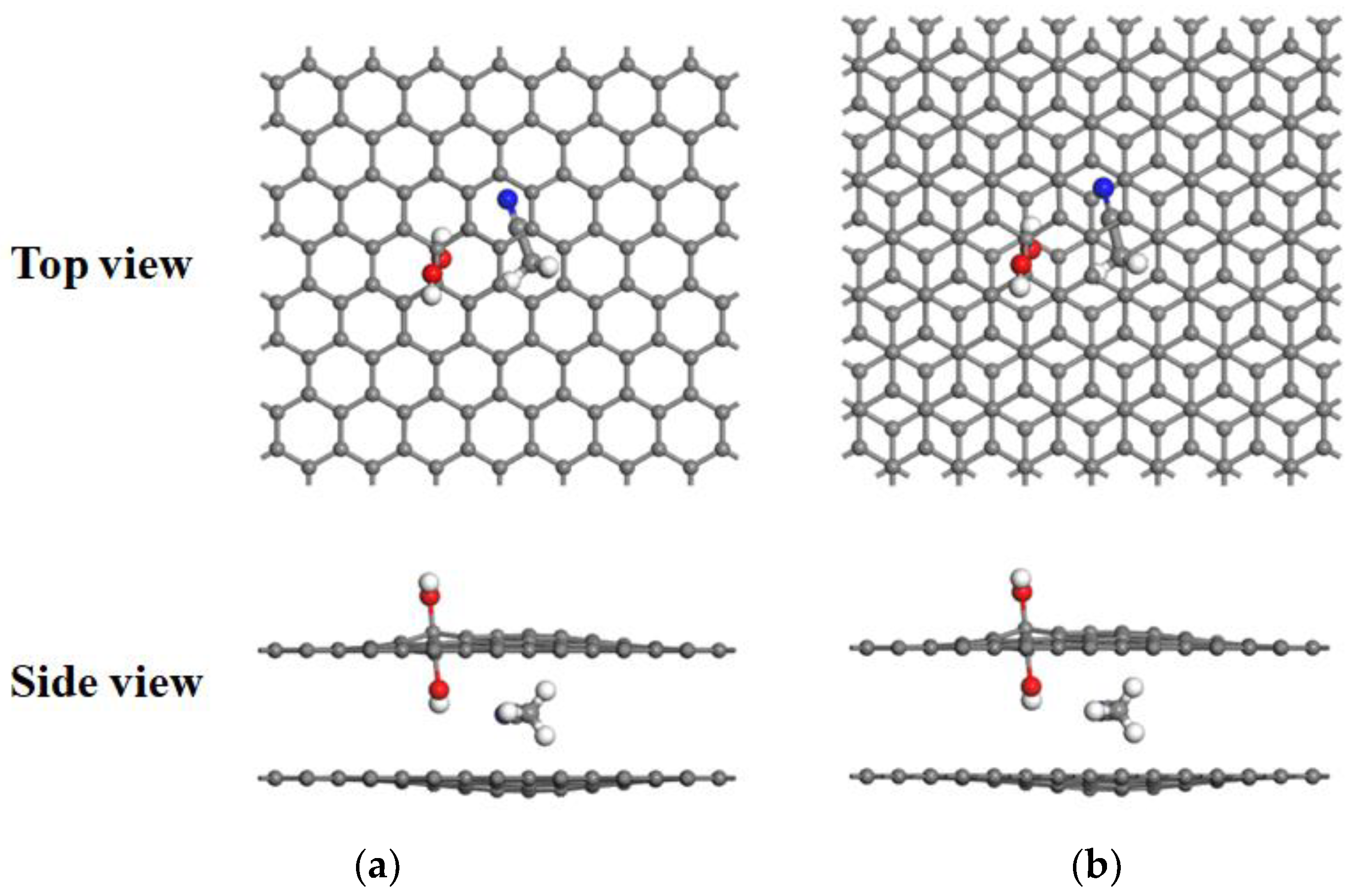
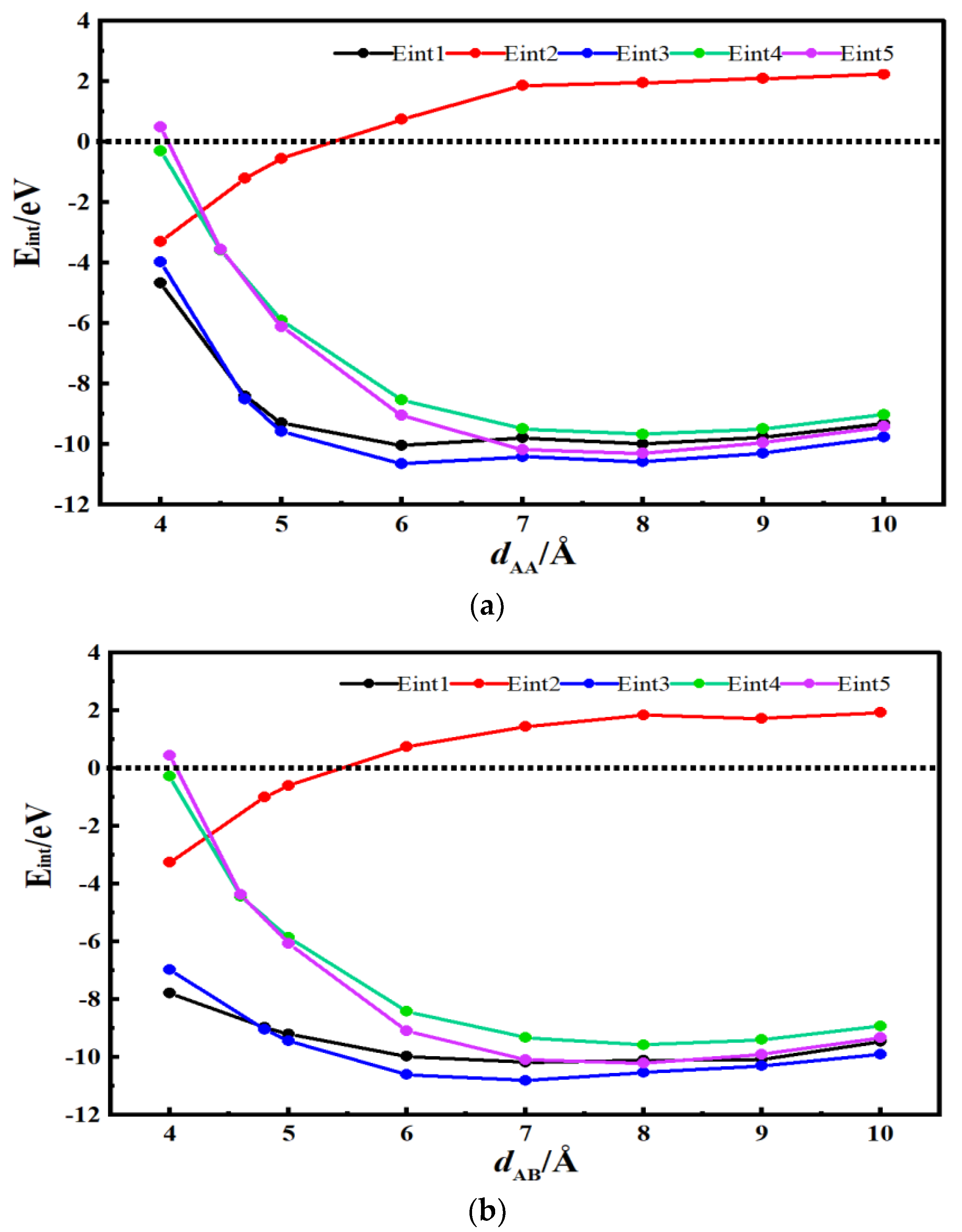
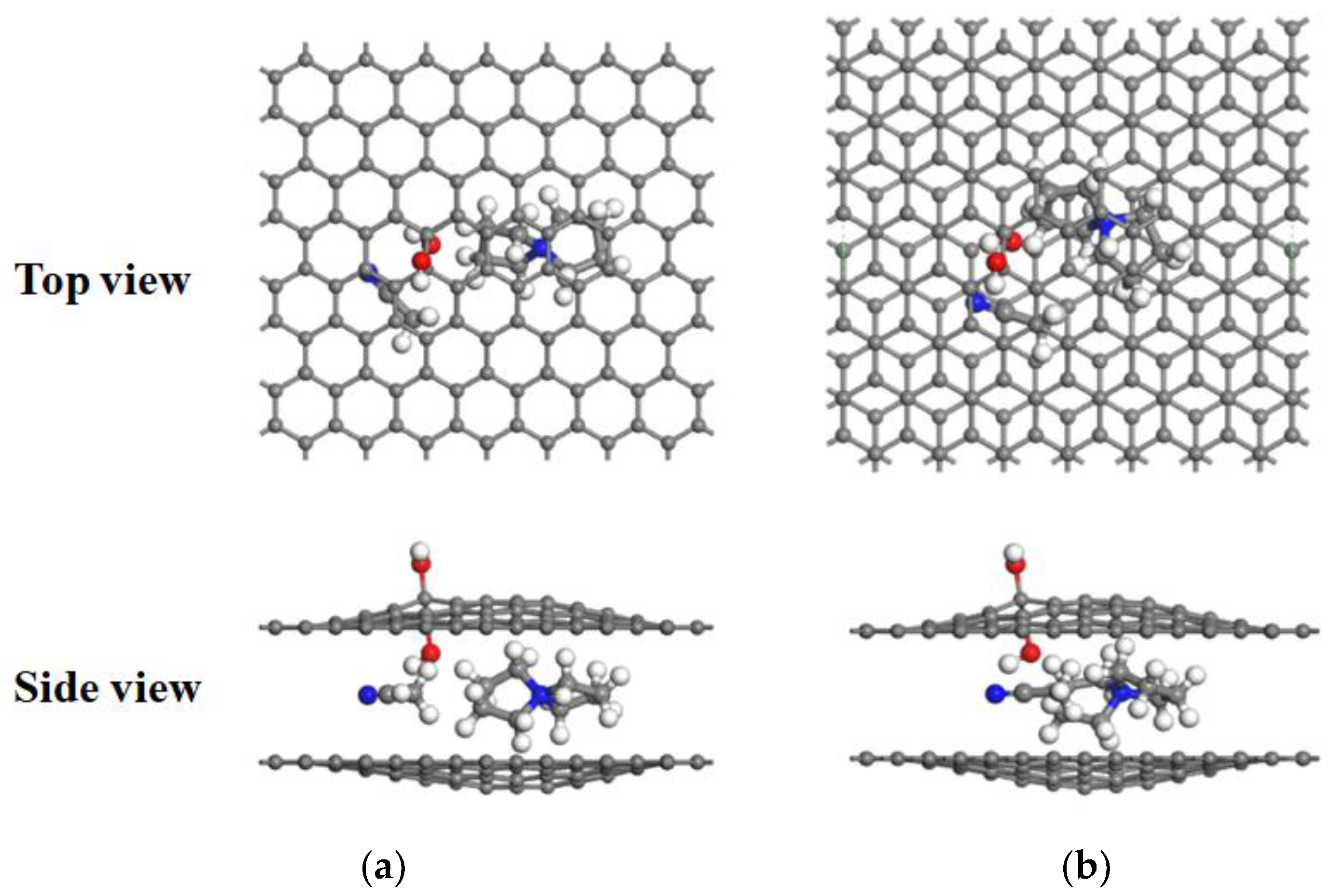
3.2. Desolvation of TEA+ Complexes
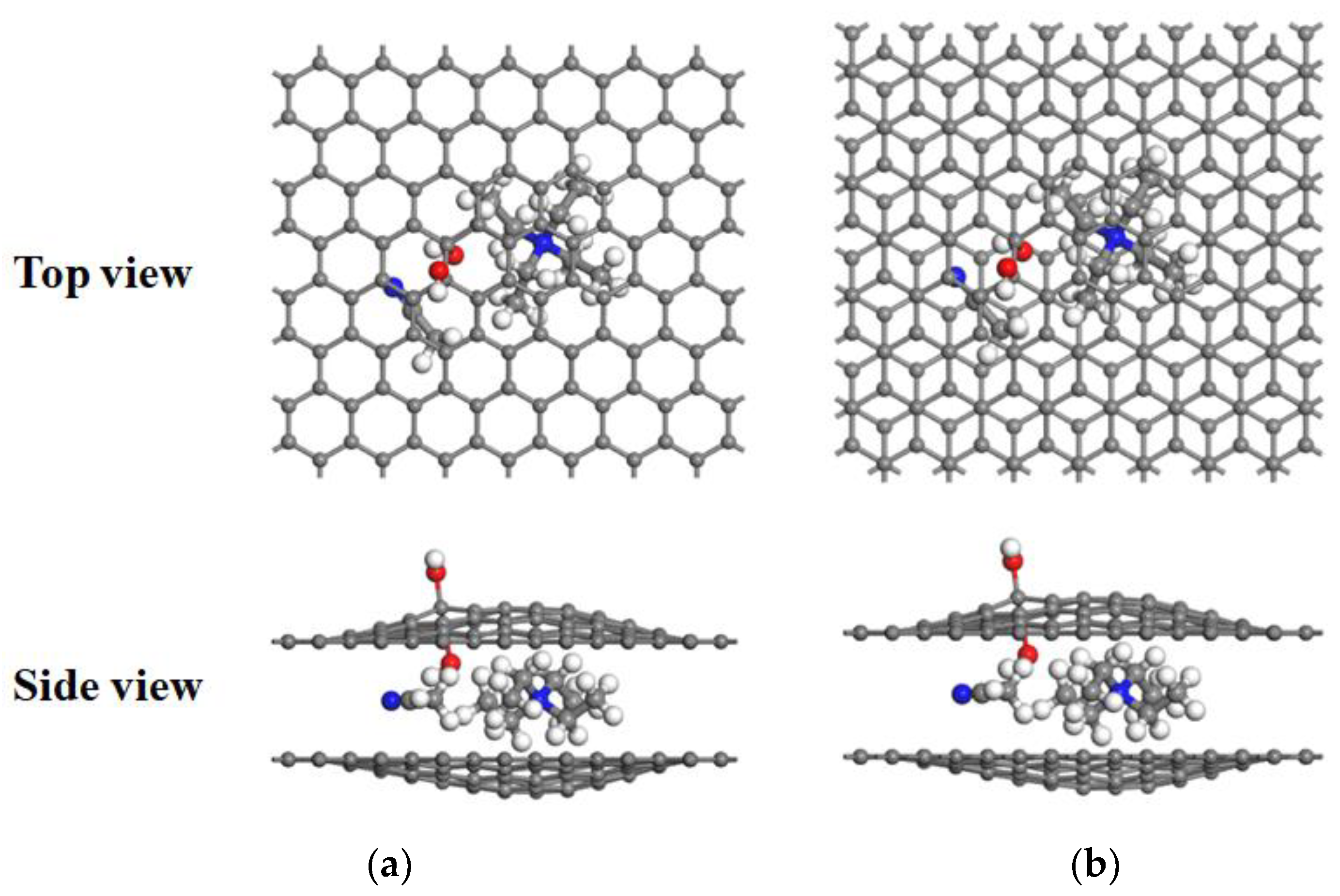
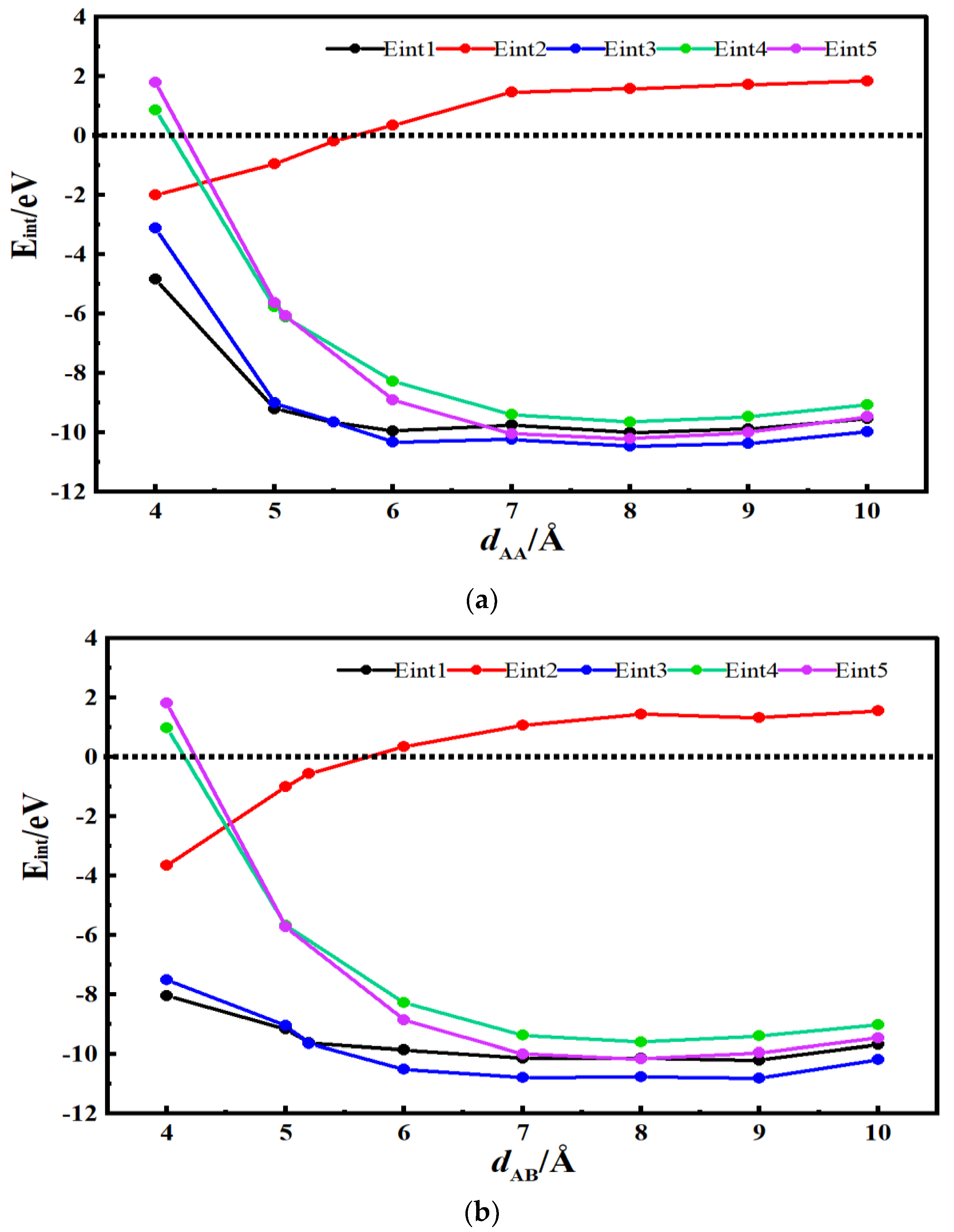
3.3. Desolvation of SBP+ Complexes
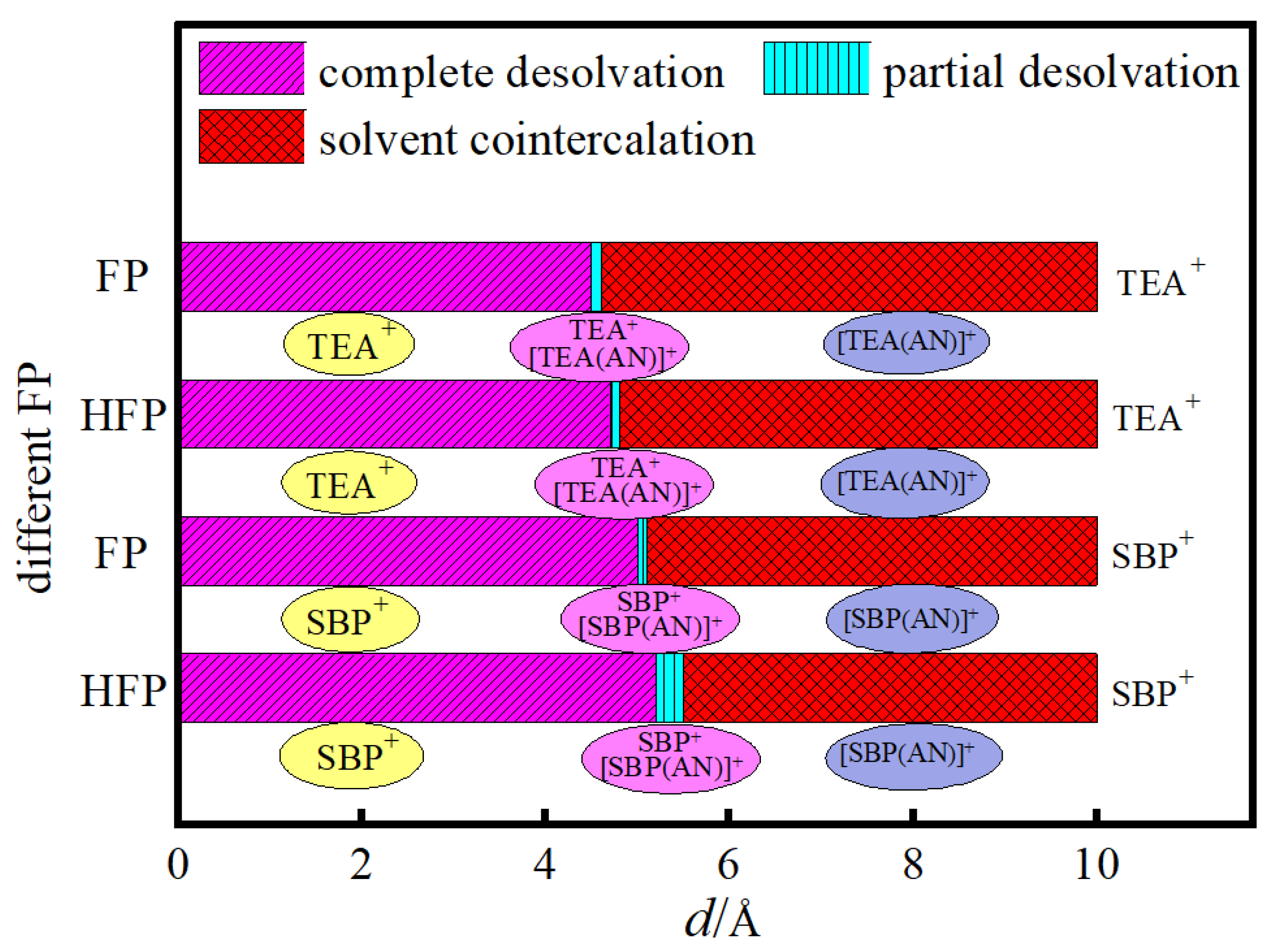
3.4. Analysis of Critical Size for Desolvation of Quaternary Ammonium Cationic Complexes
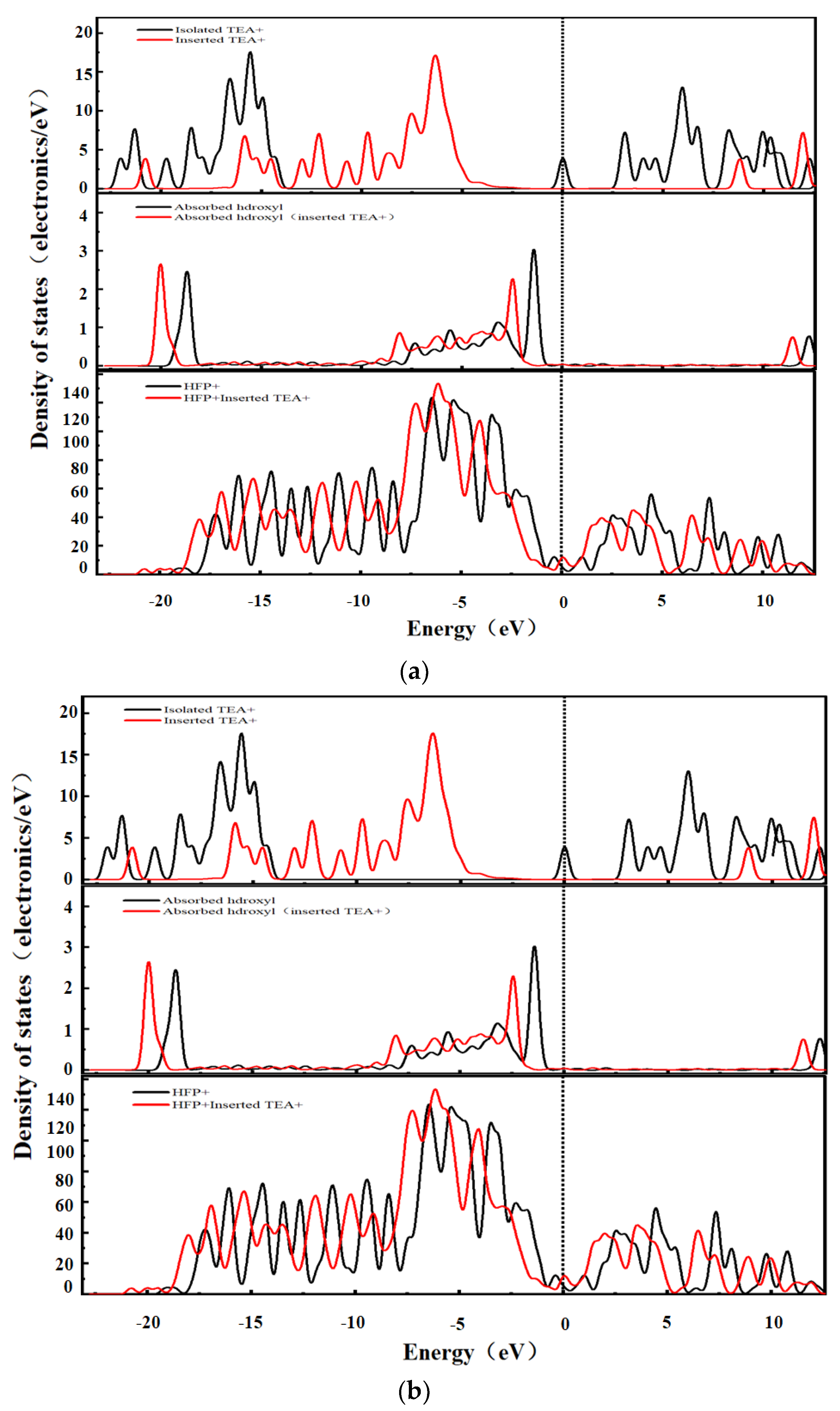
3.5. Density of States (DOS) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.C.; Ding, M.J.; Wang, X.C. Research on control of bidirectional DC-DC converter based on a supercapacitor. J. Power Supply 2021, 19, 129–139. [Google Scholar]
- Ghouri, A.S.; Aslam, R.; Siddiqui, M.S. Recent progress in textile-based flexible supercapacitor. Front. Mater. 2020, 7, 58. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Jiao, Y.; Yang, H.; Li, Y.; Zhang, J.; Gao, P. The grapheme/lanthanum oxide nanocomposites as electrode materials of supercapacitor. J. Power Sources 2019, 419, 99–105. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Korenblit, Y.; Kajdos, A.; West, W.C. In Situ studies of ion transport in microporous supercapacitor electrodes at ultralow temperatures. Adv. Funct. Mater. 2012, 22, 1655–1662. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Beguin, F.; Presser, V.; Balduccl, A. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Mayer, C.J.; Geim, K.A.; Katanelson, I.M. The structure of suspended grapheme sheets. Nat. Mater. 2007, 446, 60–63. [Google Scholar]
- Novoselov, S.K.; Geim, K.A.; Morozov, V.S. Two-dimensional gas of massless dirac fermions in grapheme. Nat. Mater. 2005, 438, 197–200. [Google Scholar]
- Fong, R.; Von, S.U.; Dahn, J.R. Studies of Lithium Intercalation into Carbons Using Nonaqueous Electrochemical Cells. J. Electrochem. Soc. 1990, 137, 2009. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Winter, M.; Yang, J.; Biberacher, W. Filming mechanism of lithium-carbon anodes in organic and inorganic electrolytes. J. Power Sources 1995, 54, 228. [Google Scholar] [CrossRef]
- Wang, H.; Yoshio, M. Effect of cation on the performance of AC/graphite capacitor. Electrochem. Commun. 2008, 10, 382–386. [Google Scholar] [CrossRef]
- Liang, C.; Guo, S.S.; Jie, S. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar]
- Yang, S.B.; Liu, X.L.; Zhang, X.; Tang, S.W. Insights into the effect of hydroxyl-, epoxy-, and carboxyl-pores on the desolvation of K+ with water as a solvent: A first-principles study. J. Phys. Condens. Matter 2021, 33, 445201. [Google Scholar] [CrossRef]
- Mattsson, A.E.; Schultz, P.A.; Desjarlais, M.P.; Mattsson, T.R.; Leung, K. Designing meaningful density functional theory calculations in materials science-a primer. Model Simul. Mater. Sci. Eng. 2004, 13, R1. [Google Scholar] [CrossRef]
- Rajagopal, A.K.; Callaway, J. Inhomogeneous Electron Gas. Phys. Rev. B 1973, 7, 1912–1919. [Google Scholar] [CrossRef]
- Seifert, G.; Porezag, D.; Frauenheim, T. Calculations of molecules, clusters, and solids with a simplified LCAO-DFT-LDA Scheme. Int. J. Quantum Chem. 1996, 58, 185–192. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Su, S.H.; Seifert, G. Self-consistent charge density functional tight-binding method for simulation of complex material properties. Phys. Rev. B 1998, 58, 7260. [Google Scholar] [CrossRef]
- Seifert, G. Tight-binding density functional theory: An approximate Kohn-Sham DFT scheme. J. Phys. Chem. A 2007, 111, 5609. [Google Scholar] [CrossRef]
- Segall, D.M.; Lindan, D.J.P.; Probert, J.M.; Pickard, J.C.; Hasnip, J.P.; Clark, J.S.; Payne, C.M. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717. [Google Scholar] [CrossRef]
- Zhao, P.; Su, Y.; Zhang, Y.; Li, S.J.; Chen, G. CO catalytic oxidation on iron-embedded hexagonal boron nitride sheets. Chem. Phys. Lett. 2011, 515, 159–162. [Google Scholar] [CrossRef]
- Wu, S.; Yan, G.; Cheng, X. First-principles investigation of the superconducting properties of thallium sulfide. Phys. C 2019, 562, 1–6. [Google Scholar] [CrossRef]
- Schira, R.; Latouche, C. DFT and hybrid-DFT calculations on the electronic properties of vanadate materials: Theory meets experiments. New J. Chem. 2020, 44, 11602. [Google Scholar] [CrossRef]
- Monkhorst, J.H.; Pack, D.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Tonel, M.Z.; Zanella, I.; Fagan, S.B. Theoretical study of small aromatic molecules adsorbed in pristineand functionalized graphene. J. Mol. Model. 2021, 27, 193. [Google Scholar] [CrossRef] [PubMed]
- Calborean, A.; Buimaga-Iarinca, L.; Graur, F. DFT charge transfer of hybrid molecular ferrocene/Si structures. Phys. Scr. 2015, 90, 055803. [Google Scholar] [CrossRef]
- Rabone, J.; Uffelen, P.V. DFT-based Metadynamics simulation of proton diffusion in tetragonal zirconia at 1500 K. J. Nucl. Mater. 2015, 459, 30–36. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.B.; Shan, X.Y. Insights into the effect of the interlayer spacings of bilayer graphene on the desolvation of H+, Li+, Na+, and K+ ions with water as a solvent: A first-principles study. Phys. Chem. 2019, 21, 23697. [Google Scholar] [CrossRef]
- Ghaderi, N.; Peressi, M. First-Principle study of hydroxyl functional groups on pristine, defected graphene, and graphene epoxide. J. Phys. Chem. C 2010, 114, 21625–21630. [Google Scholar] [CrossRef]
- Yan, J.A.; Chou, M.Y. Lithiation of the Two-Dimensional Silicon Carbide-Graphene vander Waals Heterostructure: A First Principles Study. J. Phys. Chem. C 2019, 123, 10738–10745. [Google Scholar]
- Leenaerts, O.; Partoens, B.; Peeters, F. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B. 2008, 77, 125416. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 5794. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.; Laguna, H.O.; Centeno, A.M. Microprocess technology for hydrogen purification. Renew. Hydrog. Technol. 2013, 10, 225–243. [Google Scholar]
- Janes, A.; Lust, E. Use of organic esters as co-solvents for electrical double layer capacitors with low temperature performance. J. Electroanal. Chem. 2006, 588, 285–295. [Google Scholar] [CrossRef]
- Yang, S.B.; Shan, X.Y.; Li, S.N. Insight into the Adsorption and Diffusion Behaviors of Na on XC3 (X=B, N and P) Doping Graphene Surfaces: A First Principle Study. Mater Rep. B Res. Pap. 2019, 33, 1640–1645. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Yang, S.; Zhang, X.; Tang, S.; Xia, Y. Insight into the Desolvation of Quaternary Ammonium Cation with Acetonitrile as a Solvent in Hydroxyl-Flat Pores: A First-Principles Calculation. Materials 2023, 16, 3858. https://doi.org/10.3390/ma16103858
Liu F, Yang S, Zhang X, Tang S, Xia Y. Insight into the Desolvation of Quaternary Ammonium Cation with Acetonitrile as a Solvent in Hydroxyl-Flat Pores: A First-Principles Calculation. Materials. 2023; 16(10):3858. https://doi.org/10.3390/ma16103858
Chicago/Turabian StyleLiu, Fudong, Shaobin Yang, Xu Zhang, Shuwei Tang, and Yingkai Xia. 2023. "Insight into the Desolvation of Quaternary Ammonium Cation with Acetonitrile as a Solvent in Hydroxyl-Flat Pores: A First-Principles Calculation" Materials 16, no. 10: 3858. https://doi.org/10.3390/ma16103858
APA StyleLiu, F., Yang, S., Zhang, X., Tang, S., & Xia, Y. (2023). Insight into the Desolvation of Quaternary Ammonium Cation with Acetonitrile as a Solvent in Hydroxyl-Flat Pores: A First-Principles Calculation. Materials, 16(10), 3858. https://doi.org/10.3390/ma16103858






