Review of Potential Drug-Eluting Contact Lens Technologies
Abstract
1. Introduction
2. Lenses
2.1. Initial Considerations
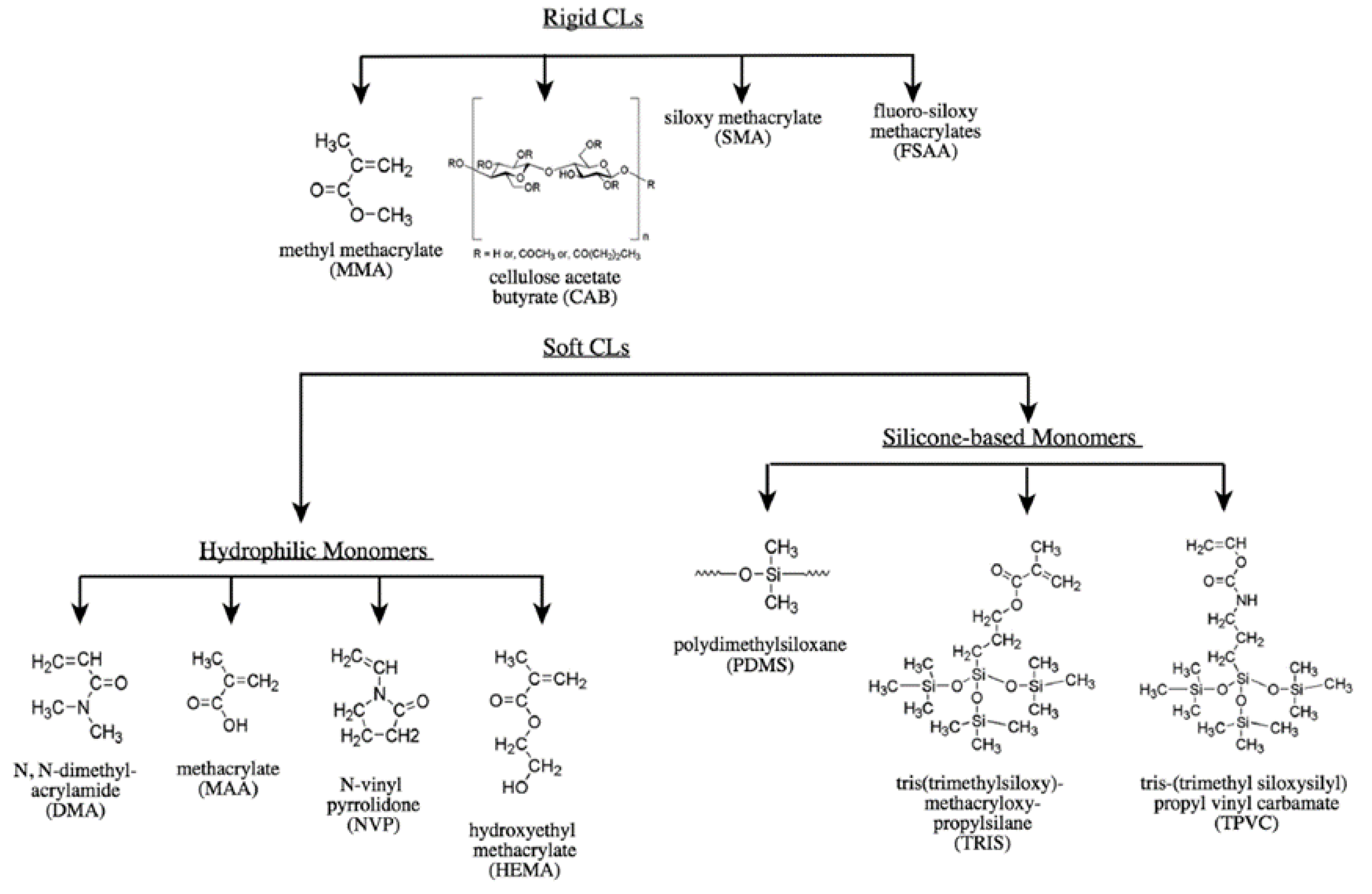
2.2. Lens Materials
2.2.1. “Classical” Hydrogels
2.2.2. Silicone Hydrogels
2.2.3. pHEMA
2.2.4. PLGA
3. Methods of Binding Drugs into/to Lenses
3.1. Layered Contact Lenses
3.2. Surface-Modified Contact Lenses
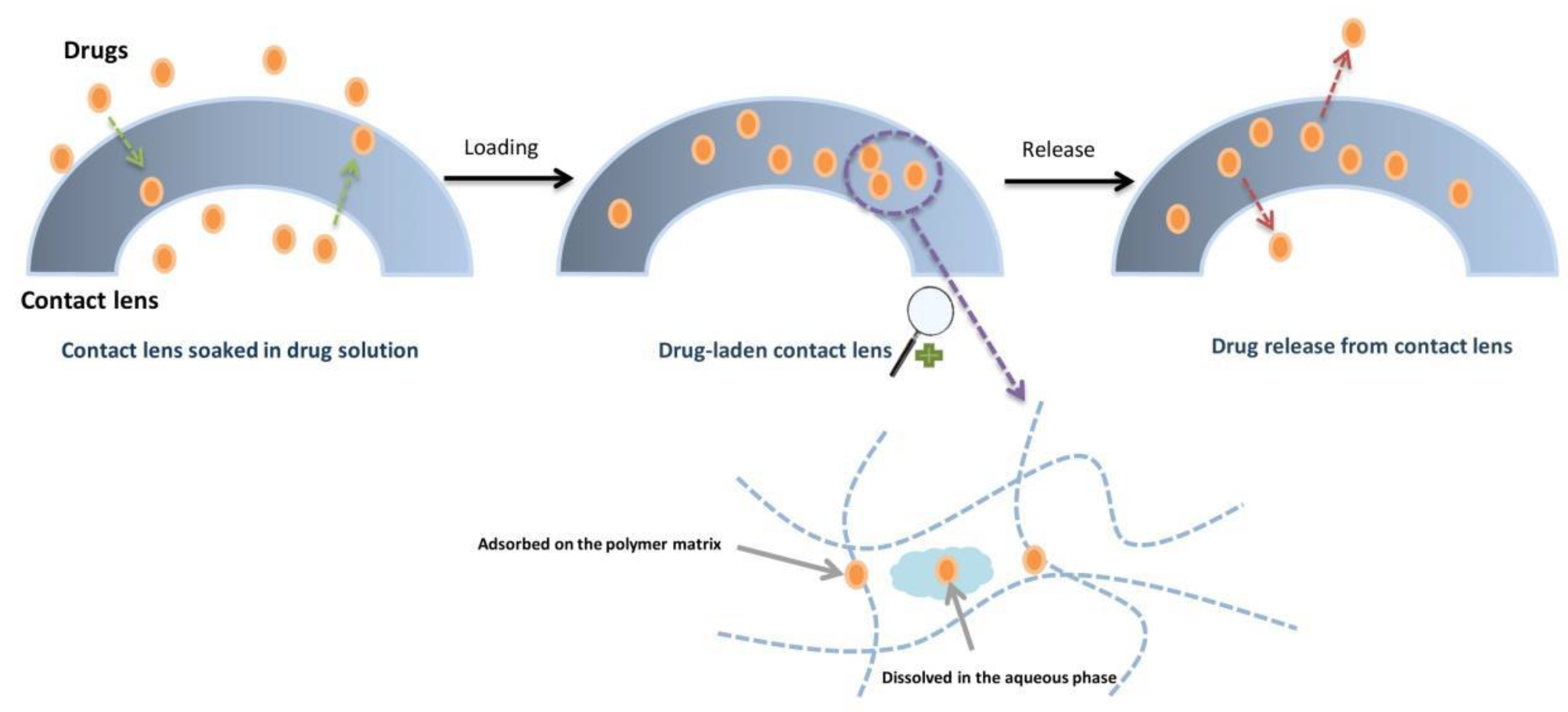
3.3. Soaking Method
3.4. Molecular Imprinting
3.5. Colloidal Nanoparticles
3.5.1. Polymeric Nanoparticles
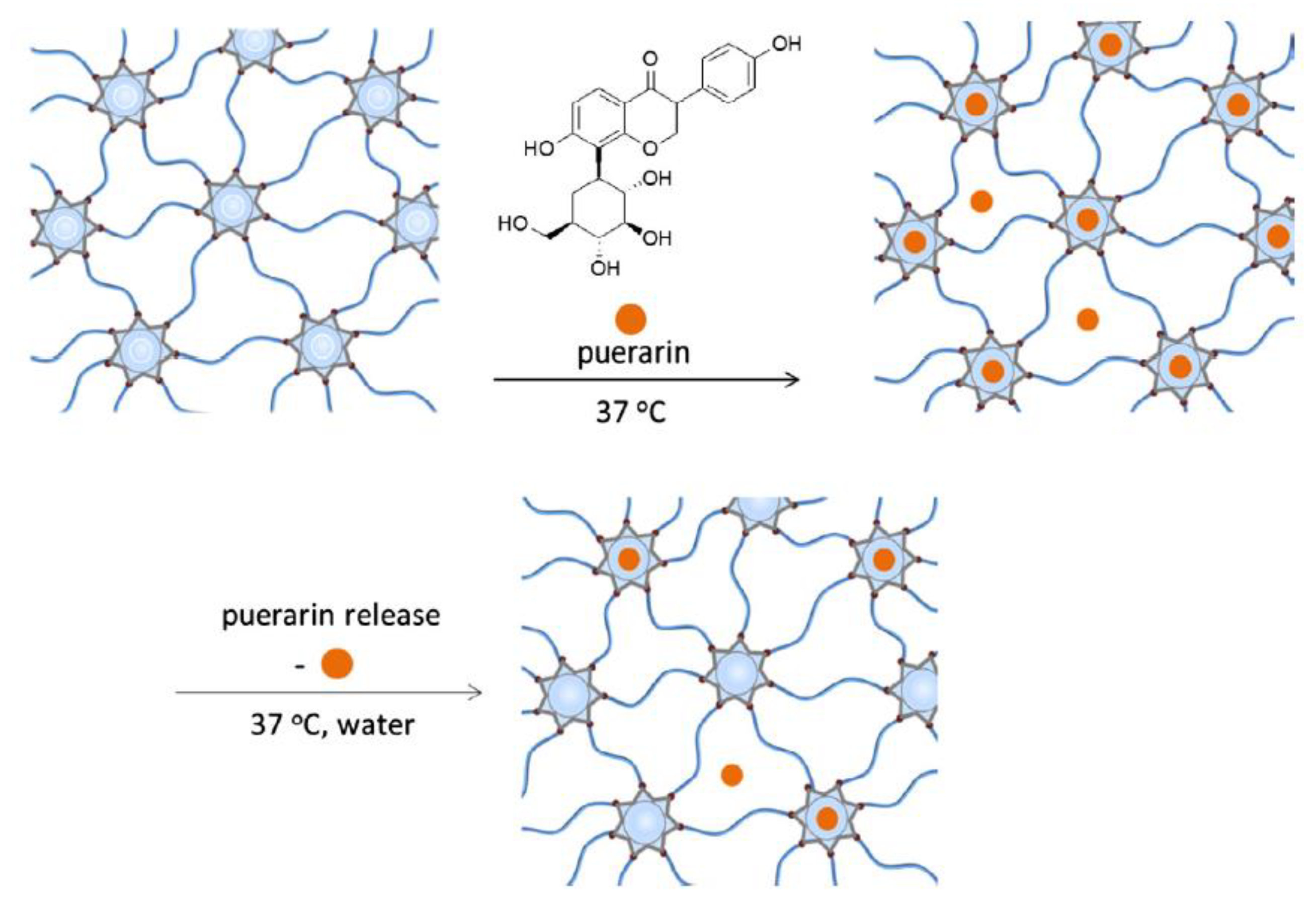
3.5.2. Cyclodextrins
3.5.3. Liposomes
3.5.4. Microemulsions and Micelles

3.6. Supercritical Fluid Technology (SCF)
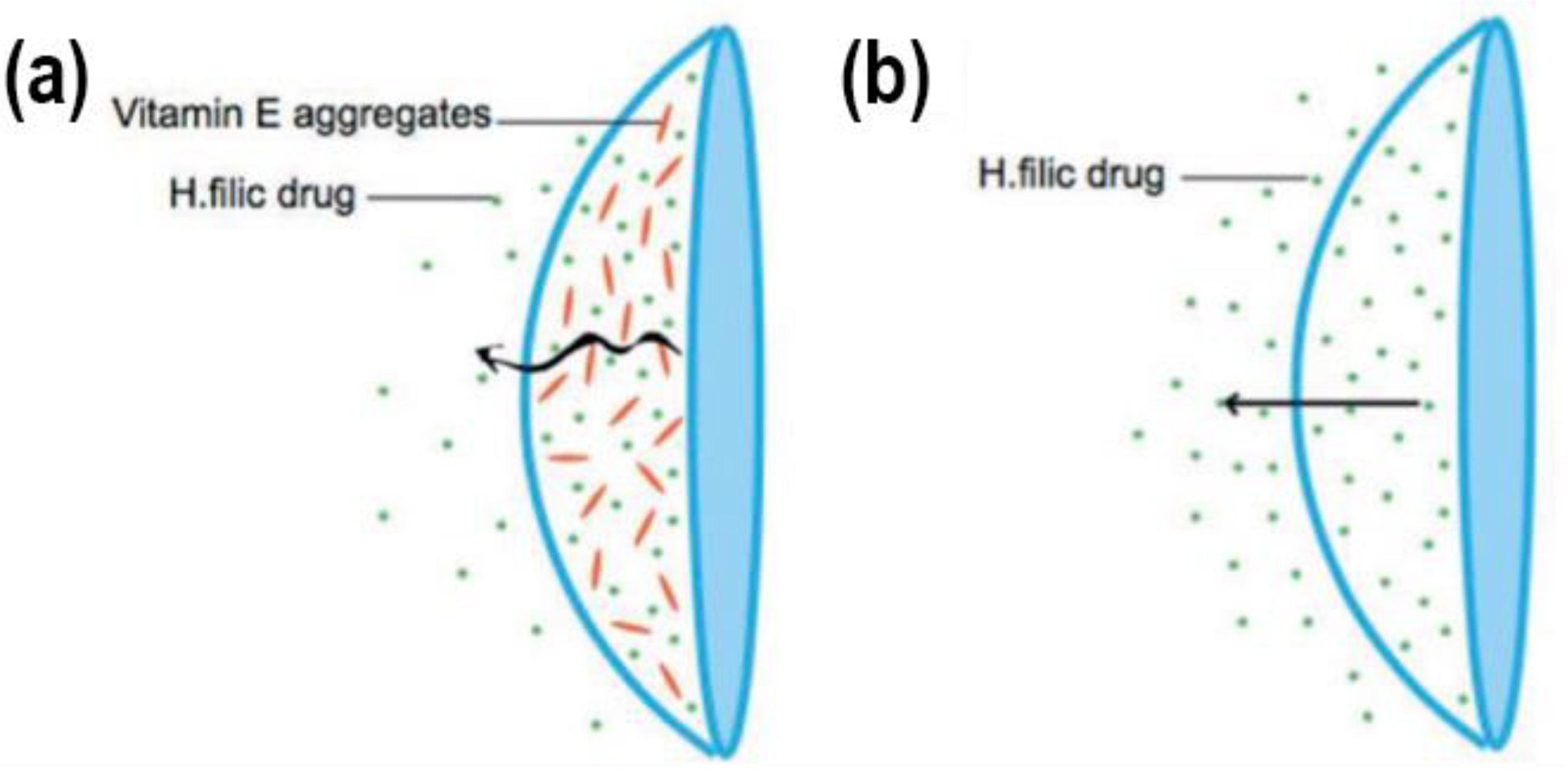
3.7. Vitamin E as a Release-Modifying Additive
3.8. Other Solutions
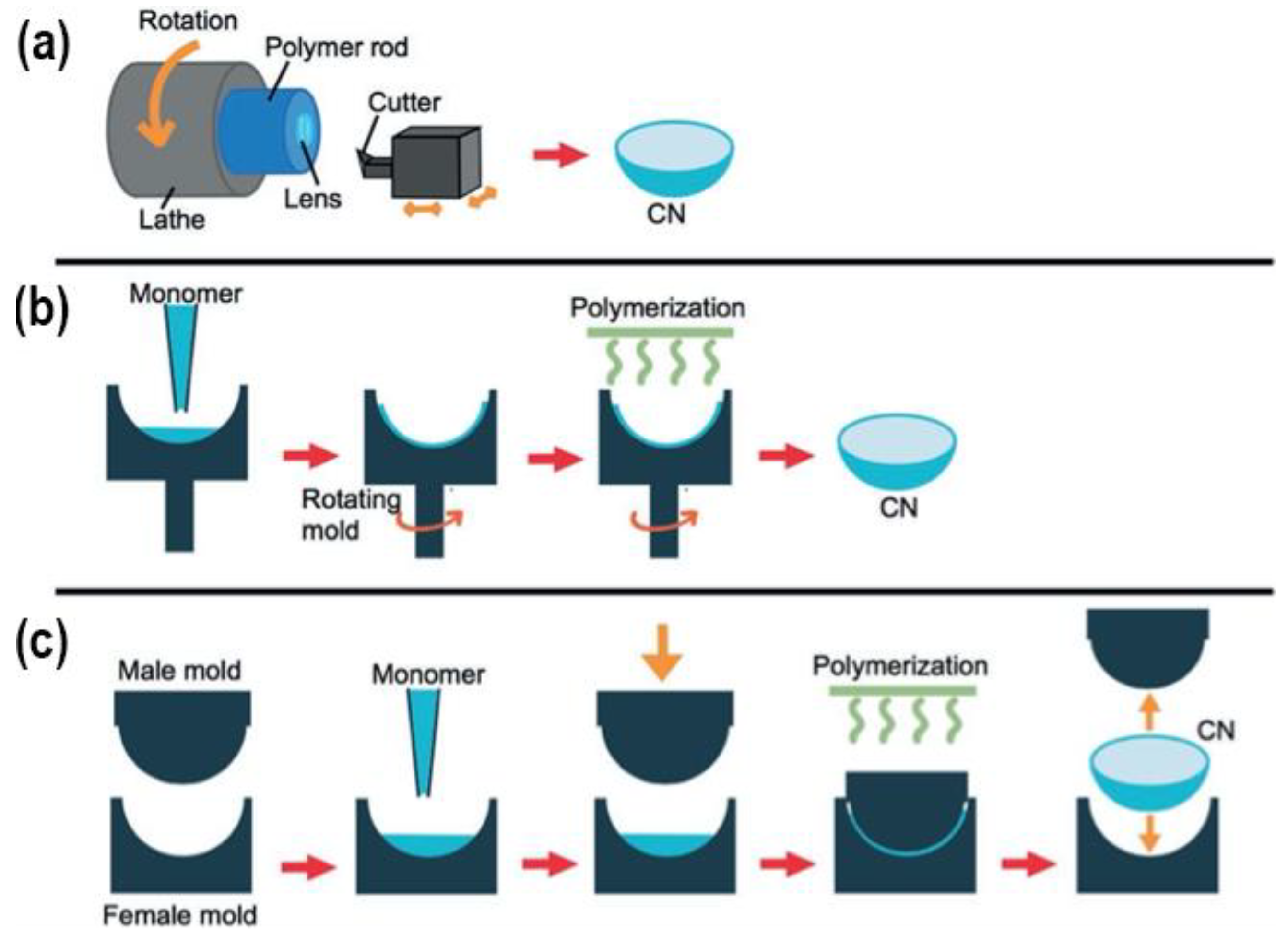
4. Contact Lenses Preparation Methods for Use in Drug-Delivery
4.1. Cast-Moulding Method
4.2. Lathe-Cutting Method
4.3. Spin-Casting Method
5. Future Prospects
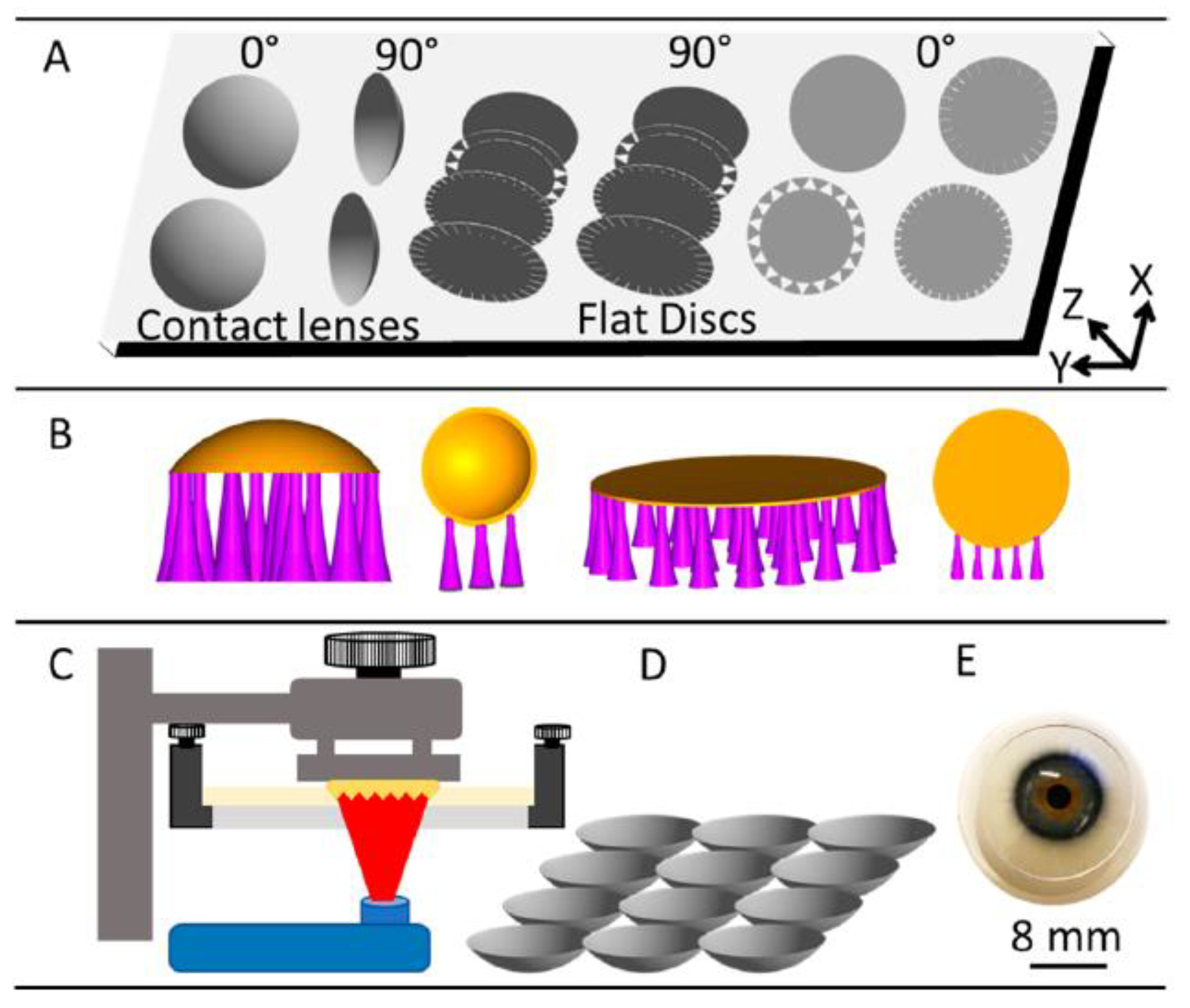
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitra, A.K. Role of Transporters in Ocular Drug Delivery System. Pharm. Res. 2009, 26, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A Comprehensive Review on Contact Lens for Ophthalmic Drug Delivery. J Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Lim, L.; Lim, E.W.L. Therapeutic Contact Lenses in the Treatment of Corneal and Ocular Surface Diseases—A Review. Asia-Pac. J. Ophthalmol. 2020, 9, 524–532. [Google Scholar] [CrossRef]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Aghabegi Moghanjoughi, A.; Khoshnevis, D.; Zarrabi, A. A Concise Review on Smart Polymers for Controlled Drug Release. Drug Deliv. Transl. Res. 2016, 6, 333–340. [Google Scholar] [CrossRef]
- Stapleton, F.; Stretton, S.; Papas, E.; Skotnitsky, C.; Sweeney, D.F. Silicone Hydrogel Contact Lenses and the Ocular Surface. Ocul. Surf. 2006, 4, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Colligris, B.; Pintor, J. Contact Lenses: Promising Devices for Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2013, 29, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Auffarth, G.U.; Apple, D.J. Zur Entwicklungsgeschichte Der Intraokularlinsen. Der Ophthalmol. 2001, 98, 1017–1031. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft Contact Lens Polymers: An Evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Center for Devices and Radiological Health. Class II Daily Wear Contact Lenses—Premarket Notification [510(k)] Guidance Document; FDA: Silver Spring, MD, USA, 2020.
- Gonzalez-Chomon, C.; Concheiro, A.; Alvarez-Lorenzo, C. Soft Contact Lenses for Controlled Ocular Delivery: 50 Years in the Making. Ther. Deliv. 2013, 4, 1141–1161. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, Z.; Shams Es-Haghi, S.; Cakmak, M. Recent Trends in Advanced Contact Lenses. Adv. Health Mater. 2019, 8, e1801390. [Google Scholar] [CrossRef] [PubMed]
- Donshik, P.C. Extended Wear Contact Lenses. Ophthalmol. Clin. N. Am. 2003, 16, 305–309. [Google Scholar] [CrossRef]
- The Editors of Encyclopaedia Britannica. PolyHEMA. Available online: https://www.britannica.com/science/polyHEMA (accessed on 10 May 2023).
- Dixon, P.; Shafor, C.; Gause, S.; Hsu, K.H.; Powell, K.C.; Chauhan, A. Therapeutic Contact Lenses: A Patent Review. Expert Opin. Ther. Pat. 2015, 25, 1117–1129. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymer 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Hudson, S.P.; Mobbs, A.N.; Hoare, T.R.; Iwata, N.G.; Fink, G.R.; Kohane, D.S. A Prototype Antifungal Contact Lens. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6286–6291. [Google Scholar] [CrossRef]
- Nakada, K.; Sugiyama, A. Process for Producing Controlled Drug-Release Contact Lens, and Controlled Drug-Release Contact Lens Thereby Produced. U.S. Patent US6027745A, 29 May 1998. [Google Scholar]
- Danion, A.; Brochu, H.; Martin, Y.; Vermette, P. Fabrication and Characterization of Contact Lenses Bearing Surface-Immobilized Layers of Intact Liposomes. J. Biomed. Mater. Res. A 2007, 82, 41–51. [Google Scholar] [CrossRef]
- Danion, A.; Arsenault, I.; Vermette, P. Antibacterial Activity of Contact Lenses Bearing Surface-Immobilized Layers of Intact Liposomes Loaded with Levofloxacin. J. Pharm. Sci. 2007, 96, 2350–2363. [Google Scholar] [CrossRef]
- Qiu, Y. Silicone Hydrogel Lens with a Crosslinked Hydrophilic Coating. U.S. Patent No. 9,708,087, 18 July 2017. [Google Scholar]
- Li, J.; Zhang, Z.; Loose, C.R.; Coury, A. Silicone Hydrogel Contact Lens Modified Using Lanthanide or Transition Metal Oxidants. U.S. Patent No. 8,870,372, 28 October 2014. [Google Scholar]
- Korogiannaki, M.; Samsom, M.; Schmidt, T.A.; Sheardown, H. Surface-Functionalized Model Contact Lenses with a Bioinspired Proteoglycan 4 (PRG4)-Grafted Layer. ACS Appl. Mater. Interfaces 2018, 10, 30125–30136. [Google Scholar] [CrossRef]
- Winterton, L.C. Method of Making Silicone Hydrogel Contact Lenses. U.S. Patent No. 7,858,000, 28 December 2010. [Google Scholar]
- Li, C.C.; Chauhan, A. Ocular Transport Model for Ophthalmic Delivery of Timolol through P-HEMA Contact Lenses. J. Drug Deliv. Sci. Technol. 2007, 17, 69–79. [Google Scholar] [CrossRef]
- Kim, J.; Chauhan, A. Dexamethasone Transport and Ocular Delivery from Poly(Hydroxyethyl Methacrylate) Gels. Int. J. Pharm. 2008, 353, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. Extended Release of Hyaluronic Acid from Hydrogel Contact Lenses for Dry Eye Syndrome. J. Biomater. Sci. Polym. Ed. 2015, 26, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.; Watkins, R. Pilocarpine Dispensation for the Soft Hydrophilic Contact Lens. Br. J. Ophthalmol. 1975, 59, 455–458. [Google Scholar] [CrossRef]
- Soluri, A.; Hui, A.; Jones, L. Delivery of Ketotifen Fumarate by Commercial Contact Lens Materials. Optom. Vis. Sci. 2012, 89, 1140–1149. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. In Vitro and in Vivo Evaluation of Ketotifen Fumarate-Loaded Silicone Hydrogel Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2011, 18, 150–158. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A Review on Therapeutic Contact Lenses for Ocular Drug Delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef]
- Schultz, C.L.; Poling, T.R.; Mint, J.O. A Medical Device/Drug Delivery System for Treatment of Glaucoma. Clin. Exp. Optom. 2009, 92, 343–348. [Google Scholar] [CrossRef]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric Hydrogels for Novel Contact Lens-Based Ophthalmic Drug Delivery Systems: A Review. Contact Lens. Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef]
- Maulvi, D.F. Effect of Timolol Maleate Concentration on Uptake and Release from Hydrogel Contact Lenses Using Soaking Method. J. Pharm. Appl. Sci. 2014, 1, 17–23. [Google Scholar]
- Franco, P.; De Marco, I. Contact Lenses as Ophthalmic Drug Delivery Systems: A Review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Oliveira, A.S.; Fernandes, A.; Nunes, T.G.; Serro, A.P.; Saramago, B. Improving Sustained Drug Delivery from Ophthalmic Lens Materials through the Control of Temperature and Time of Loading. Eur. J. Pharm. Sci. 2018, 117, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Ishida, W.; Kishimoto, T.; Nakajima, I.; Hino, S.; Arai, R.; Matsunaga, T.; Fukushima, A.; Yamagami, S. In Vitro and in Vivo Performance of Epinastine Hydrochloride-Releasing Contact Lenses. PLoS ONE 2019, 14, e0210362. [Google Scholar] [CrossRef] [PubMed]
- Zidarič, T.; Finšgar, M.; Maver, U.; Maver, T. Artificial Biomimetic Electrochemical Assemblies. Biosensors 2022, 12, 44. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Hiratani, H.; Gomez-Amoza, J.L.; Martinez-Pacheco, R.; Souto, C.; Concheiro, A. Soft Contact Lenses Capable of Sustained Delivery of Timolol. J. Pharm. Sci. 2002, 91, 2182–2192. [Google Scholar] [CrossRef]
- Hiratani, H.; Mizutani, Y.; Alvarez-Lorenzo, C. Controlling Drug Release from Imprinted Hydrogels by Modifying the Characteristics of the Imprinted Cavities. Macromol. Biosci. 2005, 5, 728–733. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yanez, F.; Barreiro-Iglesias, R.; Concheiro, A. Imprinted Soft Contact Lenses as Norfloxacin Delivery Systems. J. Control. Release 2006, 113, 236–244. [Google Scholar] [CrossRef]
- Tieppo, A.; Pate, K.M.; Byrne, M.E. In Vitro Controlled Release of an Anti-Inflammatory from Daily Disposable Therapeutic Contact Lenses under Physiological Ocular Tear Flow. Eur. J. Pharm. Biopharm. 2012, 81, 170–177. [Google Scholar] [CrossRef]
- Venkatesh, S.; Saha, J.; Pass, S.; Byrne, M.E. Transport and Structural Analysis of Molecular Imprinted Hydrogels for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2008, 69, 852–860. [Google Scholar] [CrossRef]
- Hiratani, H.; Fujiwara, A.; Tamiya, Y.; Mizutani, Y.; Alvarez-Lorenzo, C. Ocular Release of Timolol from Molecularly Imprinted Soft Contact Lenses. Biomaterials 2005, 26, 1293–1298. [Google Scholar] [CrossRef]
- White, C.J.; McBride, M.K.; Pate, K.M.; Tieppo, A.; Byrne, M.E. Extended Release of High Molecular Weight Hydroxypropyl Methylcellulose from Molecularly Imprinted, Extended Wear Silicone Hydrogel Contact Lenses. Biomaterials 2011, 32, 5698–5705. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Wedel, T.; Moll, R.; Geerling, G. Combination of Serum Eye Drops with Hydrogel Bandage Contact Lenses in the Treatment of Persistent Epithelial Defects. Graefes. Arch. Clin. Exp. Ophthalmol. 2006, 244, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.E.; Salian, V. Molecular Imprinting within Hydrogels II: Progress and Analysis of the Field. Int. J. Pharm. 2008, 364, 188–212. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, C.Y.; Wang, X.H.; Li, R.S.; Yang, J.R.; Huang, Y.P.; Liu, Z.S. Macromolecular Crowding of Molecular Imprinting: A Facile Pathway to Produce Drug Delivery Devices for Zero-Order Sustained Release. Int. J. Pharm. 2015, 496, 822–833. [Google Scholar] [CrossRef]
- Malakooti, N.; Alexander, C.; Alvarez-Lorenzo, C. Imprinted Contact Lenses for Sustained Release of Polymyxin B and Related Antimicrobial Peptides. J. Pharm. Sci. 2015, 104, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, F.; Concheiro, A.; Alvarez-Lorenzo, C. Epalrestat-Loaded Silicone Hydrogels as Contact Lenses to Address Diabetic-Eye Complications. Eur. J. Pharm. Biopharm. 2018, 122, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nair, A.S.; Parvathy, J. Extended Wear Therapeutic Contact Lens Fabricated from Timolol Imprinted Carboxymethyl Chitosan-g-Hydroxy Ethyl Methacrylate-g-Poly Acrylamide as a Onetime Medication for Glaucoma. Eur. J. Pharm. Biopharm. 2016, 109, 61–71. [Google Scholar] [CrossRef]
- Gupta, C.; Chauhan, A. Drug Transport in HEMA Conjunctival Inserts Containing Precipitated Drug Particles. J. Colloid Interface Sci. 2010, 347, 31–42. [Google Scholar] [CrossRef]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma Therapy by Extended Release of Timolol from Nanoparticle Loaded Silicone-Hydrogel Contact Lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Hsu, K.H.; Gause, S.; Chauhan, A. Review of Ophthalmic Drug Delivery by Contact Lenses. J. Drug Deliv. Sci. Technol. 2014, 24, 123–135. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Khameneh, B.; Jalili-Behabadi, M.M.; Malaekeh-Nikouei, B.; Mohajeri, S.A. Preparation, Characterization and Antimicrobial Study of a Hydrogel (Soft Contact Lens) Material Impregnated with Silver Nanoparticles. Contact Lens. Anterior Eye 2014, 37, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Chauhan, A. Ophthalmic Drug Delivery through Contact Lenses. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Li, C.C.; Chauhan, A. Dispersion of DMPC Liposomes in Contact Lenses for Ophthalmic Drug Delivery. Curr. Eye Res. 2005, 30, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Chauhan, A. Dispersion of Microemulsion Drops in HEMA Hydrogel: A Potential Ophthalmic Drug Delivery Vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef]
- Jung, H.J.; Chauhan, A. Temperature Sensitive Contact Lenses for Triggered Ophthalmic Drug Delivery. Biomaterials 2012, 33, 2289–2300. [Google Scholar] [CrossRef]
- Zhang, W.; Zu, D.; Chen, J.; Peng, J.; Liu, Y.; Zhang, H.; Li, S.; Pan, W. Bovine Serum Albumin-Meloxicam Nanoaggregates Laden Contact Lenses for Ophthalmic Drug Delivery in Treatment of Postcataract Endophthalmitis. Int. J. Pharm. 2014, 475, 25–34. [Google Scholar] [CrossRef]
- Loftsson, T.; Masson, M.; Brewster, M.E. Self-Association of Cyclodextrins and Cyclodextrin Complexes. J. Pharm. Sci. 2004, 93, 1091–1099. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Couceiro, R.; Concheiro, A.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C. Poly(Hydroxyethyl Methacrylate-Co-Methacrylated-Beta-Cyclodextrin) Hydrogels: Synthesis, Cytocompatibility, Mechanical Properties and Drug Loading/Release Properties. Acta Biomater. 2008, 4, 745–755. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Alvarez-Lorenzo, C.; Silva, M.; Balsa, L.; Couceiro, J.; Torres-Labandeira, J.J.; Concheiro, A. Soft Contact Lenses Functionalized with Pendant Cyclodextrins for Controlled Drug Delivery. Biomaterials 2009, 30, 1348–1355. [Google Scholar] [CrossRef]
- Jain, R.L.; Shastri, J.P. Study of Ocular Drug Delivery System Using Drug-Loaded Liposomes. Int. J. Pharm. Investig. 2011, 1, 35–41. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.M.; Lewis, S.A. Next-Generation Contact Lenses: Towards Bioresponsive Drug Delivery and Smart Technologies in Ocular Therapeutics. Eur. J. Pharm. Biopharm. 2021, 161, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Abrahamson, M.; Kapoor, Y.; Chauhan, A. Timolol Transport from Microemulsions Trapped in HEMA Gels. J. Colloid Interface Sci. 2007, 315, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Chauhan, A. Ophthalmic Delivery of Cyclosporine A from Brij-97 Microemulsion and Surfactant-Laden p-HEMA Hydrogels. Int. J. Pharm. 2008, 361, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yoganathan, R.B.; Kociolek, M.; Allen, C. Hydrogel Containing Silica Shell Cross-Linked Micelles for Ocular Drug Delivery. J. Pharm. Sci. 2013, 102, 627–637. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Mangukiya, M.A.; Patel, P.A.; Vaidya, R.J.; Koli, A.R.; Ranch, K.M.; Shah, D.O. Extended Release of Ketotifen from Silica Shell Nanoparticle-Laden Hydrogel Contact Lenses: In Vitro and in Vivo Evaluation. J. Mater. Sci. Mater. Med. 2016, 27, 113. [Google Scholar] [CrossRef]
- Shayani Rad, M.; Khameneh, B.; Sabeti, Z.; Mohajeri, S.A.; Fazly Bazzaz, B.S. Antibacterial Activity of Silver Nanoparticle-Loaded Soft Contact Lens Materials: The Effect of Monomer Composition. Curr. Eye Res. 2016, 41, 1286–1293. [Google Scholar] [CrossRef]
- Chandasana, H.; Prasad, Y.D.; Chhonker, Y.S.; Chaitanya, T.K.; Mishra, N.N.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Corneal Targeted Nanoparticles for Sustained Natamycin Delivery and Their PK/PD Indices: An Approach to Reduce Dose and Dosing Frequency. Int. J. Pharm. 2014, 477, 317–325. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Sun, F. Cyclodextrin-Containing Hydrogels for Contact Lenses as a Platform for Drug Incorporation and Release. Acta Biomater. 2010, 6, 486–493. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Tabary, N.; Martel, B.; Cazaux, F.; Oliva, A.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Poly-(Cyclo)Dextrins as Ethoxzolamide Carriers in Ophthalmic Solutions and in Contact Lenses. Carbohydr. Polym. 2013, 98, 1343–1352. [Google Scholar] [CrossRef]
- Glisoni, R.J.; Garcia-Fernandez, M.J.; Pino, M.; Gutkind, G.; Moglioni, A.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Sosnik, A. Beta-Cyclodextrin Hydrogels for the Ocular Release of Antibacterial Thiosemicarbazones. Carbohydr. Polym. 2013, 93, 449–457. [Google Scholar] [CrossRef]
- Arslan, M.; Gevrek, T.N.; Sanyal, R.; Sanyal, A. Fabrication of Poly(Ethylene Glycol)-Based Cyclodextrin Containing Hydrogels via Thiol-Ene Click Reaction. Eur. Polym. J. 2015, 62, 426–434. [Google Scholar] [CrossRef]
- Hu, X.; Tan, H.; Wang, X.; Chen, P. Surface Functionalization of Hydrogel by Thiol-Yne Click Chemistry for Drug Delivery. Colloids Surf. Physicochem. Eng. Asp. 2016, 489, 297–304. [Google Scholar] [CrossRef]
- Prakash, M.; Dhesingh, R.S. Nanoparticle Modified Drug Loaded Biodegradable Polymeric Contact Lenses for Sustainable Ocular Drug Delivery. Curr. Drug Deliv. 2017, 14, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Danion, A.; Doillon, C.J.; Giasson, C.J.; Djouahra, S.; Sauvageau, P.; Paradis, R.; Vermette, P. Biocompatibility and Light Transmission of Liposomal Lenses. Optom. Vis. Sci. 2007, 84, 954–961. [Google Scholar] [CrossRef]
- Bengani, L.C.; Chauhan, A. Extended Delivery of an Anionic Drug by Contact Lens Loaded with a Cationic Surfactant. Biomaterials 2013, 34, 2814–2821. [Google Scholar] [CrossRef]
- Kapoor, Y.; Thomas, J.C.; Tan, G.; John, V.T.; Chauhan, A. Surfactant-Laden Soft Contact Lenses for Extended Delivery of Ophthalmic Drugs. Biomaterials 2009, 30, 867–878. [Google Scholar] [CrossRef]
- Costa, V.P.; Braga, M.E.M.; Duarte, C.M.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Gil, M.H.; de Sousa, H.C. Anti-Glaucoma Drug-Loaded Contact Lenses Prepared Using Supercritical Solvent Impregnation. J. Supercrit. Fluids 2010, 53, 165–173. [Google Scholar] [CrossRef]
- Yanez, F.; Martikainen, L.; Braga, M.E.; Alvarez-Lorenzo, C.; Concheiro, A.; Duarte, C.M.; Gil, M.H.; de Sousa, H.C. Supercritical Fluid-Assisted Preparation of Imprinted Contact Lenses for Drug Delivery. Acta Biomater. 2011, 7, 1019–1030. [Google Scholar] [CrossRef]
- Zaidi, S.A. Molecular Imprinting: A Useful Approach for Drug Delivery. Mater. Sci. Energy Technol. 2020, 3, 72–77. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Simplicio, A.L.; Vega-González, A.; Subra-Paternault, P.; Coimbra, P.; Gil, M.H.; de Sousa, H.C.; Duarte, C.M.M. Supercritical Fluid Impregnation of a Biocompatible Polymer for Ophthalmic Drug Delivery. J. Supercrit. Fluids 2007, 42, 373–377. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Casimiro, T.; Aguiar-Ricardo, A.; Simplício, A.L.; Duarte, C.M.M. Supercritical Fluid Polymerisation and Impregnation of Molecularly Imprinted Polymers for Drug Delivery. J. Supercrit. Fluids 2006, 39, 102–106. [Google Scholar] [CrossRef]
- Yokozaki, Y.; Sakabe, J.; Ng, B.; Shimoyama, Y. Effect of Temperature, Pressure and Depressurization Rate on Release Profile of Salicylic Acid from Contact Lenses Prepared by Supercritical Carbon Dioxide Impregnation. Chem. Eng. Res. Des. 2015, 100, 89–94. [Google Scholar] [CrossRef]
- Peng, C.C.; Kim, J.; Chauhan, A. Extended Delivery of Hydrophilic Drugs from Silicone-Hydrogel Contact Lenses Containing Vitamin E Diffusion Barriers. Biomaterials 2010, 31, 4032–4047. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Peng, C.C.; Chauhan, A. Extended Release of Dexamethasone from Silicone-Hydrogel Contact Lenses Containing Vitamin E. J. Control. Release 2010, 148, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Chauhan, A. Extended Cyclosporine Delivery by Silicone-Hydrogel Contact Lenses. J. Control. Release 2011, 154, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.H.; Fentzke, R.C.; Chauhan, A. Feasibility of Corneal Drug Delivery of Cysteamine Using Vitamin E Modified Silicone Hydrogel Contact Lenses. Eur. J. Pharm. Biopharm. 2013, 85, 531–540. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Chauhan, A. Transport of Topical Anesthetics in Vitamin E Loaded Silicone Hydrogel Contact Lenses. Langmuir 2012, 28, 1478–1487. [Google Scholar] [CrossRef]
- Peng, C.C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended Drug Delivery by Contact Lenses for Glaucoma Therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef]
- Hsu, K.H.; Carbia, B.E.; Plummer, C.; Chauhan, A. Dual Drug Delivery from Vitamin E Loaded Contact Lenses for Glaucoma Therapy. Eur. J. Pharm. Biopharm. 2015, 94, 312–321. [Google Scholar] [CrossRef]
- Dixon, P.; Fentzke, R.C.; Bhattacharya, A.; Konar, A.; Hazra, S.; Chauhan, A. In Vitro Drug Release and in Vivo Safety of Vitamin E and Cysteamine Loaded Contact Lenses. Int. J. Pharm. 2018, 544, 380–391. [Google Scholar] [CrossRef]
- Dixon, P.; Chauhan, A. Carbon Black Tinted Contact Lenses for Reduction of Photophobia in Cystinosis Patients. Curr. Eye Res. 2019, 44, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Sekar, P.; Chauhan, A. Effect of Vitamin-E Integration on Delivery of Prostaglandin Analogs from Therapeutic Lenses. J. Colloid Interface Sci. 2019, 539, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Torres-Luna, C.; Hu, N.; Tammareddy, T.; Domszy, R.; Yang, J.; Wang, N.S.; Yang, A. Extended Delivery of Non-Steroidal Anti-Inflammatory Drugs through Contact Lenses Loaded with Vitamin E and Cationic Surfactants. Contact Lens Anterior Eye 2019, 42, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II Pathophysiology Report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Li, P.; Beachley, V.; McDonnell, P.; Elisseeff, J.H. A Hyaluronic Acid-Binding Contact Lens with Enhanced Water Retention. Contact Lens Anterior Eye 2015, 38, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Codina, C.; Efron, N. Impact of Manufacturing Technology and Material Composition on the Mechanical Properties of Hydrogel Contact Lenses. Ophthalmic. Physiol. Opt. 2004, 24, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Singhania, S.S.; Desai, A.R.; Shukla, M.R.; Tannk, A.S.; Ranch, K.M.; Vyas, B.A.; Shah, D.O. Contact Lenses with Dual Drug Delivery for the Treatment of Bacterial Conjunctivitis. Int. J. Pharm. 2018, 548, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Sammons, W.A. The Nissel Memorial LectureManufacturing—Materials, Methods and Measurements. J. Br. Contact Lens Assoc. 1989, 12, 12–19. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical Properties of Hydrogels and Their Experimental Determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Baker, J.; Blanch, H.; Prausnitz, J. Equilibrium Swelling Properties of Weakly Lonizable 2-Hydroxyethyl Methacrylate (HEMA)-Based Hydrogels. J. Appl. Polym. Sci. 1994, 52, 783–788. [Google Scholar] [CrossRef]
- Milojević, M.; Gradišnik, L.; Stergar, J.; Skelin Klemen, M.; Stožer, A.; Vesenjak, M.; Dobnik Dubrovski, P.; Maver, T.; Mohan, T.; Stana Kleinschek, K.; et al. Development of Multifunctional 3D Printed Bioscaffolds from Polysaccharides and NiCu Nanoparticles and Their Application. Appl. Surf. Sci. 2019, 488, 836–852. [Google Scholar] [CrossRef]
- Milojević, M.; Harih, G.; Vihar, B.; Vajda, J.; Gradišnik, L.; Zidarič, T.; Stana Kleinschek, K.; Maver, U.; Maver, T. Hybrid 3D Printing of Advanced Hydrogel-Based Wound Dressings with Tailorable Properties. Pharmaceutics 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Štiglic, A.D.; Gürer, F.; Lackner, F.; Bračič, D.; Winter, A.; Gradišnik, L.; Makuc, D.; Kargl, R.; Duarte, I.; Plavec, J.; et al. Organic Acid Cross-Linked 3D Printed Cellulose Nanocomposite Bioscaffolds with Controlled Porosity, Mechanical Strength, and Biocompatibility. iScience 2022, 25, 104263. [Google Scholar] [CrossRef] [PubMed]
- Vajda, J.; Vihar, B.; Ćurić, L.Č.; Maver, U.; Vesenjak, M.; Dubrovski, P.D.; Milojević, M. Sr2+ vs. Ca2+ as Post-Processing Ionic Crosslinkers: Implications for 3D Bioprinting of Polysaccharide Hydrogels in Tissue Engineering. J. Mater. Res. Technol. 2023, 23, 1805–1820. [Google Scholar] [CrossRef]
- Tan, G.; Ioannou, N.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D Printing in Ophthalmology: From Medical Implants to Personalised Medicine. Int. J. Pharm. 2022, 625, 122094. [Google Scholar] [CrossRef] [PubMed]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D Printed Drug-Eluting Contact Lenses. J. Pharm. Pharmacol. 2022, 74, 1467–1476. [Google Scholar] [CrossRef]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling Hot Melt Extrusion and Fused Deposition Modeling: Critical Properties for Successful Performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef]
- Alam, F.; Elsherif, M.; AlQattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater. Sci. Eng. 2021, 7, 794–803. [Google Scholar] [CrossRef]
- Gruene, M.; Pflaum, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Wilhelmi, M.; Haverich, A.; Chichkov, B.N. Adipogenic Differentiation of Laser-Printed 3D Tissue Grafts Consisting of Human Adipose-Derived Stem Cells. Biofabrication 2011, 3, 015005. [Google Scholar] [CrossRef]
- Phan, C.-M.; Bajgrowicz, M.; Gao, H.; Subbaraman, L.N.; Jones, L.W. Release of Fluconazole from Contact Lenses Using a Novel In Vitro Eye Model. Optom. Vis. Sci. 2016, 93, 387–394. [Google Scholar] [CrossRef]
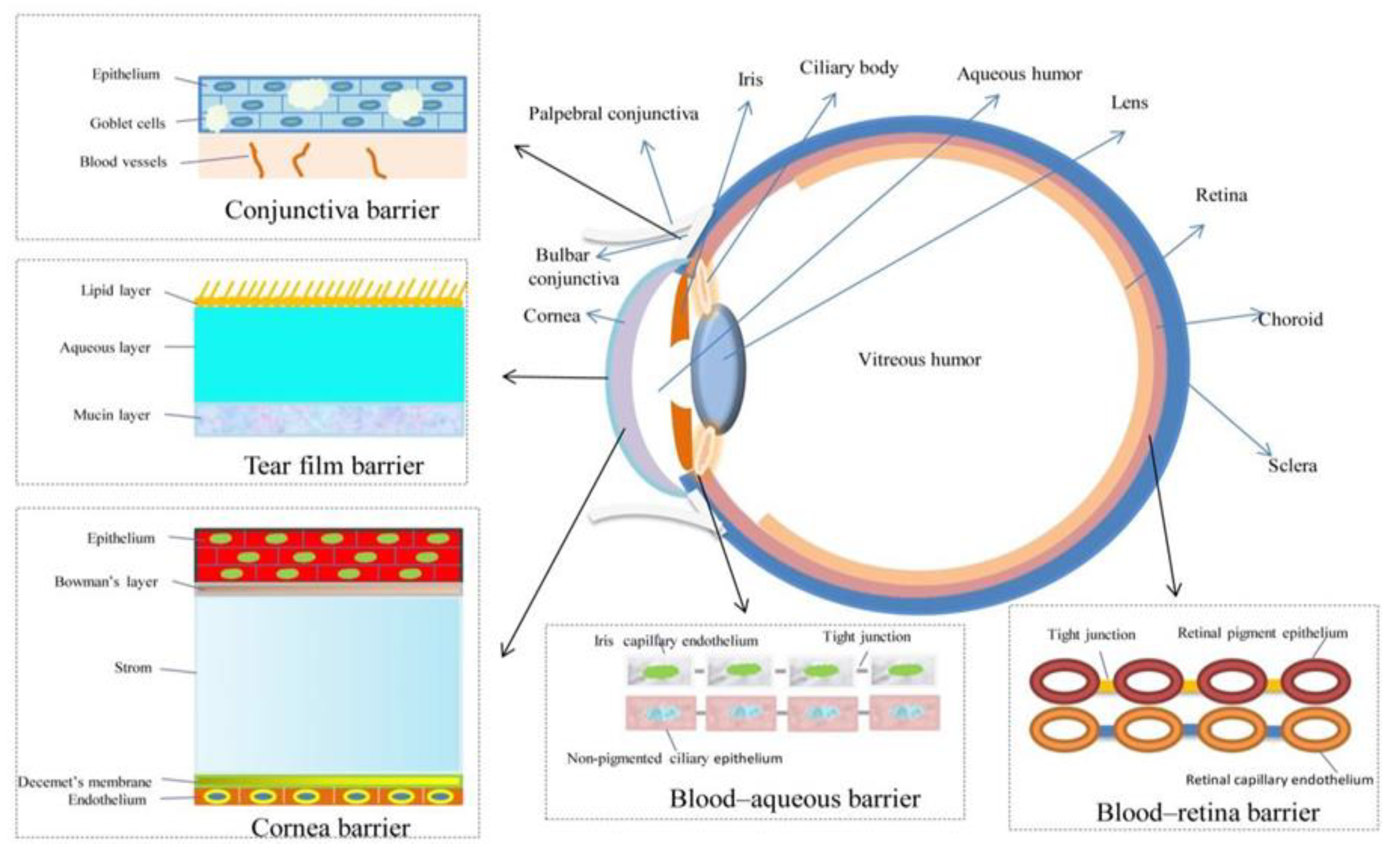




| Colloidal Nanoparticles | Examples | Characteristics | References |
|---|---|---|---|
| Polymeric nanoparticles |
|
| [55,61,62] |
| Cyclodextrins |
|
| [63,64,65] |
| Liposomes |
|
| [9,21,33,59,66] |
| Microemulsions and micelles |
|
| [60,67,68,69,70,71] |
| Method | Mechanism | Advantages | Limitations | References |
|---|---|---|---|---|
| Layered contact lenses | Approaches:
|
|
| [17,22,33] |
| Surface-modified contact lenses |
|
|
| [21,25] |
| Soaking method |
|
|
| [27,28,29,30,33,34,35,36] |
| Molecular imprinting |
|
|
| [33,40,41,48,49] |
| Colloidal nanoparticles |
|
|
| [33,54,55,56,59,60] |
| Supercritical fluid technology |
|
|
| [33,83,84] |
| Vitamin E as a release-modifying additive |
|
|
| [17,33,89,92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lovrec-Krstič, T.; Orthaber, K.; Maver, U.; Sarenac, T. Review of Potential Drug-Eluting Contact Lens Technologies. Materials 2023, 16, 3653. https://doi.org/10.3390/ma16103653
Lovrec-Krstič T, Orthaber K, Maver U, Sarenac T. Review of Potential Drug-Eluting Contact Lens Technologies. Materials. 2023; 16(10):3653. https://doi.org/10.3390/ma16103653
Chicago/Turabian StyleLovrec-Krstič, Tina, Kristjan Orthaber, Uroš Maver, and Tomislav Sarenac. 2023. "Review of Potential Drug-Eluting Contact Lens Technologies" Materials 16, no. 10: 3653. https://doi.org/10.3390/ma16103653
APA StyleLovrec-Krstič, T., Orthaber, K., Maver, U., & Sarenac, T. (2023). Review of Potential Drug-Eluting Contact Lens Technologies. Materials, 16(10), 3653. https://doi.org/10.3390/ma16103653








