Multilayer Methacrylate-Based Wound Dressing as a Therapeutic Tool for Targeted Pain Relief
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of Solutions
2.1.2. Substrate Preparation
2.1.3. Preparation of Multilayer Dressings
2.2. Characterisation
2.2.1. Contact Angle Measurement
2.2.2. Attenuated Total Reflectance Infrared Spectroscopy (AFT-IR)
2.2.3. Atomic Force Microscopy
2.3. Functional Testing
2.3.1. In Vitro Drug Release Testing
2.3.2. Cell Cultures and Viability Testing
3. Results and Discussion
3.1. Material Preparation
3.2. Characterisation
3.2.1. Hydrophilicity Evaluation through Water Contact Angle Measurement
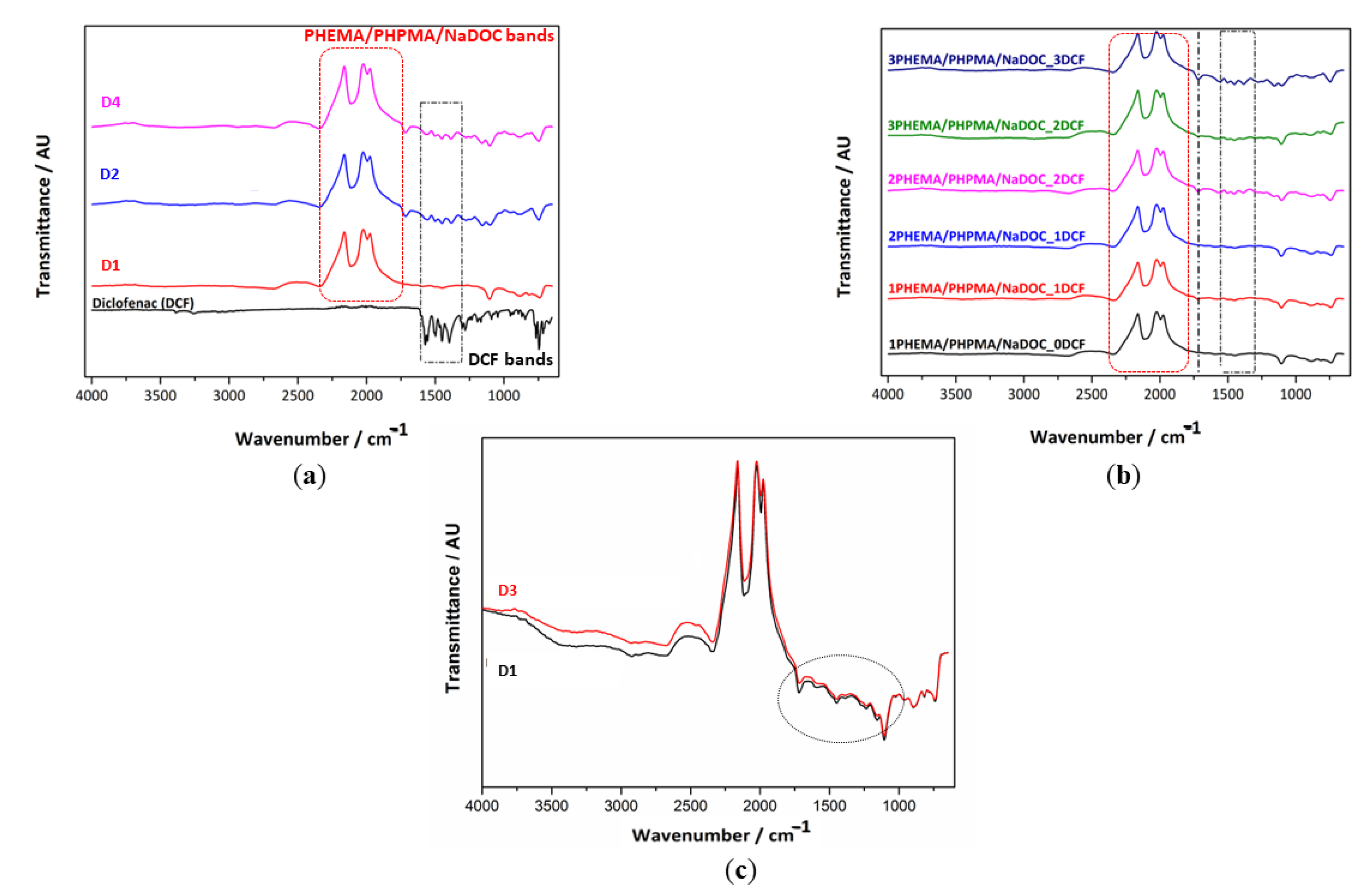
3.2.2. Structural Properties by AFT-IR Spectroscopy
3.2.3. Sample Morphology and Roughness
3.3. Functional Testing
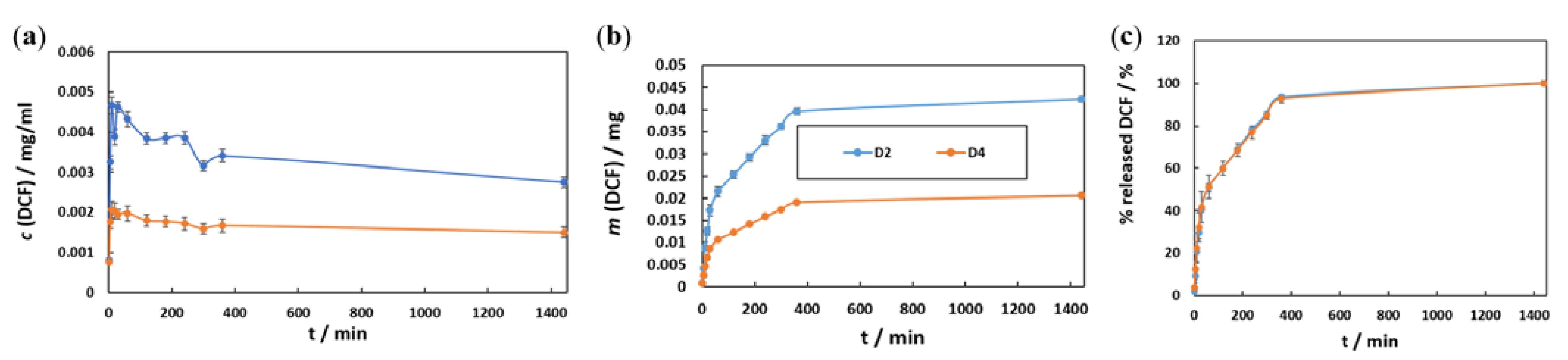
3.3.1. In Vitro Drug Release Testing
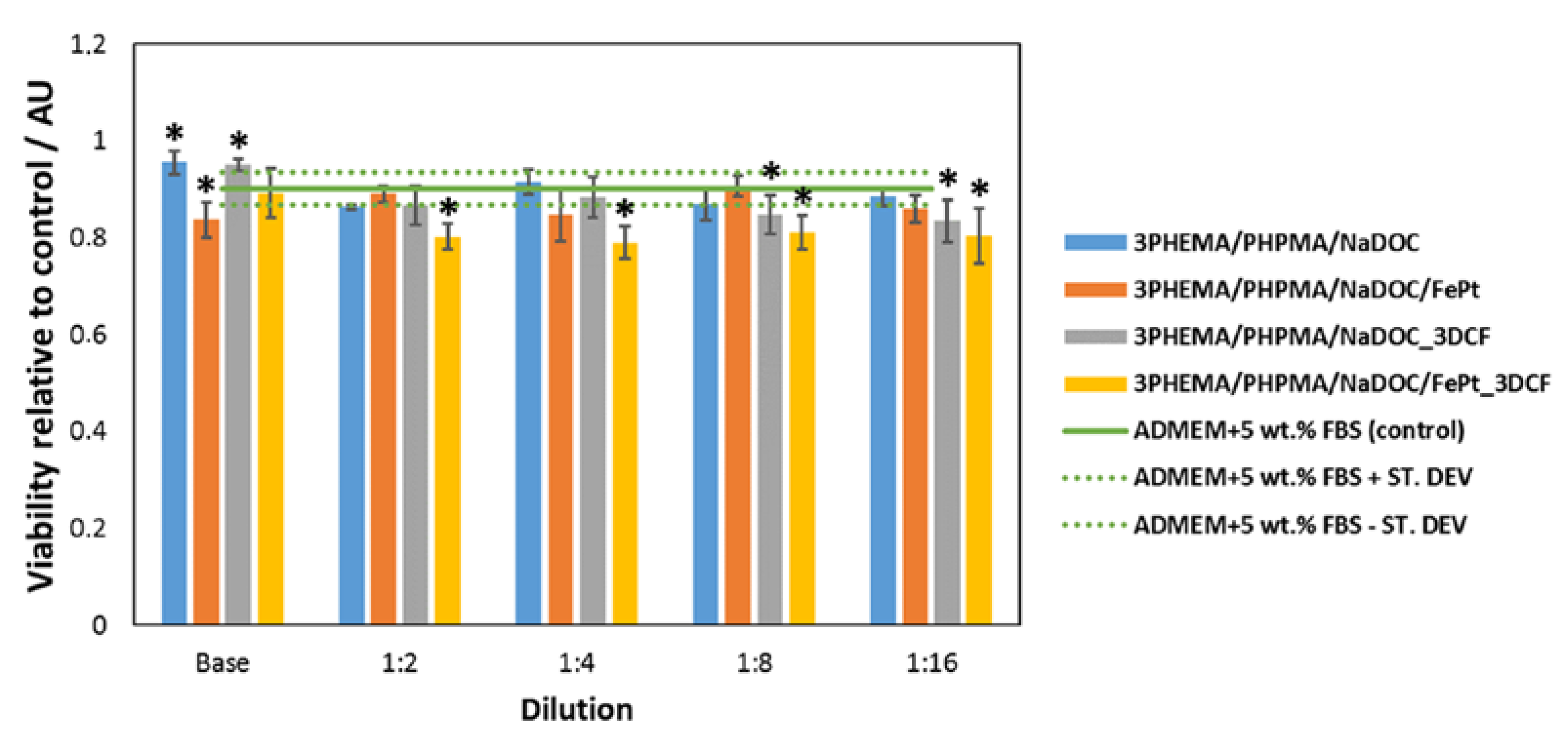
3.3.2. Biocompatibility Testing Using Human-Derived Skin Fibroblasts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gouin, J.P.; Kiecolt-Glaser, J.K. The Impact of Psychological Stress on Wound Healing: Methods and Mechanisms. Immunol. Allergy Clin. N. Am. 2011, 31, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Das, S.; Baker, A.B. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Mostegel, F.; Griesser, T.; Spirk, S.; Smrke, D.; Stana-Kleinschek, K. Cellulose based thin films as a platform for drug release studies to mimick wound dressing materials. Cellulose 2015, 22, 749–761. [Google Scholar] [CrossRef]
- Resmi, R.; Unnikrishnan, S.; Krishnan, L.K.; Kalliyana Krishnan, V. Synthesis and characterisation of silver nanoparticle incorporated gelatin-hydroxypropyl methacrylate hydrogels for wound dressing applications. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Yamane, T.; Nakagami, G.; Yoshino, S.; Muramatsu, A.; Matsui, S.; Oishi, Y.; Kanazawa, T.; Minematsu, T.; Sanada, H. Hydrocellular Foam Dressing Promotes Wound Healing along with Increases in Hyaluronan Synthase 3 and PPARα Gene Expression in Epidermis. PLoS ONE 2013, 8, e0073988. [Google Scholar] [CrossRef]
- Maver, T.; Mohan, T.; Gradisnik, L.; Finsgar, M.; Stana Kleinschek, K.; Maver, U. Polysaccharide Thin Solid Films for Analgesic Drug Delivery and Growth of Human Skin Cells. Front. Chem. 2019, 7, 217. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing—A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, W.; Sun, M.; He, P.; Lv, H.; Wang, Q.; Zhang, S.; Wu, Q.; Ling, P.; Chen, S. Novel bi-layered dressing patches constructed with radially-oriented nanofibrous pattern and herbal compound-loaded hydrogel for accelerated diabetic wound healing. Appl. Mater. Today 2022, 28, 101542. [Google Scholar] [CrossRef]
- Maver, T.; Gradišnik, L.; Smrke, D.M.; Kleinschek, K.S.; Maver, U. Systematic evaluation of a diclofenac-loaded carboxymethyl cellulose-based wound dressing and its release performance with changing ph and temperature. AAPS PharmSciTech 2019, 20, 29. [Google Scholar] [CrossRef]
- He, M.; Ou, F.; Wu, Y.; Sun, X.; Chen, X.; Li, H.; Sun, D.; Zhang, L. Smart multilayer PVA foam/CMC mesh dressing with integrated multi-functions for wound management and infection monitoring. Mater. Des. 2020, 194, 108913. [Google Scholar] [CrossRef]
- Tamayol, A.; Akbari, M.; Zilberman, Y.; Comotto, M.; Lesha, E.; Serex, L.; Bagherifard, S.; Chen, Y.; Fu, G.; Ameri, S.K. Flexible pH-sensing hydrogel fibers for epidermal applications. Adv. Healthc. Mater. 2016, 5, 711–719. [Google Scholar] [CrossRef]
- Pan, N.; Qin, J.; Feng, P.; Li, Z.; Song, B. Color-changing smart fibrous materials for naked eye real-time monitoring of wound pH. J. Mater. Chem. B 2019, 7, 2626–2633. [Google Scholar] [CrossRef]
- Hattori, Y.; Falgout, L.; Lee, W.; Jung, S.Y.; Poon, E.; Lee, J.W.; Na, I.; Geisler, A.; Sadhwani, D.; Zhang, Y. Multifunctional skin-like electronics for quantitative, clinical monitoring of cutaneous wound healing. Adv. Healthc. Mater. 2014, 3, 1597–1607. [Google Scholar] [CrossRef]
- Milne, S.D.; Seoudi, I.; Al Hamad, H.; Talal, T.K.; Anoop, A.A.; Allahverdi, N.; Zakaria, Z.; Menzies, R.; Connolly, P. A wearable wound moisture sensor as an indicator for wound dressing change: An observational study of wound moisture and status. Int. Wound J. 2016, 13, 1309–1314. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Skok, K.; Zidarič, T.; Orthaber, K.; Pristovnik, M.; Kostevšek, N.; Rožman, K.Ž.; Šturm, S.; Gradišnik, L.; Maver, U.; Maver, T. Novel Methacrylate-Based Multilayer Nanofilms with Incorporated FePt-Based Nanoparticles and the Anticancer Drug 5-Fluorouracil for Skin Cancer Treatment. Pharmaceutics 2022, 14, 689. [Google Scholar] [CrossRef]
- Perale, G.; Rossi, F.; Sundstrom, E.; Bacchiega, S.; Masi, M.; Forloni, G.; Veglianese, P. Hydrogels in spinal cord injury repair strategies. ACS Chem. Neurosci. 2011, 2, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, B.; Ding, J.; Yan, L.; Thawani, J.P.; Fu, C.; Chen, X. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019, 88, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Wu, C.-M. Switchable Wettability of Poly (NIPAAm-co-HEMA-co-NMA) Coated PET Fabric for Moisture Management. Polymers 2020, 12, 100. [Google Scholar] [CrossRef]
- Gok, Z.G.; Inal, M.; Bozkaya, O.; Yigitoglu, M.; Vargel, I. Production of 2-hydroxyethyl methacrylate-g-poly (ethylene terephthalate) nanofibers by electrospinning and evaluation of the properties of the obtained nanofibers. J. Appl. Polym. Sci. 2020, 137, 49257. [Google Scholar] [CrossRef]
- Huang, Y.; Dan, Y.; Dan, N.; Chen, Y.J. Controlled-release of indomethacin trigged by inflammation-response for wound care. Polym. Test. 2021, 97, 107129. [Google Scholar] [CrossRef]
- Finšgar, M.; Uzunalić, A.P.; Stergar, J.; Gradišnik, L.; Maver, U. Novel chitosan/diclofenac coatings on medical grade stainless steel for hip replacement applications. Sci. Rep. 2016, 6, 26653. [Google Scholar] [CrossRef]
- Moreira, J.; Vale, A.C.; Alves, N.M. Spin-coated freestanding films for biomedical applications. J. Mater. Chem. B 2021, 9, 3778–3799. [Google Scholar] [CrossRef]
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W.; Bai, Z.; Chen, Y.; Yang, C. Facile fabrication of gelatin and polycaprolactone based bilayered membranes via spin coating method with antibacterial and cyto-compatible properties. Int. J. Biol. Macromol. 2019, 124, 699–707. [Google Scholar] [CrossRef]
- Maver, T.; Kurečič, M.; Smrke, D.M.; Kleinschek, K.S.; Maver, U. Electrospun nanofibrous CMC/PEO as a part of an effective pain-relieving wound dressing. J. Sol-Gel Sci. Technol. 2016, 79, 475–486. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Wang, C.; Feng, L.; Li, Y.; Liu, Z. Drug-induced self-assembly of modified albumins as nano-theranostics for tumor-targeted combination therapy. ACS Nano 2015, 9, 5223–5233. [Google Scholar] [CrossRef]
- Labala, S.; Mandapalli, P.K.; Kurumaddali, A.; Venuganti, V.V.K. Layer-by-layer polymer coated gold nanoparticles for topical delivery of imatinib mesylate to treat melanoma. Mol. Pharm. 2015, 12, 878–888. [Google Scholar] [CrossRef]
- Stana, J.; Stergar, J.; Gradisnik, L.; Flis, V.; Kargl, R.; Frohlich, E.; Stana Kleinschek, K.; Mohan, T.; Maver, U. Multilayered Polysaccharide Nanofilms for Controlled Delivery of Pentoxifylline and Possible Treatment of Chronic Venous Ulceration. Biomacromolecules 2017, 18, 2732–2746. [Google Scholar] [CrossRef]
- Wu, G.; Ma, X.; Fan, L.; Gao, Y.; Deng, H.; Wang, Y. Accelerating dermal wound healing and mitigating excessive scar formation using LBL modified nanofibrous mats. Mater. Des. 2020, 185, 108265. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin Loaded Poly(ε-Caprolactone) Nanofibers: Diabetic Wound Dressing with Antioxidant and Anti-inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef]
- Rao, Y.-F.; Chen, W.; Liang, X.-G.; Huang, Y.-Z.; Miao, J.; Liu, L.; Lou, Y.; Zhang, X.-G.; Wang, B.; Tang, R.-K.; et al. Epirubicin-Loaded Superparamagnetic Iron-Oxide Nanoparticles for Transdermal Delivery: Cancer Therapy by Circumventing the Skin Barrier. Small 2015, 11, 239–247. [Google Scholar] [CrossRef]
- Burke, L.; Mortimer, C.J.; Curtis, D.J.; Lewis, A.R.; Williams, R.; Hawkins, K.; Maffeis, T.G.; Wright, C.J. In-situ synthesis of magnetic iron-oxide nanoparticle-nanofibre composites using electrospinning. Mater. Sci. Eng. C 2017, 70, 512–519. [Google Scholar] [CrossRef]
- Rožman, K.Ž.; Pečko, D.; Šturm, S.; Maver, U.; Nadrah, P.; Bele, M.; Kobe, S. Electrochemical synthesis and characterization of Fe70Pd30 nanotubes for drug-delivery applications. Mater. Chem. Phys. 2012, 133, 218–224. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef]
- Raviraj, V.; Pham, B.T.; Kim, B.J.; Pham, N.T.; Kok, L.F.; Painter, N.; Delic, N.C.; Jones, S.K.; Hawkett, B.S.; Lyons, J.G. Non-invasive transdermal delivery of chemotherapeutic molecules in vivo using superparamagnetic iron oxide nanoparticles. Cancer Nanotechnol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.J. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Marepally, S.; Vemula, P.; Xu, C. Inorganic nanoparticles for transdermal drug delivery and topical application. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–72. [Google Scholar]
- Seynhaeve, A.; Amin, M.; Haemmerich, D.; van Rhoon, G.; Ten Hagen, T. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Chiang, W.-H.; Parvin, N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 52, 205–224. [Google Scholar]
- Kang, Z.; Peng, Y.; Zhou, L.; Li, Z.; Wang, T.; Zhang, Z.; Liao, Q.; Gao, J.; Li, Y.; Zhang, Y. Thermo-responsive phase-transition polymer grafted magnetic FePt nanoparticles with tunable critical temperature for controlled drug release. Mater. Chem. Front. 2018, 2, 1609–1617. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E.; Carvalho, M.; Ferreira, L.; Cruz, M.; Arat, R. Preparation of magnetite nanoparticle and fatty acid incorporated poly (methacrylic acid-ethyl acrylate) nanowebs via electrospinning for magnetic hyperthermia application. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Lesvos, Greece, 5–7 September 2018; IOP Publishing: Bristol, UK, 2018; p. 012025. [Google Scholar]
- Kostevšek, N.; Žužek Rožman, K.; Arshad, M.S.; Spreitzer, M.; Kobe, S.; Šturm, S.O. Multimodal hybrid FePt/SiO2/Au nanoparticles for nanomedical applications: Combining photothermal stimulation and manipulation with an external magnetic field. J. Phys. Chem. C 2015, 119, 16374–16382. [Google Scholar] [CrossRef]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Sakamoto, W.; Yogo, T.; Ishimura, K. Magnetically responsive smart nanoparticles for cancer treatment with a combination of magnetic hyperthermia and remote-control drug release. Theranostics 2014, 4, 834–844. [Google Scholar] [CrossRef]
- Sasikala, A.R.; GhavamiNejad, A.; Unnithan, A.R.; Thomas, R.G.; Moon, M.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. A smart magnetic nanoplatform for synergistic anticancer therapy: Manoeuvring mussel-inspired functional magnetic nanoparticles for pH responsive anticancer drug delivery and hyperthermia. Nanoscale 2015, 7, 18119–18128. [Google Scholar] [CrossRef]
- Yang, H.Y.; Li, Y.; Lee, D.S. Multifunctional and stimuli-responsive magnetic nanoparticle-based delivery systems for biomedical applications. Adv. Ther. 2018, 1, 1800011. [Google Scholar] [CrossRef]
- Russo, J.; Fiegel, J.; Brogden, N.K. Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia. Pharmaceutics 2020, 12, 1214. [Google Scholar] [CrossRef]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing inflammation in skin wound healing: A review. Int. J. Inflamm. 2015, 2015, 316235. [Google Scholar] [CrossRef]
- Trevisol, T.C.; Scartazzini, L.; Valerio, A.; Guelli Ulson de Souza, S.M.A.; Bierhalz, A.C.K.; Valle, J.A.B. Diclofenac release from alginate/carboxymethyl cellulose mono and bilayer films for wound dressing applications. Cellulose 2020, 27, 6629–6642. [Google Scholar] [CrossRef]
- Singer, K.; Dettmer, K.; Unger, P.; Schönhammer, G.; Renner, K.; Peter, K.; Siska, P.; Berneburg, M.; Herr, W.; Oefner, P. Topical diclofenac reprograms metabolism and immune cell infiltration in actinic keratosis. Front. Oncol. 2019, 9, 605. [Google Scholar] [CrossRef]
- Voiculescu, V.M.; Lisievici, C.V.; Lupu, M.; Vajaitu, C.; Draghici, C.C.; Popa, A.V.; Solomon, I.; Sebe, T.I.; Constantin, M.M.; Caruntu, C. Mediators of inflammation in topical therapy of skin cancers. Mediat. Inflamm. 2019, 2019, 8369690. [Google Scholar] [CrossRef]
- Maver, U.; Xhanari, K.; Žižek, M.; Gradišnik, L.; Repnik, K.; Potočnik, U.; Finšgar, M. Carboxymethyl cellulose/diclofenac bioactive coatings on AISI 316LVM for controlled drug delivery, and improved osteogenic potential. Carbohydr. Polym. 2020, 230, 115612. [Google Scholar] [CrossRef]
- Rosenthal, A.; Stoddard, M.; Chipps, L.; Herrmann, J. Venereology. Skin cancer prevention: A review of current topical options complementary to sunscreens. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1261–1267. [Google Scholar] [CrossRef]
- Pavel, T.I.; Chircov, C.; Rădulescu, M.; Grumezescu, A.M. Regenerative Wound Dressings for Skin Cancer. Cancer 2020, 12, 2954. [Google Scholar] [CrossRef]
- Milojević, M.; Gradišnik, L.; Stergar, J.; Klemen, M.S.; Stožer, A.; Vesenjak, M.; Dubrovski, P.D.; Maver, T.; Mohan, T.; Kleinschek, K.S. Development of multifunctional 3D printed bioscaffolds from polysaccharides and NiCu nanoparticles and their application. Appl. Surf. Sci. 2019, 488, 836–852. [Google Scholar] [CrossRef]
- Maver, U.; Planinsek, O.; Jamnik, J.; Hassanien, A.I.; Gaberscek, M. Preparation of Atomically Flat Gold Substrates for AFM Measurements. Acta Chim. Slov. 2012, 59, 212–219. [Google Scholar] [PubMed]
- Tyona, M. A theoritical study on spin coating technique. Adv. Mater. Res. 2013, 2, 195. [Google Scholar] [CrossRef]
- Maver, U.; Xhanari, K.; Žižek, M.; Korte, D.; Gradišnik, L.; Franko, M.; Finšgar, M. A combination of interdisciplinary analytical tools for evaluation of multilayered coatings on medical grade stainless steel for biomedical applications. Eur. J. Pharm. Biopharm. 2018, 128, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Finšgar, M.; Kovač, J.; Maver, U. The development and characterisation of bioactive coatings for local drug delivery in orthopedic applications. Prog. Org. Coat. 2021, 158, 106350. [Google Scholar] [CrossRef]
- Maver, U.; Godec, A.; Bele, M.; Planinšek, O.; Gaberšček, M.; Srčič, S.; Jamnik, J. Novel hybrid silica xerogels for stabilisation and controlled release of drug. Int. J. Pharm. 2007, 330, 164–174. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ferrari, M.; Fornasiero, M.C.; Isetta, A.M. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J. Immunol. Methods 1990, 131, 165–172. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Liu, Y.; Gao, J.; Wang, K.; Liu, W. An injectable and antifouling self-fused supramolecular hydrogel for preventing postoperative and recurrent adhesions. Chem. Eng. J. 2021, 404, 127096. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Xu, M.; Zhang, L.; Zhang, J.; Dong, W. Development of photocrosslinked salecan composite hydrogel embedding titanium carbide nanoparticles as cell scaffold. Int. J. Biol. Macromol. 2019, 123, 549–557. [Google Scholar] [CrossRef]
- Andrade-Vivero, P.; Fernandez-Gabriel, E.; Alvarez-Lorenzo, C.; Concheiro, A. Improving the loading and release of NSAIDs from pHEMA hydrogels by copolymerisation with functionalised monomers. J. Pharm. Sci. 2007, 96, 802–813. [Google Scholar] [CrossRef]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Multilayer assembly. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.; Bhaduri, S. Biomedical applications of electrospun nanofibers: Drug and nanoparticle delivery. Pharmaceutics 2019, 11, 5. [Google Scholar] [CrossRef]
- Khodir, A.; Wan, W.K.; Razak, A.; Hakim, A.; Ng, M.H.; Guarino, V.; Susanti, D. Encapsulation and characterisation of gentamicin sulfate in the collagen added electrospun nanofibers for skin regeneration. J. Funct. Biomater. 2018, 9, 36. [Google Scholar] [CrossRef]
- Billa, N.; Yuen, K.-H.; Peh, K.-K. Diclofenac release from Eudragit-containing matrices and effects of thermal treatment. Drug Dev. Ind. Pharm. 1998, 24, 45–50. [Google Scholar] [CrossRef]
- Adeyeye, M.; Mwangi, E.; Katondo, B.; Jain, A.; Ichikawa, H.; Fukumori, Y. Dissolution stability studies of suspensions of prolonged-release diclofenac microcapsules prepared by the Wurster process: I. Eudragit-based formulation and possible drug-excipient interaction. J. Microencapsul. 2005, 22, 333–342. [Google Scholar] [CrossRef]
- Sipos, P.; Szűcs, M.; Szabó, A.; Erős, I.; Szabó-Révész, P. An assessment of the interactions between diclofenac sodium and ammonio methacrylate copolymer using thermal analysis and Raman spectroscopy. J. Pharm. Biomed. Anal. 2008, 46, 288–294. [Google Scholar] [CrossRef]
- Yu, S.Y.; Ullrich, P.J.; Weissman, J.P.; Joshi, C.J.; Taylor, R.; Patel, A.; El Hoseny, S.; Galiano, R.D. Evaluation of Altrazeal transforming powder dressing on stage 2–4 pressure ulcers: A clinical case series. J. Wound Care 2022, 31, S6–S12. [Google Scholar] [CrossRef]
- Maver, T.; Gradisnik, L.; Kurecic, M.; Hribernik, S.; Smrke, D.M.; Maver, U.; Kleinschek, K.S. Layering of different materials to achieve optimal conditions for treatment of painful wounds. Int. J. Pharm. 2017, 529, 576–588. [Google Scholar] [CrossRef]
- Dissemond, J.; Augustin, M.; Eming, S.A.; Goerge, T.; Horn, T.; Karrer, S.; Schumann, H.; Stücker, M.; Working Group for Wound Healing (AGW) of the German Society of Dermatology (DDG). Modern wound care–practical aspects of non-interventional topical treatment of patients with chronic wounds. JDDG J. Der Dtsch. Dermatol. Ges. 2014, 12, 541–554. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Maibach, H.I. Degenerative Changes in Aging Skin. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–18. [Google Scholar] [CrossRef]
- Maver, T.; Hribernik, S.; Mohan, T.; Smrke, D.M.; Maver, U.; Stana-Kleinschek, K. Functional wound dressing materials with highly tunable drug release properties. RSC Adv. 2015, 5, 77873–77884. [Google Scholar] [CrossRef]
- Elkhyat, A.; Sainthillier, J.; Ferial, F.; Lihoreau, T.; Mary, S.M.; Jeudy, A.; Humbert, P. Skin wettability and friction coefficient: An in vivo and in vitro study. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 167–168. [Google Scholar] [CrossRef]
- Zidarič, T.; Milojević, M.; Gradišnik, L.; Stana Kleinschek, K.; Maver, U.; Maver, T. Polysaccharide-Based Bioink Formulation for 3D Bioprinting of an In Vitro Model of the Human Dermis. Nanomaterials 2020, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar] [CrossRef]
- Sinha, M.; Gupte, T. Design and evaluation of artificial cornea with core–skirt design using polyhydroxyethyl methacrylate and graphite. Int. Ophthalmol. 2018, 38, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Mercan, E.S.; Karaman, M. Coating of hydrophilic poly (hydroxypropyl methacrylate) thin films via pulsed-initiated chemical vapor deposition method. J. Coat. Technol. Res. 2021, 18, 1261–1268. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Kleinschek, K.S.; Raščan, I.M.; Smrke, D.M. Advanced therapies of skin injuries. Wien. Klin. Wochenschr. 2015, 127, 1–12. [Google Scholar] [CrossRef]
- Naves, L.B.; Dhand, C.; Venugopal, J.R.; Rajamani, L.; Ramakrishna, S.; Almeida, L. Nanotechnology for the treatment of melanoma skin cancer. Prog. Biomater. 2017, 6, 13–26. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Y.; Zhang, J.; Zhang, H.; Guo, B. Structural and Functional Design of Electrospun Nanofibers for Hemostasis and Wound Healing. Adv. Fiber Mater. 2022, 4, 1027–1057. [Google Scholar] [CrossRef]
- Toledo, L.; Palacio, D.A.; Urbano, B.F. Tuning the softness of poly (2-hydroxyethyl methacrylate) nanocomposite hydrogels through the addition of PEG coated nanoparticles. J. Colloid Interface Sci. 2020, 578, 749–757. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Ghosal, A.; Bala, J.; Nikkhah-Moshaie, R.; Wani, W.A.; Manickam, P.; Nair, M. Nanocomposite hydrogels: Advances in nanofillers used for nanomedicine. Gels 2018, 4, 75. [Google Scholar] [CrossRef]
- Idumah, C.I.; Obele, C.M.; Emmanuel, E.O.; Hassan, A. Recently emerging nanotechnological advancements in polymer nanocomposite coatings for anti-corrosion, anti-fouling and self-healing. Surf. Interfaces 2020, 21, 100734. [Google Scholar] [CrossRef]
- Bayram, C.; Demirbilek, M.; Çalışkan, N.; Demirbilek, M.E.; Denkbcş, E.B. Osteoblast activity on anodised titania nanotubes: Effect of simulated body fluid soaking time. J. Biomed. Nanotechnol. 2012, 8, 482–490. [Google Scholar] [CrossRef]
- Mokhtari, H.; Ghasemi, Z.; Kharaziha, M.; Karimzadeh, F.; Alihosseini, F. Chitosan-58S bioactive glass nanocomposite coatings on TiO2 nanotube: Structural and biological properties. Appl. Surf. Sci. 2018, 441, 138–149. [Google Scholar] [CrossRef]
- Capra, P.; Musitelli, G.; Perugini, P. Wetting and adhesion evaluation of cosmetic ingredients and products: Correlation of in vitro–in vivo contact angle measurements. Int. J. Cosmet. Sci. 2017, 39, 393–401. [Google Scholar] [CrossRef]
- Sethi, S.; Kaith, B.S.; Kaur, M.; Sharma, N.; Kumar, V. Cross-linked xanthan gum–starch hydrogels as promising materials for controlled drug delivery. Cellulose 2020, 27, 4565–4589. [Google Scholar] [CrossRef]
- Berkey, C.; Biniek, K.; Dauskardt, R.H. Predicting hydration and moisturiser ingredient effects on mechanical behavior of human stratum corneum. Extrem. Mech. Lett. 2021, 46, 101327. [Google Scholar] [CrossRef]
- Iliescu, T.; Baia, M.; Kiefer, W. FT-Raman, surface-enhanced Raman spectroscopy and theoretical investigations of diclofenac sodium. Chem. Phys. 2004, 298, 167–174. [Google Scholar] [CrossRef]
- França, D.; Trigueiro, P.; Silva Filho, E.; Fonseca, M.; Jaber, M. Monitoring diclofenac adsorption by organophilic alkylpyridinium bentonites. Chemosphere 2020, 242, 125109. [Google Scholar] [CrossRef]
- Sarwar, M.N.; Ullah, A.; Haider, M.K.; Hussain, N.; Ullah, S.; Hashmi, M.; Khan, M.Q.; Kim, I.S. Evaluating antibacterial efficacy and biocompatibility of PAN nanofibers loaded with diclofenac sodium salt. Polymers 2021, 13, 510. [Google Scholar] [CrossRef]
- Hobzova, R.; Hrib, J.; Sirc, J.; Karpushkin, E.; Michalek, J.; Janouskova, O.; Gatenholm, P. Embedding of bacterial cellulose nanofibers within PHEMA hydrogel matrices: Tunable stiffness composites with potential for biomedical applications. J. Nanomater. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Aljubailah, A.; Alqahtani, S.M.; Al-Garni, T.S.; Saeed, W.S.; Semlali, A.; Aouak, T. Naproxen-Loaded Poly (2-hydroxyalkyl methacrylates): Preparation and Drug Release Dynamics. Polymers 2022, 14, 450. [Google Scholar] [CrossRef]
- Li, X.; Feng, H.; Mei, Y.; Wu, Z.; Cai, X.; Xu, Z.; Hui, J.; Wu, Z. Describing a modern therapeutic drug prepared by in situ decorated gold nanoparticles on starch-modified magnetic nanoparticles to treat the cutaneous wound: A preclinical trial study. J. Exp. Nanosci. 2022, 17, 150–162. [Google Scholar] [CrossRef]
- Kareem, S.H.; Naji, A.M.; Taqi, Z.J.; Jabir, M.S. Polyvinylpyrrolidone loaded-MnZnFe2O4 magnetic nanocomposites induce apoptosis in cancer cells through mitochondrial damage and P53 pathway. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5009–5023. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Usman Minhas, M.; Barkat, K.; Sohail, M. Cross-linked sodium alginate-g-poly (acrylic acid) structure: A potential hydrogel network for controlled delivery of loxoprofen sodium. Adv. Polym. Technol. 2018, 37, 985–995. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Rojewska, M.; Biadasz, A.; Lulek, J.; Prochaska, K. Surface properties and morphology of selected polymers and their blends designed to mucoadhesive dosage forms. React. Funct. Polym. 2017, 118, 10–19. [Google Scholar] [CrossRef]
- Koopaie, M.; Bordbar-Khiabani, A.; Kolahdooz, S.; Darbandsari, A.K.; Mozafari, M. Advanced surface treatment techniques counteract biofilm-associated infections on dental implants: A comparative study. Mater. Res. Express 2020, 7, 015417. [Google Scholar] [CrossRef]
- Chouhan, S.; Bajpai, A.K.; Bhatt, R. Analysis of topographical parameters and interfacial interaction of zinc oxide reinforced poly (vinyl alcohol-g-acrylonitrile) nanocomposite film surfaces using atomic force microscopy. Nano-Struct. Nano-Objects 2019, 18, 100308. [Google Scholar] [CrossRef]
- Zhou, M.; Xiong, X.; Drummer, D.; Jiang, B. Interfacial interaction and joining property of direct injection-molded polymer-metal hybrid structures: A molecular dynamics simulation study. Appl. Surf. Sci. 2019, 478, 680–689. [Google Scholar] [CrossRef]
- Ailincai, D.; Dorobanțu, A.M.; Dima, B.; Irimiciuc, Ș.A.; Lupașcu, C.; Agop, M.; Olguta, O. Poly (vinyl alcohol boric acid)-diclofenac sodium salt drug delivery systems: Experimental and theoretical studies. J. Immunol. Res. 2020, 2020, 3124304. [Google Scholar] [CrossRef]
- Maver, U.; Velnar, T.; Gaberšček, M.; Planinšek, O.; Finšgar, M. Recent progressive use of atomic force microscopy in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 96–111. [Google Scholar] [CrossRef]
- Reddy, P.L.; Deshmukh, K.; Chidambaram, K.; Ali, M.M.N.; Sadasivuni, K.K.; Kumar, Y.R.; Lakshmipathy, R.; Pasha, S. Dielectric properties of polyvinyl alcohol (PVA) nanocomposites filled with green synthesised zinc sulphide (ZnS) nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 4676–4687. [Google Scholar] [CrossRef]
- Swick, L.; Mencke, D.; Suah, M. Novel Liquid Bandage as a Topical Treatment for Post-Surgical Wound Care. J. Clin. Exp. Dermatol. Res. 2017, 8, 2. [Google Scholar] [CrossRef]
- Maver, T.; Smrke, D.; Kurečič, M.; Gradišnik, L.; Maver, U.; Kleinschek, K.S. Combining 3D printing and electrospinning for preparation of pain-relieving wound-dressing materials. J. Sol-Gel Sci. Technol. 2018, 88, 33–48. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Kebdani, M.; Shirinbayan, M.; Champmartin, S.; Tcharkhtchi, A.; Kouidri, S.; Bakir, F. Development of a model based on physical mechanisms for the explanation of drug release: Application to diclofenac release from polyurethane films. Polymers 2021, 13, 1230. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Khandelwal, M. Ex-situ modification of bacterial cellulose for immediate and sustained drug release with insights into release mechanism. Carbohydr. Polym. 2020, 249, 116816. [Google Scholar] [CrossRef] [PubMed]
- Kurdtabar, M.; Rezanejade Bardajee, G. Drug release and swelling behavior of magnetic iron oxide nanocomposite hydrogels based on poly (acrylic acid) grafted onto sodium alginate. Polym. Bull. 2020, 77, 3001–3015. [Google Scholar] [CrossRef]
- Viscusi, G.; Gorrasi, G. Facile preparation of layered double hydroxide (LDH)-alginate beads as sustainable system for the triggered release of diclofenac: Effect of pH and temperature on release rate. Int. J. Biol. Macromol. 2021, 184, 271–281. [Google Scholar] [CrossRef]
- Barkhordari, S.; Alizadeh, A. Fabrication of pH-sensitive chitosan/layered double hydroxide (LDH)/Fe3O4 nanocomposite hydrogel beads for controlled release of diclofenac. Polym. Bull. 2022, 79, 5533–5548. [Google Scholar] [CrossRef]
- Hervault, A.; Dunn, A.E.; Lim, M.; Boyer, C.; Mott, D.; Maenosono, S.; Thanh, N.T. Doxorubicin loaded dual pH-and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale 2016, 8, 12152–12161. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Kazemi-Bagsangani, S.; Jamili, M.; Zavareh, S. Evaluation of magnetic nanoparticles coated by 5-fluorouracil imprinted polymer for controlled drug delivery in mouse breast cancer model. Int. J. Pharm. 2016, 497, 228–238. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Peng, Z.; She, F.; Kong, L. Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr. Polym. 2011, 85, 698–704. [Google Scholar] [CrossRef]
- Kostevšek, N.; Šturm, S.; Serša, I.; Sepe, A.; Bloemen, M.; Verbiest, T.; Kobe, S.; Rožman, K.Ž. “Single-” and “multi-core” FePt nanoparticles: From controlled synthesis via zwitterionic and silica bio-functionalisation to MRI applications. J. Nanoparticle Res. 2015, 17, 464. [Google Scholar] [CrossRef]
- Nowok, A.; Dulski, M.; Grelska, J.; Szeremeta, A.Z.; Jurkiewicz, K.; Grzybowska, K.; Musiał, M.; Pawlus, S. Phenyl Ring: A Steric Hindrance or a Source of Different Hydrogen Bonding Patterns in Self-Organizing Systems? J. Phys. Chem. Lett. 2021, 12, 2142–2147. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.; Asyraf, M.; Razman, M. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Du, Y.; Jia, S.; Chen, Y.; Zhang, L.; Tan, J. Type I photoinitiator-functionalised block copolymer nanoparticles prepared by RAFT-mediated polymerisation-induced self-assembly. ACS Macro Lett. 2021, 10, 297–306. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yetter, A.B. Correlation of visual in vitro cytotoxicity ratings of biomaterials with quantitative in vitro cell viability measurements. Cell Biol. Toxicol. 2008, 24, 315–319. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Amanullah, A.; Upadhyay, A.; Dhiman, R.; Singh, S.; Kumar, A.; Ahirwar, D.K.; Gutti, R.K.; Mishra, A. Development and Challenges of Diclofenac-Based Novel Therapeutics: Targeting Cancer and Complex Diseases. Cancers 2022, 14, 4385. [Google Scholar] [CrossRef]
- Pawłowska, B.; Telesiński, A.; Biczak, R. Effect of diclofenac and naproxen and their mixture on spring barley seedlings and Heterocypris incongruens. Environ. Toxicol. Pharmacol. 2021, 88, 103746. [Google Scholar] [CrossRef]
- Saranya, S.; Vijayaranai, K.; Pavithra, S.; Raihana, N.; Kumanan, K. In vitro cytotoxicity of zinc oxide, iron oxide and copper nanopowders prepared by green synthesis. Toxicol. Rep. 2017, 4, 427–430. [Google Scholar]
- Sgouras, D.; Duncan, R. Methods for the evaluation of biocompatibility of soluble synthetic polymers which have potential for biomedical use: 1—Use of the tetrazolium-based colorimetric assay (MTT) as a preliminary screen for evaluation ofin vitro cytotoxicity. J. Mater. Sci. Mater. Med. 1990, 1, 61–68. [Google Scholar] [CrossRef]
- González, G.; Baruffaldi, D.; Martinengo, C.; Angelini, A.; Chiappone, A.; Roppolo, I.; Pirri, C.F.; Frascella, F. Materials testing for the development of biocompatible devices through vat-polymerisation 3D printing. Nanomaterials 2020, 10, 1788. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Gorrita, M.; Herráez-Galindo, C.; Torres-Lagares, D.; Serrera-Figallo, M.-Á.; Gutiérre-Pérez, J.-L. Biocompatibility of polymer and ceramic CAD/CAM materials with human gingival fibroblasts (HGFs). Polymers 2019, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
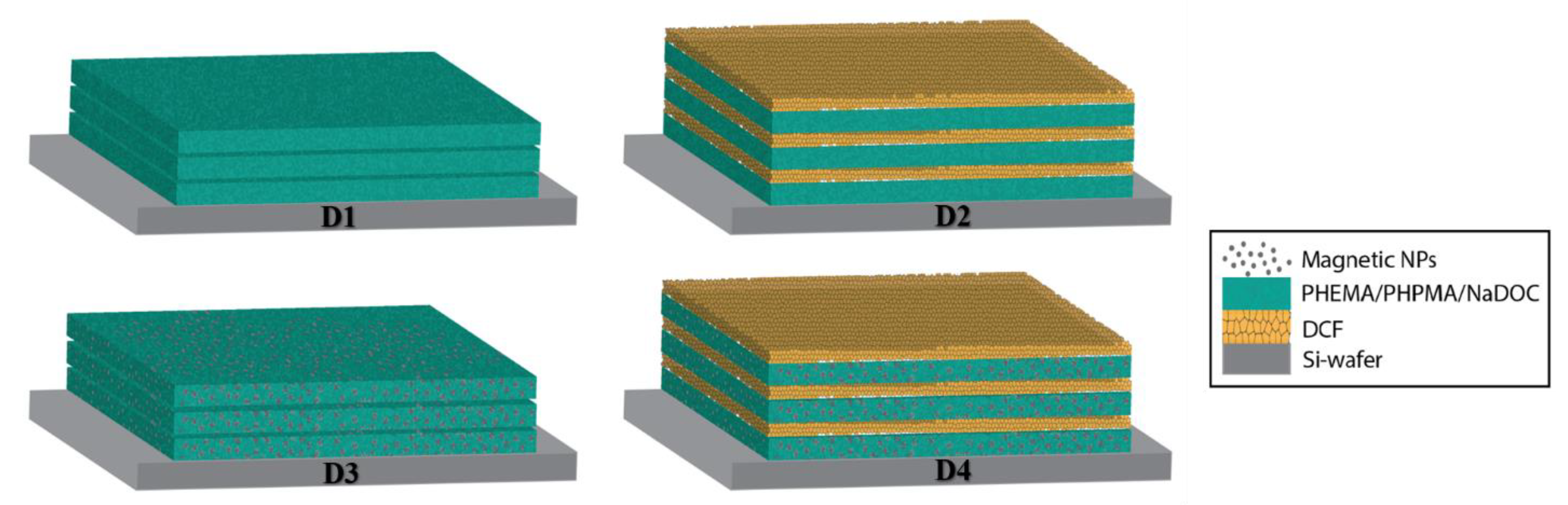

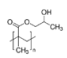
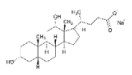
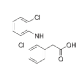
| PHEMA | PHPMA | NaDOC | DCF | FePt NPs | |
|---|---|---|---|---|---|
 |  |  |  |  | |
| FORMULATION 1 (F1) | 1 | 0.1 | 1 | ||
| FORMULATION 2 (F2) | 1 | 0.1 | 1 | 1 | |
| FORMULATION 3 (F3) | 1 | 0.1 | 1 | 1 | |
| FORMULATION 4 (F4) | 1 | 0.1 | 1 | 1 | 1 |
| Preparation | |
|---|---|
| DRESSING 1 (D1) | 3 layers of F1 |
| DRESSING 2 (D2) | 3 layers of F1 with alternating 3 layers of F2 |
| DRESSING 3 (D3) | 3 layers of F3 |
| DRESSING 4 (D4) | 3 layers of F3 with alternating 3 layers of F4 |
| Sample | Average Contact Angle Value (°) | Standard Deviation (°) | Photographs |
|---|---|---|---|
| D1 | 25.53 | 1.51 |  |
| D2 | 39.03 | 0.64 | |
| D3 | 21.89 | 1.18 | |
| D4 | 32.93 | 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zidarič, T.; Skok, K.; Orthaber, K.; Pristovnik, M.; Gradišnik, L.; Maver, T.; Maver, U. Multilayer Methacrylate-Based Wound Dressing as a Therapeutic Tool for Targeted Pain Relief. Materials 2023, 16, 2361. https://doi.org/10.3390/ma16062361
Zidarič T, Skok K, Orthaber K, Pristovnik M, Gradišnik L, Maver T, Maver U. Multilayer Methacrylate-Based Wound Dressing as a Therapeutic Tool for Targeted Pain Relief. Materials. 2023; 16(6):2361. https://doi.org/10.3390/ma16062361
Chicago/Turabian StyleZidarič, Tanja, Kristijan Skok, Kristjan Orthaber, Matevž Pristovnik, Lidija Gradišnik, Tina Maver, and Uroš Maver. 2023. "Multilayer Methacrylate-Based Wound Dressing as a Therapeutic Tool for Targeted Pain Relief" Materials 16, no. 6: 2361. https://doi.org/10.3390/ma16062361
APA StyleZidarič, T., Skok, K., Orthaber, K., Pristovnik, M., Gradišnik, L., Maver, T., & Maver, U. (2023). Multilayer Methacrylate-Based Wound Dressing as a Therapeutic Tool for Targeted Pain Relief. Materials, 16(6), 2361. https://doi.org/10.3390/ma16062361










