Development of New Azomethine Metal Chelates Derived from Isatin: DFT and Pharmaceutical Studies
Abstract
1. Introduction
2. Employed Experiments for the Study
2.1. Starting Materials, Reagents Instruments and Solutions
2.2. Synthesis of Schiff Base Ligand
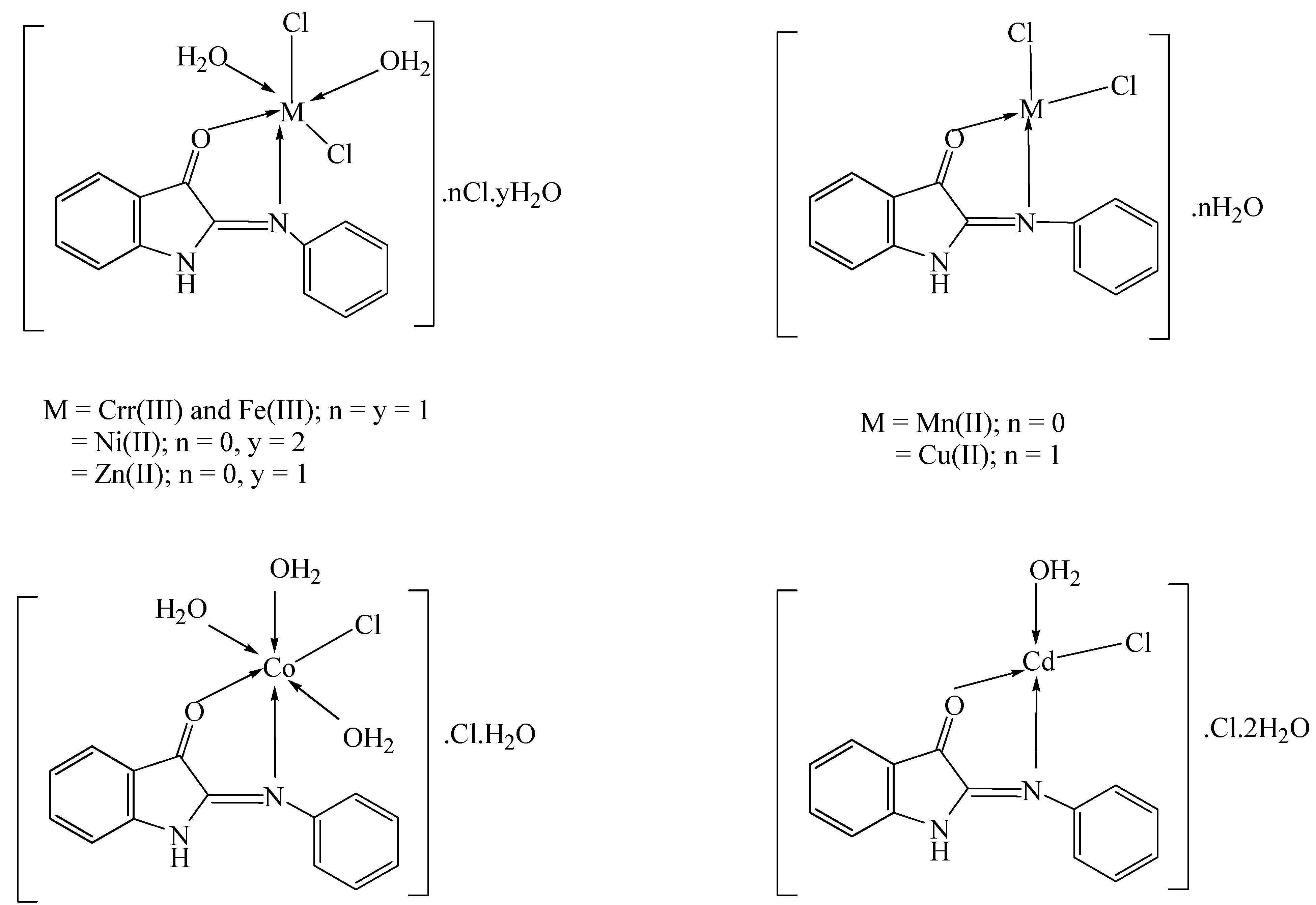
2.3. Synthesis of Metal Complexes
2.4. DFT Studies
2.5. Antimicrobial Activity
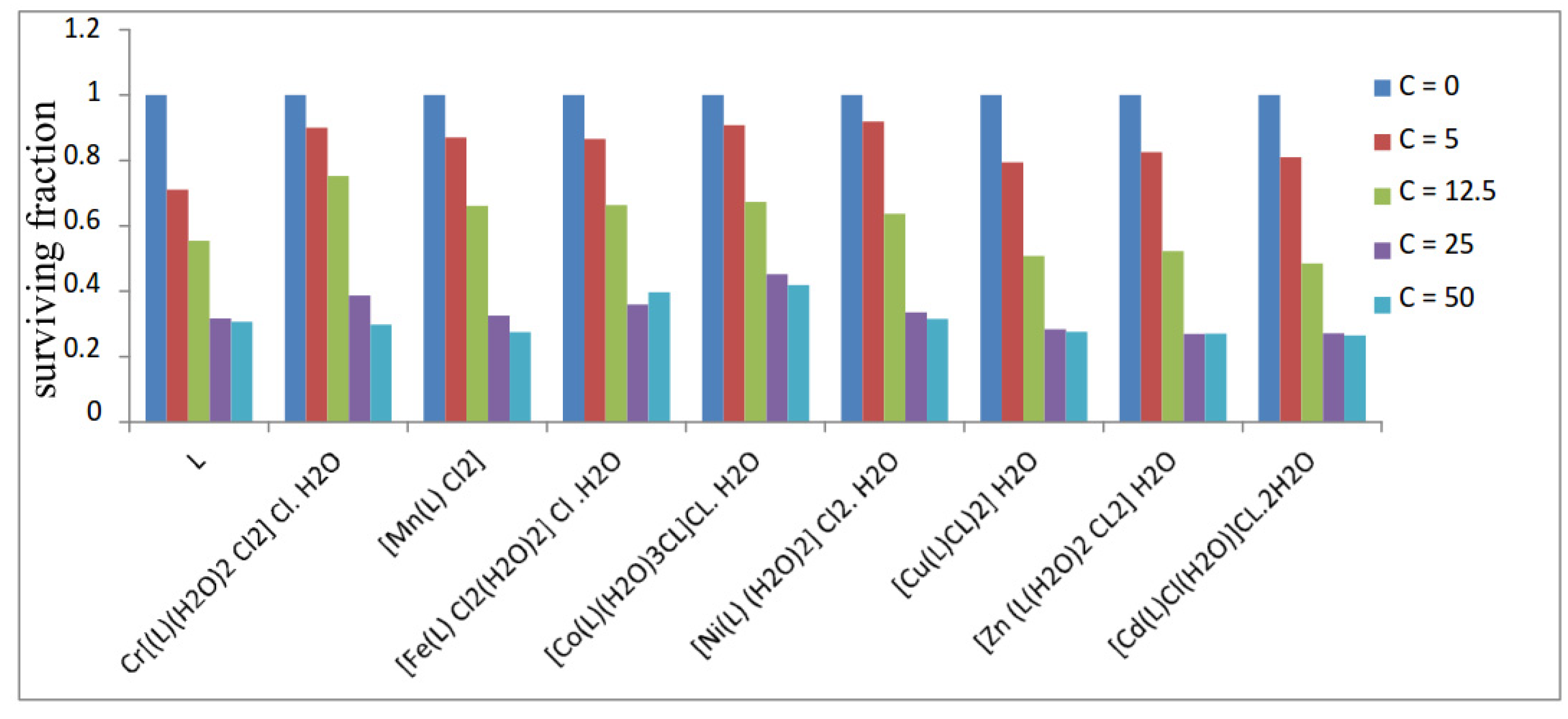
2.6. Anticancer Activity
3. Results and Discussion
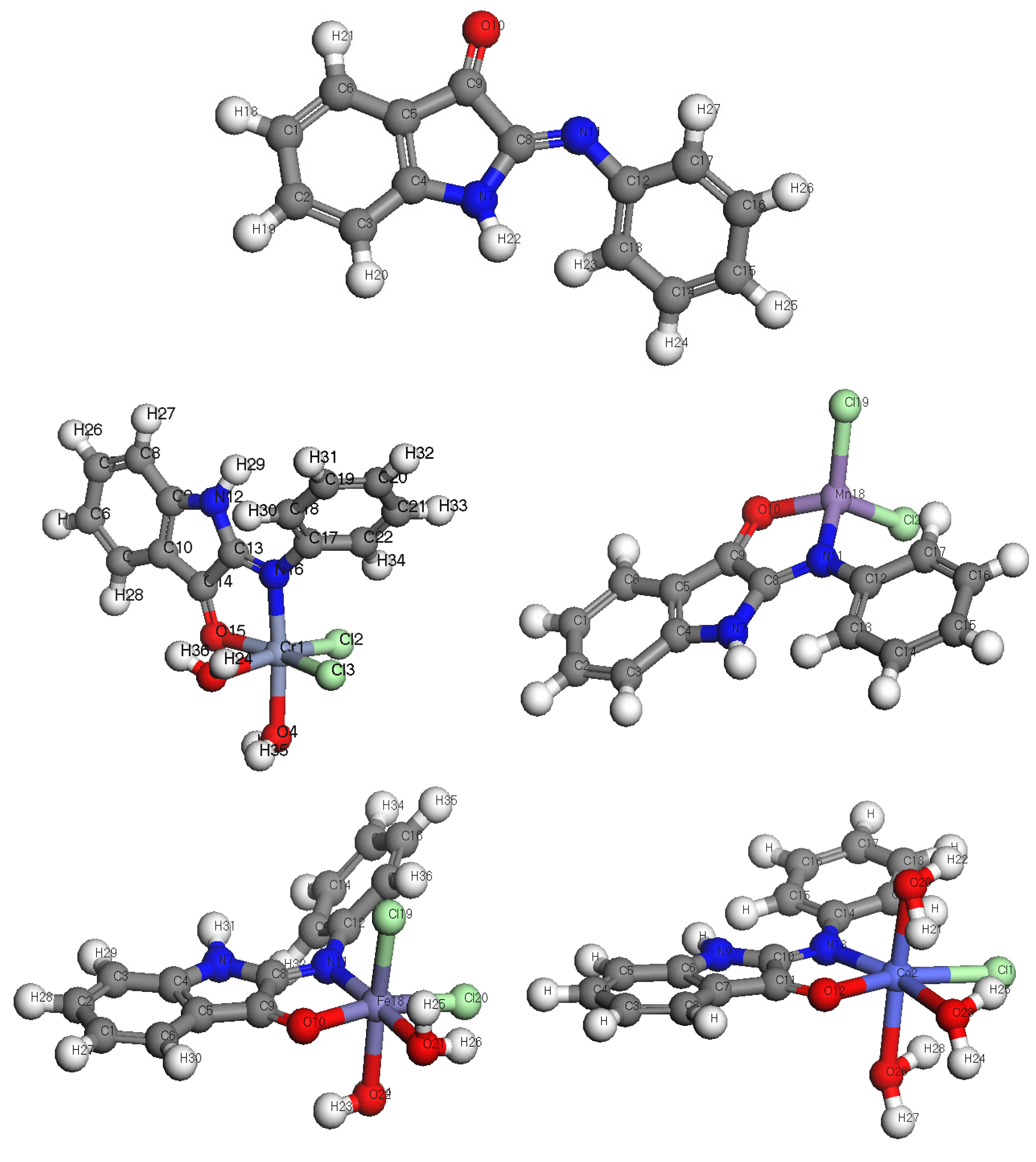
3.1. Characterization of Schiff Base Ligand (L)
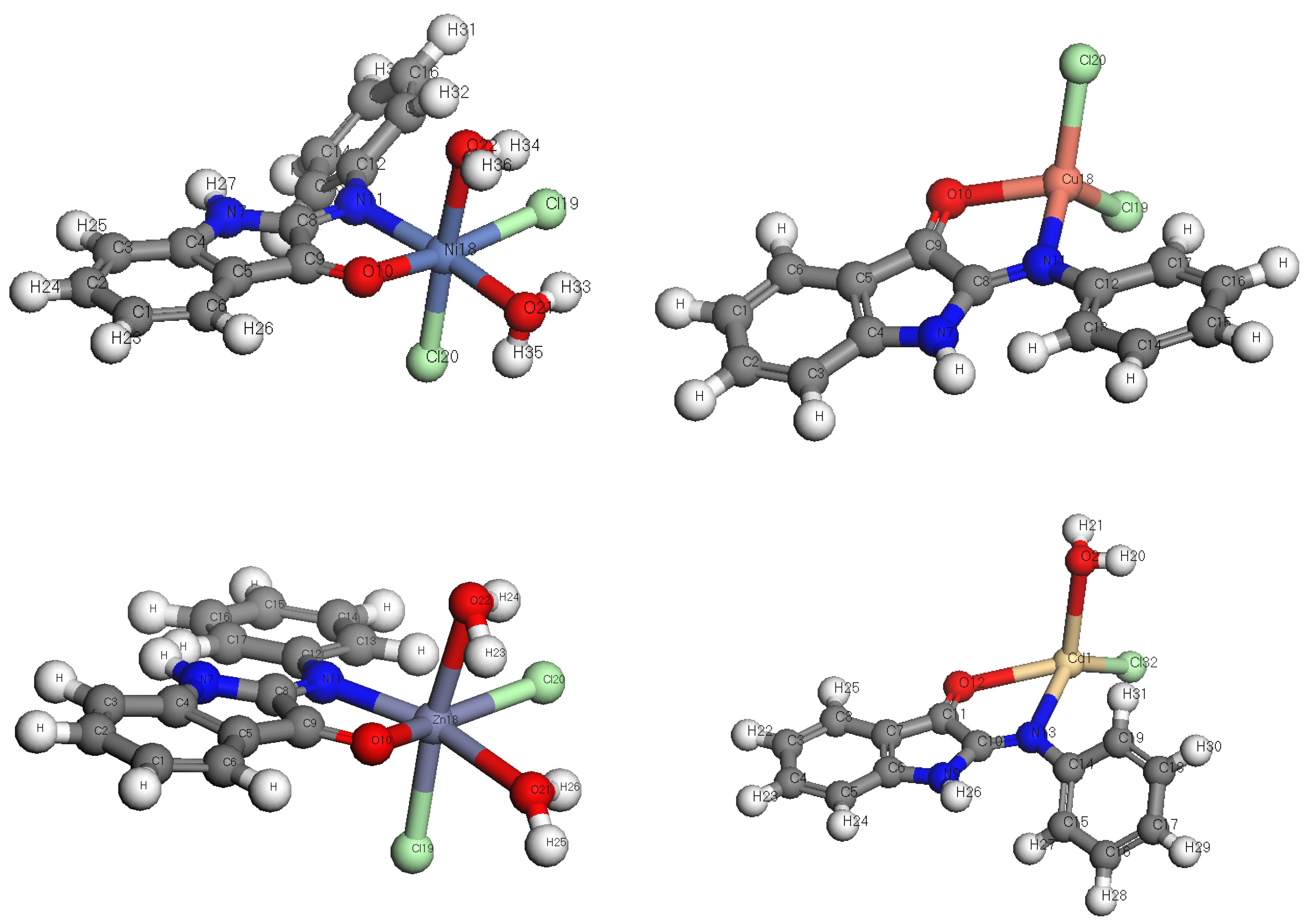
3.2. Elemental Analyses of Complexes
3.3. IR Spectral Studies
3.4. Molar Conductivity Measurements
3.5. UV–Vis Spectra
3.6. Electronic Spectra and Magnetic Moments
3.7. Thermal Studies
3.8. SEM Study
3.9. Calculation of Quantum Chemical Parameters
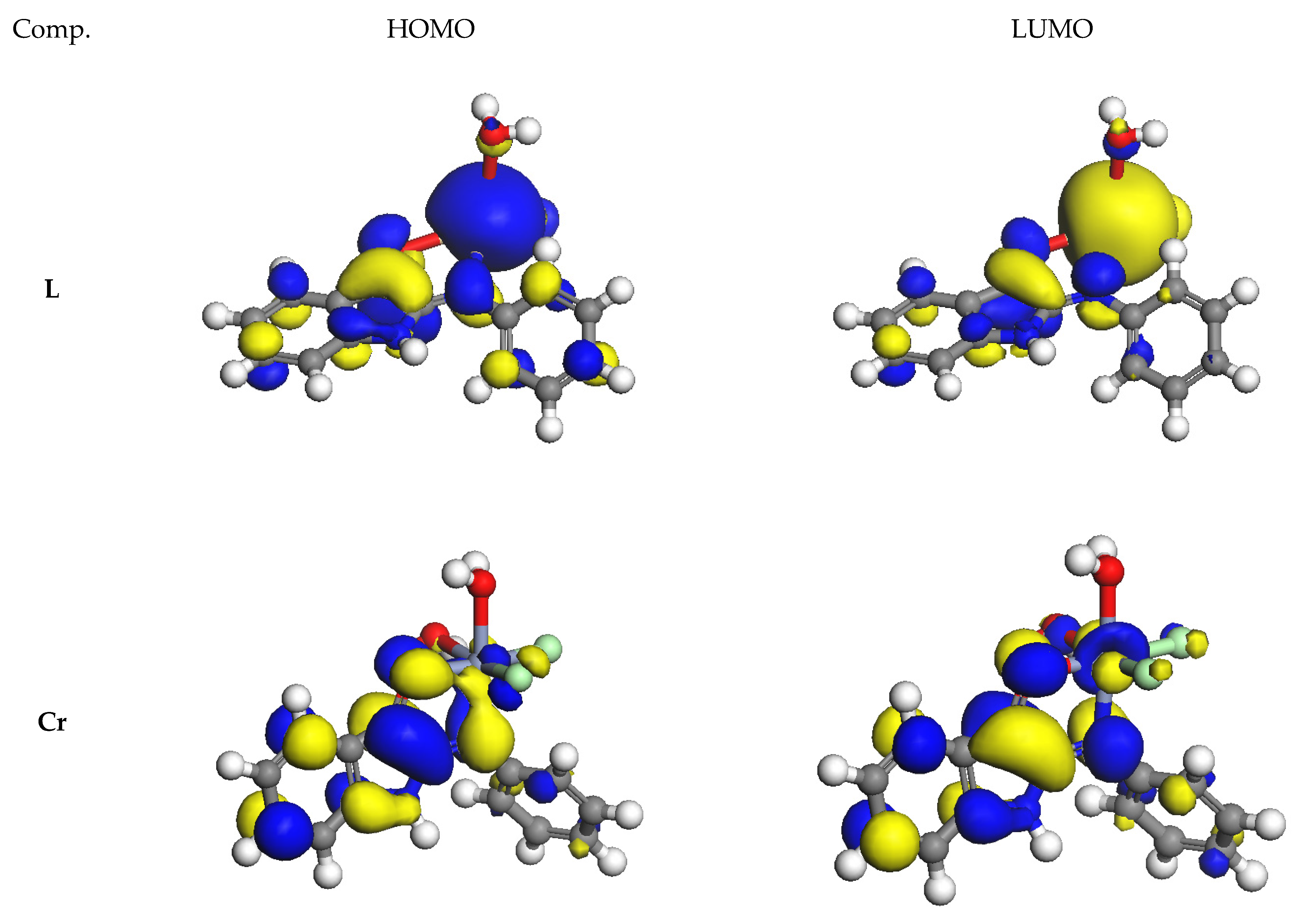
3.9.1. DFT Calculations Studies
3.9.2. Study of Frontier Orbitals
3.9.3. Chemical Reactivity Descriptors
3.10. In Silico ADME Predictions
3.11. Antimicrobial Activity
- Against Streptococcus pneumonia
- Ni(II) > Cr(III) > Cd(III) > Cu(II) > Co(II) > Fe(III) > Mn(II)
- Against Bacillus subtilis
- Cr(III) > Cu(II) > Fe(III) > Ni(II) > Cd(III) > Mn(II)
- Against Pseudomonas aeruginosa
- Mn(II) > Cr(III) >Co(II) > Cd(III) > Cu(II) > Fe(III) > Ni(II)
- Against Escherichia coli
- Fe(III) > Cu(II) > Cr(III) > Mn(II) = Co(II) > Zn(II) = Cd(III) > Ni(II)
- Against Aspergillus fumigatus
- Cd(III) > Ni(II) > Cr(III) > Co(II) > Zn(II)
- Against Candida albicans
- Cr(III) > Co(II) > Cd(III) >Zn(II) > Ni(II) > Cu(II) > Mn(II) > Fe(III)
3.12. Anticancer Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arulmurugan, S.; Kavitha, P.H.; Venkatraman, R.P. Biological activities of Schiff base and its complexes: A review. Rasayan J. Chem. 2010, 3, 385–410. [Google Scholar]
- Abu-Dief, A.M.; El-Khatib, R.M.; Aljohani, F.S.; Al-Abdulkarim, H.A.; Alzahrani, S.; El-Sarrag, G.; Ismael, M. Synthesis, structuralelucidation, DFT calculation, biological studies and DNA inter-action of some aryl hydrazone Cr3+, Fe3+, and Cu2+ chelates. Comput. Biol. Chem. 2022, 97, 107643. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, M.S.; Prasad, D.J.; Poojary, B.; Bhat, K.S.; Holla, B.S.; Kumari, N.S. Synthesis and biological activity of Schiff and Mannich bases bearing 2, 4-dichloro-5-fluorophenyl moiety. Bioorg. Med. Chem. 2006, 14, 7482–7489. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dief, A.M.; El-Sagher, H.M.; Shehata, M.R. Fabrication, spectroscopic characterization, calf thymus DNA binding investigation, antioxidant and anticancer activities of some antibiotic azomethine Cu (II), Pd (II), Zn (II) and Cr (III) complexes. Appl. Organomet. Chem. 2019, 33, e4943. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Nair, R.R.; Vijayalakshmi, G.; Subramanian, E.H.; Sridhar, S.K. Synthesis of Schiff bases of 4-(4-aminophenyl)-morpholine as potential antimicrobial agents. Eur. J. Med. Chem. 2005, 4, 225–229. [Google Scholar] [CrossRef]
- Aljohani, E.T.; Shehata, M.R.; Alkhatib, F.; Alzahrani, S.O.; Abu-Dief, A.M. Development and structure elucidation of new VO2+, Mn2+, Zn2+, and Pd2+ complexes based on azomethine ferrocenyl ligand: DNA interaction, antimicrobial, antioxidant, anticancer activities, and molecular docking. Appl. Organomet. Chem. 2021, 35, e6154. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Khalaf, M.M.; Shehata, M.R.; Abu-Dief, A.M. Fabrication, DFT calculation, and molecular docking of two Fe (III) imine chelates as anti-COVID-19 and pharmaceutical drug candidate. Int. J. Mol. Sci. 2022, 23, 3994. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Khatib, R.M.; Salah, M.E.; Alzahrani, S.; Alkhatib, F.; El-Sarrag, G.; Ismael, M. Tailoring, structural elucidation, DFT calculation, DNA interaction and pharmaceutical applications of some aryl hydrazone Mn (II), Cu (II) and Fe (III) complexes. J. Mol. Struct. 2021, 1244, 131017. [Google Scholar] [CrossRef]
- Qasem, H.A.; Aouad, M.R.; Al-Abdulkarim, H.A.; Al-Farraj, E.S.; Attar, R.M.; El-Metwaly, N.M.; Abu-Dief, A.M. Tailoring of some novel bis-hydrazone metal chelates, spectral based characterization and DFT calculations for pharmaceutical applications and in-silico treatments for verification. J. Mol. Struct. 2022, 1264, 133263. [Google Scholar] [CrossRef]
- Rezaeivala, M.; Keypour, H. Schiff base and non-Schiff base macrocyclic ligands and complexes incorporating the pyridine moiety–The first 50 years. Coord. Chem. Rev. 2014, 280, 203–253. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Deghadi, R.G.; Mohamed, G.G. Novel Schiff base ligand and its metal complexes with some transition elements. Synthesis, spectroscopic, thermal analysis, antimicrobial and in vitro anticancer activity. Appl. Organom. Chem. 2016, 30, 221–230. [Google Scholar] [CrossRef]
- Ouyang, X.M.; Fei, B.L.; Okamuro, T.A.; Sun, W.Y.; Tang, W.X.; Ueyama, N. Synthesis, crystal structure and superoxide dismutase (SOD) activity of novel seven-coordinated manganese (II) complex with multidentate di-Schiff base ligands. Chem. Lett. 2002, 3, 362–363. [Google Scholar] [CrossRef]
- El-Remaily, M.A.E.A.A.A.; Soliman, A.M.; Khalifa, M.E.; El-Metwaly, N.M.; Alsoliemy, A.; El-Dabea, T.; Abu-Dief, A.M. Rapidly, highly yielded and green synthesis of dihydrotetrazolo [1, 5-a] pyrimidine derivatives in aqueous media using recoverable Pd (II) thiazole catalyst accelerated by ultrasonic: Computational studies. Appl. Organomet. Chem. 2022, 36, e6320. [Google Scholar] [CrossRef]
- Ali El-Remaily, M.A.E.A.A.; El-Dabea, T.; Alsawat, M.; Mahmoud, M.H.; Alfi, A.A.; El-Metwaly, N.; Abu-Dief, A.M. Development of new thiazole complexes as powerful catalysts for synthesis of pyrazole-4-carbonitrile derivatives under ultrasonic irradiation condition supported by DFT studies. ACS Omega 2021, 6, 21071–21086. [Google Scholar] [CrossRef] [PubMed]
- El-Remaily, M.A.E.A.A.A.; El-Metwaly, N.M.; Bawazeer, T.M.; Khalifa, M.E.; El-Dabea, T.; Abu-Dief, A.M. Efficient and recoverable novel pyranothiazol Pd (II), Cu (II) and Fe (III) catalysts in simple synthesis of polyfunctionalized pyrroles: Under mild conditions using ultrasonic irradiation. Appl. Organomet. Chem. 2021, 11, e6370. [Google Scholar] [CrossRef]
- Jouad, E.M.; Larcher, G.; Allain, M.; Riou, A.; Bouet, G.M.; Khan, M.A.; Do Thanh, X. Synthesis, structure and biological activity of nickel (II) complexes of 5-methyl 2-furfural thiosemicarbazone. J. Inorg. Biochem. 2001, 86, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Sriram, D.; Yogeeswari, P.; Ananthan, S. Antituberculous activity of norfloxacin mannich bases with isatin derivatives. Chemotherapy 2001, 47, 266–269. [Google Scholar] [CrossRef]
- Konstantinovic, S.S.; Radovanović, B.; Cakic, Z.; Vasić, V.M. Synthesis and characterization of Co (II), Ni (II), Cu (II) and Zn (II) complexes with 3-salicylidenehydrazono-2-indolinone. J. Serb. Chem. Soc. 2003, 68, 641–647. [Google Scholar] [CrossRef]
- Silva, B.V. Isatin, a versatile molecule: Studies in Brazil. J. Braz. Chem. Soc. 2013, 24, 707–720. [Google Scholar] [CrossRef]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Ranson, M.; Skropeta, D. Cytotoxic and anticancer activities of isatin and its derivatives: A comprehensive review from 2000–2008. Anti-Cancer Agents Med. Chem. 2009, 9, 397. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Clow, A.; Sandler, M.; Glover, V. Isatin—A link between natriuretic peptides and monoamines? Biochem. Pharmacol. 1996, 52, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.V.; Fahmi, N.; Biyala, M.K. Coordination behavior and biopotency of N and S/O donor ligands with their Paladium(II) and Platinum(II) complexes. J. Iran. Chem. Soc. 2005, 2, 40–46. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Shaikh, A.U.; Naseer, M.M. Metal-based isatin-bearing Sulfonamides: Their synthesis, characterization and biological properties. Appl. Organometal. Chem. 2006, 20, 729–739. [Google Scholar] [CrossRef]
- Ikotun, A.A.; Coogan, M.P.; Owoseni, A.A.; Egharevba, G.O. Design, synthesis, physicochemical and antimicrobial properties of rhenium (I) tricarbonyl complexes of 3-(phenylimino)indole-2-one. J. Chem. Soc. Nigeria 2019, 44, 948–958. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.02; Gaussian Inc.: Wallingfort, UK, 2009. [Google Scholar]
- Niu, Y.; Feng, S.; Ding, Y.; Qu, R.; Wang, D.; Han, J. Theoretical investigation on sulfur-containing chelating resin-divalent metal complexes. Int. J. Quantum Chem. 2010, 110, 1982–1993. [Google Scholar] [CrossRef]
- Ekennia, A.C.; Onwudiwe, D.C.; Olasunkanmi, L.O.; Osowole, A.A.; Ebenso, E.E. Synthesis, DFT calculation, and antimicrobial studies of novel Zn (II), Co (II), Cu (II), and Mn (II) heteroleptic complexes containing benzoylacetone and dithiocarbamate. Bioinorg. Chem. Appl. 2015, 2015, 789063. [Google Scholar] [CrossRef]
- Al-Janabi, A.S.; Al-Bayati, A.F.; ALtaie, O.A.; Elzupir, A.O.; Yousef, T.A. Co(II), Ni(II), and Zn(II) complexes of 5-methyl-1,3,4-oxadiazol-2-amine Schiff base as potential heat shock protein 90 inhibitors: Spectroscopic, biological activity, density functional theory, and molecular docking studies. Appl. Organomet. Chem. 2022, 36, e6899. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Moustafa, H.; Abdel-Mawgoud, A.A.H. Design and nonlinear optical properties (NLO) using DFT approach of new Cr (III), VO (II), and Ni (II) chelates incorporating tri-dentate imine ligand for DNA interaction, antimicrobial, anticancer activities and molecular docking studies. Arab. J. Chem. 2020, 13, 649–670. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Shehata, M.R.; Abdel-Mawgoud, A.A.H. Novel azomethine Pd (II)-and VO (II)-based metallo-pharmaceuticals as anticancer, antimicrobial, and antioxidant agents: Design, structural inspection, DFT investigation, and DNA interaction. J. Phys. Org. Chem. 2019, 32, e4009. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Mahmoud, N.F.; Mohamed, G.G.; El-Bindary, A.A.; El-Sonbati, A.Z. Supramolecular structural, thermal properties and biological activity of 3-(2-methoxyphenoxy) propane-1, 2-diol metal complexes. J. Mol. Struct. 2015, 1086, 266–275. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abdelhamid, A.A.; Abu-Dief, A.M.; Shehata, M.R.; Bakhe, M.A. Facile synthesis, X-Ray structure of new multi-substituted aryl imidazole ligand, biological screening and DNA binding of its Cr (III), Fe (III) and Cu (II) coordination compounds as potential antibiotic and anticancer drugs. J. Mol. Struct. 2020, 1200, 127034. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Mahmoud, N.F.; Mohamed, G.G.; El-Sonbati, A.Z.; El-Bindary, A.A. Ternary metal complexes of guaifenesin drug: Synthesis, spectroscopic characterization and in vitro anticancer activity of the metal complexes. Spectrochim. Acta A 2015, 150, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Yousef, T.A.; El-Reash, G.M.A.; El-Gammal, O.A.; Bedier, R.A. Synthesis, characterization, optical band gap, in vitro antimicrobial activity and DNA cleavage studies of some metal complexes of pyridyl thiosemicarbazone. J. Mol. Struct. 2013, 1035, 307–317. [Google Scholar] [CrossRef]
- Netalkar, P.P.; Netalkar, S.P.; Revankar, V.K. Synthesis, crystal structures and characterization of late first row transition metal complexes derived from benzothiazole core: Anti-tuberculosis activity and special emphasis on DNA binding and cleavage property. Eur. J. Med. Chem. 2014, 79, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeedi, S.I.; Abdel-Rahman, L.H.; Abu-Dief, A.M.; Abdel-Fatah, S.M.; Alotaibi, T.M.; Alsalme, A.M.; Nafady, A. Catalytic Oxidation of Benzyl Alcohol Using Nanosized Cu/Ni xzSchiff-Base Complexes and Their Metal Oxide Nanoparticles. Catalysts 2018, 8, 452. [Google Scholar] [CrossRef]
- Aljohani, E.T.; Shehata, M.R.; Abu-Dief, A.M. Design, synthesis, structural inspection of Pd2+, VO2+, Mn2+, and Zn2+ chelates incorporating ferrocenyl thiophenol ligand: DNA interaction and pharmaceutical studies. Appl. Organomet. Chem. 2021, 35, e6169. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdel-Mawgoud, A.A.H. A robust in vitro anticancer, antioxidant and antimicrobial agents based on new metal-azomethine chelates incorporating Ag (I), Pd (II) and VO (II) cations: Probing the aspects of DNA interaction. Appl. Organomet. Chem. 2020, 34, e5373. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Vlad, A.; Vasiliu, M. Chelate Polymers. V. Novel Alternating Copolymers Based on Transition Metal–Azomethine Complexes and Siloxanes. J. Macromol. Sci. A 2004, 41, 565–575. [Google Scholar] [CrossRef]
- Salehi, M.; Rahimifar, F.; Kubicki, M.; Asadi, A. Structural, spectroscopic, electrochemical and antibacterial studies of some new nickel (II) Schiff base complexes. J. Inorg. Chim. Acta 2016, 443, 28–35. [Google Scholar] [CrossRef]
- Abdel-Monem, Y.K.; El-Enein, S.A.A.; El-Sheikh-Amer, M.M. Design of new metal complexes of 2-(3-amino-4, 6-dimethyl-1H-pyrazolo [3, 4-b] pyridin-1-yl) aceto-hydrazide: Synthesis, characterization, modelling and antioxidant activity. J. Mol. Struct. 2017, 1127, 386–396. [Google Scholar] [CrossRef]
- El-Halim, H.F.A.; Mohamed, G.G.; El-Dessouky, M.M.I.; Mahmoud, W.H. Ligational behaviour of lomefloxacin drug towards Cr (III), Mn (II), Fe (III), Co (II), Ni (II), Cu (II), Zn (II), Th (IV) and UO2 (VI) ions: Synthesis, structural characterization and biological activity studies. Spectrochim. Acta A 2011, 82, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.G.; Omar, M.M.; Hindy, A.M. Synthesis, characterization and biological activity of some transition metals with Schiff base derived from 2-thiophene carboxaldehyde and aminobenzoic acid. Spectrochim. Acta A 2005, 62, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.P.; Hadi, J.S.; Abdulnabi, Z.A.; Bolandnazar, Z. Spectroscopic, thermal analysis and DFT computational studies of salen-type Schiff base complexes. Spectrochim. Acta A 2014, 117, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Gup, R.; Kirkan, B. Synthesis and spectroscopic studies of mixed-ligand and polymeric dinuclear transition metal complexes with bis-acylhydrazone tetradentate ligands and 1, 10-phenanthroline. Spectrochim. Acta 2006, 64, 809. [Google Scholar] [CrossRef]
- Khalaf, M.M.; El-Lateef, H.M.A.; Alhadhrami, A.; Sayed, F.N.; Mohamed, G.G.; Gouda, M.; Shaaban, S.; Abu-Dief, A.M. Synthesis, Spectroscopic, Structural and Molecular Docking Studies of Some New Nano-Sized Ferrocene-Based Imine Chelates as Antimicrobial and Anticancer Agents. Materials 2022, 15, 3678. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Abd El-Lateef, H.M.; Gouda, M.; Sayed, F.N.; Mohamed, G.G.; Abu-Dief, A.M. Design, Structural Inspection and Bio-Medicinal Applications of Some Novel Imine Metal Complexes Based on Acetylferrocene. Materials 2022, 15, 4842. [Google Scholar] [CrossRef]
- Nawaz, M.; Abbasi, M.W.; Hisaindee, S.; Zaki, M.J.; Abbas, H.F.; Mengting, H.; Ahmed, M.A. Synthesis, spectral studies and biological evaluation of 2-aminonicotinic acid metal complexes. Spectrochim. Acta 2016, 161, 39–43. [Google Scholar] [CrossRef]
- Al-Janabi, A.S.; Elzupir, A.O.; Yousef, T.A. Synthesis, anti-bacterial evaluation, DFT study and molecular docking as a potential 3-chymotrypsin-like protease (3CLpro) of SARS-CoV-2 inhibitors of a novel Schiff bases. J. Mol. Struct. 2021, 1228, 129454. [Google Scholar] [CrossRef]
- Lewis, D.F.V.; Loannides, C.; Parke, D.V. Interaction of a series of nitriles with the alcohol-inducible isoform of P450: Computer analysis of structure-activity relationships. Xenobiotica 1994, 24, 401–408. [Google Scholar] [CrossRef]
- Shaaban, S.; Ferjani, H.; Abd El-Lateef, H.M.; Khalaf, M.M.; Gouda, M.; Alaasar, M.; Yousef, T.A. Unexpected kinetically controlled organoselenium-based isomaleimide: X-ray structure, hirshfeld surface analysis, 3D energy framework approach, and density functional theory calculation. Front. Chem. 2022, 10, 961787. [Google Scholar] [CrossRef]
- Shaaban, S.; Ferjani, H.; Yousef, T.; Abdel-Motaal, M. Supramolecular Self-Assembly Built by Hydrogen, Stacking and Br···Br Interactions in 4-((4-Bromobenzyl)Selanyl)Aniline: Structure, Hirshfeld Surface Analysis, 3D Energy Framework Approach and Global Reactivity Descriptors. J. Inorg. Organomet. Polym. Mat. 2022, 32, 1878–1890. [Google Scholar] [CrossRef]
- Shaaban, S.; Ferjani, H.; Althagafi, I.; Yousef, T. Crystal structure, Hirshfeld surface analysis, and DFT calculations of methyl (Z)-4-((4-((4-bromobenzyl)selanyl) phenyl) amino)-4-oxobut-2-enoate. J. Mol. Struct. 2021, 1245, 131072. [Google Scholar] [CrossRef]
- Arif, R.; Rana, M.; Yasmeen, S.; Khan, M.S.; Abid, M.; Khan, M.S.; Rahisuddin. Facile synthesis of chalcone derivatives as antibacterial agents: Synthesis, DNA binding, molecular docking, DFT and antioxidant studies. J. Mol. Struct. 2020, 1208, 127905. [Google Scholar] [CrossRef]
- Yousef, T.A.; Khairy, M. Synthesis, Characterization, Optical, DFT, TD DFT Studies and in Silico ADME Predictions of Thiosemicarbazone Ligand and its Au(III) Complex. Orient. J. Chem. 2022, 38. [Google Scholar] [CrossRef]
- El-Shamy, N.T.; Alkaoud, A.M.; Hussein, R.K.; Ibrahim, M.A.; Alhamzani, A.G.; Abou-Krisha, M.M. DFT, ADMET and Molecular Docking Investigations for the Antimicrobial Activity of 6,6′-Diamino-1,1′,3,3′-tetramethyl-5,5′-(4-chlorobenzylidene)bis[pyrimidine-2,4(1H,3H)-dione]. Molecules 2022, 27, 620. [Google Scholar] [CrossRef]
- Psomas, G.; Dendrinou-Samara, C.; Philippakopoulos, P.; Tangoulis, V.; Raptopoulou, C.P.; Samaras, H.; Kessissoglou, D.P. CuII-herbicide complexes: Structure and bioactivity. Inorg. Chim. Acta 1998, 272, 24–32. [Google Scholar] [CrossRef]
- Dendrinou-Samara, C.; Psomas, G.; Raptopoulou, C.P.; Kessissoglou, D.P. Copper (II) complexes with phenoxyalkanoic acids and nitrogen donor heterocyclic ligands: Structure and bioactivity. J. Inorg. Biochem. 2001, 83, 7–16. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Adam, M.S.; Abu-Dief, A.M.; Ahmed, H.E.; Nafady, A. Non-linear optical property and biological assays of therapeutic potentials under in vitro conditions of Pd (II), Ag (I) and Cu (II) complexes of 5-diethyl amino-2-({2-[(2-hydroxy-Benzylidene)-amino]-phenylimino}-methyl)-phenol. Molecules 2020, 25, 5089. [Google Scholar] [CrossRef]
- Al-Abdulkarim, H.A.; El-Khatib, R.M.; Aljohani, F.S.; Mahran, A.; Alharbi, A.; Mersal, G.A.M.; El-Metwaly, N.M.; Abu-Dief, A.M. Optimization for synthesized quinoline-based Cr3+, VO2+, Zn2+ and Pd2+ complexes: DNA interaction, bio-logical assay and in-silico treatments for verification. J. Mol. Liq. 2021, 339, 116797. [Google Scholar] [CrossRef]
- Rehman, M.; Baloch, M.K.; Badshah, A. Synthesis, spectral characterization and bio-analysis of some organotin (IV) complexes. Eur. J. Med. Chem. 2008, 43, 2380–2385. [Google Scholar] [CrossRef]
- Santoso, S.P.; Ismadji, S.; Angkawijayaa, A.E.; Soetaredjo, F.E.; Go, A.W.; Jua, Y.H. Complexes of 2, 6-dihydroxybenzoic acid with divalent metal ions: Synthesis, crystal structure, spectral studies, and biological activity enhancement. J. Mol. Liq. 2016, 221, 617–623. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Alkhatib, F.M.; Abualnaja, M.; El-Dabea, T.; Ali, M.A.A. Synthesis and characterization of Fe (III), Pd (II) and Cu (II)-thiazole complexes; DFT, pharmacophore modeling, in-vitro assay and DNA binding studies. J. Mol. Liq. 2021, 326, 115277. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdel-Rahman, L.H.; Abdelhamid, A.A.; Marzouk, A.A.; Shehata, M.R.; Bakheet, M.A.; Almaghrabi, O.A.; Nafady, A. Synthesis and characterization of new Cr (III), Fe (III) and Cu (II) complexes incorporating multi-substituted aryl imidazole ligand: Structural, DFT, DNA binding, and biological implications. Spectrochim. Acta A 2020, 228, 117700. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, W.H.; Deghadi, R.G.; Mohamed, G.G. Preparation, geometric structure, molecular docking thermal and spectroscopic characterization of novel Schiff base ligand and its metal chelates. J. Therm. Anal. Calorim. 2017, 127, 2149–2171. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Mahmoud, N.F.; Mohamed, G.G.; El-Sonbati, A.Z.; El-Bindary, A.A. Synthesis, spectroscopic, thermogravimetric and antimicrobial studies of mixed ligands complexes. J. Mol. Struct. 2015, 1095, 15–25. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Moustafa, H.; Hamdan, S.K. Ni (II) and Cu (II) complexes with ONNO asymmetric tetradentate Schiff base ligand: Synthesis, spectroscopic characterization, theoretical calculations, DNA interaction and antimicrobial studies. Appl. Organomet. Chem. 2017, 31, e3555. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Abdel-Mawgoud, A.A.H. Development, structural investigation, DNA binding, anti-microbial screening and anticancer activities of two novel quari-dentate VO (II) and Mn (II) mononuclear complexes. J. King Saud Univ.-Sci. 2019, 31, 52–60. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Khatib, R.M.; Aljohani, F.S.; Alzahrani, S.O.; Khalifa, A.M.; Mohamed, E.; El-Metwaly, N.M. Synthesis and intensive characterization for novel Zn (II), Pd (II), Cr (III) and VO (II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J. Mol. Struct. 2021, 1242, 130693. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Adam, M.S.; Abu-Dief, A.M.; Moustafa, H.; Basha, M.T.; Aboraia, A.S.; Al-Farhan, B.S.; El-Sayed Ahmed, H. Synthesis, theoretical investigations, biocidal screening, DNA binding, in vitro cytotoxicity and molecular docking of novel Cu (II), Pd (II) and Ag (I) complexes of chlorobenzylidene Schiff base: Promising antibiotic and anticancer agents. Appl. Organomet. Chem. 2018, 32, e4527. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Alkhatib, F.; Abumelha, H.M.; El-Dabea, T.; El-Remaily, M.A.E.A.A.A. Structural, conformational and therapeutic studies on new thiazole complexes: Drug-likeness and MOE-simulation assessments. Res. Chem. Intermed. 2021, 47, 1979–2002. [Google Scholar] [CrossRef]

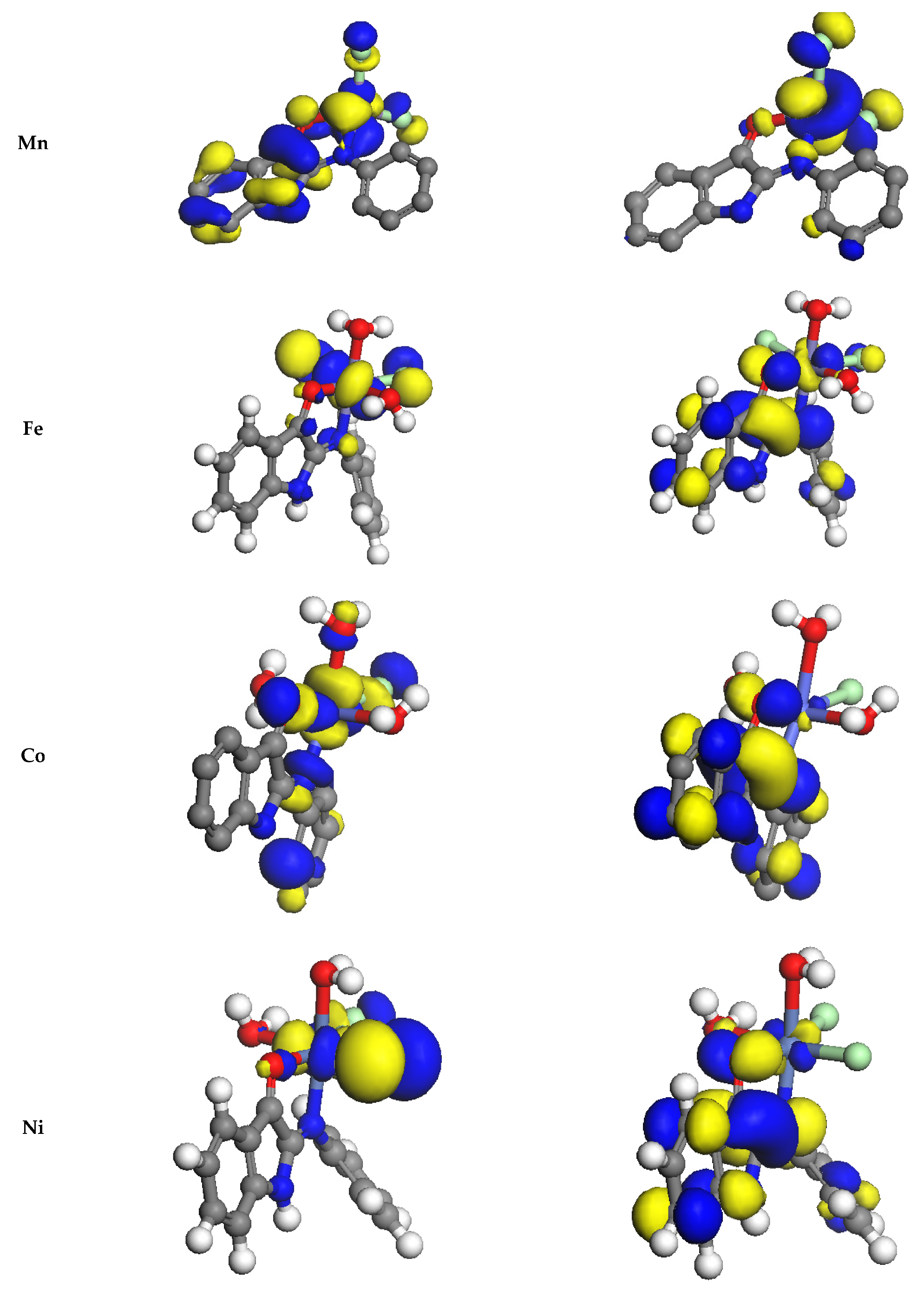


| Compound | Colour (%Yield) | m.p. (°C) | % Found (Calcd.) | μeff. (B.M.) | Λm Ω−1mol−1 cm2 | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | M | |||||
| L | Yellow | 190 | 75.45 (75.66) | 4.49 (4.54) | 12.67 (12.60) | - | - | - |
| Cr[(L)(H2O)2Cl2]Cl·H2O | Green 86 | >300 | 38.65 (38.72) | 3.48 (3.54) | 5.84 (6.00) | 10.94 (11.00) | 4.14 | 60.10 |
| [Mn(L)Cl2] | Dark brown 85 | >300 | 48.23 (48.25) | 3.27 (3.40) | 7.60 (7.90) | 14.76 (15.00) | 5.63 | 9.70 |
| [Fe(L)Cl2(H2O)2]Cl·H2O | Yellowish brown 80 | >300 | 38.50 (38.90) | 3.92 (4.03) | 6.18 (6.26) | 12.12 (12.17) | 5.08 | 66.50 |
| [Co(L)(H2O)3Cl]Cl·H2O | Faint pink 90 | >300 | 39.09 (39.20) | 3.64 (3.70) | 5.89 (6.00) | 13.10 (13.50) | 4.81 | 58.60 |
| [Ni(L)(H2O)2Cl2]·2H2O | Light green 84 | >300 | 39.10 (39.33) | 4.04 (4.10) | 6.09 (6.19) | 12.90 (13.00) | 3.44 | 10.50 |
| [Cu(L)Cl2]H2O | Brown 83 | >300 | 47.09 (47.60) | 3.60 (3.80) | 7.17 (7.25) | 16.04 (16.15) | 1.88 | 8.60 |
| [Zn(L)(H2O)2Cl2]H2O | Yellowish white 87 | >300 | 40.32 (40.55) | 3.27 (3.50) | 6.18 (6.24) | 15.02 (15.25) | Dia. | 12.00 |
| [Cd(L)Cl(H2O)]Cl·2H2O | Brown 86 | >300 | 33.19 (33.50) | 2.92 (3.00) | 4.94 (5.00) | 22.17 (22.50) | Dia. | 78.20 |
| Complex | TG Range (°C) | DTGmax (°C) | n | Mass Loss Total Mass Loss Estim (Calcd) % | Assignment | Residues |
|---|---|---|---|---|---|---|
| L | 70–365 365–490 | 2 1 | 40.19 (40.99) 59.63(59.00) 99.82(99.99) | - Loss of C6H5N. - Loss of C8H5NO. | - | |
| [Cr(L)(H2O)2Cl2]Cl·H2O | 90–165 165–265 265–515 | 102 232 471 | 1 1 1 | 4.76 (4.13) 26.48 (26.85) 56.51 (56.29) 87.75 (87.27) | - Loss of H2O. - Loss of 2H2O and C6H6. - Loss of C8H5Cl3N2 | ½ Cr2O3 |
| [Mn(L)Cl2] | 295–365 365–560 | 309, 352 496, 541 | 2 2 | 47.50 (46.67) 31.90 (32.76) 79.40 (79.43) | - Loss of C8H6ClN2. - Loss of C6H4Cl. | MnO |
| [Fe(L)Cl2(H2O)2]Cl·H2O | 60–100 100–380 380–590 | 85 284 543, 532 | 1 1 2 | 4.18 (4.08) 16.54 (17.12) 62.60 (62.00) 83.32 (83.20) | - Loss of H2O. - Loss of 2H2O and 2HCl. - Loss of C14H9Cl2N2. | ½ Fe2O3 |
| [Co(L)(H2O)3Cl]Cl·H2O | 50–80 80–230 230–495 495–580 | 74 204 289, 445 549 | 1 1 2 1 | 4.63 (4.00) 12.73 (12.00) 33.20 (33.84) 35.14(35.20) 85.70 (85.04) | - Loss of H2O. - Loss of 3H2O. - Loss of C8H5N2. - Loss of C6H5Cl2. | CoO |
| [Ni(L)(H2O)2Cl2]2H2O | 80–120 120–440 440–590 | 105 187, 371 525, 570 | 1 2 2 | 12.38 (12.70) 26.61 (26.77) 47.10 (47.20) 86.09 (86.67) | - Loss of 3H2O. - Loss of H2O and C6H5. - Loss of C8H5N2Cl2. | NiO |
| [Cu(L)(Cl)2]H2O | 105–435 435–740 | 135, 347 673 | 2 1 | 14.64 (14.55) 64.00 (64.21) 78.64(78.76) | - Loss of H2O and HCl - Loss of C14H9Cl2N2. | CuO |
| [Zn(L)(H2O)2Cl2] H2O | 70–225 225–365 365–660 | 106, 197 321 606 | 2 1 1 | 8.36 (7.00) 8.36(8.00) 64.30 (66.00) 81.02 (81.00) | - Loss of 2H2O. - Loss of H2O. - Loss of C14H10N2Cl2. | ZnO |
| [Cd(L)(Cl)(H2O)]Cl·2H2O | 70–165 165–430 430–700 | 126 384 650 | 1 1 1 | 7.80 (7.90) 20.67(21.71) 43.35(45.00) 71.82(71.61) | - Loss of 2H2O. - Loss of H2O and C6H5. - Loss of C8H5N2Cl2. | CdO |
| Parameter | L | Cr | Mn | Fe | Co | Ni | Cu | Zn | Cd |
|---|---|---|---|---|---|---|---|---|---|
| Total Energy | −454,624 | −4,991,837 | −4,623,610 | −1,218,134 | −988,478 | −1,249,679 | −1,168,178 | −1,284,154 | −848,041 |
| Binding Energy | −3315.80 | −3982.04 | −2992.27 | −3978.27 | −3676.29 | −3996.55 | −2903.37 | −3324.26 | −3803.25 |
| Total Dipole Moment | 5.35 | 6.51 | 1.09 | 7.45 | 8.48 | 8.89 | 0.72 | 7.75 | 4.66 |
| Enthalpy | 8.00 | 14.56 | 11.59 | 13.79 | 14.96 | 14.69 | 10.66 | 16.04 | 12.65 |
| Free Energy | −23.89 | −31.92 | −30.09 | −30.55 | -32.68 | −32.32 | −27.99 | −37.69 | −31.02 |
| Heat Capacity (Cp) | 51.61 | 86.12 | 63.84 | 83.53 | 82.25 | 85.92 | 60.61 | 82.72 | 74.16 |
| Entropy (S) | 106.99 | 155.91 | 139.82 | 148.72 | 159.78 | 157.67 | 129.66 | 180.18 | 146.49 |
| Comp. | EH/eV | EL eV | (EL-EH)/Ev | χ/eV | μ/eV | η/eV | S/eV−1 | ω/eV | σ/eV−1 |
|---|---|---|---|---|---|---|---|---|---|
| L | −2.9 | −4.86 | 1.96 | 3.88 | −3.88 | 0.98 | 1.02 | 7.68 | 0.49 |
| Cr | −3.41 | −4.06 | 0.65 | 3.74 | −3.74 | 0.33 | 3.08 | 21.46 | 0.16 |
| Mn | −5.19 | −5.39 | 0.2 | 5.29 | −5.29 | 0.1 | 10.0 | 139.92 | 0.05 |
| Fe | −3.69 | −4.18 | 0.49 | 3.94 | −3.94 | 0.25 | 4.08 | 31.60 | 0.12 |
| Co | −4.22 | −5.48 | 1.26 | 4.85 | −4.85 | 0.63 | 1.58 | 18.66 | 0.32 |
| Ni | −3.91 | −4.65 | 0.74 | 4.28 | −4.28 | 0.37 | 2.70 | 24.75 | 0.19 |
| Cu | −4.88 | −5.95 | 1.07 | 5.42 | −5.42 | 0.54 | 1.86 | 27.40 | 0.27 |
| Zn | −4.46 | −5.42 | 0.96 | 4.94 | −4.94 | 0.48 | 2.08 | 25.42 | 0.24 |
| Cd | −3.65 | −3.79 | 0.14 | 3.72 | −3.72 | 0.07 | 14.28 | 98.84 | 0.04 |
| L | Cr | Mn | Fe | Co | Ni | Cu | Zn | Cd | |
|---|---|---|---|---|---|---|---|---|---|
| GI absorption | High | High | High | High | High | High | High | High | High |
| BBB permeant | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| P-gp substrate | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| CYP1A2 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| CYP3A4 inhibitor | No | Yes | No | Yes | No | No | No | Yes | No |
| Log Kp (skin permeation) | −5.76 cm/s | −6.50 cm/s | −5.62 cm/s | −6.52 cm/s | −5.67 cm/s | −6.54 cm/s | −5.67 cm/s | −6.58 cm/s | −6.76 cm/s |
| Lipinski | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation |
| Ghose | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Veber | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Egan | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Muegge | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shamry, A.A.; Khalaf, M.M.; El-Lateef, H.M.A.; Yousef, T.A.; Mohamed, G.G.; El-Deen, K.M.K.; Gouda, M.; Abu-Dief, A.M. Development of New Azomethine Metal Chelates Derived from Isatin: DFT and Pharmaceutical Studies. Materials 2023, 16, 83. https://doi.org/10.3390/ma16010083
Al-Shamry AA, Khalaf MM, El-Lateef HMA, Yousef TA, Mohamed GG, El-Deen KMK, Gouda M, Abu-Dief AM. Development of New Azomethine Metal Chelates Derived from Isatin: DFT and Pharmaceutical Studies. Materials. 2023; 16(1):83. https://doi.org/10.3390/ma16010083
Chicago/Turabian StyleAl-Shamry, Abdulrhman A., Mai M. Khalaf, Hany M. Abd El-Lateef, Tarek A. Yousef, Gehad G. Mohamed, Kariman M. Kamal El-Deen, Mohamed Gouda, and Ahmed M. Abu-Dief. 2023. "Development of New Azomethine Metal Chelates Derived from Isatin: DFT and Pharmaceutical Studies" Materials 16, no. 1: 83. https://doi.org/10.3390/ma16010083
APA StyleAl-Shamry, A. A., Khalaf, M. M., El-Lateef, H. M. A., Yousef, T. A., Mohamed, G. G., El-Deen, K. M. K., Gouda, M., & Abu-Dief, A. M. (2023). Development of New Azomethine Metal Chelates Derived from Isatin: DFT and Pharmaceutical Studies. Materials, 16(1), 83. https://doi.org/10.3390/ma16010083










