Immunohistochemical and Histopathological Features of Persistent Gingival Enlargement in Relation to Metal Allergic Sensitisation during Orthodontic Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Allergy Testing
2.3. Histopathological Analyses
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Pathohistological Characteristics of Gingival Tissue Samples
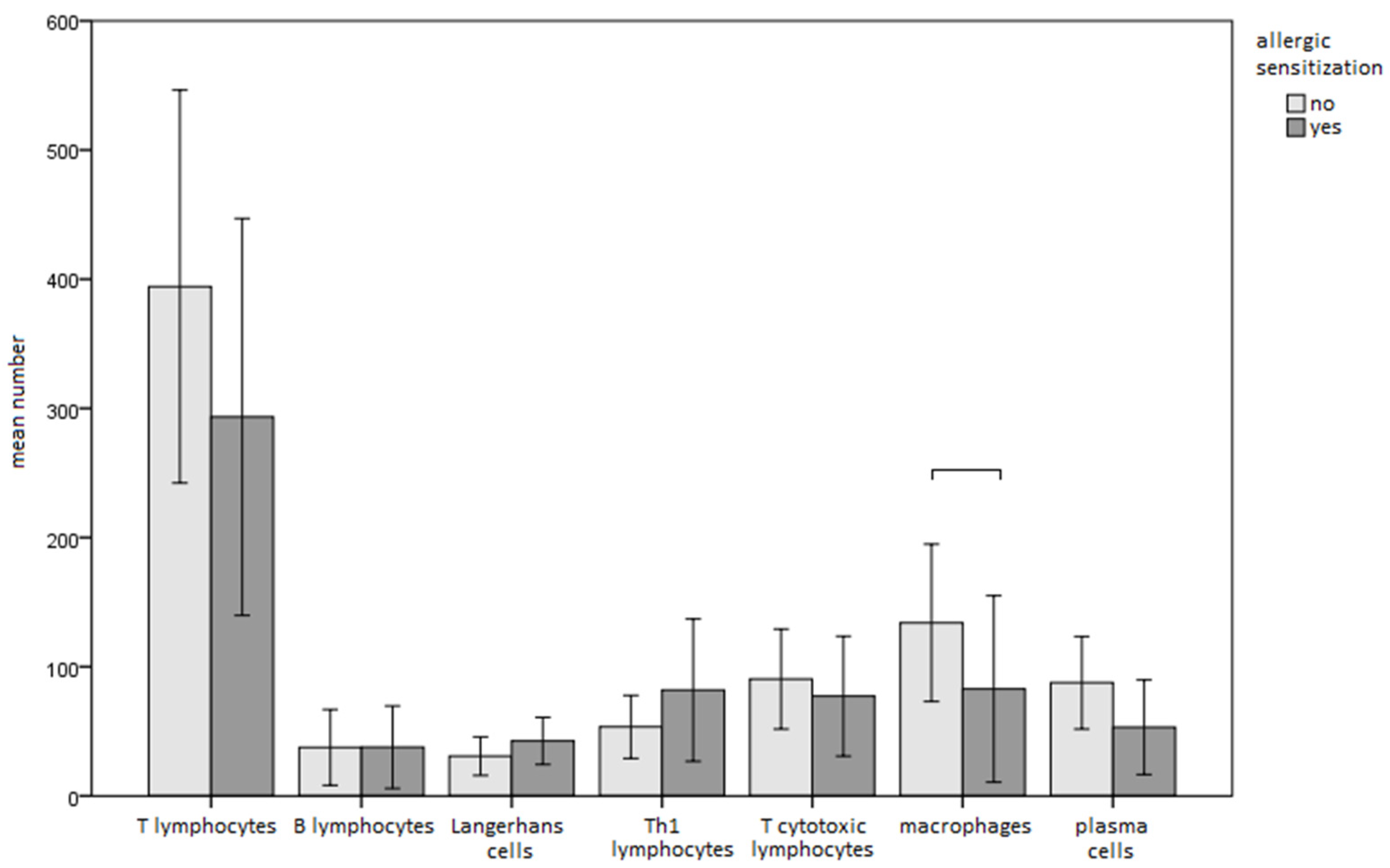
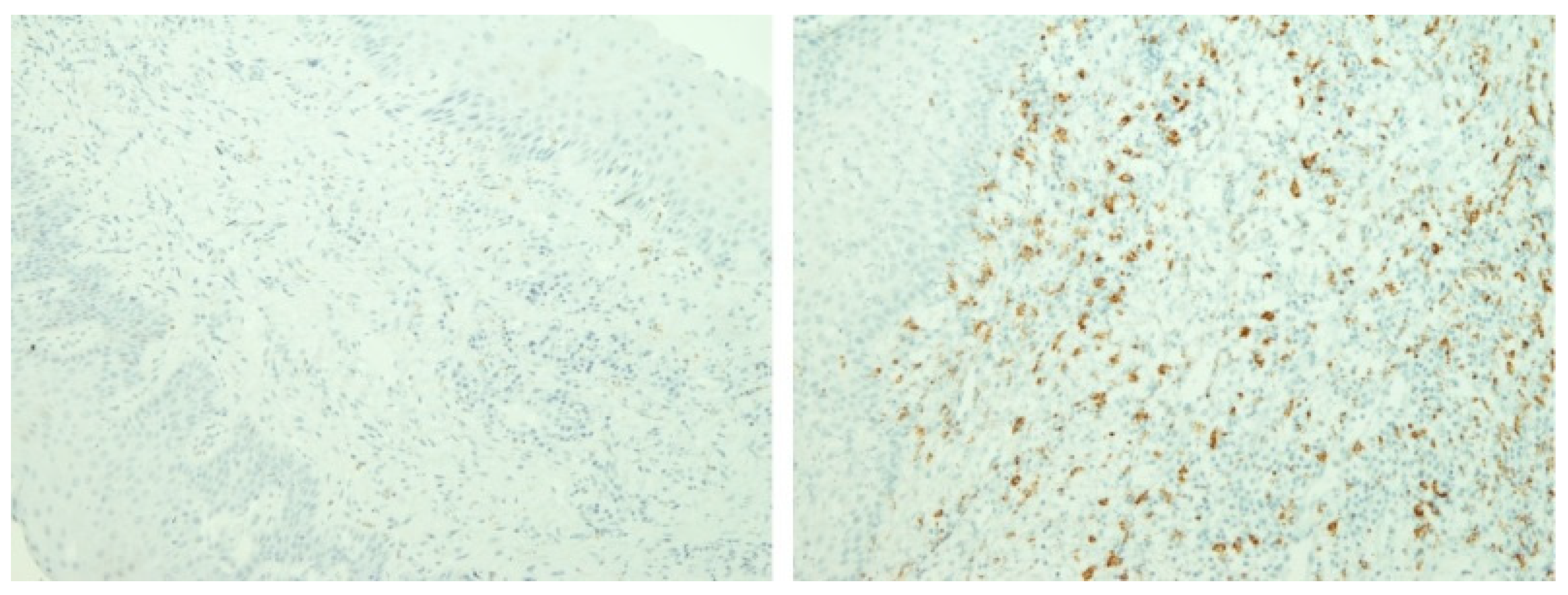
3.2. Immunohistochemical Features of Gingival Tissue Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez, C.; Abellán, R.; Palma, J.C. Efficacy of photodynamic therapy vs ultrasonic scaler for preventing gingival inflammation and white spot lesions during orthodontic treatment. Photodiagnosis Photodyn. Ther. 2018, 24, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Periodontol. 2018, 89 (Suppl. S1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, P.; Plemons, J.; Meyle, J. Non-plaque-induced gingival diseases. J. Periodontol. 2018, 89 (Suppl. S1), S28–S45. [Google Scholar] [CrossRef]
- Hostynek, J.J. Sensitization to nickel: Etiology, epidemiology, immune reactions, prevention, and therapy. Rev. Environ. Health 2006, 21, 253–280. [Google Scholar] [CrossRef]
- Miniculo, P.L.; Paolino, G.; Vacca, M.; Gangemi, S.; Nettis, E. Unmet diagnostic needs in contact oral mucosal allergies. Clin. Mol. Allergy 2016, 14, 10. [Google Scholar] [CrossRef]
- Koch, P.; Bahmer, F.A. Oral lichenoid lesions, mercury hypersensitivity and combined hypersensitivity to mercury and other metals: Histologically-proven reproduction of the reaction by patch testing with metal salts. Contact Dermat. 1995, 33, 323–328. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K. Trace metal release from orthodontic appliances by in vivo studies: A systematic literature review. Biol. Trace Elem. Res. 2010, 137, 127–138. [Google Scholar] [CrossRef]
- Menne, T. Quantitative aspects of nickel dermatitis: Sensitization and eliciting threshold concentrations. Sci. Total Environ. 1994, 148, 275–281. [Google Scholar] [CrossRef]
- Roblegg, E.; Fröhlich, E.; Meindl, C.; Teubl, B.; Zaversky, M.; Zimmer, A. Evaluation of a physiological in vitro system to study the transport of nanoparticles through the buccal mucosa. Nanotoxicology 2012, 6, 399–413. [Google Scholar] [CrossRef]
- Lalor, P.A.; Revell, P.A.; Gray, A.B.; Wright, S.; Railton, G.T.; Freeman, M.A. Sensitivity to titanium. A cause of implant failure? J. Bone Jt. Surg. Br. 1991, 73, 25–28. [Google Scholar] [CrossRef]
- Van Opstal, N.; Verheyden, F. Revision of a tibial baseplate using a customized oxinium component in a case of suspected metal allergy. A case report. Acta Orthop. Belg. 2011, 77, 691–695. [Google Scholar] [PubMed]
- Janeway, C.A.J.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Zigante, M.; Peternel, S.; Muhvic Urek, M.; Rincic Mlinaric, M.; Pop Acev, D.; Spalj, S. Smell and taste in titanium and nickel allergic sensitization in orthodontic patients. Orthod. Craniofac. Res. 2020, 23, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Boenisch, T. Staining methods. In Immunochemical Staining Methods, 3rd ed.; Boenisch, T., Ed.; DAKO Corporation: Carpinteria, CA, USA, 2001. [Google Scholar]
- Thierse, H.J.; Gamerdinger, K.; Junkes, C.; Guerreiro, N.; Weltzien, H.U. T cell receptor (TCR) interaction with haptens: Metal ions as non-classical haptens. Toxicology 2005, 209, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermat. 2016, 74, 323–345. [Google Scholar] [CrossRef]
- Pazzini, C.A.; Pereira, L.J.; Marques, L.S.; Generoso, R.; de Oliveira, G., Jr. Allergy to nickel in orthodontic patients: Clinical and histopathologic evaluation. Gen. Dent. 2010, 58, 58–61. [Google Scholar]
- Pazzini, C.A.; Júnior, G.O.; Marques, L.S.; Pereira, C.V.; Pereira, L.J. Prevalence of nickel allergy and longitudinal evaluation of periodontal abnormalities in orthodontic allergic patients. Angle Orthod. 2009, 79, 922–927. [Google Scholar] [CrossRef]
- Pereira, C.V.; Kaminagakura, E.; Bonan, P.R.; Bastos, R.A.; Pereira, L.J. Cellular, humoral, and histopathologic analysis in rats implanted with orthodontic nickel brackets. Angle Orthod. 2008, 78, 114–119. [Google Scholar] [CrossRef]
- Fernandez-Botran, R.; Sanders, V.M.; Mosmann, T.R.; Vitetta, E.S. Lymphokine-mediated regulation of the proliferative response of clones of T helper 1 and T helper 2 cells. J. Exp. Med. 1988, 168, 543–558. [Google Scholar] [CrossRef]
- Gawish, A.; Gamal-Eldeen, A.M.; Sherif, S.H.; Neamat, A. Influence of the etiological factors for gingival enlargement on some angiogenic and inflammatory mediators: An immunohistochemical study. J. Am. Sci. 2010, 6, 1754–1760. [Google Scholar]
- Gursoy, U.K.; Sokucu, O.; Uitto, V.-J.; Aydin, A.; Demirer, S.; Toker, H.; Erdem, O.; Sayal, A. The role of nickel accumulation and epithelial cell proliferation in orthodontic treatment-induced gingival overgrowth. Eur. J. Orthod. 2007, 29, 555–558. [Google Scholar] [CrossRef]
- Koch, P.; Bahmer, F.A. Oral lesions and symptoms related to metals used in dental restorations: A clinical, allergological, and histologic study. J. Am. Acad. Dermatol. 1999, 41, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Sun, D.-H.; Trindade, M.C.D.; Maloney, W.J.; Goodman, S.B.; Schurman, D.J.; Smith, R.L. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J. Bone Jt. Surg. Am. 1999, 81, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Valentine-Thon, E. Hypersensitivity to titanium: Clinical and laboratory evidence. Neuro Endocrinol. Lett. 2006, 27, 31–35. [Google Scholar] [PubMed]
- Nakashima, Y.; Sun, D.-H.; Trindade, M.C.D.; Chun, L.E.; Goodman, S.; Schurman, D.J.; Maloney, W.J.; Smith, R.L.; Song, Y. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J. Bone Jt. Br. 1999, 81, 155–162. [Google Scholar] [CrossRef]
- Flatebø, R.S.; Johannessen, A.C.; Grønningsæter, A.G.; Bøe, O.E.; Gjerdet, N.R.; Grung, B.; Leknes, K.N. Host response to titanium dental implant placement evaluated in a human oral model. J. Periodontol. 2006, 77, 1201–1210. [Google Scholar] [CrossRef]
- Trombetta, D.; Mondello, M.R.; Cimino, F.; Cristiani, M.; Pergolizzi, S.; Saija, A. Toxic effect of nickel in an in vitro model of human oral epithelium. Toxicol. Lett. 2005, 159, 219–225. [Google Scholar] [CrossRef]
- Brasch, J.; Burgard, J.; Sterry, W. Common pathogenetic pathways in allergic and irritant contact dermatitis. J. Investig. Dermatol. 1992, 98, 166–170. [Google Scholar] [CrossRef][Green Version]
- Shelly, W.B.; Juhlin, L. Selective uptake of contact allergens by Langerhans cells. Arch. Dermatol. 1977, 113, 187. [Google Scholar] [CrossRef]
- Christensesn, O.B.; Daniels, T.E.; Maibach, H.I. Expression of the OKT6 antigen by Langerhans cells in the patch test reaction. Contact Dermat. 1986, 14, 26–31. [Google Scholar] [CrossRef]
- van Loon, L.A.; van Elsas, P.W.; Bos, J.D.; Harkel-Hagenaar, H.C.; Krieg, S.R.; Davidson, C.L. T-lymphocyte and Langerhans cell distribution in normal and allergically induced oral mucosa in contact with nickel containing dental alloys. J. Oral Pathol. 1988, 17, 129–137. [Google Scholar] [CrossRef]

| Antibody | Source | Clone and Manufacturer | Dilution and Incubation | Positive Control |
|---|---|---|---|---|
| Anti-CD1a | Mouse monoclonal IgG1 | 010, Agilent Dako, Santa Clara, CA, SAD | 1:40, 30 min | Langerhans cells |
| Anti-CD3 | Rabbit polyclonal | Agilent Dako, Santa Clara, CA, SAD | 1:100, 30 min | T-lymphocytes |
| Anti-CD4 | Rabbit monoclonal IgG | SP35, Cell Marque, Rocklin, CA, SAD | 1:75, 60 min | T-helper lymphocytes |
| Anti-CD8 | Mouse monoclonal IgG1 | C8/144B, Agilent Dako, Santa Clara, CA, SAD | Ready to use, 30 min | T-cytotoxic lymphocytes |
| Anti-CD20 | Mouse monoclonal IgG2a | L26, Agilent Dako, Santa Clara, CA, SAD | 1:200, 30 min | B-lymphocytes |
| Anti-CD68 | Mouse monoclonal IgG3 | PG-M1, Agilent Dako, Santa Clara, CA, SAD | 1:200, 30 min | Macrophages |
| Anti-CD138 | Mouse monoclonal IgG1 | MI15, Agilent Dako, Santa Clara, CA, SAD | 1:100, 30 min | Plasma cells |
| Sensitisation Present (N (% When Compared to Non-Sensitised Subjects)) | p * | ||
|---|---|---|---|
| Epithelium without changes | No | 22 (53.7%) | |
| Yes | 0 (0%) | 0.233 | |
| Mild epithelial hyperplasia | No | 10 (40%) | |
| Yes | 12 (63.2%) | 0.223 | |
| Pronounced epithelial hyperplasia | No | 13 (54.2%) | |
| Yes | 9 (45%) | 0.763 | |
| Spongiosis | No | 19 (51.4%) | |
| Yes | 3 (42.9%) | 1.000 | |
| Colliquation | No | 17 (45.9%) | |
| Yes | 5 (71.4%) | 0.412 | |
| Mild fibrosis | No | 12 (44.4%) | |
| Yes | 10 (58.8%) | 0.537 | |
| Moderate fibrosis | No | 18 (52.9%) | |
| Yes | 4 (40%) | 0.721 | |
| Single inflammatory cells | No | 12 (42.9%) | |
| Yes | 10 (62.5%) | 0.347 | |
| Focal inflammation | No | 18 (52.9%) | |
| Yes | 4 (40%) | 0.721 | |
| Multifocal inflammation | No | 19 (61.3%) | |
| Yes | 3 (23.1%) | 0.045 | |
| Band-like inflammatory infiltrates | No | 17 (45.9%) | |
| Yes | 5 (71.4%) | 0.412 |
| Sensitisation Present (N (% When Compared to Non-Sensitised Subjects)) | p * | ||
|---|---|---|---|
| Mild exocytosis | No | 21 (53.8%) | |
| Yes | 1 (20%) | 0.345 | |
| Pronounced exocytosis | No | 18 (45%) | |
| Yes | 4 (100%) | 0.108 | |
| Inflammation along the basement membrane | No | 14 (56%) | |
| Yes | 8 (42.1%) | 0.543 | |
| Inflammation in the upper parts of the stroma | No | 1 (50%) | |
| Yes | 21 (50%) | 1.000 | |
| Inflammation in the deeper parts of the stroma | No | 18 (56.2%) | |
| Yes | 4 (33.3%) | 0.310 | |
| Inflammation with predominance of lymphocytes and histiocytes | No | 13 (23.1%) | |
| Yes | 9 (42.9%) | 0.547 | |
| Mixed inflammatory infiltrate | No | 10 (23.1%) | |
| Yes | 12 (57.1%) | 0.547 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zigante, M.; Spalj, S.; Prpic, J.; Pavlic, A.; Katic, V.; Matusan Ilijas, K. Immunohistochemical and Histopathological Features of Persistent Gingival Enlargement in Relation to Metal Allergic Sensitisation during Orthodontic Treatment. Materials 2023, 16, 81. https://doi.org/10.3390/ma16010081
Zigante M, Spalj S, Prpic J, Pavlic A, Katic V, Matusan Ilijas K. Immunohistochemical and Histopathological Features of Persistent Gingival Enlargement in Relation to Metal Allergic Sensitisation during Orthodontic Treatment. Materials. 2023; 16(1):81. https://doi.org/10.3390/ma16010081
Chicago/Turabian StyleZigante, Martina, Stjepan Spalj, Jelena Prpic, Andrej Pavlic, Visnja Katic, and Koviljka Matusan Ilijas. 2023. "Immunohistochemical and Histopathological Features of Persistent Gingival Enlargement in Relation to Metal Allergic Sensitisation during Orthodontic Treatment" Materials 16, no. 1: 81. https://doi.org/10.3390/ma16010081
APA StyleZigante, M., Spalj, S., Prpic, J., Pavlic, A., Katic, V., & Matusan Ilijas, K. (2023). Immunohistochemical and Histopathological Features of Persistent Gingival Enlargement in Relation to Metal Allergic Sensitisation during Orthodontic Treatment. Materials, 16(1), 81. https://doi.org/10.3390/ma16010081






