Synthesis, Characterization, and Electronic Properties of ZnO/ZnS Core/Shell Nanostructures Investigated Using a Multidisciplinary Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.1.1. Synthesis
2.1.2. Crystallographic Studies
2.1.3. Electron Microscopy
2.1.4. UV/Vis Diffuse-Reflectance Spectroscopy
2.2. Computational Details
2.2.1. Ab Initio Structure Optimization
2.2.2. Electronic Band Structure Calculations
3. Results
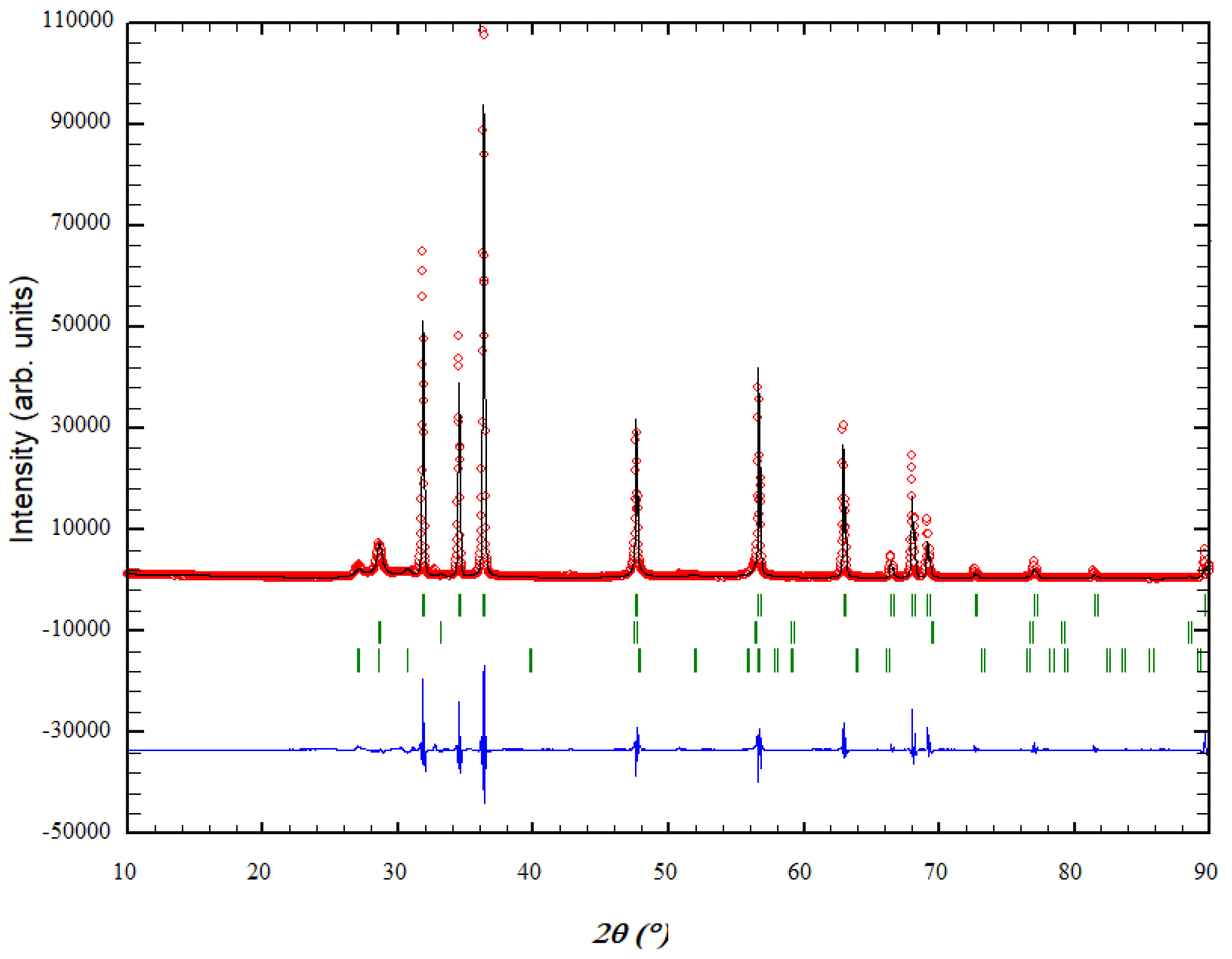
3.1. XRD Analysis and Rietveld Refinement of ZnO/ZnS Core–Shell Nanoparticles
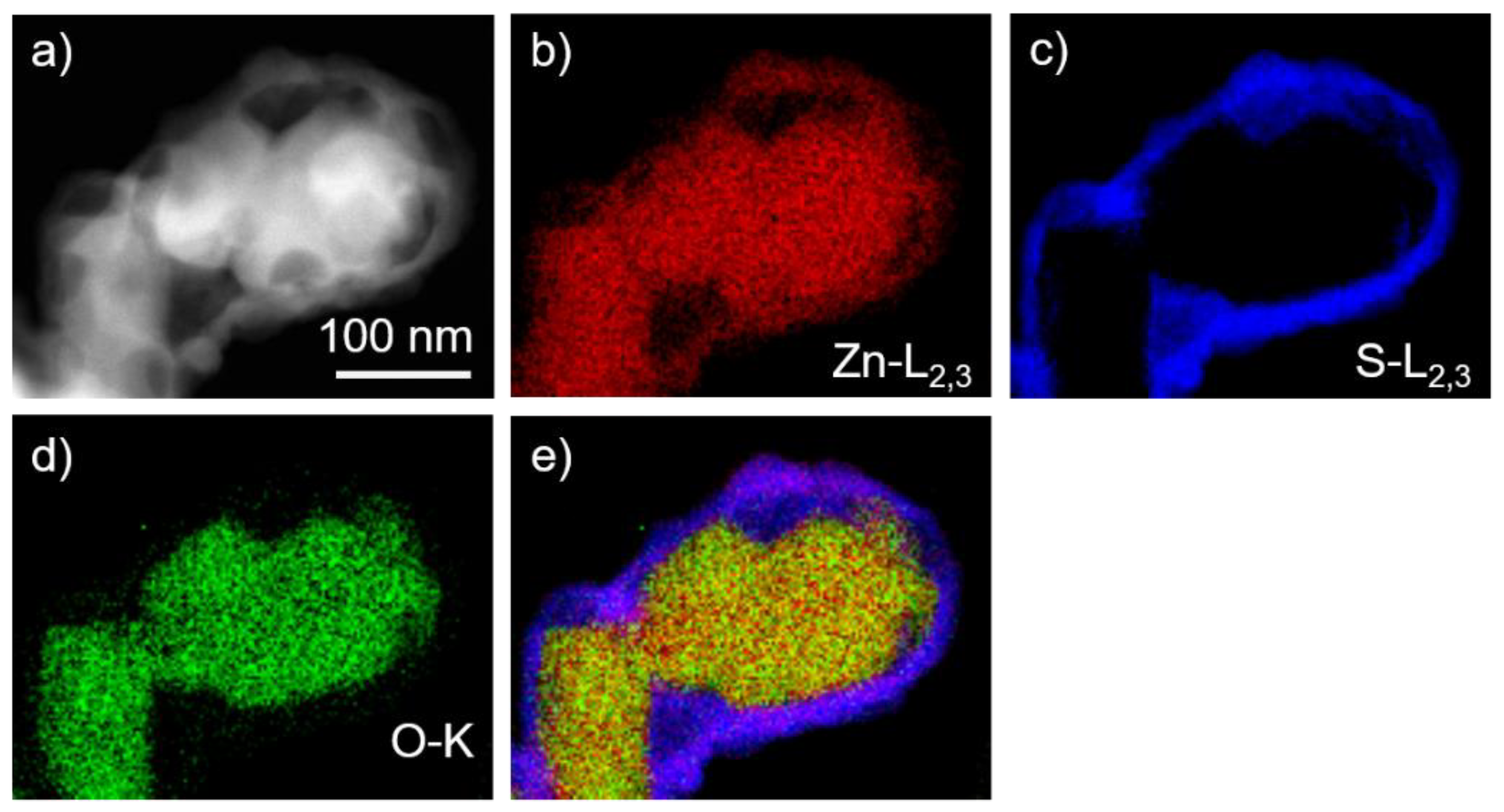
3.2. Imaging and Analytical TEM Analysis of the Core–Shell Sample
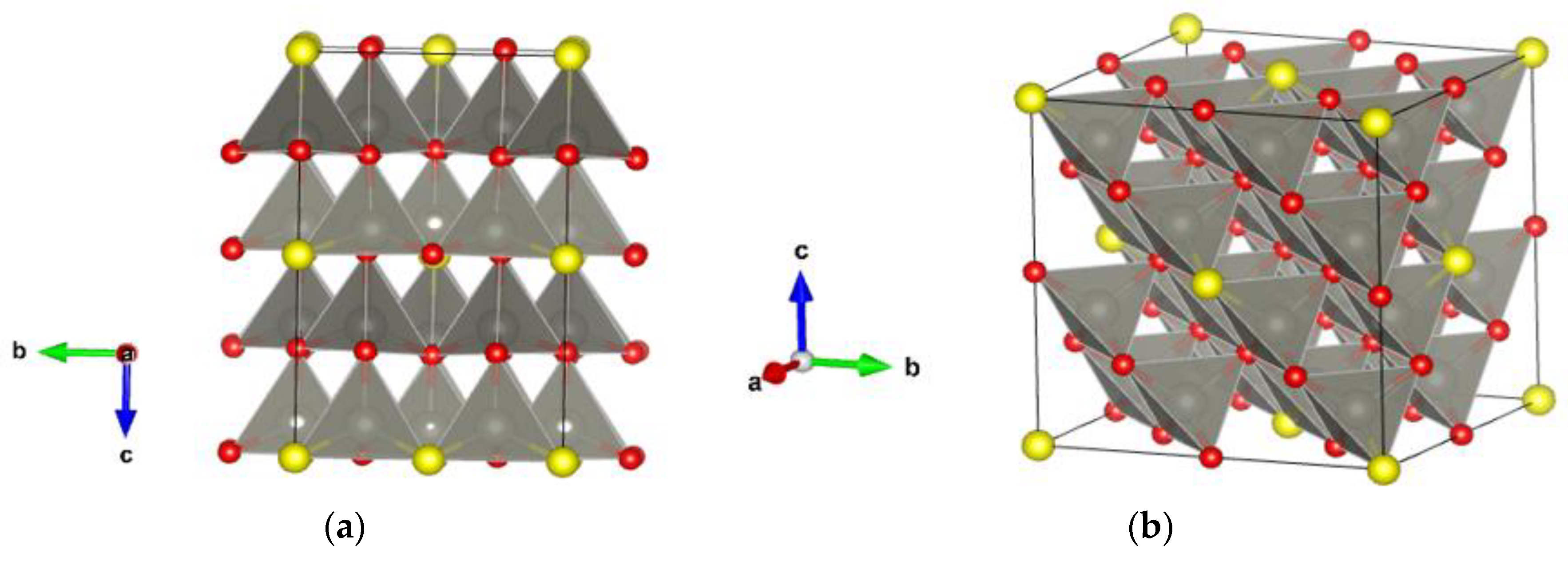
3.3. Ab Initio Modeling and Structure Analysis
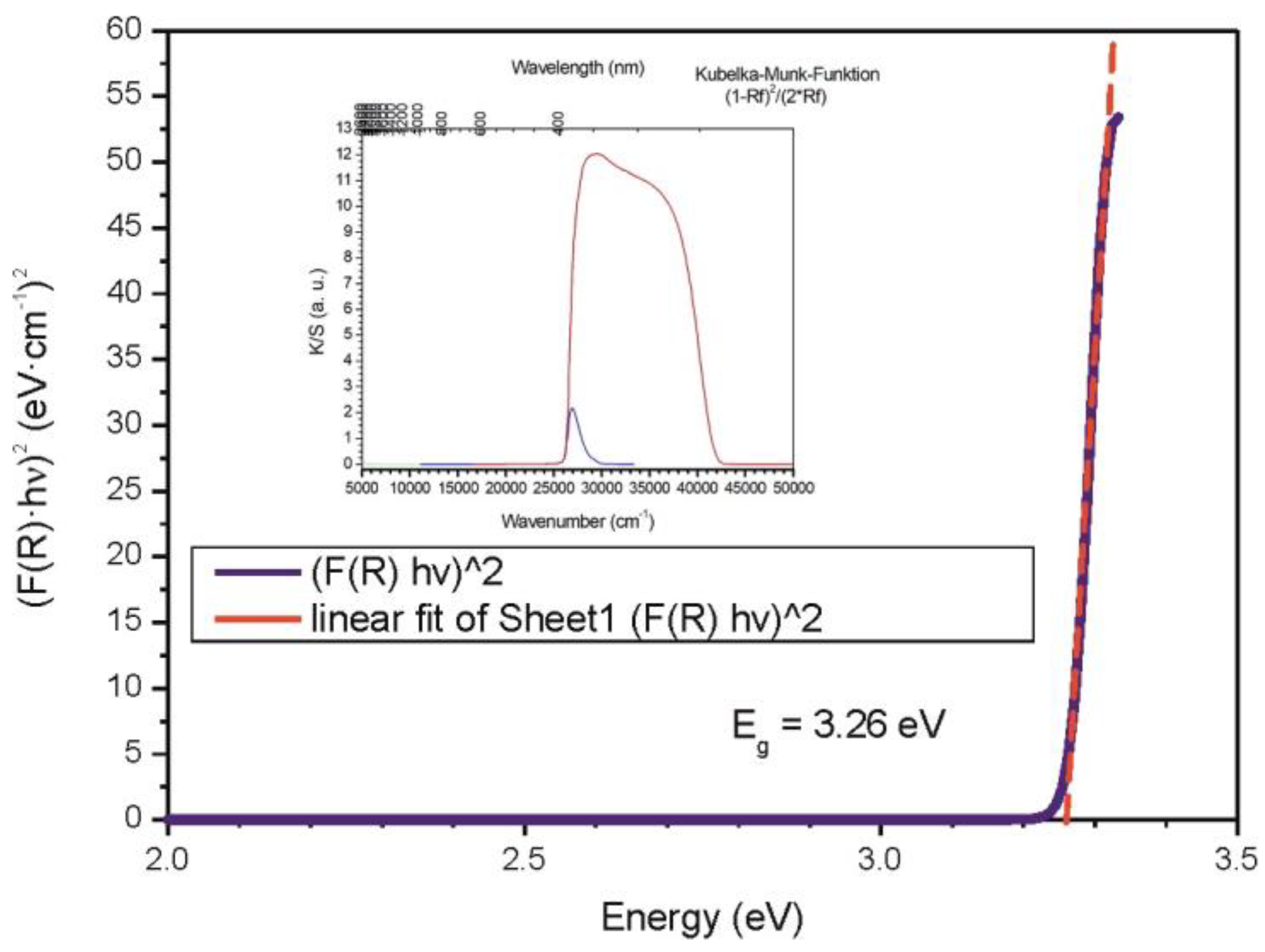
3.4. Band-Gap Measurements of Core–Shell ZnO/ZnS (Nano)particles
3.5. First-Principles Band Structure Calculations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stinn, C.; Allanore, A. Selective sulfidation of metal compounds. Nature 2022, 602, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, K.E.; Brennan, T.P.; Bent, S.F. Interface Engineering in Inorganic-Absorber Nanostructured Solar Cells. J. Phys. Chem. Lett. 2014, 5, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.M.; Ahmad, K.S. Nanocomposite Zr2S3-BaS-Cr2S3 ternary-metal chalcogenide: An impressive supercapacitor electrode and environmental remediant of toxic pollutants. Int. J. Energy Res. 2022, 46, 18697–18710. [Google Scholar] [CrossRef]
- Bertolotti, F.; Dirin, D.N.; Ibáñez, M.; Krumeich, F.; Cervellino, A.; Frison, R.; Voznyy, O.; Sargent, E.H.; Kovalenko, M.V.; Guagliardi, A.; et al. Crystal symmetry breaking and vacancies in colloidal lead chalcogenide quantum dots. Nat. Mater. 2016, 15, 987–994. [Google Scholar] [CrossRef]
- Xiong, Y.; Yao, S.; Karni, M.; Kostenko, A.; Burchert, A.; Apeloig, Y.; Driess, M. Heavier congeners of CO and CO2 as ligands: From zero-valent germanium (‘germylone’) to isolable monomeric GeX and GeX2 complexes (X = S, Se, Te). Chem. Sci. 2016, 7, 5462–5469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, G.; Wang, J.; Wang, Q.; Zhu, H.; Liu, C. Structural and electrical transport properties of PbS quantum dots under high pressure. J. Alloys Compd. 2021, 857, 157482. [Google Scholar] [CrossRef]
- Thiele, G.; Franzke, Y.; Weigend, F.; Dehnen, S. {μ-PbSe}: A Heavy CO Homologue as an Unexpected Ligand. Angew. Chem. Int. Ed. 2015, 54, 11283–11288. [Google Scholar] [CrossRef]
- Zeuthen, C.M.; Thorup, P.S.; Roth, N.; Iversen, B.B. Reconciling Crystallographic and Physical Property Measurements on Thermoelectric Lead Sulfide. J. Am. Chem. Soc. 2019, 141, 8146–8157. [Google Scholar] [CrossRef]
- Jovanovic, D.; Zagorac, D.; Matovic, B.; Zarubica, A.; Zagorac, J. Anion substitution and influence of sulfur on the crystal structures, phase transitions, and electronic properties of mixed TiO2/TiS2 compounds. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2021, 77, 833–847. [Google Scholar] [CrossRef]
- Ul Haq, B.; AlFaify, S.; Ahmed, R.; Butt, F.K.; Tahir, M.; Ur Rehman, S.; Alsardia, M.M.; Kim, S.-H. Physical properties of novel Tin-chalcogenides heterostructures: A first-principles study. Mater. Sci. Semicond. Process. 2022, 149, 106820. [Google Scholar] [CrossRef]
- Huso, J.; Ritter, J.R.; Bergman, L.; McCluskey, M.D. High Order Oxygen Local Vibrational Modes in ZnS1−xOx. Phys. Status Solidi B 2019, 256, 1800607. [Google Scholar] [CrossRef]
- Gao, M.-R.; Xu, Y.-F.; Jiang, J.; Yu, S.-H. Nanostructured metal chalcogenides: Synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 2013, 42, 2986–3017. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Sadollahkhani, A.; Kazeminezhad, I.; Lu, J.; Nur, O.; Hultman, L.; Willander, M. Synthesis, structural characterization and photocatalytic application of ZnO@ZnS core-shell nanoparticles. RSC Adv. 2014, 4, 36940–36950. [Google Scholar] [CrossRef]
- Tarish, S.; Xu, Y.; Wang, Z.; Mate, F.; Al-Haddad, A.; Wang, W.; Lei, Y. Highly efficient biosensors by using well-ordered ZnO/ZnS core/shell nanotube arrays. Nanotechnology 2017, 28, 405501. [Google Scholar] [CrossRef]
- Wu, D.; Jiang, Y.; Yuan, Y.; Wu, J.; Jiang, K. ZnO–ZnS heterostructures with enhanced optical and photocatalytic properties. J. Nanoparticle Res. 2011, 13, 2875–2886. [Google Scholar] [CrossRef]
- Tai, S.-T.; Tsai, Y.-S.; Sermon Wu, Y.; Wang, J., Jr.; Chen, H. Material characterizations of ZnO/ZnS core-shell structures on glass substrate. Results Phys. 2019, 15, 102703. [Google Scholar] [CrossRef]
- Schrier, J.; Demchenko, D.O.; Lin, W.; Alivisatos, A.P. Optical Properties of ZnO/ZnS and ZnO/ZnTe Heterostructures for Photovoltaic Applications. Nano Lett. 2007, 7, 2377–2382. [Google Scholar] [CrossRef]
- Sukkabot, W. Manipulation of structural and optical properties in ZnO/ZnS type-II and ZnS/ZnO inverted type-II core/shell nanocrystals: Tight-binding theory. J. Comput. Electron. 2017, 16, 756–764. [Google Scholar] [CrossRef]
- Scott, S.D.; Barnes, H.L. Sphalerite-wurtzite equilibria and stoichiometry. Geochim. Cosmochim. Acta 1972, 36, 1275–1295. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, J.H.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Logar, M.; Jančar, B.; Rečnik, A.; Suvorov, D. Controlled synthesis of pure and doped ZnS nanoparticles in weak polyion assemblies: Growth characteristics and fluorescence properties. Nanotechnology 2009, 20, 275601. [Google Scholar] [CrossRef] [PubMed]
- Zagorac, D.; Schön, J.C.; Jansen, M. Energy Landscape Investigations Using the Prescribed Path Method in the ZnO System. J. Phys. Chem. C 2012, 116, 16726–16739. [Google Scholar] [CrossRef]
- Luković Golić, D.; Branković, G.; Počuča Nešić, M.; Vojisavljević, K.; Rečnik, A.; Daneu, N.; Bernik, S.; Šćepanović, M.; Poleti, D.; Branković, Z. Structural characterization of self-assembled ZnO nanoparticles obtained by the sol–gel method from Zn(CH3COO)2·2H2O. Nanotechnology 2011, 22, 395603. [Google Scholar] [CrossRef]
- Catlow, C.R.A.; French, S.A.; Sokol, A.A.; Al-Sunaidi, A.A.; Woodley, S.M. Zinc oxide: A case study in contemporary computational solid state chemistry. J. Comput. Chem. 2008, 29, 2234–2249. [Google Scholar] [CrossRef]
- Zagorac, D.; Schön, J.C. Chapter 8—Energy landscapes of pure and doped ZnO: From bulk crystals to nanostructures. In Frontiers of Nanoscience; Wales, D.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 21, pp. 151–193. [Google Scholar]
- Olejnik, M.; Kersting, M.; Rosenkranz, N.; Loza, K.; Breisch, M.; Rostek, A.; Prymak, O.; Schürmeyer, L.; Westphal, G.; Köller, M.; et al. Cell-biological effects of zinc oxide spheres and rods from the nano- to the microscale at sub-toxic levels. Cell Biol. Toxicol. 2021, 37, 573–593. [Google Scholar] [CrossRef]
- Shabbir, S.; Shaari, A.; Ul Haq, B.; Ahmed, R.; Ahmed, M. Investigations of novel polymorphs of ZnO for optoelectronic applications. Optik 2020, 206, 164285. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, M.N. First-Principles Study of Structural, Electronic and Optical Properties of wz-Zinc Oxide. Adv. Sci. Lett. 2015, 21, 2688–2691. [Google Scholar] [CrossRef]
- Ul Haq, B.; AlFaify, S.; Alshahrani, T.; Ahmed, R.; Butt, F.K.; Ur Rehman, S.; Tariq, Z. Devising square- and hexagonal-shaped monolayers of ZnO for nanoscale electronic and optoelectronic applications. Sol. Energy 2020, 211, 920–927. [Google Scholar] [CrossRef]
- Kang, K.; Kononov, A.; Lee, C.-W.; Leveillee, J.A.; Shapera, E.P.; Zhang, X.; Schleife, A. Pushing the frontiers of modeling excited electronic states and dynamics to accelerate materials engineering and design. Comput. Mater. Sci. 2019, 160, 207–216. [Google Scholar] [CrossRef]
- Sponza, L.; Goniakowski, J.; Noguera, C. Confinement effects in ultrathin ZnO polymorph films: Electronic and optical properties. Phys. Rev. B 2016, 93, 195435. [Google Scholar] [CrossRef]
- Menad, A.; Benmalti, M.E.; Zaoui, A.; Ferhat, M. Impact of polytypism on the ground state properties of zinc oxide: A first-principles study. Results Phys. 2020, 18, 103316. [Google Scholar] [CrossRef]
- Fang, X.; Bando, Y.; Gautam, U.K.; Zhai, T.; Zeng, H.; Xu, X.; Liao, M.; Golberg, D. ZnO and ZnS Nanostructures: Ultraviolet-Light Emitters, Lasers, and Sensors. Crit. Rev. Solid State Mater. Sci. 2009, 34, 190–223. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Liang, B.; Liu, Z.; Chen, D.; Shen, G. ZnS Nanostructures: Synthesis, Properties, and Applications. Crit. Rev. Solid State Mater. Sci. 2013, 38, 57–90. [Google Scholar] [CrossRef]
- Roychowdhury, A.; Pati, S.P.; Kumar, S.; Das, D. Effects of magnetite nanoparticles on optical properties of zinc sulfide in fluorescent-magnetic Fe3O4/ZnS nanocomposites. Powder Technol. 2014, 254, 583–590. [Google Scholar] [CrossRef]
- Amakali, T.; Živković, A.; Warwick, M.E.A.; Jones, D.R.; Dunnill, C.W.; Daniel, L.S.; Uahengo, V.; Mitchell, C.E.; Dzade, N.Y.; de Leeuw, N.H. Photocatalytic Degradation of Rhodamine B Dye and Hydrogen Evolution by Hydrothermally Synthesized NaBH4—Spiked ZnS Nanostructures. Front. Chem. 2022, 10, 835832. [Google Scholar] [CrossRef]
- Jaquez, M.; Yu, K.M.; Ting, M.; Hettick, M.; Sánchez-Royo, J.F.; Wełna, M.; Javey, A.; Dubon, O.D.; Walukiewicz, W. Growth and characterization of ZnO1−xSx highly mismatched alloys over the entire composition. J. Appl. Phys. 2015, 118, 215702. [Google Scholar] [CrossRef]
- Larquet, C.; Carenco, S. Metal Oxysulfides: From Bulk Compounds to Nanomaterials. Front. Chem. 2020, 8, 179. [Google Scholar] [CrossRef]
- Zagorac, D.; Zagorac, J.; Pejić, M.; Matović, B.; Schön, J.C. Band Gap Engineering of Newly Discovered ZnO/ZnS Polytypic Nanomaterials. Nanomaterials 2022, 12, 1595. [Google Scholar] [CrossRef]
- Bakke, J.R.; Tanskanen, J.T.; Hägglund, C.; Pakkanen, T.A.; Bent, S.F. Growth characteristics, material properties, and optical properties of zinc oxysulfide films deposited by atomic layer deposition. J. Vac. Sci. Technol. A 2012, 30, 01A135. [Google Scholar] [CrossRef]
- Deulkar, S.H.; Huang, J.-L.; Neumann-Spallart, M. Zinc Oxysulfide Thin Films Grown by Pulsed Laser Deposition. J. Electron. Mater. 2010, 39, 589–594. [Google Scholar] [CrossRef]
- Thankalekshmi, R.R.; Rastogi, A.C. Structure and optical band gap of ZnO1−xSx thin films synthesized by chemical spray pyrolysis for application in solar cells. J. Appl. Phys. 2012, 112, 063708. [Google Scholar] [CrossRef]
- Wang, X.; Gao, P.; Li, J.; Summers, C.J.; Wang, Z.L. Rectangular Porous ZnO–ZnS Nanocables and ZnS Nanotubes. Adv. Mater. 2002, 14, 1732–1735. [Google Scholar] [CrossRef]
- Yan, C.; Xue, D. Room Temperature Fabrication of Hollow ZnS and ZnO Architectures by a Sacrificial Template Route. J. Phys. Chem. B 2006, 110, 7102–7106. [Google Scholar] [CrossRef]
- Hu, Y.; Qian, H.; Liu, Y.; Du, G.; Zhang, F.; Wang, L.; Hu, X. A microwave-assisted rapid route to synthesize ZnO/ZnS core-shell nanostructures via controllable surface sulfidation of ZnO nanorods. CrystEngComm 2011, 13, 3438–3443. [Google Scholar] [CrossRef]
- Jia, W.; Jia, B.; Qu, F.; Wu, X. Towards a highly efficient simulated sunlight driven photocatalyst: A case of heterostructured ZnO/ZnS hybrid structure. Dalton Trans. 2013, 42, 14178–14187. [Google Scholar] [CrossRef]
- Daiko, Y.; Schmidt, J.; Kawamura, G.; Romeis, S.; Segets, D.; Iwamoto, Y.; Peukert, W. Mechanochemically induced sulfur doping in ZnO via oxygen vacancy formation. Phys. Chem. Chem. Phys. 2017, 19, 13838–13845. [Google Scholar] [CrossRef]
- Skinner, B.J.; Barton, P.B. The substitution of oxygen for sulfur in wurtzite and sphalerite. Am. Mineral. 1960, 4, 612–615. [Google Scholar]
- Patil, A.B.; Patil, K.R.; Pardeshi, S.K. Ecofriendly synthesis and solar photocatalytic activity of S-doped ZnO. J. Hazard. Mater. 2010, 183, 315–323. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tu, Y.-J.; Wang, Y.-S.; Kan, R.-S.; Huang, C.-M. Characterization and photoreactivity of N-, S-, and C-doped ZnO under UV and visible light illumination. J. Photochem. Photobiol. A Chem. 2008, 199, 170–178. [Google Scholar] [CrossRef]
- Shen, G.; Cho, J.H.; Yoo, J.K.; Yi, G.-C.; Lee, C.J. Synthesis and Optical Properties of S-Doped ZnO Nanostructures: Nanonails and Nanowires. J. Phys. Chem. B 2005, 109, 5491–5496. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Ahmed, S.; Shakil, M.; Choudhary, M.A. First-principles calculations of structural, electronic, and thermodynamic properties of ZnO1−xSx alloys. Chin. Phys. B 2014, 23, 106108. [Google Scholar] [CrossRef]
- Luo, K.; Sun, Y.; Zhou, L.; Wang, F.; Wu, F. Theoretical simulation of performances in CIGS thin-film solar cells with cadmium-free buffer layer. J. Semicond. 2017, 38, 084006. [Google Scholar] [CrossRef]
- Srot, V.; Recnik, A.; Scheu, C.; Sturm, S.; Mirtic, B. Stacking faults and twin boundaries in sphalerite crystals from the Trepča mines in Kosovo. Am. Mineral. 2003, 88, 1809–1816. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kokh, S.N.; Seryotkin, Y.V.; Deviatiiarova, A.S.; Goryainov, S.V.; Sharygin, V.V.; Khoury, H.N.; Karmanov, N.S.; Danilovsky, V.A.; Artemyev, D.A. Ultrahigh-Temperature Sphalerite from Zn-Cd-Se-Rich Combustion Metamorphic Marbles, Daba Complex, Central Jordan: Paragenesis, Chemistry, and Structure. Minerals 2020, 10, 822. [Google Scholar] [CrossRef]
- Ru, F.; Xia, J.; Li, X.; Liu, P.; Qiao, P.; Li, Y.; Cao, J.; Tian, L.; Zhang, W.; Meng, X.-M. Epitaxial growth of structure-tunable ZnO/ZnS core/shell nanowire arrays using HfO2 as the buffer layer. Nanoscale 2022, 14, 7579–7588. [Google Scholar] [CrossRef]
- Hitkari, G.; Singh, S.; Pandey, G. Structural, optical and photocatalytic study of ZnO and ZnO–ZnS synthesized by chemical method. Nano-Struct. Nano-Objects 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Lu, M.-Y.; Song, J.; Lu, M.-P.; Lee, C.-Y.; Chen, L.-J.; Wang, Z.L. ZnO−ZnS Heterojunction and ZnS Nanowire Arrays for Electricity Generation. ACS Nano 2009, 3, 357–362. [Google Scholar] [CrossRef]
- Lin, D.; Wu, H.; Zhang, R.; Zhang, W.; Pan, W. Facile Synthesis of Heterostructured ZnO–ZnS Nanocables and Enhanced Photocatalytic Activity. J. Am. Ceram. Soc. 2010, 93, 3384–3389. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, P.; Ding, Y.; Cai, W.; Xie, S.; Wang, Z.L. Mismatch Strain Induced Formation of ZnO/ZnS Heterostructured Rings. Adv. Mater. 2007, 19, 2319–2323. [Google Scholar] [CrossRef]
- Sharma, S.; Chawla, S. Enhanced UV emission in ZnO/ZnS core shell nanoparticles prepared by epitaxial growth in solution. Electron. Mater. Lett. 2013, 9, 267–271. [Google Scholar] [CrossRef]
- Swati, G.; Morampudi, M. Size-selective and facile synthesis of ZnO/ZnS core-shell nanostructure and its characterization. Appl. Phys. A 2021, 127, 456. [Google Scholar] [CrossRef]
- Shabbir, S.; Shaari, A.; Ul Haq, B.; Ahmed, R.; AlFaify, S.; Ahmed, M.; Laref, A. First-principles investigations of electronic structures and optical spectra of wurtzite and sphalerite types of ZnO1−xSx (x = 0, 0.25, 0.50, 0.75 &1) alloys. Mater. Sci. Semicond. Process. 2021, 121, 105326. [Google Scholar] [CrossRef]
- Khomyak, V.; Shtepliuk, I.; Khranovskyy, V.; Yakimova, R. Band-gap engineering of ZnO1−xSx films grown by rf magnetron sputtering of ZnS target. Vacuum 2015, 121, 120–124. [Google Scholar] [CrossRef]
- Alqahtani, S.M.; Usman, M.; Ahmed, S.S. Unusual bandgap bowing in highly mismatched ZnOS alloys: Atomistic tight-binding band anti-crossing model. J. Appl. Phys. 2019, 125, 235704. [Google Scholar] [CrossRef]
- Huso, J.; Bergman, L.; McCluskey, M.D. Bandgap of cubic ZnS1−xOx from optical transmission spectroscopy. J. Appl. Phys. 2019, 125, 075704. [Google Scholar] [CrossRef]
- Torabi, A.; Staroverov, V.N. Band Gap Reduction in ZnO and ZnS by Creating Layered ZnO/ZnS Heterostructures. J. Phys. Chem. Lett. 2015, 6, 2075–2080. [Google Scholar] [CrossRef]
- Shabbir, S.; Shaari, A.; Ul Haq, B.; Ahmed, R.; AlFaify, S.; Ahmed, M.; Laref, A. First-principles investigations of structural parameters, electronic structures and optical spectra of 5–5- and BeO-type of ZnO1−xSx alloys. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 262, 114697. [Google Scholar] [CrossRef]
- Giri, A.K.; Charan, C.; Saha, A.; Shahi, V.K.; Panda, A.B. An amperometric cholesterol biosensor with excellent sensitivity and limit of detection based on an enzyme-immobilized microtubular ZnO@ZnS heterostructure. J. Mater. Chem. A 2014, 2, 16997–17004. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, C.; Zhai, T.; Li, S.L.; Wang, X.; Liu, J.; Jie, X.; Liu, D.; Liao, M.; Koide, Y.; et al. Flexible Ultraviolet Photodetectors with Broad Photoresponse Based on Branched ZnS-ZnO Heterostructure Nanofilms. Adv. Mater. 2014, 26, 3088–3093. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Fan, D.H.; Shen, W.Z. A General Chemical Conversion Route To Synthesize Various ZnO-Based Core/Shell Structures. J. Phys. Chem. C 2008, 112, 10402–10406. [Google Scholar] [CrossRef]
- Chen, P.; Gu, L.; Cao, X. From single ZnO multipods to heterostructured ZnO/ZnS, ZnO/ZnSe, ZnO/Bi2S3 and ZnO/Cu2S multipods: Controlled synthesis and tunable optical and photoelectrochemical properties. CrystEngComm 2010, 12, 3950–3958. [Google Scholar] [CrossRef]
- Xitao, W.; Rong, L.; Kang, W. Synthesis of ZnO@ZnS-Bi2S3 core-shell nanorod grown on reduced graphene oxide sheets and its enhanced photocatalytic performance. J. Mater. Chem. A 2014, 2, 8304–8313. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sakthivel, P.; Fernandez, A.C. Structural, optical and electrical properties of ZnO-ZnS nanocomposites prepared by simple hydrothermal method. J. Alloys Compd. 2018, 768, 553–562. [Google Scholar] [CrossRef]
- Zagorac, D.; Schön, J.C.; Rosić, M.; Zagorac, J.; Jordanov, D.; Luković, J.; Matović, B. Theoretical and Experimental Study of Structural Phases in CoMoO4. Cryst. Res. Technol. 2017, 52, 1700069. [Google Scholar] [CrossRef]
- Čebela, M.; Zagorac, D.; Batalović, K.; Radaković, J.; Stojadinović, B.; Spasojević, V.; Hercigonja, R. BiFeO3 perovskites: A multidisciplinary approach to multiferroics. Ceram. Int. 2017, 43, 1256–1264. [Google Scholar] [CrossRef]
- Zagorac, D.; Mueller, H.; Ruehl, S.; Zagorac, J.; Rehme, S. Recent developments in the Inorganic Crystal Structure Database: Theoretical crystal structure data and related features. J. Appl. Cryst. 2019, 52, 918–925. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Dovesi, R.; Saunders, V.R.; Roetti, C.; Orlando, R.; Zicovich-Wilson, C.M.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.J.; et al. CRYSTAL14 User’s Manual; University of Torino: Torino, Italy, 2009. [Google Scholar]
- Dovesi, R.; Orlando, R.; Civalleri, B.; Roetti, C.; Saunders Victor, R.; Zicovich-Wilson Claudio, M. CRYSTAL: A computational tool for the ab initio study of the electronic properties of crystals. Z. Krist.—Cryst. Mater. 2005, 220, 571–573. [Google Scholar] [CrossRef]
- Zagorac, D.; Doll, K.; Schön, J.C.; Jansen, M. Sterically Active Electron Pairs in Lead Sulfide? An Investigation of the Electronic and Vibrational Properties of PbS in the Transition Region between the Rock Salt and the α-GeTe-Type Modifications. Chem.—A Eur. J. 2012, 18, 10929–10936. [Google Scholar] [CrossRef]
- Matović, B.; Zagorac, D.; Cvijović-Alagić, I.; Zagorac, J.; Butulija, S.; Erčić, J.; Hanzel, O.; Sedlák, R.; Lisnichuk, M.; Tatarko, P. Fabrication and Characterization of High Entropy Pyrochlore Ceramics. Boletín Soc. Española Cerámica Vidr. 2021; in press. [Google Scholar] [CrossRef]
- Zagorac, D.; Zagorac, J.; Schön, J.C.; Stojanovic, N.; Matovic, B. ZnO/ZnS (hetero)structures: Ab initio investigations of polytypic behavior of mixed ZnO and ZnS compounds. Acta Crystallogr. B 2018, 74, 628–642. [Google Scholar] [CrossRef]
- Škundrić, T.; Zagorac, D.; Schön, J.C.; Pejić, M.; Matović, B. Crystal Structure Prediction of the Novel Cr2SiN4 Compound via Global Optimization, Data Mining, and the PCAE Method. Crystals 2021, 11, 891. [Google Scholar] [CrossRef]
- Doll, K.; Dovesi, R.; Orlando, R. Analytical Hartree–Fock gradients with respect to the cell parameter for systems periodic in three dimensions. Theor. Chem. Acc. 2004, 112, 394–402. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Zagorac, D.; Schön, J.C.; Zagorac, J.; Jansen, M. Prediction of structure candidates for zinc oxide as a function of pressure and investigation of their electronic properties. Phys. Rev. B 2014, 89, 075201. [Google Scholar] [CrossRef]
- Zagorac, D.; Schon, J.C.; Zagorac, J.; Jansen, M. Theoretical investigations of novel zinc oxide polytypes and in-depth study of their electronic properties. RSC Adv. 2015, 5, 25929–25935. [Google Scholar] [CrossRef]
- Fischer, D.; Zagorac, D.; Schön, J.C. The presence of superoxide ions and related dioxygen species in zinc oxide—A structural characterization by in situ Raman spectroscopy. J. Raman Spectrosc. 2022, 53, 2137–2146. [Google Scholar] [CrossRef]
- Schön, J.C.; Čančarević, Ž.; Jansen, M. Structure prediction of high-pressure phases for alkali metal sulfides. J. Chem. Phys. 2004, 121, 2289–2304. [Google Scholar] [CrossRef]
- Zagorac, D.; Doll, K.; Zagorac, J.; Jordanov, D.; Matović, B. Barium Sulfide under Pressure: Discovery of Metastable Polymorphs and Investigation of Electronic Properties on ab Initio Level. Inorg. Chem. 2017, 56, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Zagorac, D.; Zagorac, J.; Fonović, M.; Pejić, M.; Schön, J.C. Computational discovery of new modifications in scandium oxychloride (ScOCl) using a multi-methodological approach. Z. Anorg. Und Allg. Chem. 2022, 648, e202200198. [Google Scholar] [CrossRef]
- Hundt, R. KPLOT, A Program for Plotting and Analyzing Crystal Structures; Technicum Scientific Publishing: Stuttgart, Germany, 2016. [Google Scholar]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Doll, K. Gaussian Basis Sets for Solid State Calculations. In Basis Sets in Computational Chemistry; Perlt, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 157–181. [Google Scholar] [CrossRef]
- Jaffe, J.E.; Hess, A.C. Hartree-Fock study of phase changes in ZnO at high pressure. Phys. Rev. B 1993, 48, 7903–7909. [Google Scholar] [CrossRef] [PubMed]
- Homann, T.; Hotje, U.; Binnewies, M.; Börger, A.; Becker, K.-D.; Bredow, T. Composition-dependent band gap in ZnSxSe1−x: A combined experimental and theoretical study. Solid State Sci. 2006, 8, 44–49. [Google Scholar] [CrossRef]
- Towler, M.D.; Allan, N.L.; Harrison, N.M.; Saunders, V.R.; Mackrodt, W.C.; Aprà, E. Ab initio study of MnO and NiO. Phys. Rev. B 1994, 50, 5041–5054. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.M.; Pisani, C. An ab Initio Periodic Study of NiO Supported at the Pd(100) Surface. Part 1: The Perfect Epitaxial Monolayer. J. Phys. Chem. B 2006, 110, 7909–7917. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.; Harrison, N.M.; Saunders, V.R.; Flavell, W.R. An ab initio Hartree-Fock investigation of galena (PbS). Chem. Phys. Lett. 1996, 257, 627–632. [Google Scholar] [CrossRef]
- Zagorac, D.; Doll, K.; Schön, J.C.; Jansen, M. Ab initio structure prediction for lead sulfide at standard and elevated pressures. Phys. Rev. B 2011, 84, 045206. [Google Scholar] [CrossRef]
- Aroyo, M.I.; Perez-Mato, J.M.; Capillas, C.; Kroumova, E.; Ivantchev, S.; Madariaga, G.; Kirov, A.; Wondratschek, H. Bilbao Crystallographic Server: I. Databases and crystallographic computing programs. Z. Krist.—Cryst. Mater. 2006, 221, 15–27. [Google Scholar] [CrossRef]
- Aroyo, M.I.; Orobengoa, D.; de la Flor, G.; Tasci, E.S.; Perez-Mato, J.M.; Wondratschek, H. Brillouin-zone database on the Bilbao Crystallographic Server. Acta Crystallogr. Sect. A 2014, 70, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Tasci, E.S.; de la Flor, G.; Orobengoa, D.; Capillas, C.; Perez-Mato, J.M.; Aroyo, M.I. An introduction to the tools hosted in the Bilbao Crystallographic Server. EPJ Web Conf. 2012, 22, 00009. [Google Scholar] [CrossRef]
- Turner, P. Xmgrace. Available online: https://plasma-gate.weizmann.ac.il/Grace/ (accessed on 15 July 2022).
- Gerlach, W. Das Ka-Dublett, nebst einer Neubestimmung der Gitterkonstanten einiger Krystalle. Z. Phys. 1922, 23, 114–120. [Google Scholar]
- Han, J.; Liu, W.; Zhang, T.; Xue, K.; Li, W.; Jiao, F.; Qin, W. Mechanism study on the sulfidation of ZnO with sulfur and iron oxide at high temperature. Sci. Rep. 2017, 7, 42536. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Sobczak, K.; Dłużewski, P.; Reszka, A.; Kowalski, B.J.; Kłopotowski, Ł.; Elbaum, D.; Fronc, K. Facile synthesis of core/shell ZnO/ZnS nanofibers by electrospinning and gas-phase sulfidation for biosensor applications. Phys. Chem. Chem. Phys. 2015, 17, 24029–24037. [Google Scholar] [CrossRef]
- Yan, B.; Wang, Y.; Wu, X. Preparation and characterization of ZnO/ZnS core/shell nanocomposites through a simple chemical method. Adv. Compos. Mater. 2018, 27, 387–396. [Google Scholar] [CrossRef]
- Neveux, L.; Chiche, D.; Bazer-Bachi, D.; Favergeon, L.; Pijolat, M. New insight on the ZnO sulfidation reaction: Evidences for an outward growth process of the ZnS phase. Chem. Eng. J. 2012, 181–182, 508–515. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Stenzel, O. The Physics of Thin Film Optical Spectra: An Introduction; Springer: Berlin/Heidelberg, Germany, 2005; Volume 44, p. 277. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Farangis, B.; He, Y.; Hasselkamp, D.; Krämer, T.; Wang, C. Structural properties and bandgap bowing of ZnO1−xSx thin films deposited by reactive sputtering. Appl. Phys. Lett. 2004, 85, 4929–4931. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Cao, Y.; Chen, J.; Shen, K.; Kumar, A.; Xu, M.; Li, Q.; Xu, Q. The magnetic and adsorption properties of ZnO1−xSx nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 26918–26925. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, N.P.; Marins, L.G.P.; de Moura Yamanaka, M.Y.; Bacani, R.; da Silva Rocha, R.; Rodrigues, L.A. Efficient photodegradation of 4-chlorophenol under solar radiation using a new ZnO/ZnS/carbon xerogel composite as a photocatalyst. J. Photochem. Photobiol. A Chem. 2021, 418, 113377. [Google Scholar] [CrossRef]
- Kumar, S.; Fossard, F.; Amiri, G.; Chauveau, J.M.; Sallet, V. Induced structural modifications in ZnS nanowires via physical state of catalyst: Highlights of 15R crystal phase. Nano Res. 2022, 15, 377–385. [Google Scholar] [CrossRef]
- Mardix, S. Polytypism: A controlled thermodynamic phenomenon. Phys. Rev. B 1986, 33, 8677–8684. [Google Scholar] [CrossRef]
- Morkel, M.; Weinhardt, L.; Lohmüller, B.; Heske, C.; Umbach, E.; Riedl, W.; Zweigart, S.; Karg, F. Flat conduction-band alignment at the CdS/CuInSe2 thin-film solar-cell heterojunction. Appl. Phys. Lett. 2001, 79, 4482–4484. [Google Scholar] [CrossRef]
- Moon, C.-Y.; Wei, S.-H.; Zhu, Y.Z.; Chen, G.D. Band-gap bowing coefficients in large size-mismatched II-VI alloys: First-principles calculations. Phys. Rev. B 2006, 74, 233202. [Google Scholar] [CrossRef]
- Haight, R.; Barkhouse, A.; Gunawan, O.; Shin, B.; Copel, M.; Hopstaken, M.; Mitzi, D.B. Band alignment at the Cu2ZnSn(SxSe1−x)4/CdS interface. Appl. Phys. Lett. 2011, 98, 253502. [Google Scholar] [CrossRef]
- Sun, L.; Haight, R.; Sinsermsuksakul, P.; Kim, S.B.; Park, H.H.; Gordon, R.G. Band alignment of SnS/Zn(O,S) heterojunctions in SnS thin film solar cells. Appl. Phys. Lett. 2013, 103, 181904. [Google Scholar] [CrossRef]
- Wang, K.; Chen, J.J.; Zeng, Z.M.; Tarr, J.; Zhou, W.L.; Zhang, Y.; Yan, Y.F.; Jiang, C.S.; Pern, J.; Mascarenhas, A. Synthesis and photovoltaic effect of vertically aligned ZnO/ZnS core/shell nanowire arrays. Appl. Phys. Lett. 2010, 96, 123105. [Google Scholar] [CrossRef]
- Sookhakian, M.; Amin, Y.M.; Basirun, W.J.; Tajabadi, M.T.; Kamarulzaman, N. Synthesis, structural, and optical properties of type-II ZnO–ZnS core-shell nanostructure. J. Lumin. 2014, 145, 244–252. [Google Scholar] [CrossRef]


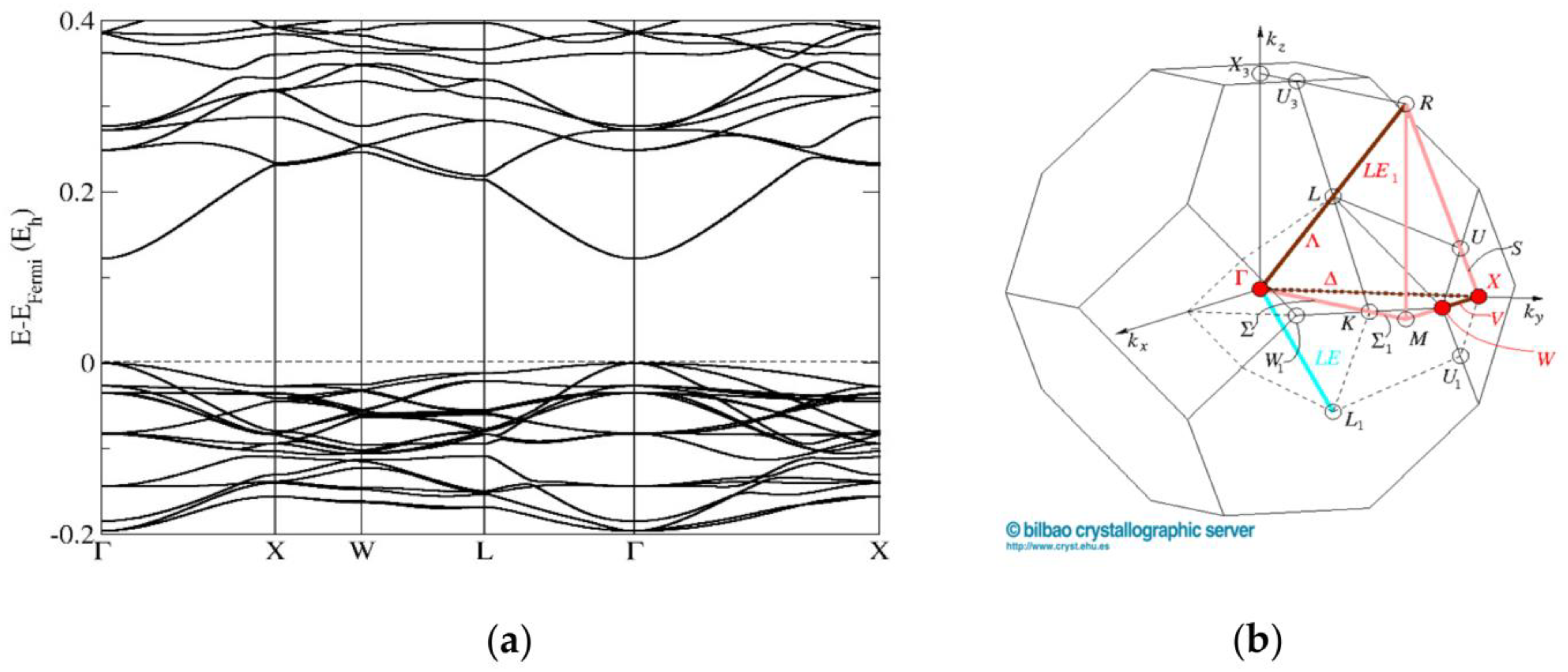
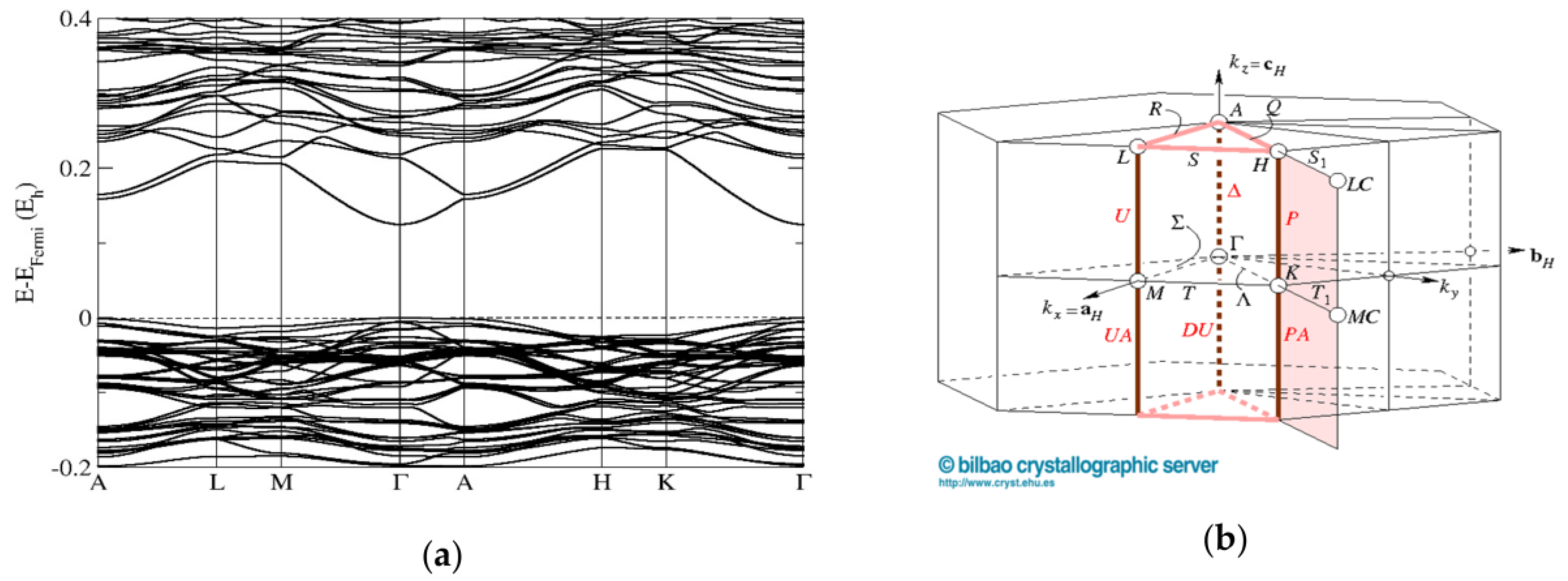

| Structure and Composition | Space Group and Unit Cell Parameters (Å) | |||
|---|---|---|---|---|
| HSE06 (Full) | HSE06 (Cell) | PBE0 (Full) | PBE0 (Cell) | |
| Sphalerite ZnO0.875S0.125 | F-43m (no. 216) a = 4.689 | F-43m (no. 216) a = 4.719 | F-43m (no. 216) a = 4.687 | F-43m (no. 216) a = 4.717 |
| Wurtzite ZnO0.813S0.187 | Cm (no. 8) a = 11.675, b = 6.727, c = 10.845, β = 90.39 | P63mc (no. 186)) a = 3.358, c = 5.379 | Cm (no. 8) a = 11.663, b = 6.724, c = 10.839, β = 90.39 | P63mc (no. 186)) a = 3.407, c = 5.451 |
| Structure and Composition | Computed Band Gap Size (eV) | |||
|---|---|---|---|---|
| HSE06 (Full) | HSE06 (Cell) | PBE0 (Full) | PBE0 (Cell) | |
| Sphalerite ZnO0.875S0.125 | 2.13 | 2.70 | 2.76 | 3.32 |
| Wurtzite ZnO0.813S0.187 | 1.53 | 2.79 | 2.17 | 3.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagorac, J.; Zagorac, D.; Šrot, V.; Ranđelović, M.; Pejić, M.; van Aken, P.A.; Matović, B.; Schön, J.C. Synthesis, Characterization, and Electronic Properties of ZnO/ZnS Core/Shell Nanostructures Investigated Using a Multidisciplinary Approach. Materials 2023, 16, 326. https://doi.org/10.3390/ma16010326
Zagorac J, Zagorac D, Šrot V, Ranđelović M, Pejić M, van Aken PA, Matović B, Schön JC. Synthesis, Characterization, and Electronic Properties of ZnO/ZnS Core/Shell Nanostructures Investigated Using a Multidisciplinary Approach. Materials. 2023; 16(1):326. https://doi.org/10.3390/ma16010326
Chicago/Turabian StyleZagorac, Jelena, Dejan Zagorac, Vesna Šrot, Marjan Ranđelović, Milan Pejić, Peter A. van Aken, Branko Matović, and J. Christian Schön. 2023. "Synthesis, Characterization, and Electronic Properties of ZnO/ZnS Core/Shell Nanostructures Investigated Using a Multidisciplinary Approach" Materials 16, no. 1: 326. https://doi.org/10.3390/ma16010326
APA StyleZagorac, J., Zagorac, D., Šrot, V., Ranđelović, M., Pejić, M., van Aken, P. A., Matović, B., & Schön, J. C. (2023). Synthesis, Characterization, and Electronic Properties of ZnO/ZnS Core/Shell Nanostructures Investigated Using a Multidisciplinary Approach. Materials, 16(1), 326. https://doi.org/10.3390/ma16010326






