Density-Controlled Growth of ZnO Nanowalls for High-Performance Photocatalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
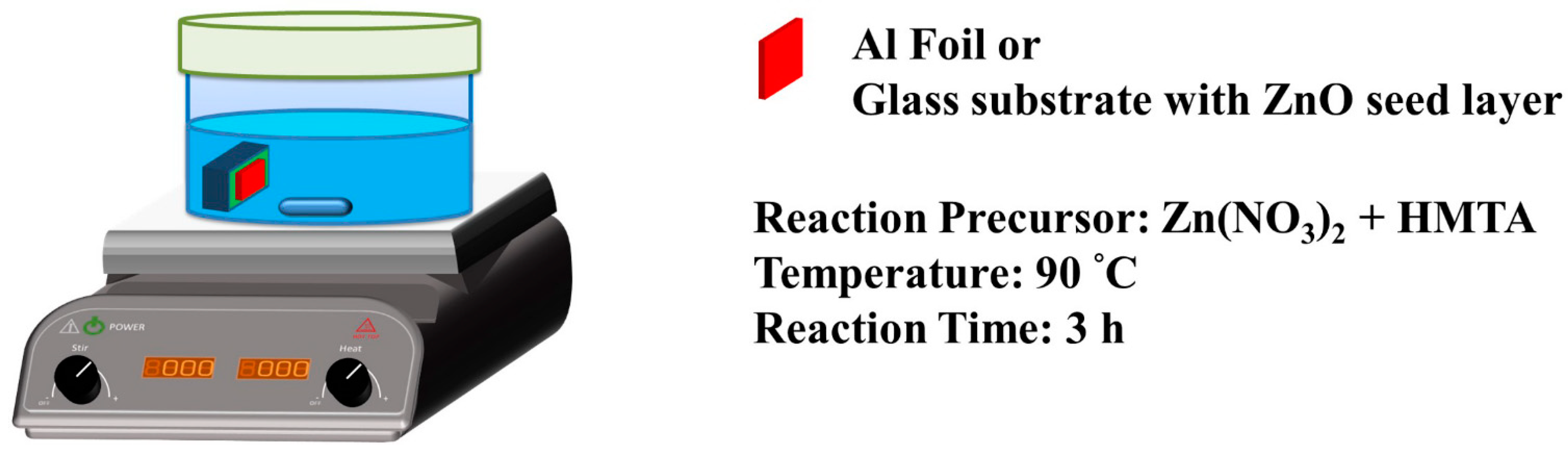
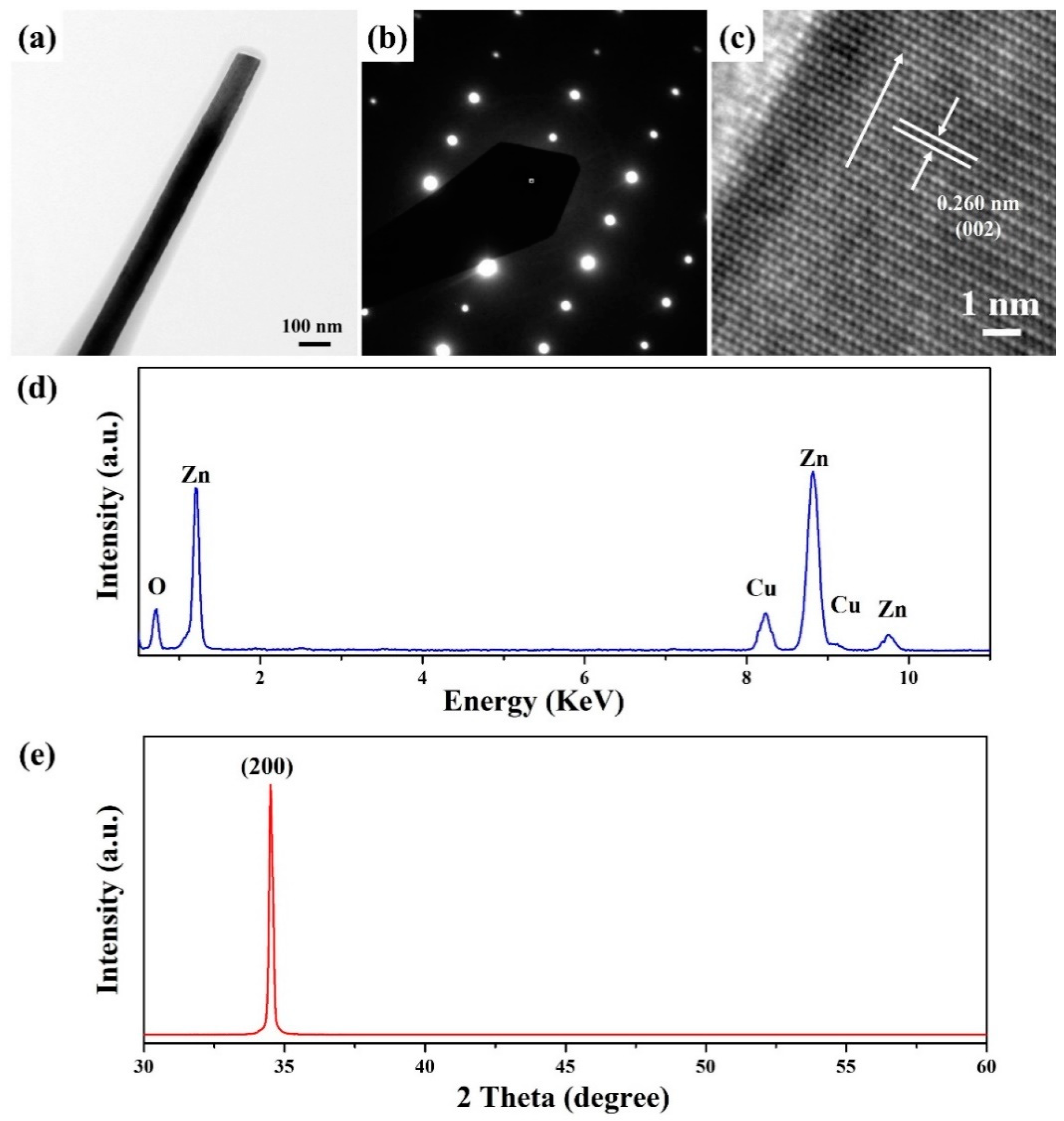
2.2. Synthesis of ZnO Nanowires
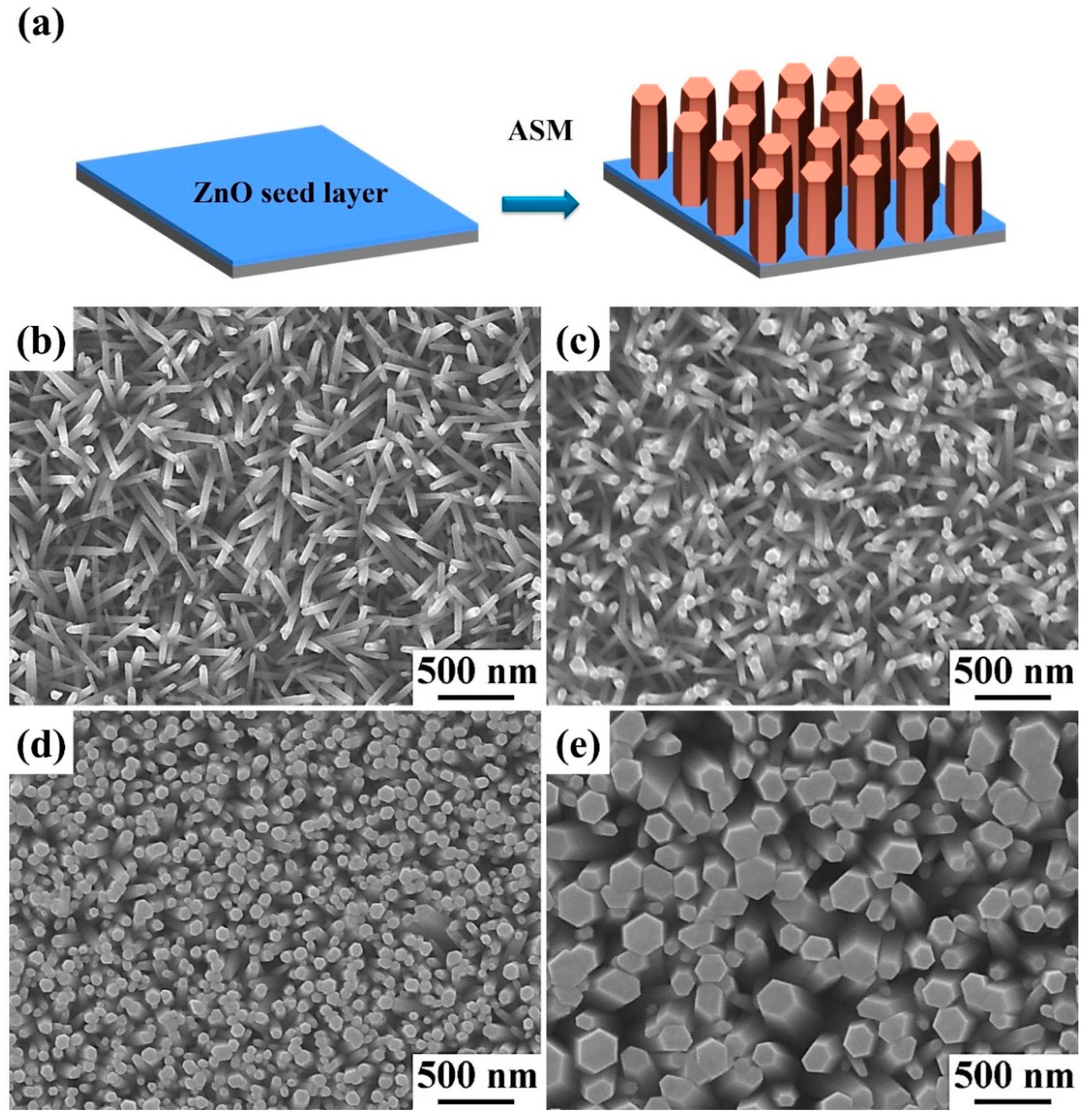
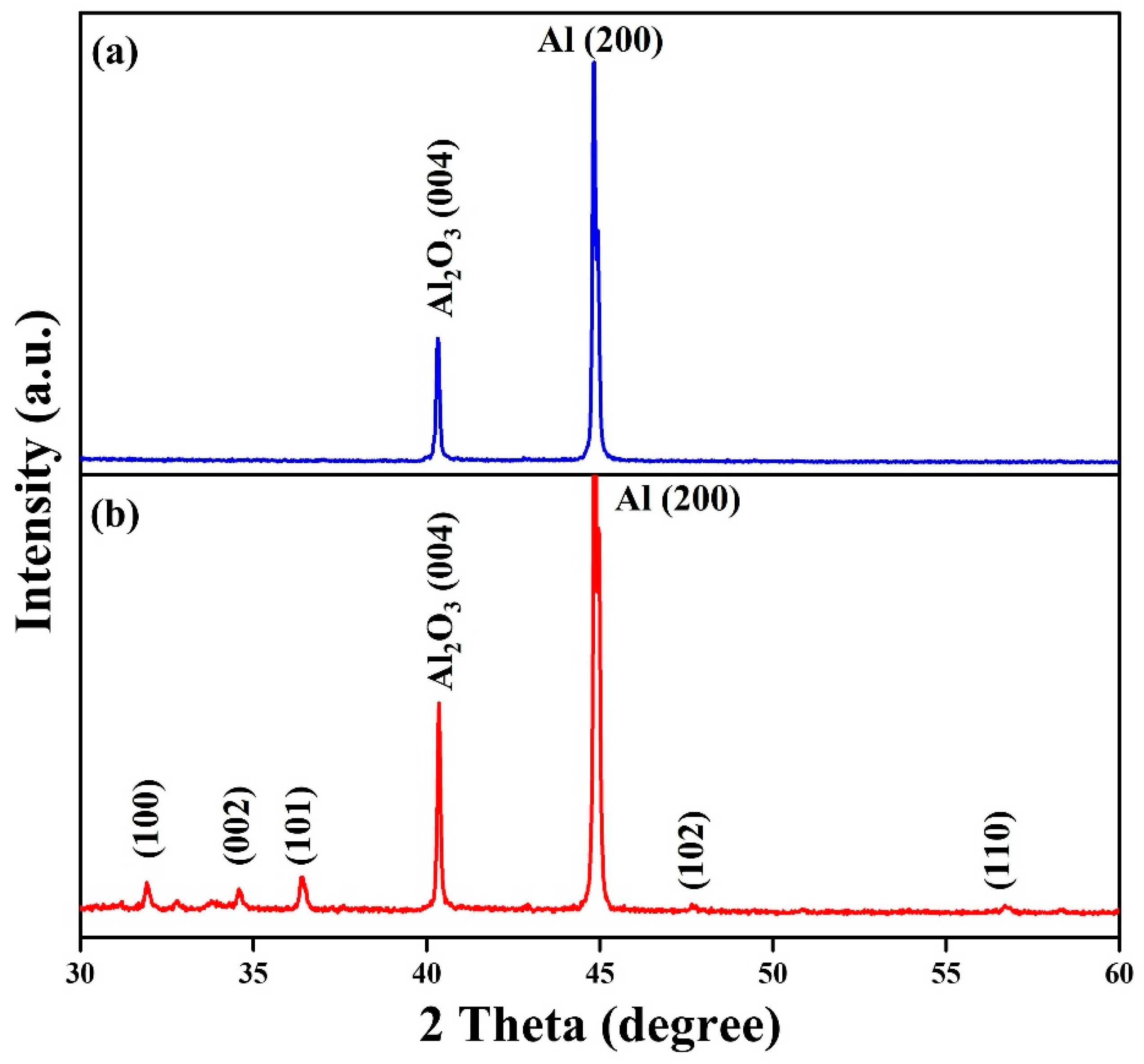
2.3. Synthesis of ZnO Nanowalls
2.4. Material Characterization
2.5. Photocatalytic Activity Test
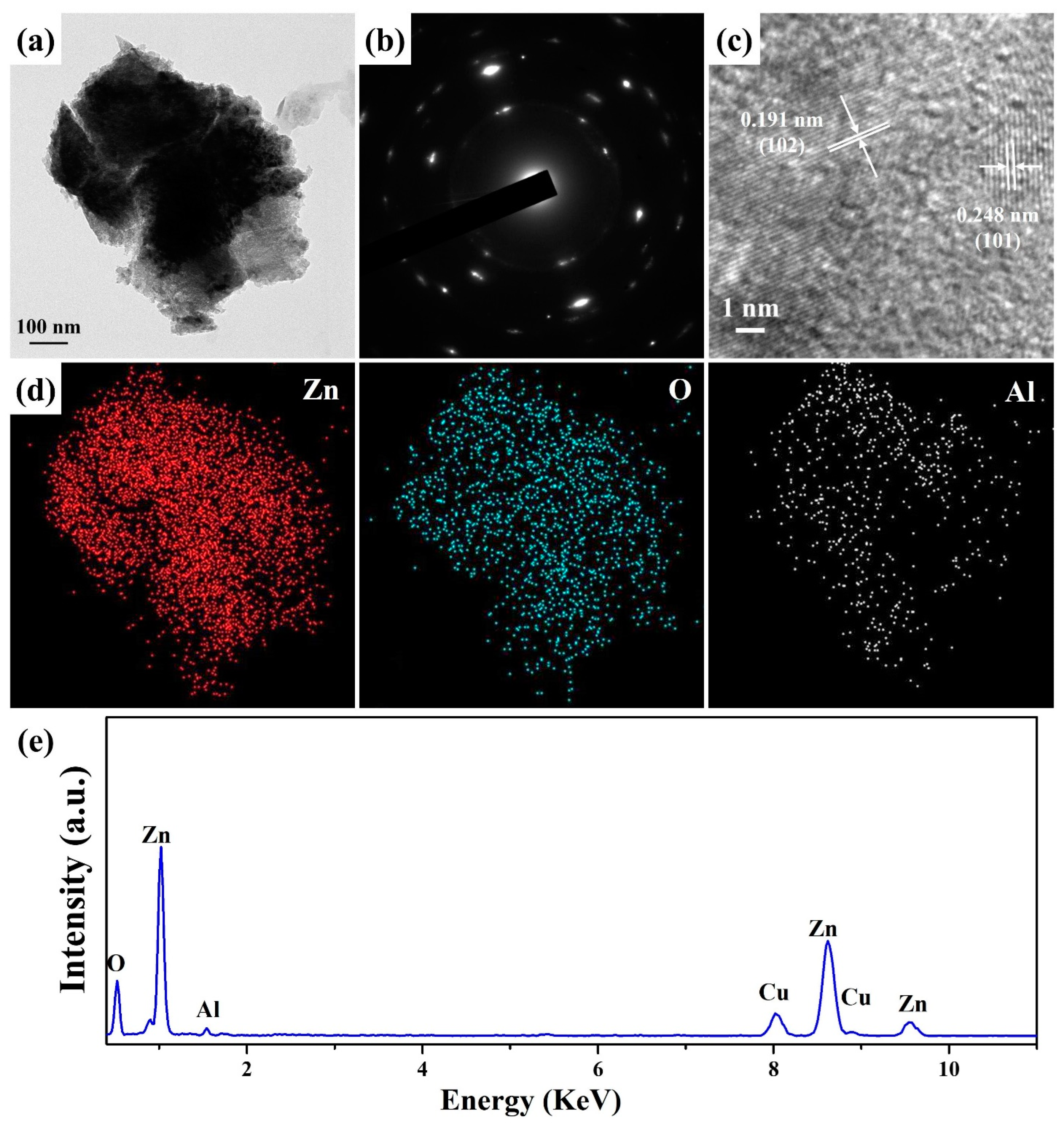
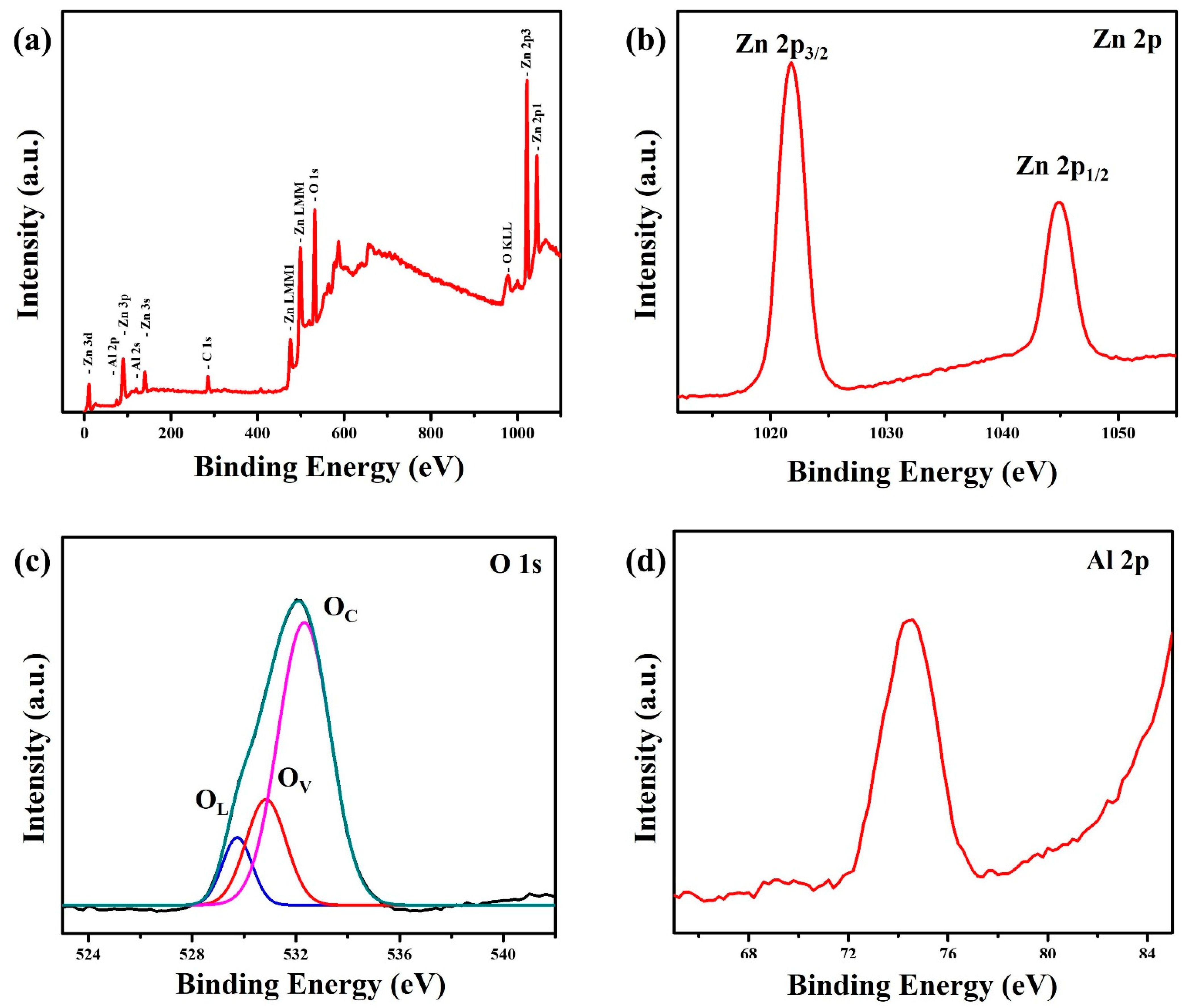
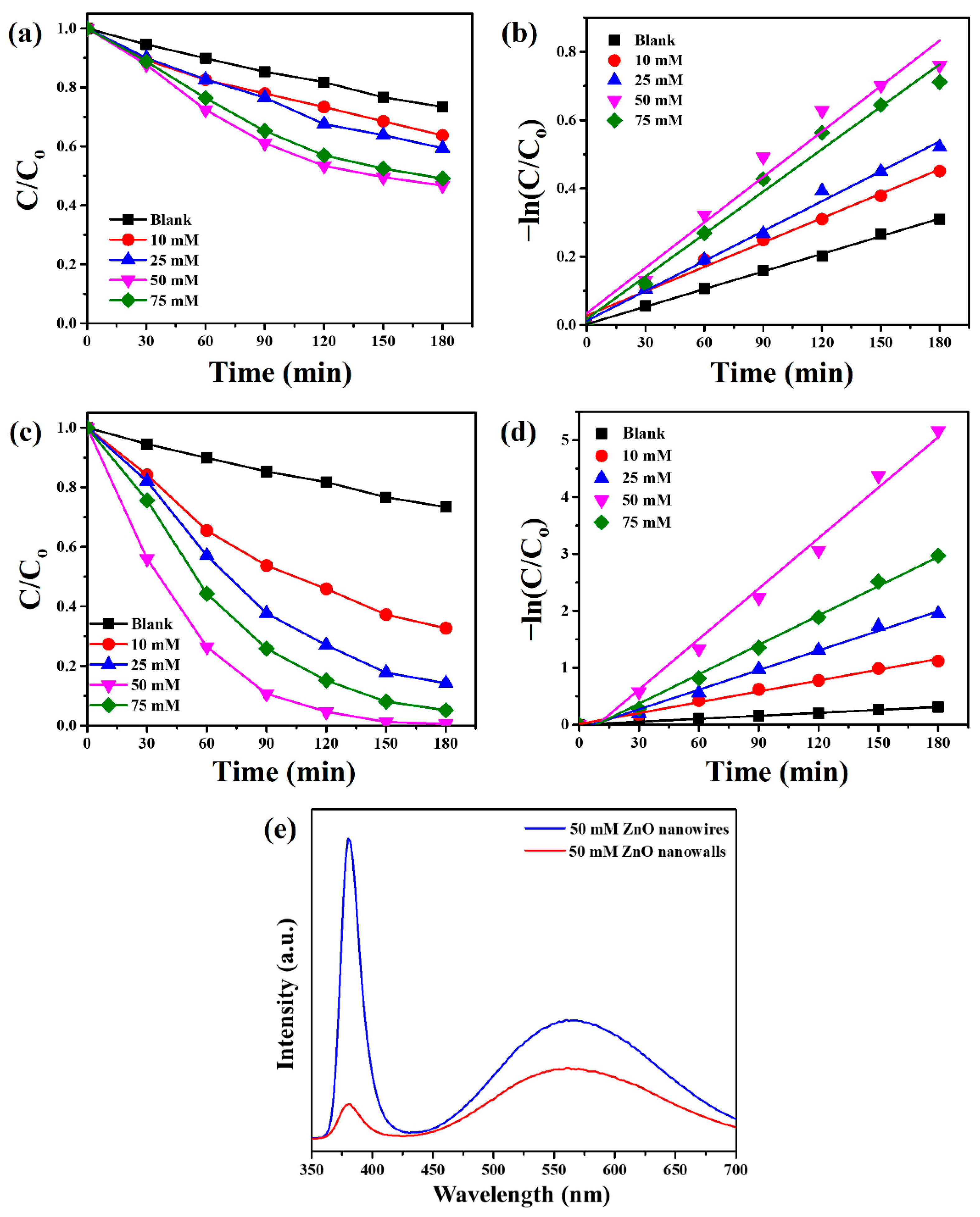
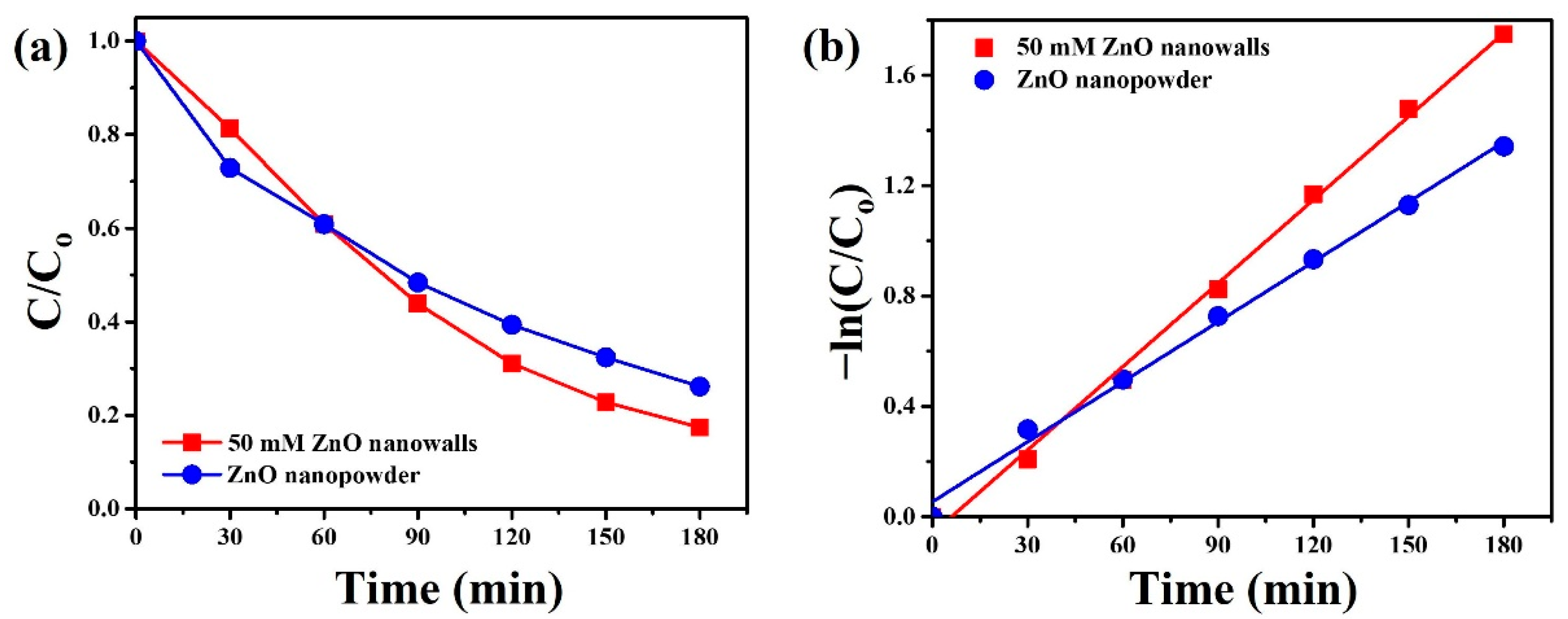
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwu, K.O.; Strano, V.; Di Mauro, A.; Impellizzeri, G.; Mirabella, S. Enhanced Quality, Growth Kinetics, and Photocatalysis of ZnO Nanowalls Prepared by Chemical Bath Deposition. Cryst. Growth Des. 2015, 15, 4206–4212. [Google Scholar] [CrossRef]
- Ye, C.; Bando, Y.; Shen, G.; Golberg, D. Thickness-Dependent Photocatalytic Performance of ZnO Nanoplatelets. J. Phys. Chem. B 2006, 110, 15146–15151. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Li, C.; Li, M.; Li, H.; Huang, X.; Yang, B. Direct growth of Al-doped ZnO ultrathin nanosheets on electrode for ethanol gas sensor application. Appl. Surf. Sci. 2018, 447, 173–181. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, G.; Fan, B.; Zhao, W.; Xie, Y.; Zhang, R. ZnS nanowall coated Ni composites: Facile preparation and enhanced electromagnetic wave absorption. RSC Adv. 2014, 4, 61219–61225. [Google Scholar] [CrossRef]
- Chang, Y.-C. Cadmium hydroxide and oxide nanoporous walls with high performance photocatalytic properties. J. Alloys Compd. 2015, 637, 112–118. [Google Scholar] [CrossRef]
- Ramesh, C.; Tyagi, P.; Yadav, B.S.; Ojha, S.; Maurya, K.K.; Senthil Kumar, M.; Kushvaha, S.S. Effect of nitridation temperature on formation and properties of GaN nanowall networks on sapphire (0 0 0 1) grown by laser MBE. Mater. Sci. Eng. B 2018, 231, 105–114. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Li, C.; Jiang, H. Dye-Sensitized Solar Cell with Energy Storage Function through PVDF/ZnO Nanocomposite Counter Electrode. Adv. Mater. 2013, 25, 4093–4096. [Google Scholar] [CrossRef]
- Udom, I.; Ram, M.K.; Stefanakos, E.K.; Hepp, A.F.; Goswami, D.Y. One dimensional-ZnO nanostructures: Synthesis, properties and environmental applications. Mater. Sci. Semicond. Process. 2013, 16, 2070–2083. [Google Scholar] [CrossRef]
- Tang, X.; Li, G.; Zhou, S. Ultraviolet Electroluminescence of Light-Emitting Diodes Based on Single n-ZnO/p-AlGaN Heterojunction Nanowires. Nano Lett. 2013, 13, 5046–5050. [Google Scholar] [CrossRef]
- Blažeka, D.; Car, J.; Klobučar, N.; Jurov, A.; Zavašnik, J.; Jagodar, A.; Kovačević, E.; Krstulović, N. Photodegradation of Methylene Blue and Rhodamine B Using Laser-Synthesized ZnO Nanoparticles. Materials 2020, 13, 4357. [Google Scholar] [CrossRef]
- Senapati, S.; Srivastava, S.K.; Singh, S.B. Synthesis, characterization and photocatalytic activity of magnetically separable hexagonal Ni/ZnO nanostructure. Nanoscale 2012, 4, 6604–6612. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.-H.; Huang, C.-W.; Hsin, C.-L.; Wang, C.-W.; Yu, S.-Y.; Yeh, P.-H.; Wu, W.-W. Well-aligned ZnO nanowires with excellent field emission and photocatalytic properties. Nanoscale 2012, 4, 1471–1475. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Lu, M.; Fan, Z.; Weng, S.; Hu, J.; Liu, P. Synergistic Effect in Polyaniline-Hybrid Defective ZnO with Enhanced Photocatalytic Activity and Stability. J. Phys. Chem. C 2014, 118, 9570–9577. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Militký, J.; Azeem, M.; Ashraf, M.A. One-Pot Sonochemical Synthesis of ZnO Nanoparticles for Photocatalytic Applications, Modelling and Optimization. Materials 2020, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Le, A.T.; Le, T.D.H.; Cheong, K.-Y.; Pung, S.-Y. Immobilization of zinc oxide-based photocatalysts for organic pollutant degradation: A review. J. Environ. Chem. Eng. 2022, 10, 108505. [Google Scholar] [CrossRef]
- Brewster, M.M.; Lu, M.-Y.; Lim, S.K.; Smith, M.J.; Zhou, X.; Gradečak, S. The Growth and Optical Properties of ZnO Nanowalls. J. Phys. Chem. Lett. 2011, 2, 1940–1945. [Google Scholar] [CrossRef]
- Labis, J.P.; Hezam, M.; Al-Anazi, A.; Al-Brithen, H.; Ansari, A.A.; Mohamed El-Toni, A.; Enriquez, R.; Jacopin, G.; Al-Hoshan, M. Pulsed laser deposition growth of 3D ZnO nanowall network in nest-like structures by two-step approach. Sol. Energy Mater. Sol. Cells 2015, 143, 539–545. [Google Scholar] [CrossRef]
- Dimova-Malinovska, D.; Lovchinov, K.; Ganchev, M.; Angelov, O.; Graff, J.S.; Ulyashin, A. Influence of the substrate material on the surface morphology of electrochemically deposited ZnO layers. Phys. Status Solidi (A) 2013, 210, 737–742. [Google Scholar] [CrossRef]
- Nayak, A.P.; Katzenmeyer, A.M.; Gosho, Y.; Tekin, B.; Islam, M.S. Sonochemical approach for rapid growth of zinc oxide nanowalls. Appl. Phys. A 2012, 107, 661–667. [Google Scholar] [CrossRef]
- Chang, T.-H.; Chang, Y.-C.; Wu, S.-H. Ag nanoparticles decorated ZnO: Al nanoneedles as a high-performance surface-enhanced Raman scattering substrate. J. Alloys Compd. 2020, 843, 156044. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Khanna, S.; Utsav; Paneliya, S.; Takhar, V.; Banerjee, R.; Mukhopadhyay, I. Fabrication of silver nanodome embedded zinc oxide nanorods for enhanced Raman spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128336. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Luo, Y.; Tao, Y.; Lei, M.; Fan, X.; Yan, W.; Peng, P. Dependence of Al3+ on the growth mechanism of vertical standing ZnO nanowalls and their NO2 gas sensing properties. Sens. Actuators B Chem. 2014, 204, 96–101. [Google Scholar] [CrossRef]
- Dao, H.T.; Makino, H. Improving electrical conductivity and its thermal stability of Al-doped ZnO polycrystalline films using ultrathin Al film as a passivation layer. Sol. Energy Mater. Sol. Cells 2019, 203, 110159. [Google Scholar] [CrossRef]
- Manjula, Y.; Rakesh Kumar, R.; Swarup Raju, P.M.; Anil Kumar, G.; Venkatappa Rao, T.; Akshaykranth, A.; Supraja, P. Piezoelectric flexible nanogenerator based on ZnO nanosheet networks for mechanical energy harvesting. Chem. Phys. 2020, 533, 110699. [Google Scholar] [CrossRef]
- Cheng, J.P.; Zhang, X.B.; Luo, Z.Q. Oriented growth of ZnO nanostructures on Si and Al substrates. Surf. Coat. Technol. 2008, 202, 4681–4686. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kumar, B.; Lee, K.Y.; Park, H.-K.; Lee, J.-H.; Lee, H.H.; Jun, H.; Lee, D.; Kim, S.-W. Piezoelectric two-dimensional nanosheets/anionic layer heterojunction for efficient direct current power generation. Sci. Rep. 2013, 3, 2017. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, V.; Kumar, R.R.; Parmar, M.; Yaddanapudi, G.R.K.; Nayak, M.M.; Rajanna, K. Morphology controlled synthesis of Al doped ZnO nanosheets on Al alloy substrate by low-temperature solution growth method. RSC Adv. 2015, 5, 13519–13524. [Google Scholar] [CrossRef]
- Gupta, M.K.; Lee, J.-H.; Lee, K.Y.; Kim, S.-W. Two-Dimensional Vanadium-Doped ZnO Nanosheet-Based Flexible Direct Current Nanogenerator. ACS Nano 2013, 7, 8932–8939. [Google Scholar] [CrossRef]
- Lai, C.; Xu, F.; Zhang, M.; Li, B.; Liu, S.; Yi, H.; Li, L.; Qin, L.; Liu, X.; Fu, Y.; et al. Facile synthesis of CeO2/carbonate doped Bi2O2CO3 Z-scheme heterojunction for improved visible-light photocatalytic performance: Photodegradation of tetracycline and photocatalytic mechanism. J. Colloid Interface Sci. 2021, 588, 283–294. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Kuo, K.-Y.; Chen, S.-H.; Hsiao, P.-H.; Lee, J.-T.; Chen, C.-Y. Day-night active photocatalysts obtained through effective incorporation of Au@CuxS nanoparticles onto ZnO nanowalls. J. Hazard. Mater. 2022, 421, 126674. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.P.; Liao, Z.M.; Shi, D.; Liu, F.; Zhang, X.B. Oriented ZnO nanoplates on Al substrate by solution growth technique. J. Alloys Compd. 2009, 480, 741–746. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hsu, C.-C.; Wu, S.-H.; Chuang, K.-W.; Chen, Y.-F. Fabrication of Cu-doped ZnO nanoneedles on different substrate via wet chemical approach: Structural characterization and photocatalytic performance. Appl. Surf. Sci. 2018, 447, 213–221. [Google Scholar] [CrossRef]
- Yun, S.; Lee, J.; Yang, J.; Lim, S. Hydrothermal synthesis of Al-doped ZnO nanorod arrays on Si substrate. Phys. B Condens. Matter 2010, 405, 413–419. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; Farahat, M.M.; El-Hout, S.I.; El-Sheikh, S.M. Preparation and characterization of magnetic photocatalyst from the banded iron formation for effective photodegradation of methylene blue under UV and visible illumination. J. Environ. Chem. Eng. 2021, 9, 105127. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Chen, S.; Dong, Q.; Zhang, X.; Di, Y.; Jiang, A.; Zhang, D.; Li, T. Synthesis of halloysite nanotubes supported Bi-modified BaSnO3 photocatalysts for the enhanced degradation of methylene blue under visible light. Opt. Mater. 2022, 134, 113202. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, N.; Chen, T.; Li, B.; Wang, Q. Fabrication of hydrangea-shaped Bi2WO6/ZIF-8 visible-light responsive photocatalysts for degradation of methylene blue. Chemosphere 2022, 307, 135949. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tasi, C.-L.; Ko, F.-H. Construction of ZnIn2S4/ZnO heterostructures with enhanced photocatalytic decomposition and hydrogen evolution under blue LED irradiation. Int. J. Hydrogen Energy 2021, 46, 10281–10292. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lin, J.-C.; Chou, C.-M. H2Ti3O7 nanowires as a high-performance photocatalytic and surface-enhanced Raman scattering substrate. J. Photochem. Photobiol. A Chem. 2020, 400, 112666. [Google Scholar] [CrossRef]
- Yu-Cheng, C.; Yu-Wen, L. MoS2@SnO2 core-shell sub-microspheres for high efficient visible-light photodegradation and photocatalytic hydrogen production. Mater. Res. Bull. 2020, 129, 110912. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Hsu, C.-C. Synergetic effect of carbon black as co-catalyst for enhanced visible-light photocatalytic activity and stability on ZnO nanoparticles. Solid State Sci. 2020, 107, 106366. [Google Scholar] [CrossRef]



| Photocatalysts | Photocatalytic Efficiency (%) | Light Sources | References |
|---|---|---|---|
| ZnO Nanowalls | 99.4 | UVC (5 W) | Present Work |
| γ-Fe2O3/Fe3O4/SiO2 Photocatalysts | 87.5 | UV (150 W) | Ref. [35] |
| Bi/BaSnO3@HNTs Nanomaterials | 90.2 | LED (200 W) | Ref. [36] |
| Cu-doped ZnO Nanoneedles | 89.5 | UVC (5 W) | Ref. [33] |
| Bi2WO6/ZIF8 Photocatalysts | 85.7 | Visible light (400 W) | Ref. [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Lin, Y.-R.; Chen, S.-W.; Chou, C.-M. Density-Controlled Growth of ZnO Nanowalls for High-Performance Photocatalysts. Materials 2022, 15, 9008. https://doi.org/10.3390/ma15249008
Chang Y-C, Lin Y-R, Chen S-W, Chou C-M. Density-Controlled Growth of ZnO Nanowalls for High-Performance Photocatalysts. Materials. 2022; 15(24):9008. https://doi.org/10.3390/ma15249008
Chicago/Turabian StyleChang, Yu-Cheng, Ying-Ru Lin, Sheng-Wen Chen, and Chia-Man Chou. 2022. "Density-Controlled Growth of ZnO Nanowalls for High-Performance Photocatalysts" Materials 15, no. 24: 9008. https://doi.org/10.3390/ma15249008
APA StyleChang, Y.-C., Lin, Y.-R., Chen, S.-W., & Chou, C.-M. (2022). Density-Controlled Growth of ZnO Nanowalls for High-Performance Photocatalysts. Materials, 15(24), 9008. https://doi.org/10.3390/ma15249008







