Effect of Al Incorporation on the Structural and Optical Properties of Sol–Gel AZO Thin Films
Abstract
1. Introduction
2. Materials and Methods
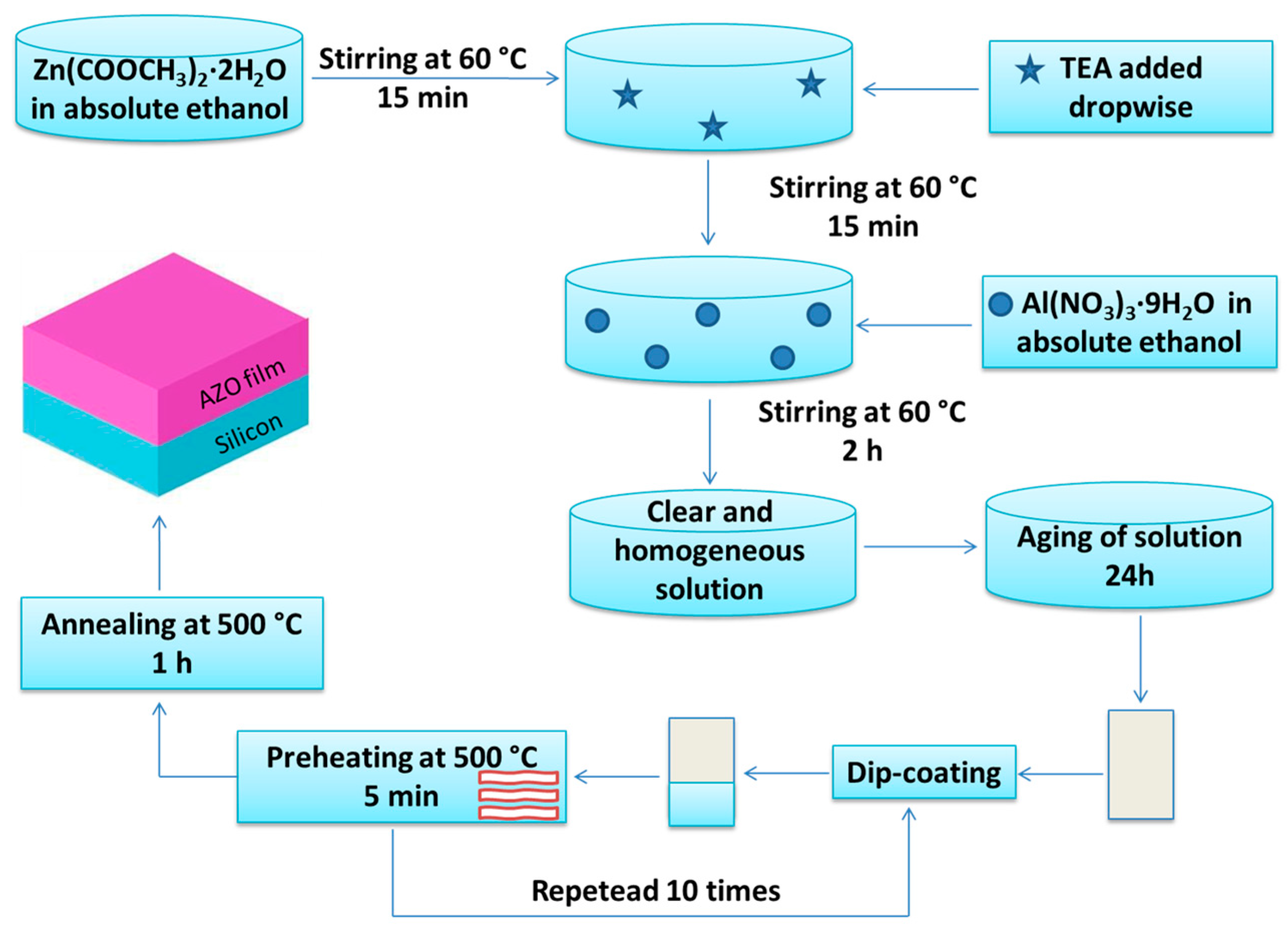
2.1. Film Preparation
2.2. Characterization Methods
3. Results and Discussion
3.1. X-ray Fluorescence (XRF)
3.2. Structure and Morphology
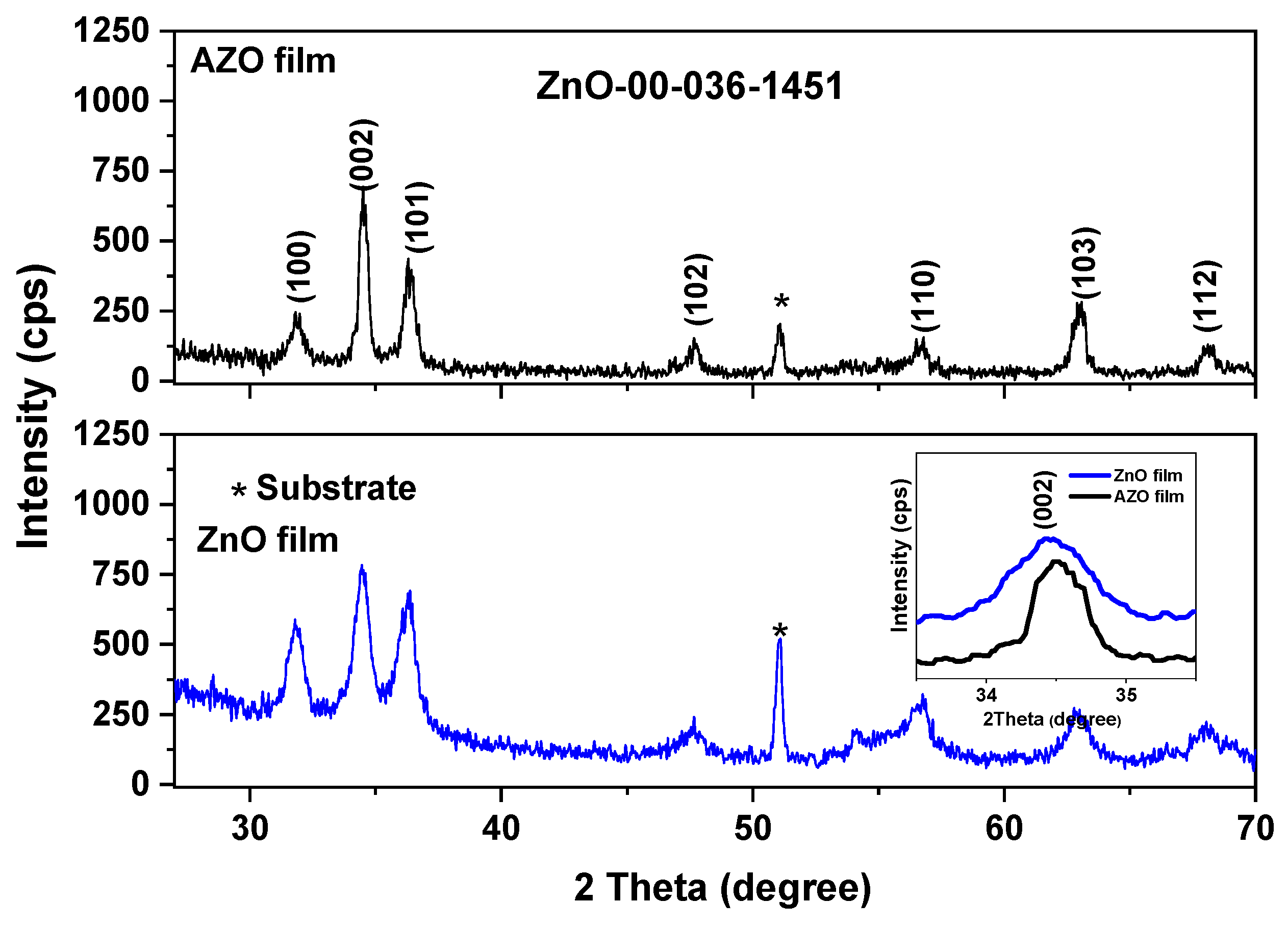
3.2.1. XRD
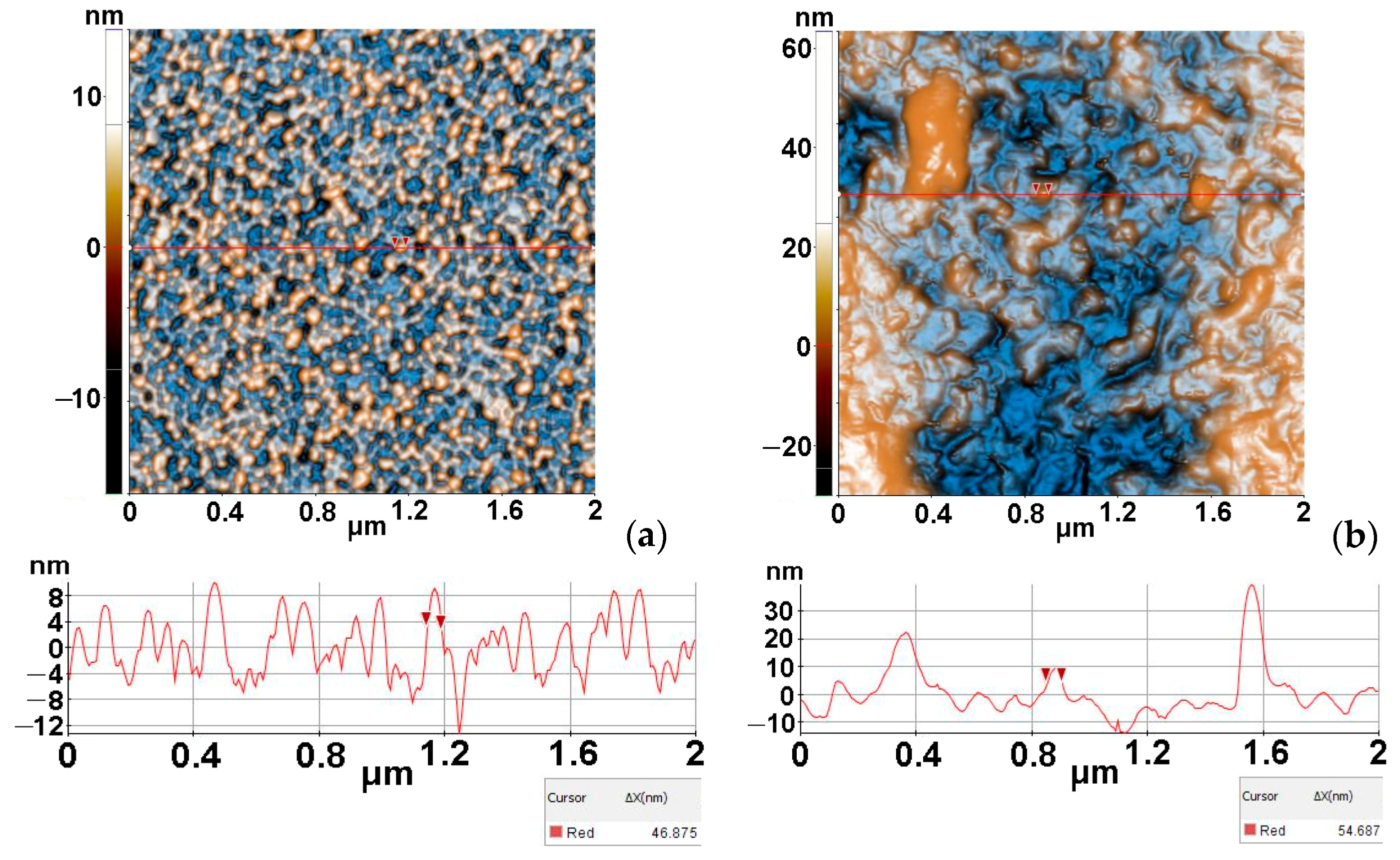
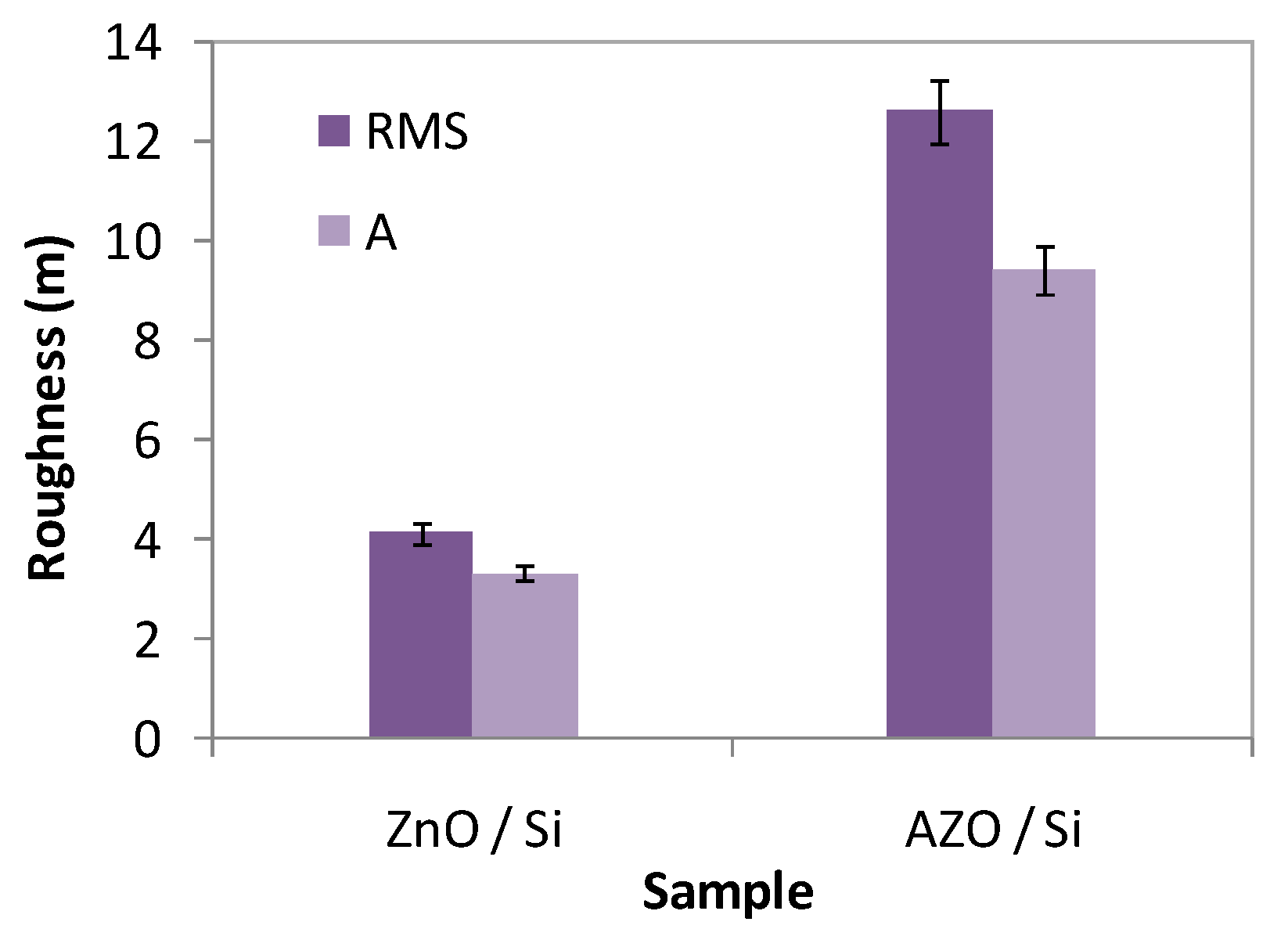
3.2.2. AFM
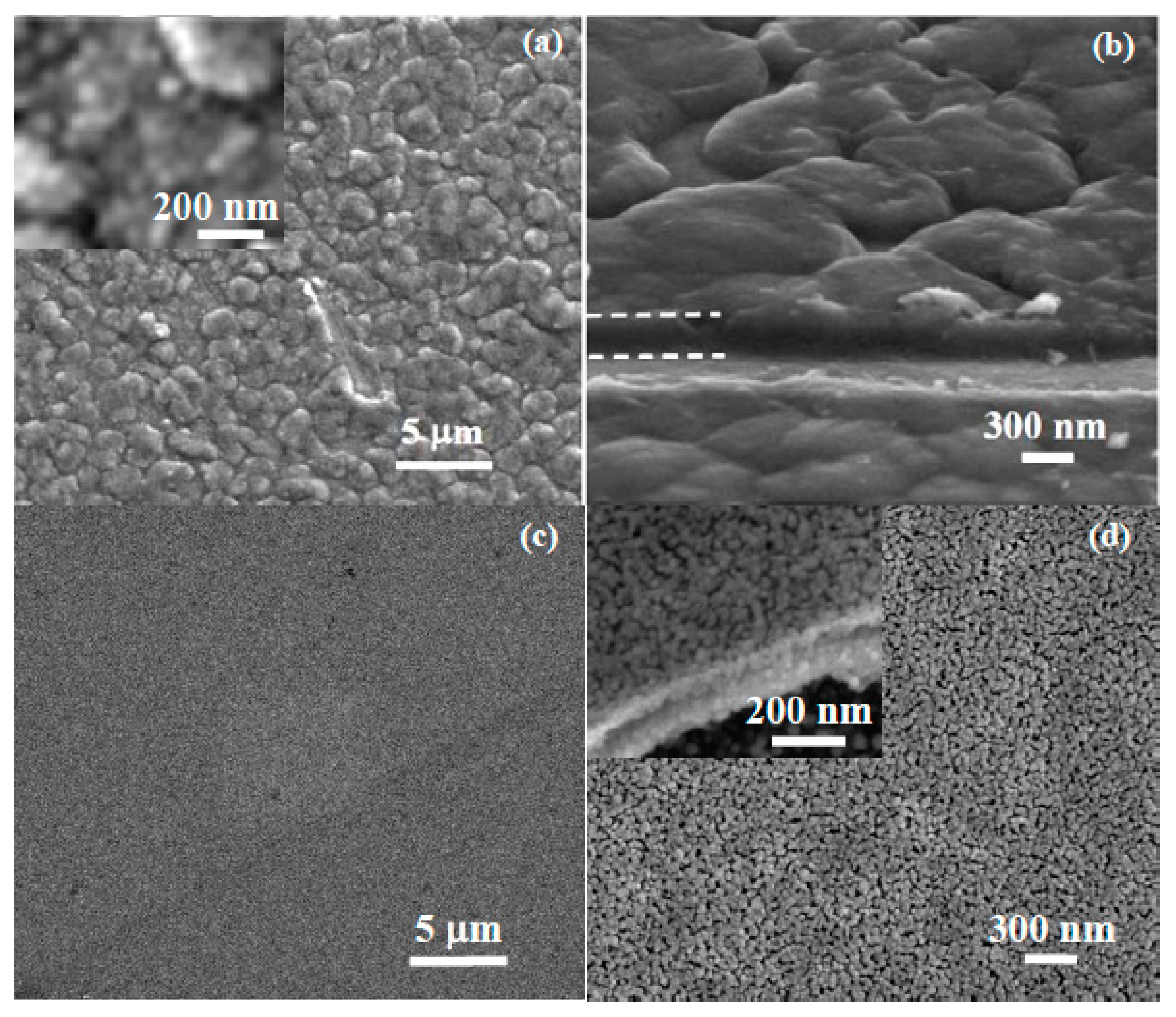
3.2.3. SEM
3.3. Optical Properties
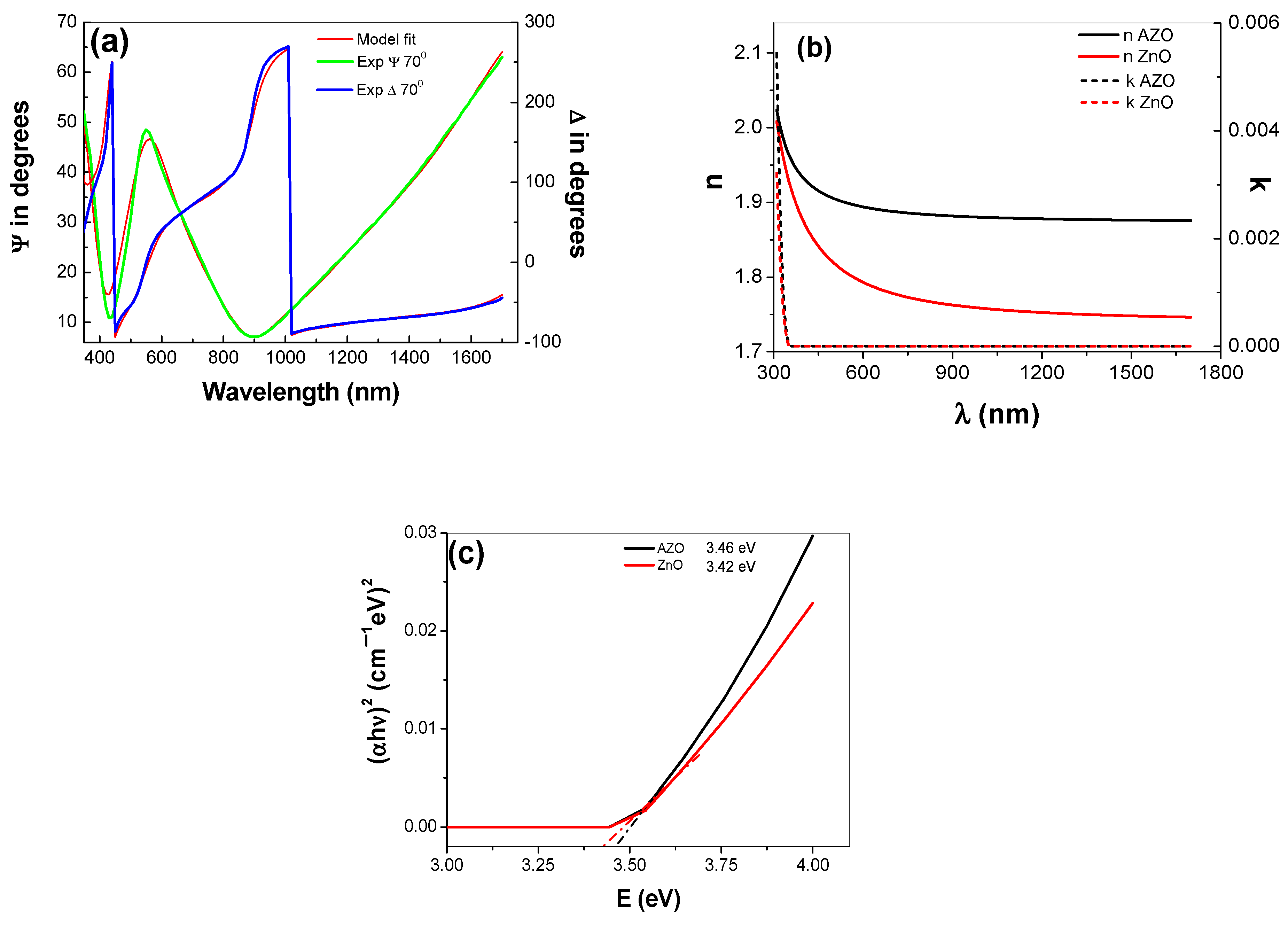
3.3.1. SE
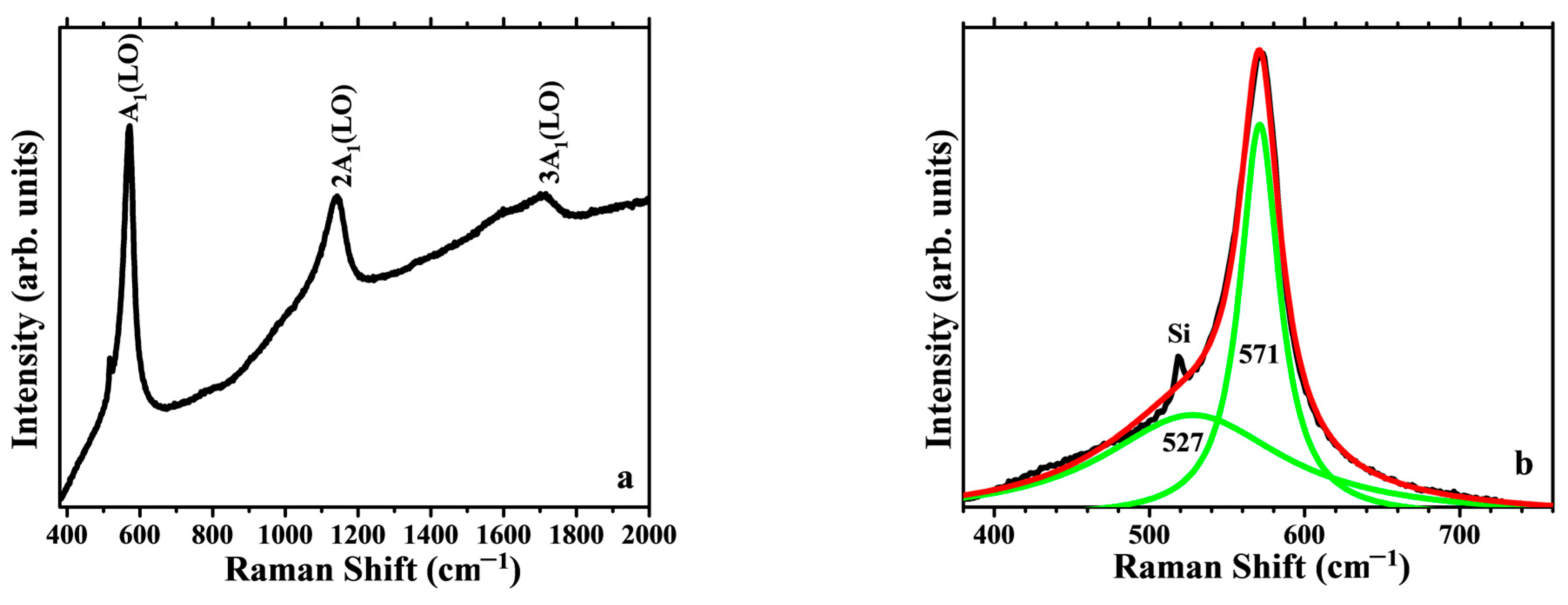
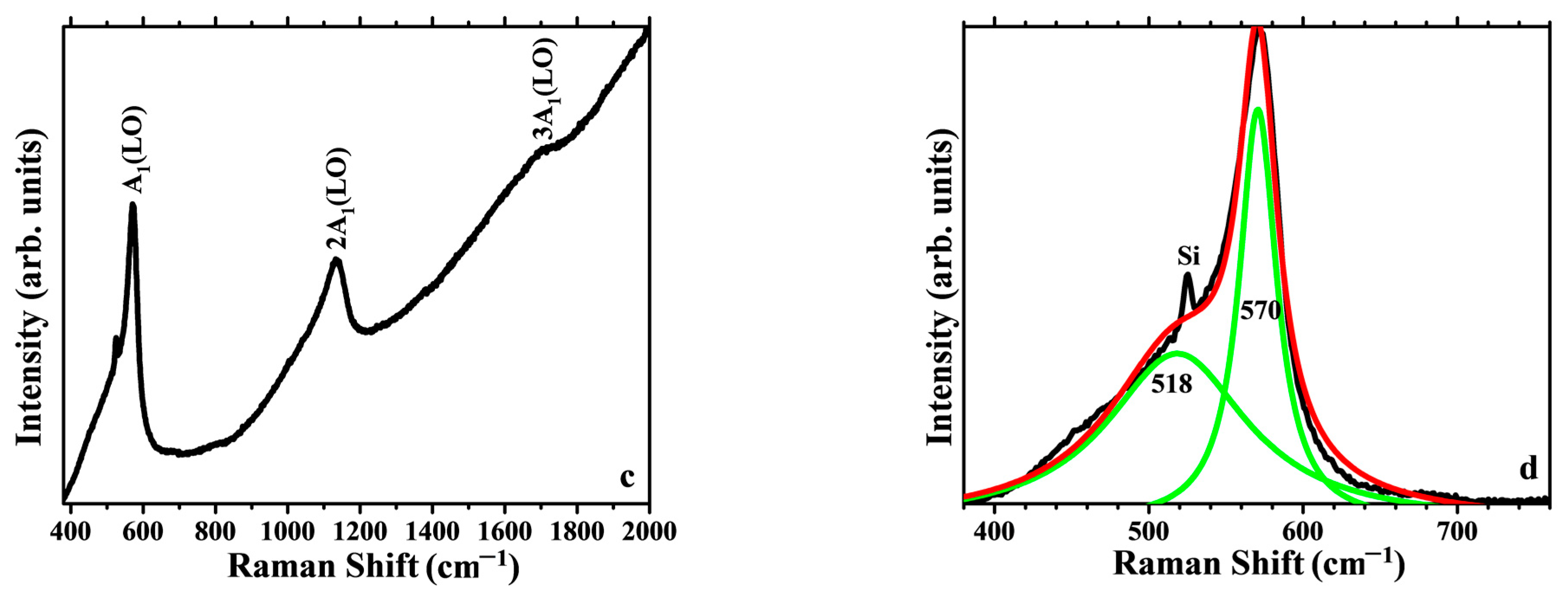
3.3.2. Raman
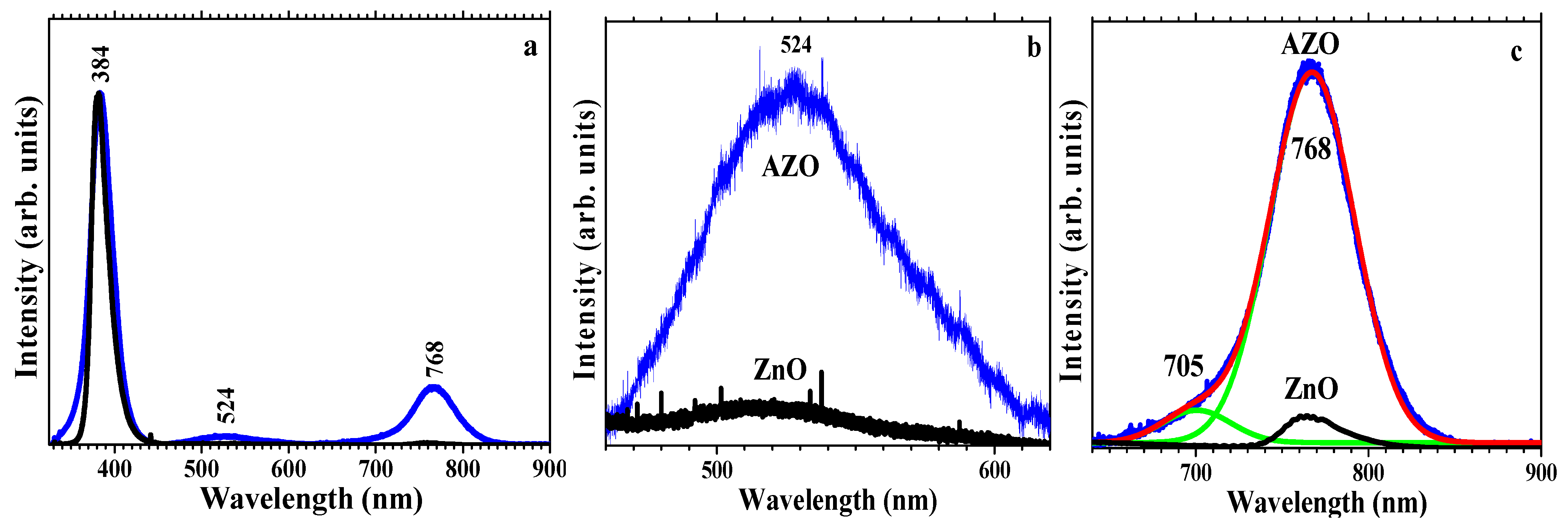
3.3.3. PL Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.; Hasan, M.R.; Mehto, N.K.; Deepak; Bishoyi, A.; Narang, J. 92 years of zinc oxide: Has been studied by the scientific community since the 1930s—An overview. Sens. Int. 2022, 3, 100182. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Malik, G.; Mourya, S.; Jaiswal, J.; Chandra, R. Effect of annealing parameters on optoelectronic properties of highly ordered ZnO thin films. Mater. Sci. Semicond. Process. 2019, 100, 200–213. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Wu, H.; Yang, J.; Wang, Y.; Zhang, J.; Bao, Q.; Wang, M.; Ma, Z.; Tress, W.; et al. A Transparent Electrode Based on Solution-Processed ZnO for Organic Optoelectronic Devices. Nat. Commun. 2022, 13, 4387. [Google Scholar] [CrossRef] [PubMed]
- Polewczyk, V.; Magrin Maffei, R.; Vinai, G.; Lo Cicero, M.; Prato, S.; Capaldo, P.; Dal Zilio, S.; di Bona, A.; Paolicelli, G.; Mescola, A.; et al. ZnO Thin Films Growth Optimization for Piezoelectric Application. Sensors 2021, 21, 6114. [Google Scholar] [CrossRef]
- Ferhati, H.; Djeffal, F.; Benhaya, A.; Martin, N. Highly sensitive, ultra-low dark current, self-powered solar-blind ultraviolet photodetector based on ZnO thin-film with an engineered rear metallic layer. Mater. Sci. Semicond. Process. 2020, 110, 104957. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Paliwal, A.; Sharma, A.; Tomar, M.; Gupta, V. Carbon monoxide (CO) optical gas sensor based on ZnO thin films. Sens. Actuators B Chem. 2017, 250, 679–685. [Google Scholar] [CrossRef]
- Nimbalkar, A.R.; Patil, M.G. Synthesis of ZnO thin film by sol-gel spin coating technique for H2S gas sensing application. Phys. B Condens. Matter 2017, 527, 7–15. [Google Scholar] [CrossRef]
- Boyadjiev, S.I.; Georgieva, V.; Yordanov, R.; Raicheva, Z.; Szilágyi, I.M. Preparation and characterization of ALD deposited ZnO thin films studied for gas sensors. Appl. Surf. Sci. 2016, 387, 1230–1235. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Pereira, S.O.; Zanoni, J.; Rodrigues, C.; Brás, M.; Costa, F.M.; Monteiro, T. ZnO Transducers for Photoluminescence-Based Biosensors: A Review. Chemosensors 2022, 10, 39. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Fedorenko, V.; Smyntyna, V.; Konup, I.; Konup, A.; Eriksson, M.; Yakimova, R.; Ramanavicius, A.; Balme, S.; Bechelany, M. ZnO films formed by atomic layer deposition as an optical biosensor platform for the detection of Grapevine virus A-type proteins. Biosens. Bioelectron. 2017, 92, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shi, X.; Wang, Z.; Hou, Z.; Xu, C.; Duan, L.; Zhao, X.; Wu, H. Defects control and origins of blue and green emissions in sol-gel ZnO thin films. Vacuum 2022, 202, 111201. [Google Scholar] [CrossRef]
- Boudjouan, F.; Chelouche, A.; Touam, T.; Djouadi, D.; Khodja, S.; Tazerout, M.; Ouerdane, Y.; Hadjoub, Z. Effects of stabilizer ratio on photoluminescence properties of sol-gel ZnO nano-structured thin films. J. Lumin. 2015, 158, 32–37. [Google Scholar] [CrossRef]
- Podia, M.; Tripathi, A.K. Structural, optical and luminescence properties of ZnO thin films: Role of hot electrons defining the luminescence mechanisms. J. Lumin. 2022, 252, 119331. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Xiao, L.; Jia, B.; Gao, Y.; Wang, Q. Effect of morphology evolution on the thermoelectric properties of oxidized ZnO thin films. Appl. Surf. Sci. 2018, 436, 354–361. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Liu, X.; Zhang, W.; Zhong, Y.; Zhao, H.; Shi, S.; Jin, S.; Amarasinghe, Y.W.R. Effect of post-annealing on microstructure and piezoelectric properties of ZnO thin film for triangular shaped vibration energy harvester. Surf. Coat. Technol. 2019, 361, 123–129. [Google Scholar] [CrossRef]
- Abu Ali, T.; Pilz, J.; Schäffner, P.; Kratzer, M.; Teichert, C.; Stadlober, B.; Coclite, A.M. Piezoelectric Properties of Zinc Oxide Thin Films Grown by Plasma-Enhanced Atomic Layer Deposition. Phys. Status Solidi 2020, 217, 2000319. [Google Scholar] [CrossRef]
- Bui, Q.C.; Salem, B.; Roussel, H.; Mescot, X.; Guerfi, Y.; Jiménez, C.; Consonni, V.; Ardila, G. Effects of thermal annealing on the structural and electrical properties of ZnO thin films for boosting their piezoelectric response. J. Alloys Compd. 2021, 870, 159512. [Google Scholar] [CrossRef]
- Stoleriu, S.; Lungu, C.; Ghitulica, C.D.; Surdu, A.; Voicu, G.; Cucuruz, A.; Turculet, C.S.; Ciocan, L.T. Influence of Dopant Nature on Biological Properties of ZnO Thin-Film Coatings on Ti Alloy Substrate. Nanomaterials 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, P.; Costa-Barbosa, A.; Costa, D.; Rodrigues, M.S.; Carvalho, P.; Borges, J.; Vaz, F.; Sampaio, P. Antifungal activirty of ZnO thin films prepared by glancing angle deposition. Thin Solid Film 2019, 687, 137461. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Maria Magdalane, C.; Kanimozhi, K.; Kennedy, J.; Siddhardha, B.; Subba Reddy, E.; Rotte, N.K.; Sharma, C.S.; Thema, F.T.; Letsholathebe, D.; et al. Elucidation of photocatalysis, photoluminescence and antibacterial studies of ZnO thin films by spin coating method. J. Photochem. Photobiol. B Biol. 2017, 173, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Zakharchuk, K.V.; Tobaldi, D.M.; Xiao, X.; Xie, W.; Mikhalev, S.M.; Martins, J.F.; Frade, J.R.; Weidenkaff, A.; Kovalevsky, A.V. Synergistic effects of zirconium- and aluminum co-doping on the thermoelectric performance of zinc oxide. J. Eur. Ceram. Soc. 2019, 39, 1222–1229. [Google Scholar] [CrossRef]
- Mia, M.N.H.; Habiba, U.; Pervez, M.F.; Kabir, H.; Nur, S.; Hossen, M.F.; Sen, S.K.; Hossain, M.K.; Iftekhar, M.A.; Rahman, M.M. Investigation of aluminum doping on structural and optical characteristics of sol–gel assisted spin-coated nano-structured zinc oxide thin films. Appl. Phys. A 2020, 126, 162. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Yu, S.-H. Melioration of Electrical and Optical Properties of Al and B Co-Doped ZnO Transparent Semiconductor Thin Films. Coatings 2021, 11, 1259. [Google Scholar] [CrossRef]
- Doni Pon, V.; Joseph Wilson, K.S.; Hariprasad, K.; Ganesh, V.; Elhosiny Ali, H.; Algarni, H.; Yahia, I.S. Enhancement of optoelectronic properties of ZnO thin films by Al doping for photodetector applications. Superlattices Microstruct. 2021, 151, 106790. [Google Scholar] [CrossRef]
- Ng, Z.-N.; Chan, K.-Y.; Low, C.-Y.; Kamaruddin, S.A.; Sahdan, M.Z. Al and Ga doped ZnO films prepared by a sol–gel spin coating technique. Ceram. Int. 2015, 41, S254–S258. [Google Scholar] [CrossRef]
- Deva Arun Kumar, K.; Ganesh, V.; Shkir, M.; AlFaify, S.; Valanarasu, S. Effect of different solvents on the key structural, optical and electronic properties of sol–gel dip coated AZO nanostructured thin films for optoelectronic applications. J. Mater. Sci. Mater. Electron. 2018, 29, 887–897. [Google Scholar] [CrossRef]
- Shahid, M.U.; Deen, K.M.; Ahmad, A.; Akram, M.A.; Aslam, M.; Akhtar, W. Formation of Al-doped ZnO thin films on glass by sol–gel process and characterization. Appl. Nanosci. 2016, 6, 235–241. [Google Scholar] [CrossRef]
- Chelouche, A.; Touam, T.; Necib, K.; Ouarez, L.; Challali, F.; Djouadi, D. Investigation of the effects of drying process on microstructural and luminescence properties of Al-doped ZnO thin films. J. Lumin. 2020, 219, 116891. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, G.; Yang, J.; Mao, W.; Wang, J.; Liu, Z.; Dong, Y.; Yang, S.; Li, J. Fabrication of AZO and FAZO films using low-cost spin-coating method. Opt. Mater. 2022, 126, 112204. [Google Scholar] [CrossRef]
- Dabir, F.; Esfahani, H.; Bakhtiargonbadi, F.; Khodadadi, Z. Study on microstructural and electro-optical properties of sol–gel derived pure and Al/Cu-doped ZnO thin films. J. Sol-Gel Sci. Technol. 2020, 96, 529–538. [Google Scholar] [CrossRef]
- Bouacheria, M.A.; Djelloul, A.; Adnane, M.; Larbah, Y.; Benharrat, L. Characterization of Pure and Al Doped ZnO Thin Films Prepared by Sol Gel Method for Solar Cell Applications. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2737–2747. [Google Scholar] [CrossRef]
- Khan, M.I.; Neha, T.R.; Billah, M.M. UV-irradiated sol-gel spin coated AZO thin films: Enhanced optoelectronic properties. Heliyon 2022, 8, e08743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.D.A.; Valanarasu, S.; Rosario, S.R.; Ganesh, V.; Shkir, M.; Sreelatha, C.J.; AlFaify, S. Evaluation of the structural, optical and electrical properties of AZO thin films prepared by chemical bath deposition for optoelectronics. Solid State Sci. 2018, 78, 58–68. [Google Scholar] [CrossRef]
- Pat, S.; Mohammadigharehbagh, R.; Özen, S.; Șenay, V.; Yudar, H.H.; Korkmaz, Ș. The Al doping effect on the surface, optical, electrical and nanomechanical properties of the ZnO and AZO thin films prepared by RF sputtering technique. Vacuum 2017, 141, 210–215. [Google Scholar] [CrossRef]
- Patel, N.P.; Chauhan, K.V. Effect of sputtering power and substrate temperature on structural, optical, wettability and anti-icing characteristics of aluminium doped zinc oxide. Mater. Res. Express 2022, 9, 076402. [Google Scholar] [CrossRef]
- Swatowska, B.; PowroŸnik, W.; Czternastek, H.; Lewiñska, G.; Stapiñski, T.; Pietruszka, R.; Witkowski, B.S.; Godlewski, M. Application Properties of ZnO and AZO Thin Films Obtained by the ALD Method. Energies 2021, 14, 6271. [Google Scholar] [CrossRef]
- Alyamani, A.; Sayari, A.; Albadri, A.; Albrithen, H.; El Mir, L. Structural, morphological and optical characterizations of ZnO:Al thin films grown on silicon substrates by pulsed laser deposition. Eur. Phys. J. Plus 2016, 131, 328. [Google Scholar] [CrossRef]
- Mahroug, A.; Boudjadar, S.; Hamrit, S.; Guerbous, L. Structural, optical and photocurrent properties of undoped and Al-doped ZnO thin films deposited by sol–gel spin coating technique. Mater. Lett. 2014, 134, 248–251. [Google Scholar] [CrossRef]
- Dimitrov, D.; Tsai, C.-L.; Petrov, S.; Marinova, V.; Petrova, D.; Napoleonov, B.; Blagoev, B.; Strijkova, V.; Hsu, K.Y.; Lin, S.H. Atomic Layer-Deposited Al-Doped ZnO Thin Films for Display Applications. Coatings 2020, 10, 539. [Google Scholar] [CrossRef]
- Dong, J.; Han, D.; Li, H.; Yu, W.; Zhang, S.; Zhang, X.; Wang, Y. Effect of Al doping on performance of ZnO thin film transistors. Appl. Surf. Sci. 2018, 433, 836–839. [Google Scholar] [CrossRef]
- Sharmin, A.; Tabassum, S.; Bashar, M.S.; Mahmood, Z.H. Depositions and characterization of sol–gel processed Al-doped ZnO (AZO) as transparent conducting oxide (TCO) for solar cell application. J. Theor. Appl. Phys. 2019, 13, 123–132. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Ding, S.; Li, Y.; Tian, Y. Preparation of highly transparent conductive aluminum-doped zinc oxide thin films using a low-temperature aqueous solution process for thin-film solar cells applications. Sol. Energy Mater. Sol. Cells 2019, 203, 110161. [Google Scholar] [CrossRef]
- Ali, D.; Muneer, I.; Butt, M.Z. Influence of aluminum precursor nature on the properties of AZO thin films and its potential application as oxygen sensor. Opt. Mater. 2021, 120, 111406. [Google Scholar] [CrossRef]
- Ghosh, J.; Ghosh, R.; Giri, P.K. Tuning the visible photoluminescence in Al doped ZnO thin film and its application in label-free glucose detection. Sens. Actuators B Chem. 2018, 254, 681–689. [Google Scholar] [CrossRef]
- Dubey, K.C.; Zaidi, A.; Awasthi, R.R. Environmentally Benign Structural, Topographic, and Sensing Properties of Pure and Al-Doped ZnO Thin Films. ACS Omega 2022, 7, 28946–28954. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Hsu, W.-T. Comparative Studies on Ultraviolet-Light-Derived Photoresponse Properties of ZnO, AZO, and GZO Transparent Semiconductor Thin Films. Materials 2017, 10, 1379. [Google Scholar] [CrossRef]
- Akin, N.; Ceren Baskose, U.; Kinaci, B.; Cakmak, M.; Ozcelik, S. AZO thin film-based UV sensors: Effects of RF power on the films. Appl. Phys. A 2015, 119, 965–970. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Li, M.; Yu, S.; Zheng, R. Optical and electrical properties of Al doped ZnO thin film with preferred orientation in situ grown at room temperature. Ceram. Int. 2019, 45, 14347–14353. [Google Scholar] [CrossRef]
- Kumar, K.D.A.; Valanarasu, S.; Kathalingam, A.; Ganesh, V.; Shkir, M.; AlFaify, S. Effect of solvents on sol–gel spin-coated nanostructured Al-doped ZnO thin films: A film for key optoelectronic applications. Appl. Phys. A 2017, 123, 801. [Google Scholar] [CrossRef]
- Sandeep, K.M.; Bhat, S.; Dharmaprakash, S.M. Structural, optical, and LED characteristics of ZnO and Al doped ZnO thin films. J. Phys. Chem. Solids 2017, 104, 36–44. [Google Scholar] [CrossRef]
- Srinatha, N.; Raghu, P.; Mahesh, H.M.; Angadi, B. Spin-coated Al-doped ZnO thin films for optical applications: Structural, micro-structural, optical and luminescence studies. J. Alloys Compd. 2017, 722, 888–895. [Google Scholar] [CrossRef]
- Lee, W.; Leem, J.-Y. Enhancement of the Ultraviolet Photoresponsivity of Al-doped ZnO Thin Films Prepared by using the Sol-gel Spin-coating Method. J. Korean Phys. Soc. 2018, 72, 610–614. [Google Scholar] [CrossRef]
- Musavi, E.; Khanlary, M.; Khakpour, Z. Red-orange photoluminescence emission of sol-gel dip-coated prepared ZnO and ZnO:Al nano-crystalline films. J. Lumin. 2019, 216, 116696. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.Y. Preparation and photoluminescence studies of high-quality AZO thin films grown on Zno buffered Si substrate. Mater. Lett. 2016, 162, 75–78. [Google Scholar] [CrossRef]
- Romanyuk, V.; Dmitruk, N.; Karpyna, V.; Lashkarev, G.; Popovych, V.; Dranchuk, M.; Pietruszka, R.; Godlewski, M.; Dovbeshko, G.; Timofeeva, I.; et al. Optical and Electrical Properties of Highly Doped ZnO:Al Films Deposited by Atomic Layer Deposition on Si Substrates in Visible and Near Infrared Region. Acta Phys. Pol. A 2016, 129, A-36–A-40. [Google Scholar] [CrossRef]
- Wen, L.; Sahu, B.B.; Kim, H.R.; Han, J.G. Study on the electrical, optical, structural, and morphological properties of highly transparent and conductive AZO thin films prepared near room temperature. Appl. Surf. Sci. 2019, 473, 649–656. [Google Scholar] [CrossRef]
- Patel, N.P.; Chauhan, K.V. Structural, optical and electrical study of ZnO:Al thin films: A review. Mater. Today Proc. 2022, 62, 3386–3396. [Google Scholar] [CrossRef]
- Alrefaee, M.; Singh, U.P.; Das, S.K. Growth of Aluminum Doped Zinc Oxide Nanostructure Thin Films by Nonconventional Sol-Gel Method. Macromol. Symp. 2022, 402, 2100350. [Google Scholar] [CrossRef]
- Speaks, D.T. Effect of concentration, aging, and annealing on sol gel ZnO and Al-doped ZnO thin films. Int. J. Mech. Mater. Eng. 2020, 15, 2. [Google Scholar] [CrossRef]
- Mohamedi, M.; Challali, F.; Touam, T.; Mendil, D.; Ouhenia, S.; Souici, A.H.; Djouadi, D.; Chelouche, A. Role of substrate and annealing on microstructural, optoelectronic and luminescence properties of RF magnetron sputtered AZO thin films in confocal configuration. J. Lumin. 2022, 244, 118739. [Google Scholar] [CrossRef]
- Nazari, H.H.; Dejam, L. Investigation of post-annealing effect on Al:ZnO thin films crystallinity and photoluminescence properties. Phys. B Condens. Matter 2022, 626, 413461. [Google Scholar] [CrossRef]
- Singh, R.; Mukherjee, S.K. Correlation of structural, electrical and optical properties of Al-doped ZnO TCOs. J. Mater. Sci. Mater. Electron. 2022, 33, 6969–6980. [Google Scholar] [CrossRef]
- Necib, K.; Touam, T.; Chelouche, A.; Ouarez, L.; Djouadi, D.; Boudine, B. Investigation of the effects of thickness on physical properties of AZO sol-gel films for photonic device applications. J. Alloys Compd. 2018, 735, 2236–2246. [Google Scholar] [CrossRef]
- Blagoev, B.S.; Dimitrov, D.Z.; Mehandzhiev, V.B.; Kovacheva, D.; Terziyska, P.; Pavlic, J.; Lovchinov, K.; Mateev, E.; Leclercq, J.; Sveshtarov, P. Electron transport in Al-doped ZnO nanolayers obtained by atomic layer deposition. J. Phys. Conf. Ser. 2016, 700, 012040. [Google Scholar] [CrossRef]
- Tompkins, H.G. WVASE32® Software Training Manual; JA Woollam Co., Inc.: Lincoln, NE, USA, 2006. [Google Scholar]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Yanagi, H. Enhanced Charge Transport in Al-Doped ZnO Nanotubes Designed via Simultaneous Etching and Al Doping of H2O-Oxidized ZnO Nanorods for Solar Cell Applications. J. Mater. Chem. C 2019, 7, 4653–4661. [Google Scholar] [CrossRef]
- Teke, A.; Özgür, Ü.; Doğan, S.; Gu, X.; Morkoç, H.; Nemeth, B.; Nause, J.; Everitt, H.O. Excitonic fine structure and recombination dynamics in single-crystalline ZnO. Phys. Rev. B 2004, 70, 195207. [Google Scholar] [CrossRef]
- Zeng, H.; Duan, G.; Li, Y.; Yang, S.; Xu, X.; Cai, W. Blue Luminescence of ZnO Nanoparticles Based on Non-Equilibrium Processes: Defect Origins and Emission Controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- ElZein, B.; Abulikemu, M.; Barham, A.S.; Al-Kilani, A.; Alkhatab, M.I.; Hamdan, S.M.; Dogheche, E.; Jabbour, G.E. In Situ Growth of PbS Nanoparticles without Organic Linker on ZnO Nanostructures via Successive Ionic Layer Adsorption and Reaction (SILAR). Coatings 2022, 12, 1486. [Google Scholar] [CrossRef]
- Mihaiu, S.; Gartner, M.; Voicescu, M.; Gabor, M.; Mocioiu, O.C.; Zaharescu, M. Characterization of the ZnO thin films obtained by chemical route. Optoelectron. Adv. Mater. Rapid Commun. 2009, 3, 884–890. [Google Scholar]
- Lin, B.; Fu, Z.; Jia, Y. Green luminescent center in undoped zinc oxide films deposited on silicon substrates. Appl. Phys. Lett. 2001, 79, 943–945. [Google Scholar] [CrossRef]
- Zaiour, A.; Benhaya, A.; Bentrcia, T. Impact of Deposition Methods and Doping on Structural, Optical and Electrical Properties of ZnO-Al Thin Films. Optik 2019, 186, 293–299. [Google Scholar] [CrossRef]
- Al Farsi, B.; Souier, T.M.; Al Marzouqi, F.; Al Maashani, M.; Bououdina, M.; Widatallah, H.M.; Al Abri, M. Structural and Optical Properties of Visible Active Photocatalytic Al Doped ZnO Nanostructured Thin Films Prepared by Dip Coating. Opt. Mater. 2021, 113, 110868. [Google Scholar] [CrossRef]
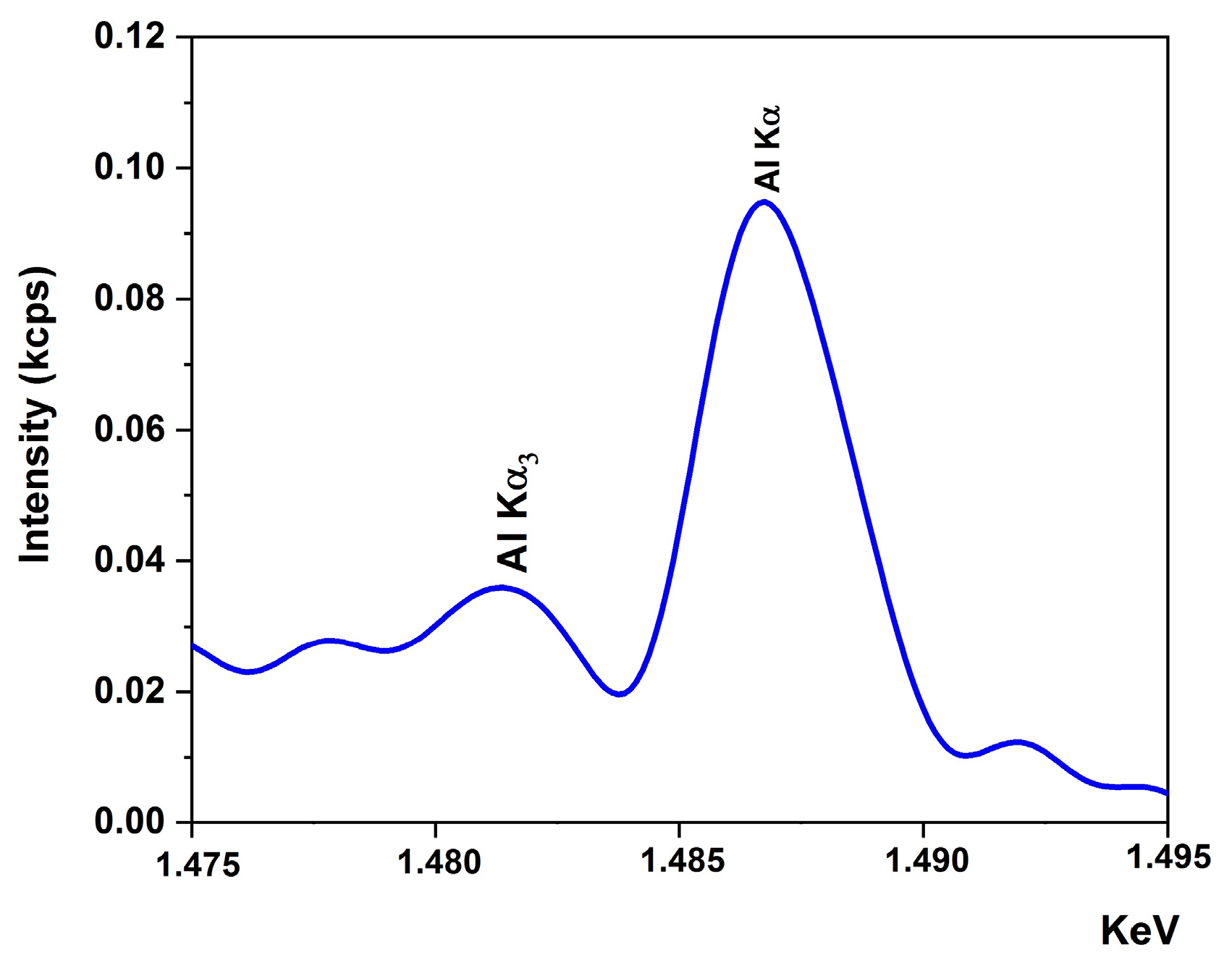
| Element | Mass % | at. % | Det. Limit | El Line |
|---|---|---|---|---|
| O | 22.91 | 54.63 | 1.69951 | O-KA |
| Al | 0.46 | 0.65 | 0.11966 | Al-KA |
| Zn | 76.63 | 44.72 | 1.57686 | Zn-KA |
| Sample | 2θ (100) (°) | FWHM (°) | 2θ (002) (°) | FWHM (°) | 2θ (101) (°) | FWHM (°) | Lattice Parameters (Å) | Average Crystallite Size (Å) | |
|---|---|---|---|---|---|---|---|---|---|
| a = b | c | ||||||||
| ZnO film | 31.80 | 0.730 | 34.45 | 0.711 | 36.33 | 0.842 | 3.253 | 5.211 | 115 |
| AZO film | 31.77 | 0.488 | 34.58 | 0.329 | 36.43 | 0.443 | 3.241 | 5.194 | 212 |
| ZnO 36-1451 | 31.77 | - | 34.42 | - | 36.25 | - | 3.250 | 5.207 | - |
| Dfilm (nm) | drough (nm) | n (λ = 630 nm) | MSE | Eg (eV) | |
|---|---|---|---|---|---|
| AZO | 332.1 | 12.4 | 1.89 | 8.2 | 3.45 |
| ZnO | 174.6 | 5.4 | 1.78 | 10.4 | 3.42 |
| Substrate | Dopant Conc. | Emission Features Depending on Various Parameters | Ref. |
|---|---|---|---|
| Glass | 3 mol % | UV emission (NBE): 387 nm; visible emission: 415 (blue) and 483 nm (blue green); effect of solvent on PL intensity: the best results were obtained for isopropanol. | [52] |
| Glass | 2 at. % | UV emission (NBE): 386 nm; visible emission: 413, 436 (blue) and 481 nm (blue green); effect of solvent on PL intensity: the best results were obtained for methanol. | [29] |
| Glass | 3 wt % | UV emission (NBE): 383 nm; visible emission: 420 nm (violet), 435, 467 nm (blue); Al doping: increase in luminous efficiency (AZO: 22.8%; ZnO: 10.8%) as a result of blue emission enhancement. | [53] |
| Glass | 0–3 mol % | UV emission (NBE): 362; near UV emission: 408 nm (NBE); visible emission: 450, 483 nm (blue emission); 530 nm (green emission); 1–2 mol% Al doping: decrease in NBE peak intensity; 3 mol% Al doping: increase in PL intensity. | [54] |
| Glass | 3 at. % | UV emission band: 396 nm (blue); visible emission: 418 (violet), 440, 450, 467 nm (blue), 480, 493 (blue green), 547 nm (green), 589 nm (yellow); PL intensity decreases with the increase in thickness of the film as follows: 6 > 9 > 12 layers. | [31] |
| Glass | 0–5 wt % | NBE: 370 nm; visible emission: 413 nm (violet), 487 nm (blue), 531 nm (green), 630 nm (orange-red); PL intensity increases as follows: 5 < 1 < 3 wt% Al. This orange-red emission appears because of Al doping which leads to a large number of defects in ZnO film. | [56] |
| Si | 0–3 vol % | UV emission: 365 nm; visible emission: 530 nm; the PL intensity of visible peak decreases with the increase in Al concentration (vol%) as follows: 3 < 2 < 1 (vol%). The corresponding visible emission band was red-shifted from 500 nm to 530 nm upon Al introduction. | [55] |
| Si | 0.46 at. % | UV emission (NBE): 384 nm; visible emission: 524 nm (green); 768 nm (red) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroescu, H.; Nicolescu, M.; Mitrea, D.; Tenea, E.; Atkinson, I.; Anastasescu, M.; Calderon-Moreno, J.M.; Gartner, M. Effect of Al Incorporation on the Structural and Optical Properties of Sol–Gel AZO Thin Films. Materials 2023, 16, 3329. https://doi.org/10.3390/ma16093329
Stroescu H, Nicolescu M, Mitrea D, Tenea E, Atkinson I, Anastasescu M, Calderon-Moreno JM, Gartner M. Effect of Al Incorporation on the Structural and Optical Properties of Sol–Gel AZO Thin Films. Materials. 2023; 16(9):3329. https://doi.org/10.3390/ma16093329
Chicago/Turabian StyleStroescu, Hermine, Madalina Nicolescu, Daiana Mitrea, Ecaterina Tenea, Irina Atkinson, Mihai Anastasescu, Jose Maria Calderon-Moreno, and Mariuca Gartner. 2023. "Effect of Al Incorporation on the Structural and Optical Properties of Sol–Gel AZO Thin Films" Materials 16, no. 9: 3329. https://doi.org/10.3390/ma16093329
APA StyleStroescu, H., Nicolescu, M., Mitrea, D., Tenea, E., Atkinson, I., Anastasescu, M., Calderon-Moreno, J. M., & Gartner, M. (2023). Effect of Al Incorporation on the Structural and Optical Properties of Sol–Gel AZO Thin Films. Materials, 16(9), 3329. https://doi.org/10.3390/ma16093329









