Forced Gradient Copolymer for Rational Design of Mussel-Inspired Adhesives and Dispersants
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Materials
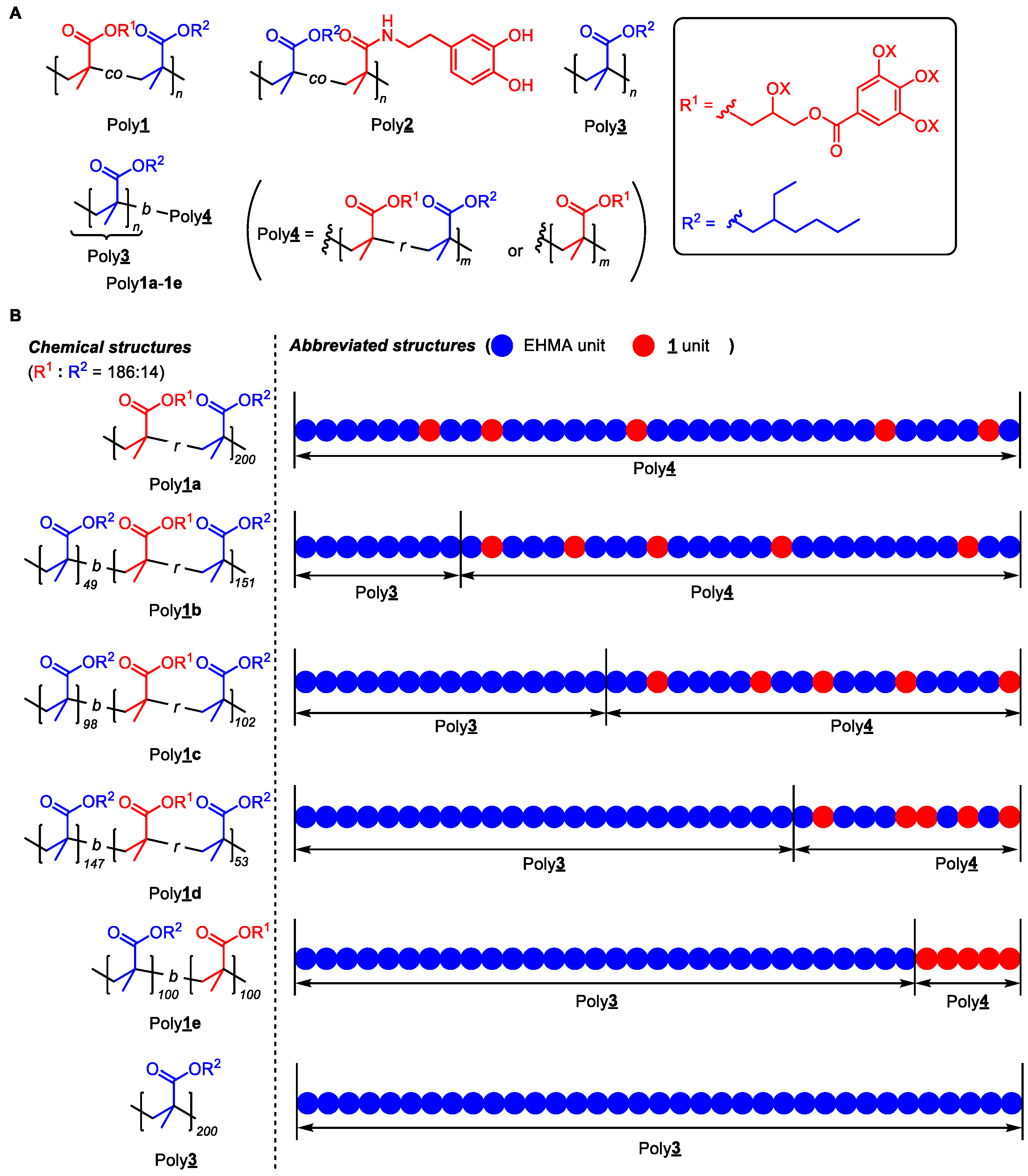
2.3. Preparation of the Mussel-Inspired Forced Gradient Copolymers
2.4. Adhesion Test
2.5. Dispersion Test
2.5.1. Dispersion Test Evaluated by the Naked Eye
2.5.2. Dispersion Test Evaluated Using a Rheometer
3. Results
3.1. Effect of the Monomer Position on the Adhesiveness
3.2. Effect of the Monomer Position on the Dispersity
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazarus, B.S.; Velasco-Hogan, A.; Río, T.G.-D.; Meyers, M.A.; Jasiuk, I. A review of impact resistant biological and bioinspired materials and structures. J. Mater. Res. Technol. 2020, 9, 15705–15738. [Google Scholar] [CrossRef]
- Wang, Y.; Naleway, S.E.; Wang, B. Biological and bioinspired materials: Structure leading to functional and mechanical performance. Bioact. Mater. 2020, 5, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Reis, R.L.; Silva, T.H. Marine invertebrates are a source of bioadhesives with biomimetic interest. Mater. Sci. Eng. C 2019, 108, 110467. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Balkenende, D.W.; Winkler, S.; Messersmith, P.B. Marine-inspired polymers in medical adhesion. Eur. Polym. J. 2019, 116, 134–143. [Google Scholar] [CrossRef]
- Yu, M.; Deming, T.J. Synthetic Polypeptide Mimics of Marine Adhesives. Macromolecules 1998, 31, 4739–4745. [Google Scholar] [CrossRef]
- Saxer, S.; Portmann, C.P.; Tosatti, S.; Gademann, K.; Züreher, S.; Textor, M. Surface Assembly of Catechol-Functionalized Poly(L-lysine)-graftpoly(ethylene glycol) Copolymer on Titanium Exploiting Combined Electrostatically Driven Self-Organization and Biomimetic Strong Adhesion. Macromolecules 2010, 43, 1050–1060. [Google Scholar] [CrossRef]
- Murphy, J.L.; Vollenweider, L.; Xu, F.; Lee, B.P. Adhesive Performance of Biomimetic Adhesive-Coated Biologic Scaffolds. Biomacromolecules 2010, 11, 2976–2984. [Google Scholar] [CrossRef]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A Water-Borne Adhesive Modeled after the Sandcastle Glue of P. californica. Macromol. Biosci. 2009, 9, 464–471. [Google Scholar] [CrossRef]
- Glass, P.; Chung, H.; Washburn, N.R.; Sittti, M. Enhanced Reversible Adhesion of Dopamine Methacrylamide-Coated Elas-tomer Microfibrillar Structures under Wet Conditions. Langmuir 2009, 25, 6607–6612. [Google Scholar] [CrossRef]
- Stepuk, A.; Halter, J.G.; Schaetz, A.; Grass, R.N.; Stark, W.J. Mussel-inspired load bearing metal–polymer glues. Chem. Commun. 2012, 48, 6238–6240. [Google Scholar] [CrossRef] [PubMed]
- Payra, D.; Naito, M.; Fujii, Y.; Yamada, N.L.; Hiromoto, S.; Singh, A. Bioinspired adhesive polymer coatings for efficient and versatile corrosion resistance. RSC Adv. 2015, 5, 15977–15984. [Google Scholar] [CrossRef]
- Lee, S.-B.; González-Cabezas, C.; Kim, K.-M.; Kim, K.-N.; Kuroda, K. Catechol-Functionalized Synthetic Polymer as a Dental Adhesive to Contaminated Dentin Surface for a Composite Restoration. Biomacromolecules 2015, 16, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Payra, D.; Fujii, Y.; Das, S.; Takaishi, J.; Naito, M. Rational design of a biomimetic glue with tunable strength and ductility. Polym. Chem. 2017, 8, 1654–1663. [Google Scholar] [CrossRef]
- Westwood, G.; Horton, T.N.; Wilker, J.J. Simplified Polymer Mimics of Cross-Linking Adhesive Proteins. Macromolecules 2007, 40, 3960–3964. [Google Scholar] [CrossRef]
- White, J.D.; Wilker, J.J. Underwater Bonding with Charged Polymer Mimics of Marine Mussel Adhesive Proteins. Macromolecules 2011, 44, 5085–5088. [Google Scholar] [CrossRef]
- Matos-Pérez, C.R.; Wilker, J.J. Ambivalent Adhesives: Combining Biomimetic Cross-Linking with Antiadhesive Oligo(ethylene glycol). Macromolecules 2012, 45, 6634–6639. [Google Scholar] [CrossRef]
- Matos-Pérez, C.R.; White, J.D.; Wilker, J.J. Polymer Composition and Substrate Influences on the Adhesive Bonding of a Biomimetic, Cross-Linking Polymer. J. Am. Chem. Soc. 2012, 134, 9498–9505. [Google Scholar] [CrossRef]
- Grewal, M.S.; Yabu, H. Biomimetic catechol-based adhesive polymers for dispersion of polytetrafluoroethylene (PTFE) na-noparticles in an aqueous medium. RSC Adv. 2020, 10, 4058–4063. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Meredith, H.J.; Wilker, J.J. Molecular Weight Effects upon the Adhesive Bonding of a Mussel Mimetic Polymer. ACS Appl. Mater. Interfaces 2013, 5, 5091–5096. [Google Scholar] [CrossRef]
- Kohri, M.; Yamazaki, S.; Irie, S.; Teramoto, N.; Taniguchi, T.; Kishikawa, K. Adhesion Control of Branched Catecholic Polymers by Acid Stimulation. ACS Omega 2018, 3, 16626–16632. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Kim, C.; Sung, K.; Ejima, H.; Yoshie, N. Tunicate-Inspired Gallol Polymers for Underwater Adhesive: A Comparative Study of Catechol and Gallol. Biomacromolecules 2017, 18, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Cheng, B.; Ejima, H. Effect of molecular weight and polymer composition on gallol-functionalized underwater adhesive. J. Mater. Chem. B 2020, 8, 6798–6801. [Google Scholar] [CrossRef]
- Hino, Y.; Ejima, H. Tissue Adhesive Properties of Functionalized Chitosan: A Comparative Study of Phenol, Catechol and Gallol. J. Photopolym. Sci. Technol. 2020, 33, 123–127. [Google Scholar] [CrossRef]
- Cheng, B.; Yu, J.; Arisawa, T.; Hayashi, K.; Richardson, J.J.; Shibuta, Y.; Ejima, H. Ultrastrong underwater adhesion on diverse substrates using non-canonical phenolic groups. Nat. Commun. 2022, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, C.; Gao, L.; Li, S.; Zhang, Y.; Zhang, J.; Cao, Y. Hidden complexity of synergistic roles of Dopa and lysine for strong wet adhesion. Mater. Chem. Front. 2017, 1, 2664–2668. [Google Scholar] [CrossRef]
- Lu, Q.; Danner, E.; Waite, J.H.; Israelachvili, J.N.; Zeng, H.; Hwang, D.S. Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface 2013, 10, 20120759. [Google Scholar] [CrossRef]
- Beginn, U. Gradient copolymers. Colloid Polym. Sci. 2008, 286, 1465–1474. [Google Scholar] [CrossRef]
- Jellema, E.; Jongerius, A.L.; van Ekenstein, G.A.; Mookhoek, S.D.; Dingemans, T.J.; Reingruber, E.M.; Chojnacka, A.; Schoenmakers, P.J.; Sprenkels, R.; van Eck, E.R.H.; et al. Rhodium-Mediated Stereospecific Carbene Polymerization: From Homopolymers to Random and Block Copolymers. Macromolecules 2010, 43, 8892–8903. [Google Scholar] [CrossRef]
- Chu, L.W.; Prakash, K.N.; Tsai, M.-T.; Lin, I.-N. Dispersion of nano-sized BaTiO3 powders in nonaqueous suspension with phosphate ester and their applications for MLCC. J. Eur. Ceram. Soc. 2008, 28, 1205–1212. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Huang, C.-W.; Wu, M.; Wei, W.-C.J.; Hsueh, C.-H. Advanced characterization of mechanical properties of mul-tilayer ceramic capacitors. J. Mater. Sci.: Mater. Electron. 2014, 25, 627–634. [Google Scholar]
- Farrokhpay, S. A review of polymeric dispersant stabilization of titania pigment. Adv. Colloid Interface Sci. 2009, 151, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Farrokhpay, S.; Morris, G.E.; Fornasiero, D.; Self, P. Effects of chemical functional groups on the polymer adsorption behavior onto titania pigment particles. J. Colloid Interface Sci. 2004, 274, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, M.; Rout, T.; Sengupta, D. Electrokinetics of TiO2 in the interpretation of its dispersion characteristics. Surf. Coatings Int. 2000, 83, 592–596. [Google Scholar] [CrossRef]
- Chen, H.T.; Ravishankar, S.A.; Farinato, R.S. Rational polymer design for solid-liquid separations in mineral processing ap-plication. Int. J. Miner. Process 2003, 72, 75–86. [Google Scholar] [CrossRef]
- Creutz, S.; Jerome, R.; Kaptijn, G.M.P.; Werf, A.W.; Akkerman, J.M. Design of polymeric dispersants for waterborne coatings. J. Coatings Technol. 1998, 70, 41–46. [Google Scholar] [CrossRef]
- Amstad, E.; Gillich, T.; Bilecka, I.; Textor, M.; Reimhult, E. Ultastable Iron Oxide Nanoparticle Colloidal Suspensions Using Dispersants with Catechol-Derived Anchor Groups. Nano Lett. 2009, 9, 4042–4048. [Google Scholar] [CrossRef]
- Ou, X.; Xue, B.; Lao, Y.; Wutthinitikornkit, Y.; Tian, R.; Zou, A.; Yang, L.; Wang, W.; Cao, Y.; Li, J. Structure and sequence features of mussel adhesive protein lead to its salt-tolerant adhesion ability. Sci. Adv. 2020, 6, eabb7620. [Google Scholar] [CrossRef]





| Polymer | EHMA:1a | Mn (×104) b, c | Đ b, c |
|---|---|---|---|
| Poly1a | 93.5:6.5 | 5.1 | 1.1 |
| Poly1b | 94.4:5.6 | 4.3 | 1.2 |
| Poly1c | 93.8:6.2 | 4.3 | 1.1 |
| Poly1d | 93.7:6.3 | 5 | 1.1 |
| Poly1e | 95.6:4.4 | 4.1 | 1.1 |
| Poly3 | 100:0 | 3.1 | 1.2 |
| Polymer | Dp of Poly3 | 1 in Poly4 (mol%) | Adhesive Strength (MPa) |
|---|---|---|---|
| Poly1a | 0 | 7 | 7.9 |
| Poly1b | 49 | 9 | 9.8 |
| Poly1c | 98 | 14 | 8.6 |
| Poly1d | 147 | 26 | 4.1 |
| Poly1e | 186 | 100 | 2.9 |
| Poly3 | 200 | 0 | 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, T.; Shuta, M.; Mano, M.; Matsumoto, S.; Nagasawa, A.; Yamada, A.; Naito, M. Forced Gradient Copolymer for Rational Design of Mussel-Inspired Adhesives and Dispersants. Materials 2023, 16, 266. https://doi.org/10.3390/ma16010266
Fujita T, Shuta M, Mano M, Matsumoto S, Nagasawa A, Yamada A, Naito M. Forced Gradient Copolymer for Rational Design of Mussel-Inspired Adhesives and Dispersants. Materials. 2023; 16(1):266. https://doi.org/10.3390/ma16010266
Chicago/Turabian StyleFujita, Takehiro, Masami Shuta, Mika Mano, Shinnosuke Matsumoto, Atsushi Nagasawa, Akihiro Yamada, and Masanobu Naito. 2023. "Forced Gradient Copolymer for Rational Design of Mussel-Inspired Adhesives and Dispersants" Materials 16, no. 1: 266. https://doi.org/10.3390/ma16010266
APA StyleFujita, T., Shuta, M., Mano, M., Matsumoto, S., Nagasawa, A., Yamada, A., & Naito, M. (2023). Forced Gradient Copolymer for Rational Design of Mussel-Inspired Adhesives and Dispersants. Materials, 16(1), 266. https://doi.org/10.3390/ma16010266






