Lead-Free MDABCO-NH4I3 Perovskite Crystals Embedded in Electrospun Nanofibers
Abstract
1. Introduction
2. Experimental Section
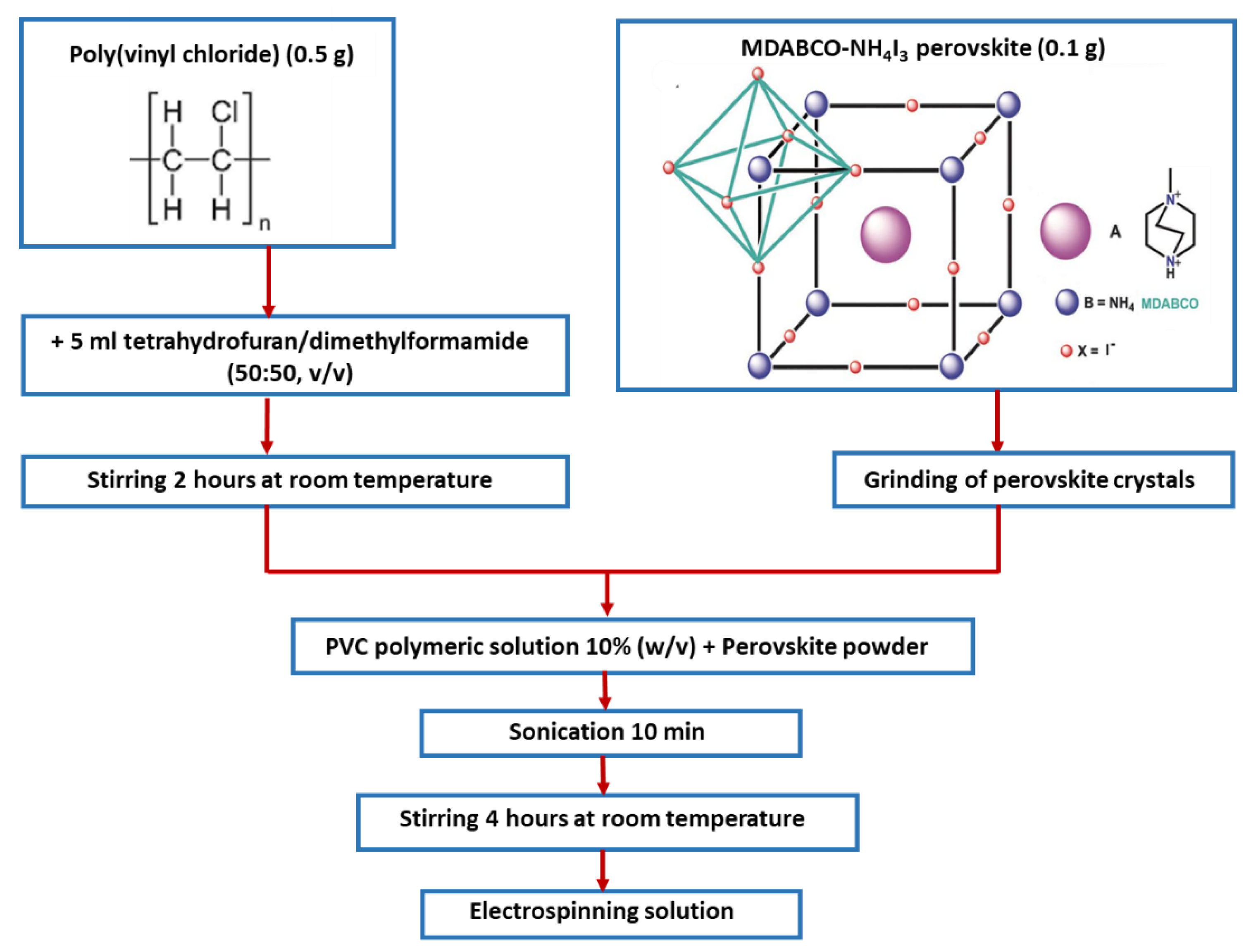
2.1. Materials and Nanofibers Preparation
2.2. Scanning Electron Microscopy (SEM)
2.3. X-ray Diffraction and Raman Spectroscopy
2.4. Mechanical Tests
2.5. Optical Absorption and Photoluminescence
2.6. Dielectric Spectroscopy
2.7. Fabrication of an MDABCO-NH4I3@PVC Piezoelectric Nanogenerator
3. Results and Discussion
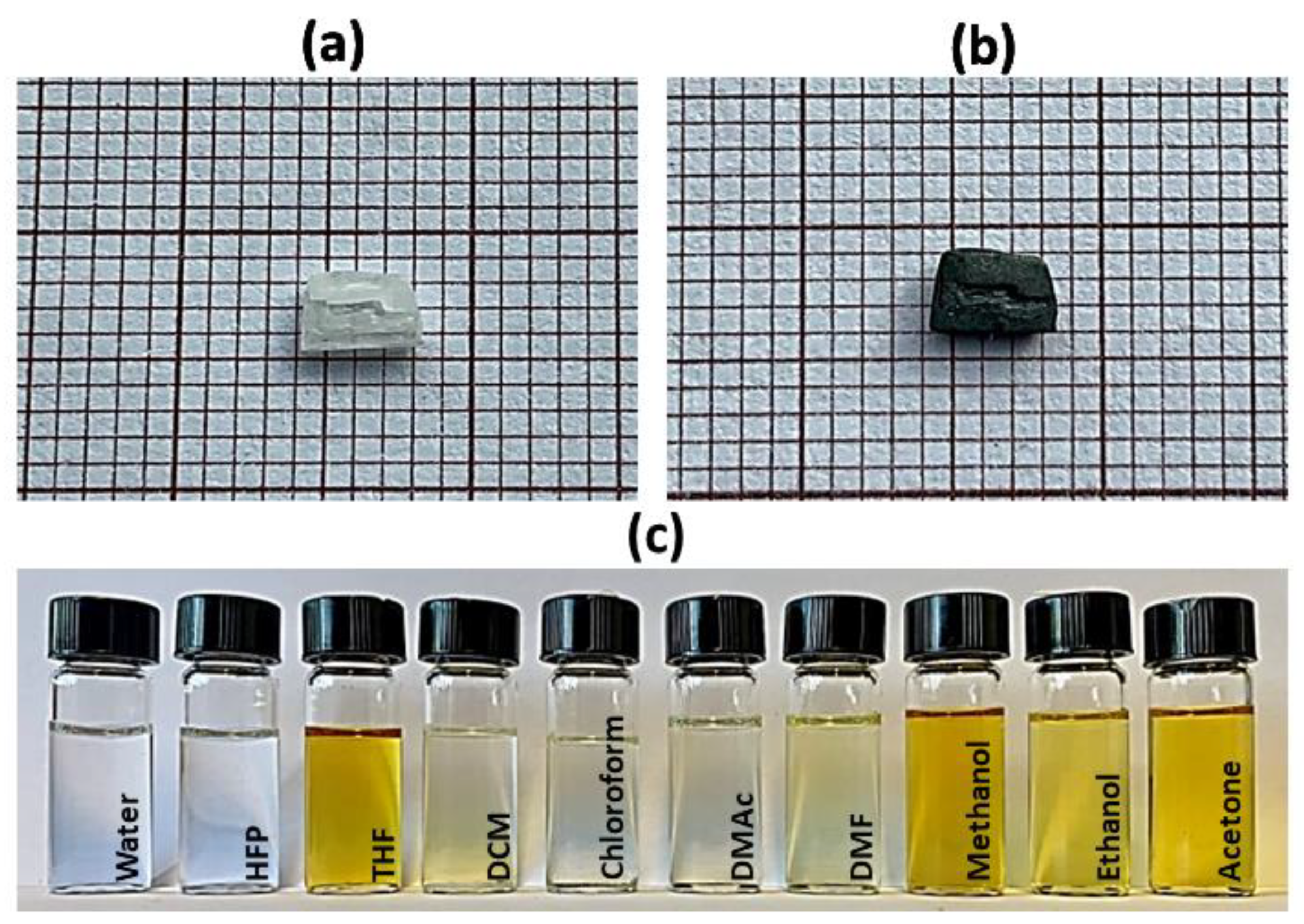
3.1. Electrospun Fibers
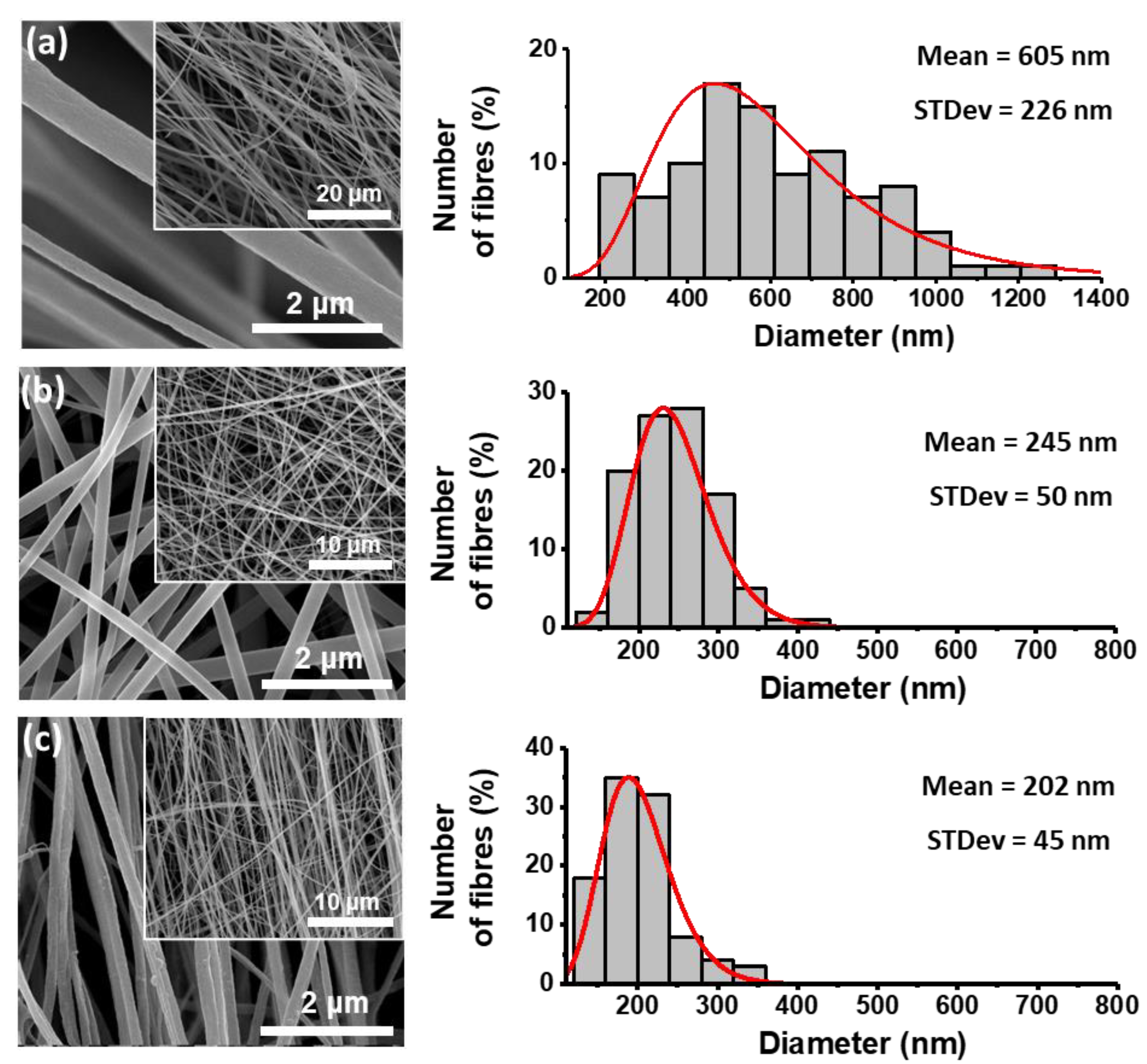
3.2. Fibers Morphology and Crystallinity
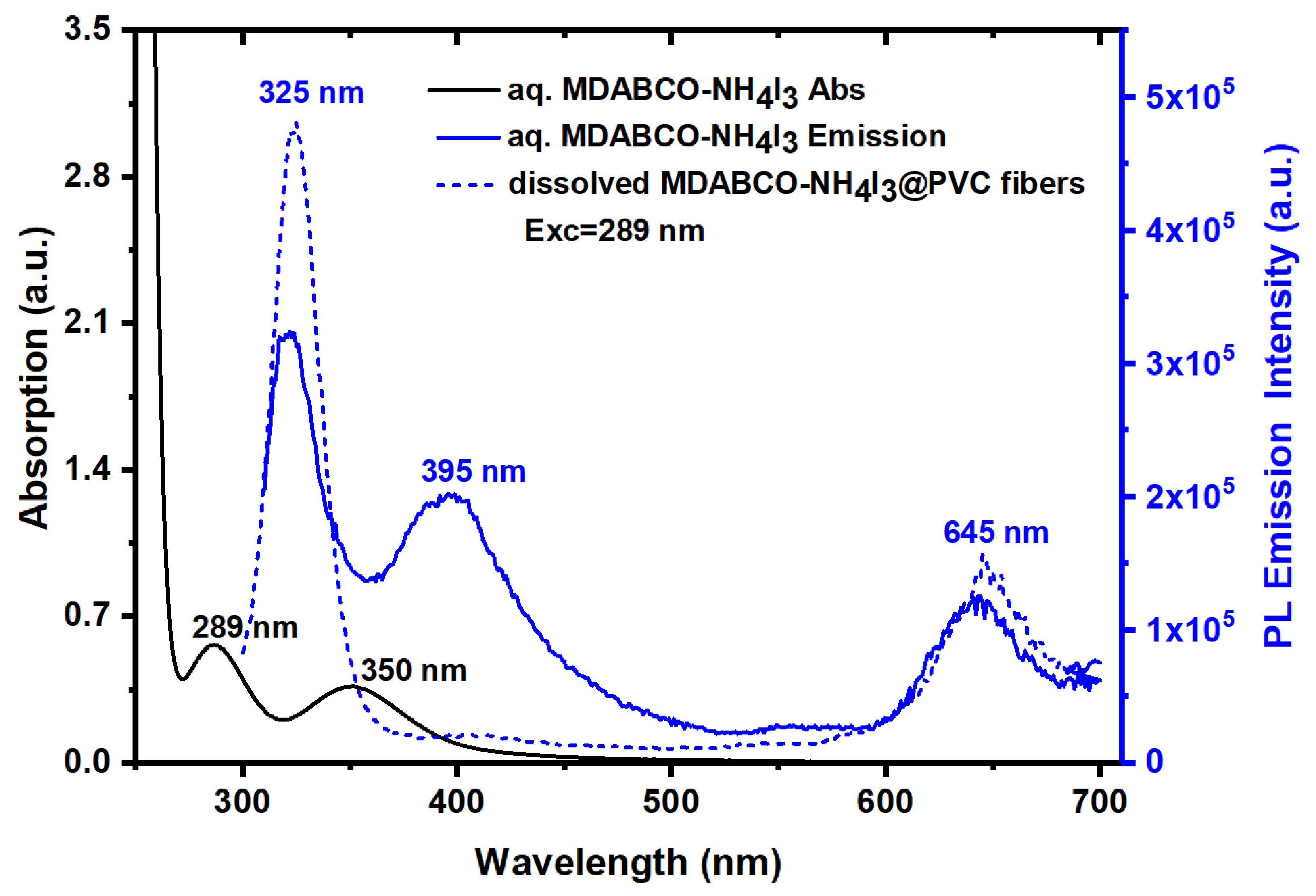
3.3. Optical Absorption and Luminescence
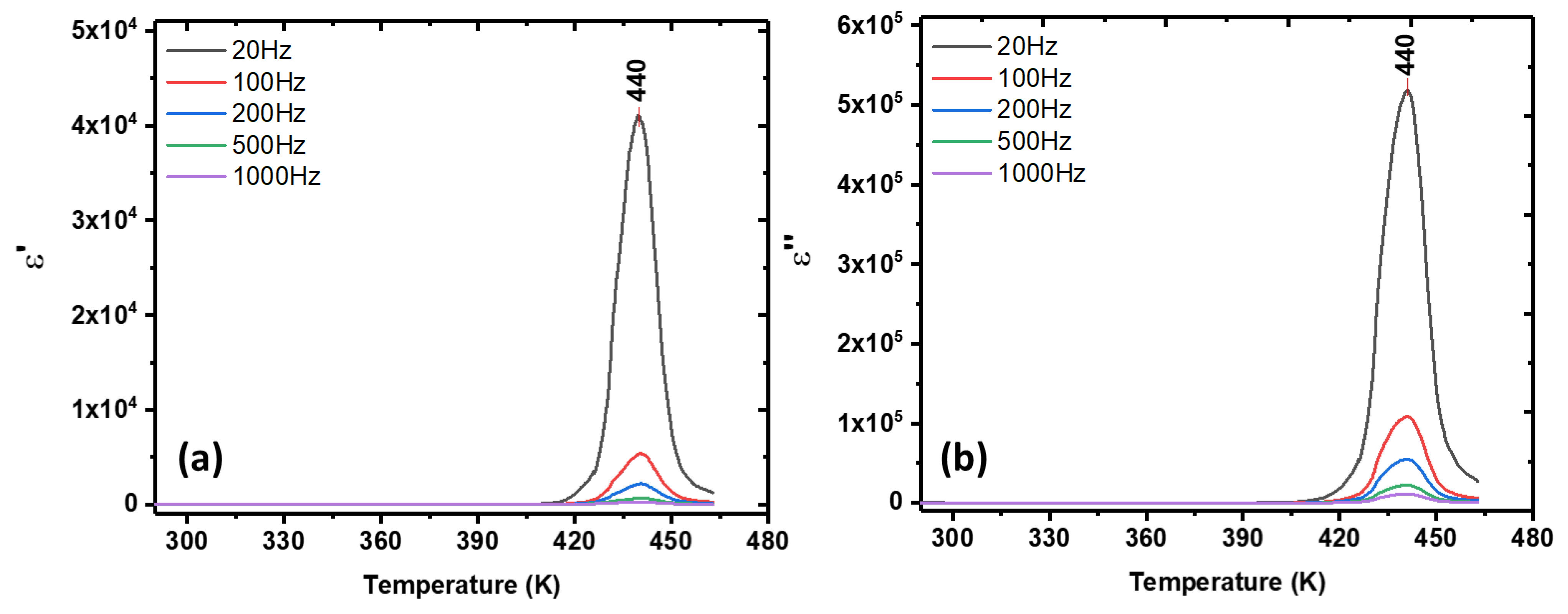
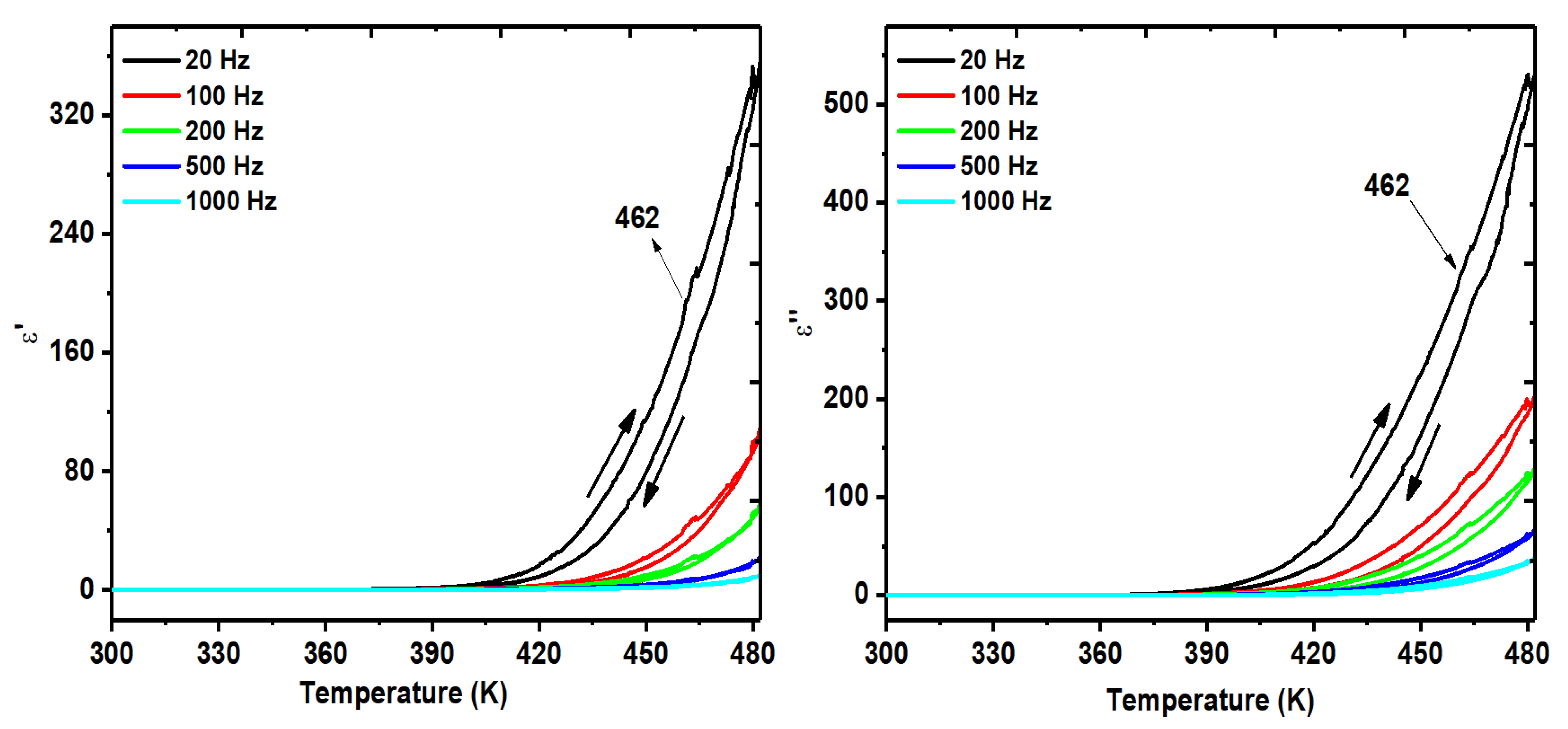
3.4. Dielectric Measurements
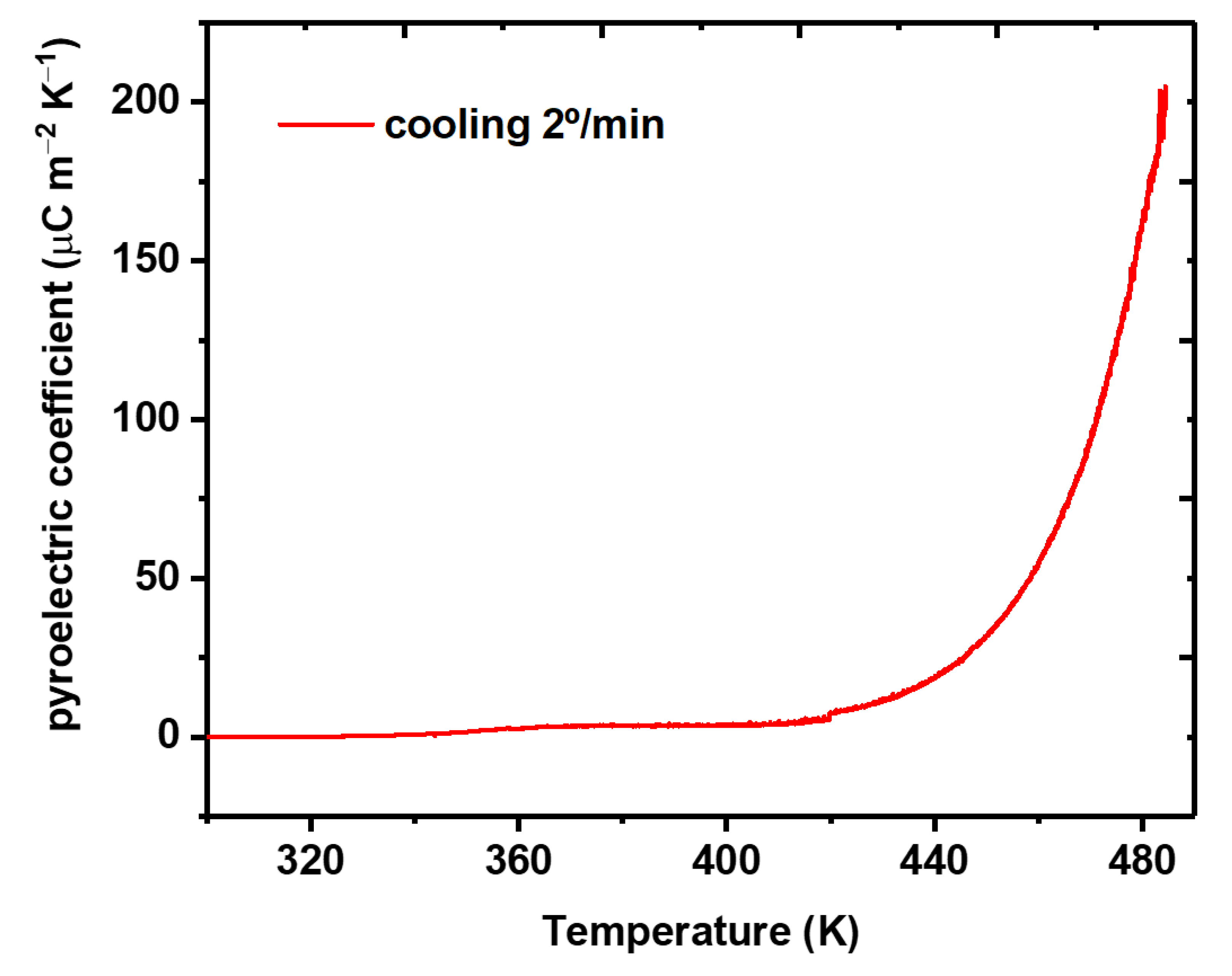
3.5. Pyroelectricity in Fibers
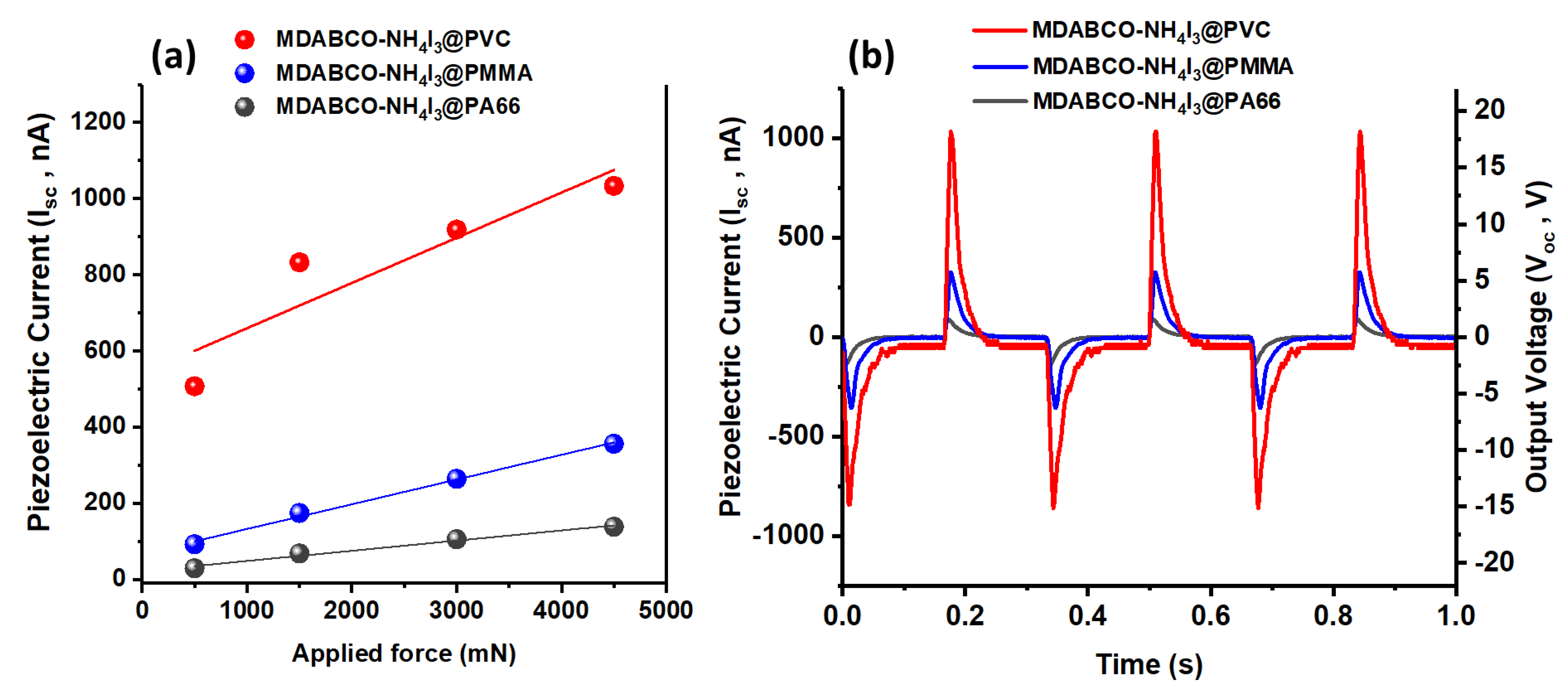
3.6. Piezoelectric Voltage and Effective Piezoelectric Coefficients in Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; Lei, T.; Qin, Y.; Yang, R. Flexible electronic skins based on piezoelectric nanogenerators and piezotronics. Nano Energy 2019, 59, 84–90. [Google Scholar] [CrossRef]
- Wang, Z.L. Towards Self-Powered Nanosystems: From Nanogenerators to Nanopiezotronics. Adv. Funct. Mater. 2008, 18, 3553–3567. [Google Scholar] [CrossRef]
- Valasek, J. Properties of Rochelle Salt Related to the Piezo-electric Effect. Phys. Rev. 1922, 20, 639–664. [Google Scholar] [CrossRef]
- Valasek, J. Piezo-Electric and Allied Phenomena in Rochelle Salt. Phys. Rev. 1921, 17, 475–481. [Google Scholar] [CrossRef]
- Lines, M.E.; Glass, A.M. Principles and Applications of Ferroelectrics and Related Materials; Oxford University Press: Oxford, UK, 2001; p. 694. [Google Scholar]
- Scott James, F.; Paz de Araujo Carlos, A. Ferroelectric Memories. Science 1989, 246, 1400–1405. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, Y.; Dou, S.X.; Liang, J. Flexible nanogenerators for wearable electronic applications based on piezoelectric materials. Mater. Today Energy 2021, 20, 100690. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. A comprehensive review on the state-of-the-art of piezoelectric energy harvesting. Nano Energy 2021, 80, 105567. [Google Scholar] [CrossRef]
- Cross, E. Lead-free at last. Nature 2004, 432, 24–25. [Google Scholar] [CrossRef]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free piezoceramics. Nature 2004, 432, 84–87. [Google Scholar] [CrossRef]
- Chen, X.-G.; Song, X.-J.; Zhang, Z.-X.; Li, P.-F.; Ge, J.-Z.; Tang, Y.-Y.; Gao, J.-X.; Zhang, W.-Y.; Fu, D.-W.; You, Y.-M.; et al. Two-Dimensional Layered Perovskite Ferroelectric with Giant Piezoelectric Voltage Coefficient. J. Am. Chem. Soc. 2020, 142, 1077–1082. [Google Scholar] [CrossRef]
- Guo, T.-M.; Gong, Y.-J.; Li, Z.-G.; Liu, Y.-M.; Li, W.; Li, Z.-Y.; Bu, X.-H. A New Hybrid Lead-Free Metal Halide Piezoelectric for Energy Harvesting and Human Motion Sensing. Small 2022, 18, 2103829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Tang, Y.-Y.; Shi, P.-P.; Xiong, R.-G. Toward the Targeted Design of Molecular Ferroelectrics: Modifying Molecular Symmetries and Homochirality. Acc. Chem. Res. 2019, 52, 1928–1938. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nat. Rev. Mater. 2017, 2, 16099. [Google Scholar] [CrossRef]
- Song, X.; Hodes, G.; Zhao, K.; Liu, S. Metal-Free Organic Halide Perovskite: A New Class for Next Optoelectronic Generation Devices. Adv. Energy Mater. 2021, 11, 2003331. [Google Scholar] [CrossRef]
- Chu, L.-L.; Zhang, T.; Zhang, W.-Y.; Shi, P.-P.; Gao, J.-X.; Ye, Q.; Fu, D.-W. Three-Dimensional Metal-Free Molecular Perovskite with a Thermally Induced Switchable Dielectric Response. J. Phys. Chem. Lett. 2020, 11, 1668–1674. [Google Scholar] [CrossRef]
- You, Y.-M.; Liao, W.-Q.; Zhao, D.; Ye, H.-Y.; Zhang, Y.; Zhou, Q.; Niu, X.; Wang, J.; Li, P.-F.; Fu, D.-W.; et al. An organic-inorganic perovskite ferroelectric with large piezoelectric response. Science 2017, 357, 306–309. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef]
- Kamat, P.V.; Bisquert, J.; Buriak, J. Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 904–905. [Google Scholar] [CrossRef]
- Boix, P.P.; Agarwala, S.; Koh, T.M.; Mathews, N.; Mhaisalkar, S.G. Perovskite Solar Cells: Beyond Methylammonium Lead Iodide. J. Phys. Chem. Lett. 2015, 6, 898–907. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Tang, Y.-Y.; Li, P.-F.; Liao, W.-Q.; Gao, J.-X.; Hua, X.-N.; Cai, H.; Shi, P.-P.; You, Y.-M.; Xiong, R.-G. Metal-free three-dimensional perovskite ferroelectrics. Science 2018, 361, 151–155. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Zhang, Z.; Liu, Z.; Lv, Z.; Li, T.; Ju, W.; Li, H.; Cai, X.; Han, H. Large piezoelectric response in a family of metal-free perovskite ferroelectric compounds from first-principles calculations. npj Comput. Mater. 2019, 5, 17. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Isakov, D.V.; de Matos Gomes, E.; Vieira, L.G.; Dekola, T.; Belsley, M.S.; Almeida, B.G. Oriented single-crystal-like molecular arrangement of optically nonlinear 2-methyl-4-nitroaniline in electrospun nanofibers. ACS Nano 2011, 5, 73–78. [Google Scholar] [CrossRef]
- Richard-Lacroix, M.; Pellerin, C. Molecular Orientation in Electrospun Fibers: From Mats to Single Fibers. Macromolecules 2013, 46, 9473–9493. [Google Scholar] [CrossRef]
- Rørvik, P.M.; Grande, T.; Einarsrud, M.-A. One-Dimensional Nanostructures of Ferroelectric Perovskites. Adv. Mater. 2011, 23, 4007–4034. [Google Scholar] [CrossRef]
- Isakov, D.; de Matos Gomes, E.; Almeida, B.; Kholkin, A.L.; Zelenovskiy, P.; Neradovskiy, M.; Shur, V.Y. Energy harvesting from nanofibers of hybrid organic ferroelectric dabcoHReO4. Appl. Phys. Lett. 2014, 104, 032907. [Google Scholar] [CrossRef]
- Sultana, A.; Ghosh, S.K.; Alam, M.M.; Sadhukhan, P.; Roy, K.; Xie, M.; Bowen, C.R.; Sarkar, S.; Das, S.; Middya, T.R.; et al. Methylammonium Lead Iodide Incorporated Poly(vinylidene fluoride) Nanofibers for Flexible Piezoelectric–Pyroelectric Nanogenerator. ACS Appl. Mater. Inter. 2019, 11, 27279–27287. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Sá, P.; Barbosa, J.; Bdikin, I.; Almeida, B.; Rolo, A.G.; Gomes, E.d.M.; Belsley, M.; Kholkin, A.L.; Isakov, D. Ferroelectric characterization of aligned barium titanate nanofibres. J. Phys. D Appl. Phys. 2013, 46, 105304. [Google Scholar] [CrossRef]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Song, X.; Li, Q.; Han, J.; Ma, C.; Xu, Z.; Li, H.; Wang, P.; Yang, Z.; Cui, Q.; Gao, L.; et al. Highly Luminescent Metal-Free Perovskite Single Crystal for Biocompatible X-Ray Detector to Attain Highest Sensitivity. Adv. Mater. 2021, 33, 2102190. [Google Scholar] [CrossRef]
- Kasel, T.W.; Deng, Z.; Mroz, A.M.; Hendon, C.H.; Butler, K.T.; Canepa, P. Metal-free perovskites for non linear optical materials. Chem. Sci. 2019, 10, 8187–8194. [Google Scholar] [CrossRef]
- Sirbu, D.; Tsui, H.C.L.; Alsaif, N.; Iglesias-Porras, S.; Zhang, Y.; Wang, M.; Liu, M.; Peacock, A.C.; Docampo, P.; Healy, N. Wide-Band-Gap Metal-Free Perovskite for Third-Order Nonlinear Optics. ACS Photonics 2021, 8, 2450–2458. [Google Scholar] [CrossRef]
- Zhao, Z.; Buscaglia, V.; Viviani, M.; Buscaglia, M.T.; Mitoseriu, L.; Testino, A.; Nygren, M.; Johnsson, M.; Nanni, P. Grain-size effects on the ferroelectric behavior of dense nanocrystalline BaTiO3 ceramics. Phys. Rev. B 2004, 70, 024107. [Google Scholar] [CrossRef]
- Choi, K.J.; Biegalski, M.; Li, Y.L.; Sharan, A.; Schubert, J.; Uecker, R.; Reiche, P.; Chen, Y.B.; Pan, X.Q.; Gopalan, V.; et al. Enhancement of Ferroelectricity in Strained BaTiO3 Thin Films. Science 2004, 306, 1005–1009. [Google Scholar] [CrossRef]
- Belhadi, J.; El Marssi, M.; Gagou, Y.; Yuzyuk, Y.I.; Raevski, I.P. Giant increase of ferroelectric phase transition temperature in highly strained ferroelectric [BaTiO3]0.7Λ/[BaZrO3]0.3Λ superlattice. Europhys. Lett. 2014, 106, 17004. [Google Scholar] [CrossRef]
- Petzelt, J. Dielectric Grain-Size Effect in High-Permittivity Ceramics. Ferroelectrics 2010, 400, 117–134. [Google Scholar] [CrossRef]
- Mao, C.; Yan, S.; Cao, S.; Yao, C.; Cao, F.; Wang, G.; Dong, X.; Hu, X.; Yang, C. Effect of grain size on phase transition, dielectric and pyroelectric properties of BST ceramics. J. Eur. Ceram. Soc. 2014, 34, 2933–2939. [Google Scholar] [CrossRef]
- Ghane-Motlagh, R.; Kroener, M.; Goldschmidtboeing, F.; Danilewsky, A.N.; Woias, P. A dynamic method for the measurement of pyroelectric properties of materials. Smart Mater. Struct. 2018, 27, 084004. [Google Scholar] [CrossRef]
- Bharti, V.; Kaura, T.; Nath, R. Improved piezoelectricity in solvent-cast PVC films. IEEE Trans. Dielectr. Electr. Insul. 1995, 2, 1106–1110. [Google Scholar] [CrossRef]
- Baptista, R.M.F.; Lopes, P.E.; Rodrigues, A.R.O.; Cerca, N.; Belsley, M.S.; de Matos Gomes, E. Self-assembly of Boc-p-nitro-l-phenylalanyl-p-nitro-l-phenylalanine and Boc-l-phenylalanyl-l-tyrosine in solution and into piezoelectric electrospun fibers. Mater. Adv. 2022, 3, 2934–2944. [Google Scholar] [CrossRef]
- Baptista, R.M.F.; de Matos Gomes, E.; Raposo, M.M.M.; Costa, S.P.G.; Lopes, P.E.; Almeida, B.; Belsley, M.S. Self-assembly of dipeptide Boc-diphenylalanine nanotubes inside electrospun polymeric fibers with strong piezoelectric response. Nanoscale Adv. 2019, 1, 4339–4346. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, C.R.; Baptista, R.M.F.; de Matos Gomes, E.; Lopes, P.E.; Raposo, M.M.M.; Costa, S.P.G.; Belsley, M.S. Anisotropic PCL nanofibers embedded with nonlinear nanocrystals as strong generators of polarized second harmonic light and piezoelectric currents. Nanoscale Adv. 2020, 2, 1206–1213. [Google Scholar] [CrossRef]
- Wu, H.-S.; Wei, S.-M.; Chen, S.-W.; Pan, H.-C.; Pan, W.-P.; Huang, S.-M.; Tsai, M.-L.; Yang, P.-K. Metal-Free Perovskite Piezoelectric Nanogenerators for Human–Machine Interfaces and Self-Powered Electrical Stimulation Applications. Adv. Sci. 2022, 9, 2105974. [Google Scholar] [CrossRef]
- Li, M.; Wondergem, H.J.; Spijkman, M.-J.; Asadi, K.; Katsouras, I.; Blom, P.W.M.; de Leeuw, D.M. Revisiting the δ-phase of poly(vinylidene fluoride) for solution-processed ferroelectric thin films. Nat. Mater. 2013, 12, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Klug, H.; Alexander, L. X-Ray Diffraction Procedures, 2nd ed.; Ch. 9; Wiley: Hoboken, NJ, USA, 1974. [Google Scholar]
- D’Errico, J. SLM—Shape Language Modeling. MATLAB Central File Exchange. 2022. Available online: https://www.mathworks.com/matlabcentral/fileexchange/24443-slm-shape-language-modeling (accessed on 5 July 2022).
- Stancik, A.L.; Brauns, E.B. A simple asymmetric lineshape for fitting infrared absorption spectra. Vib. Spectrosc. 2008, 47, 66–69. [Google Scholar] [CrossRef]
- Keve, E.T.; Abrahams, S.C.; Bernstein, J.L. Pyroelectric Ammonium Iodate, a Potential Ferroelastic: Crystal Structure. J. Chem. Phys. 1971, 54, 2556–2563. [Google Scholar] [CrossRef]
- Lee, D.W.; De Los Santos, V.L.; Seo, J.W.; Félix, L.L.; Bustamante, D.A.; Cole, J.M.; Barnes, C.H.W. The Structure of Graphite Oxide: Investigation of Its Surface Chemical Groups. J. Phys. Chem. B 2010, 114, 5723–5728. [Google Scholar] [CrossRef] [PubMed]




| MDABCO-NH4I3 in | Size (nm) |
|---|---|
| PVC | 62 ± 12 |
| PMMA | 77 ± 06 |
| PA66 | 83 ± 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, R.M.F.; Moreira, G.; Silva, B.; Oliveira, J.; Almeida, B.; Castro, C.; Rodrigues, P.V.; Machado, A.; Belsley, M.; de Matos Gomes, E. Lead-Free MDABCO-NH4I3 Perovskite Crystals Embedded in Electrospun Nanofibers. Materials 2022, 15, 8397. https://doi.org/10.3390/ma15238397
Baptista RMF, Moreira G, Silva B, Oliveira J, Almeida B, Castro C, Rodrigues PV, Machado A, Belsley M, de Matos Gomes E. Lead-Free MDABCO-NH4I3 Perovskite Crystals Embedded in Electrospun Nanofibers. Materials. 2022; 15(23):8397. https://doi.org/10.3390/ma15238397
Chicago/Turabian StyleBaptista, Rosa M. F., Gonçalo Moreira, Bruna Silva, João Oliveira, Bernardo Almeida, Cidália Castro, Pedro V. Rodrigues, Ana Machado, Michael Belsley, and Etelvina de Matos Gomes. 2022. "Lead-Free MDABCO-NH4I3 Perovskite Crystals Embedded in Electrospun Nanofibers" Materials 15, no. 23: 8397. https://doi.org/10.3390/ma15238397
APA StyleBaptista, R. M. F., Moreira, G., Silva, B., Oliveira, J., Almeida, B., Castro, C., Rodrigues, P. V., Machado, A., Belsley, M., & de Matos Gomes, E. (2022). Lead-Free MDABCO-NH4I3 Perovskite Crystals Embedded in Electrospun Nanofibers. Materials, 15(23), 8397. https://doi.org/10.3390/ma15238397









