Waterborne Polyurethane Acrylates Preparation towards 3D Printing for Sewage Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
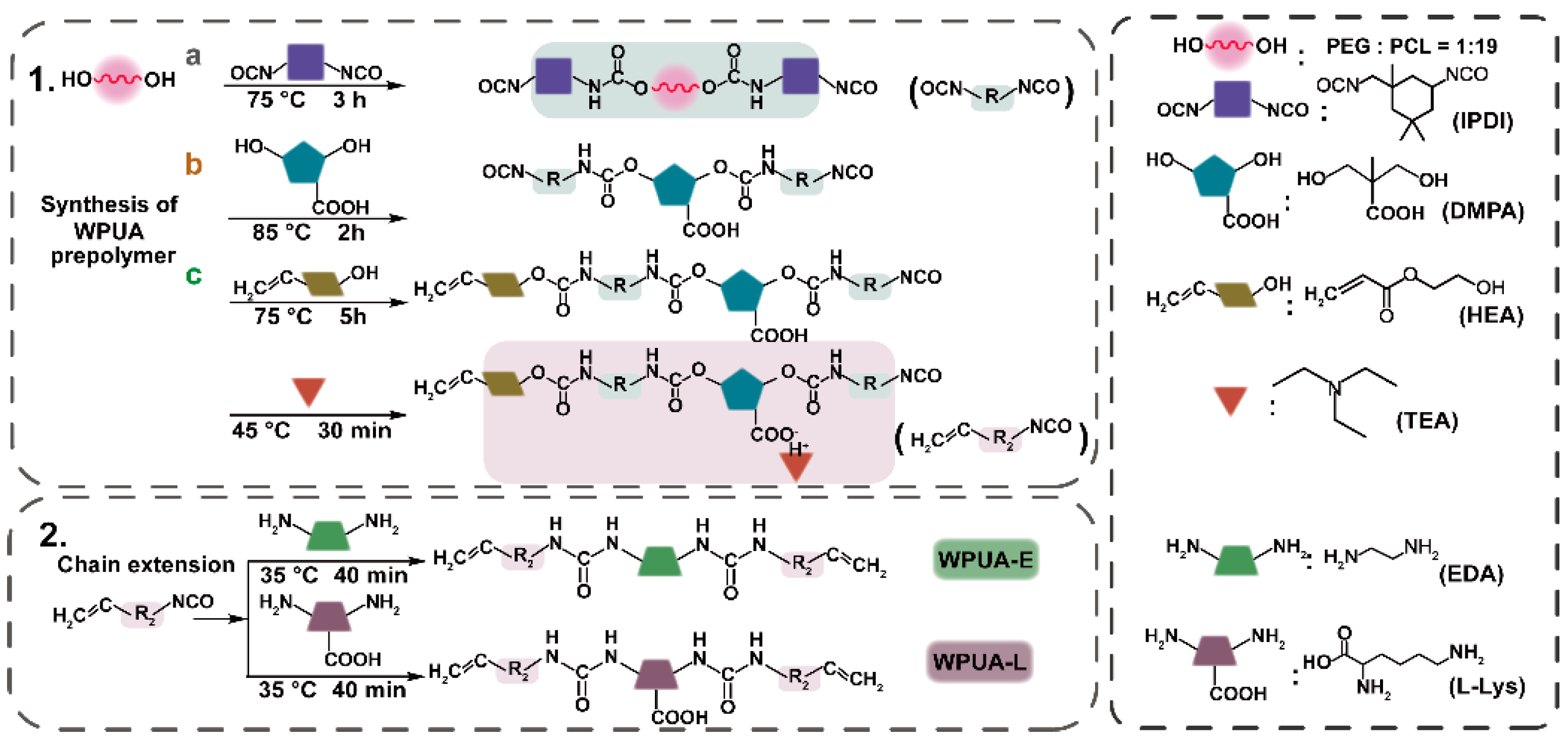
2.2. Preparation of WPUA Emulsion and Ink Matrix
2.3. Cultivation of Heterotrophic Nitrifying Bacteria
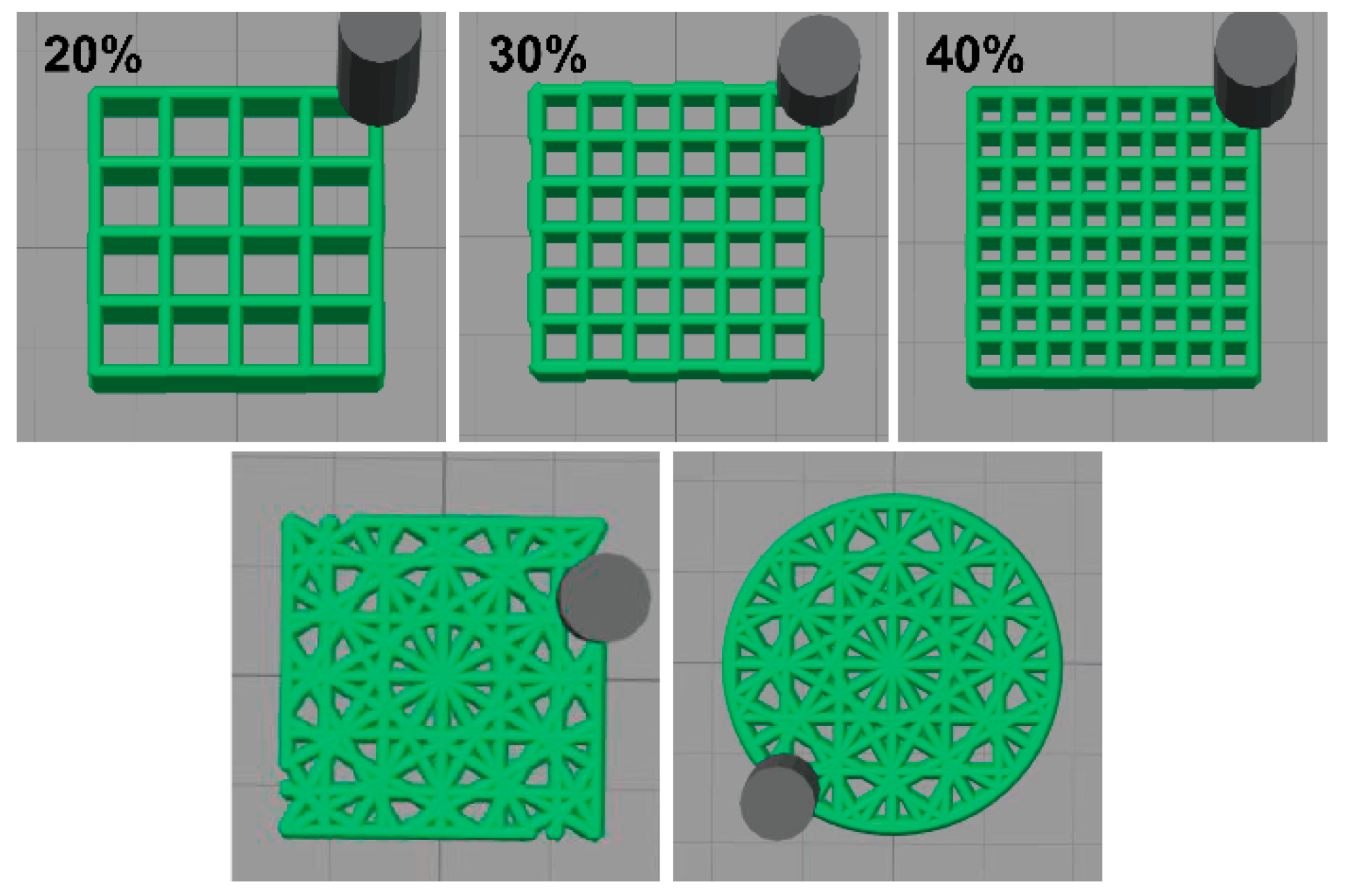
2.4. DIW 3D Bioprinting
2.5. Cyclic Processes and Simulation of Refrigerated Storage Experiment
2.6. Characterization
2.6.1. Physical–Chemical Characterization
2.6.2. The Biological Study of Nitrifying Bacteria Growth in the Scaffold and Corresponding Nitrification Effect
3. Results and Discussion
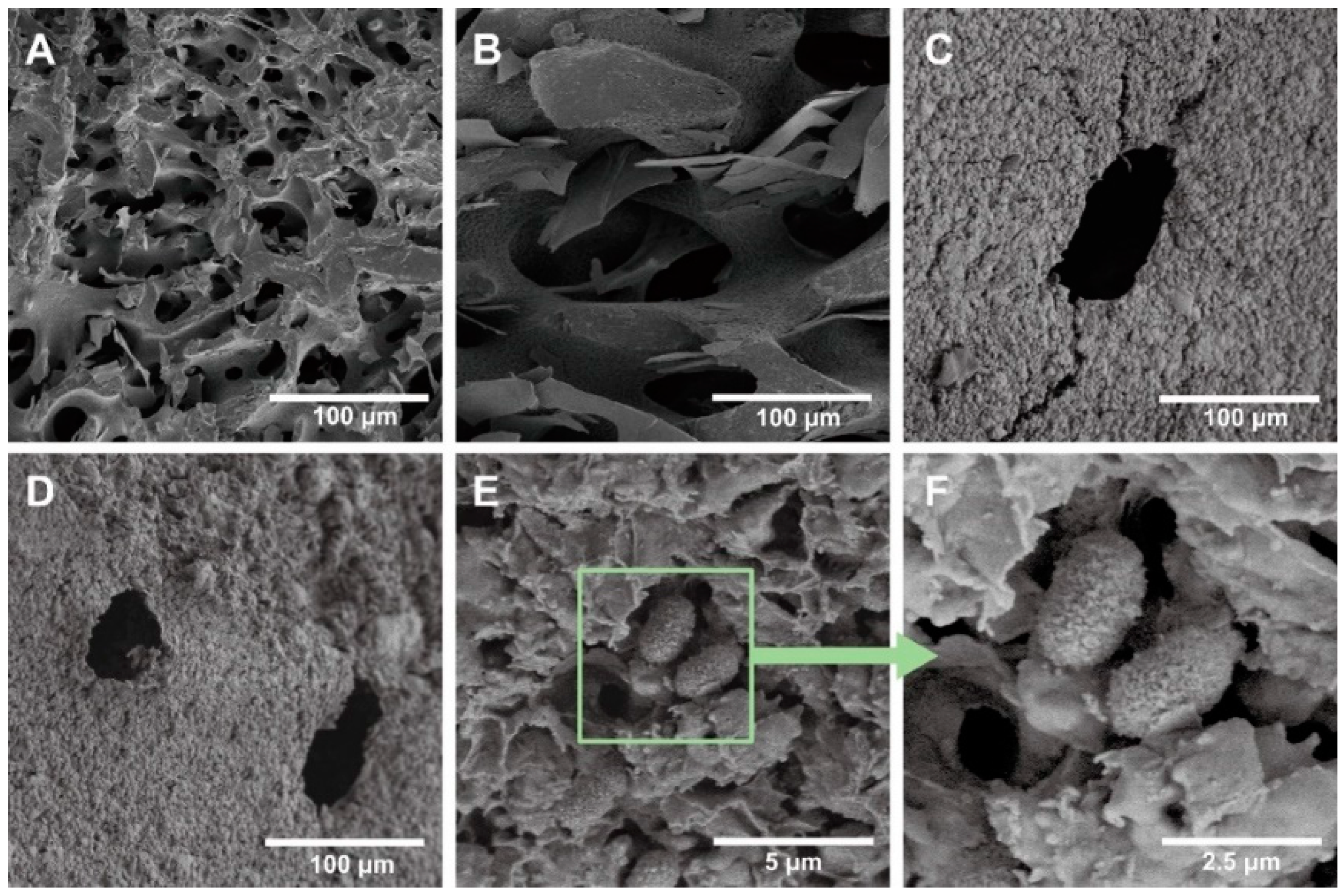
3.1. Characterization of WPUAs
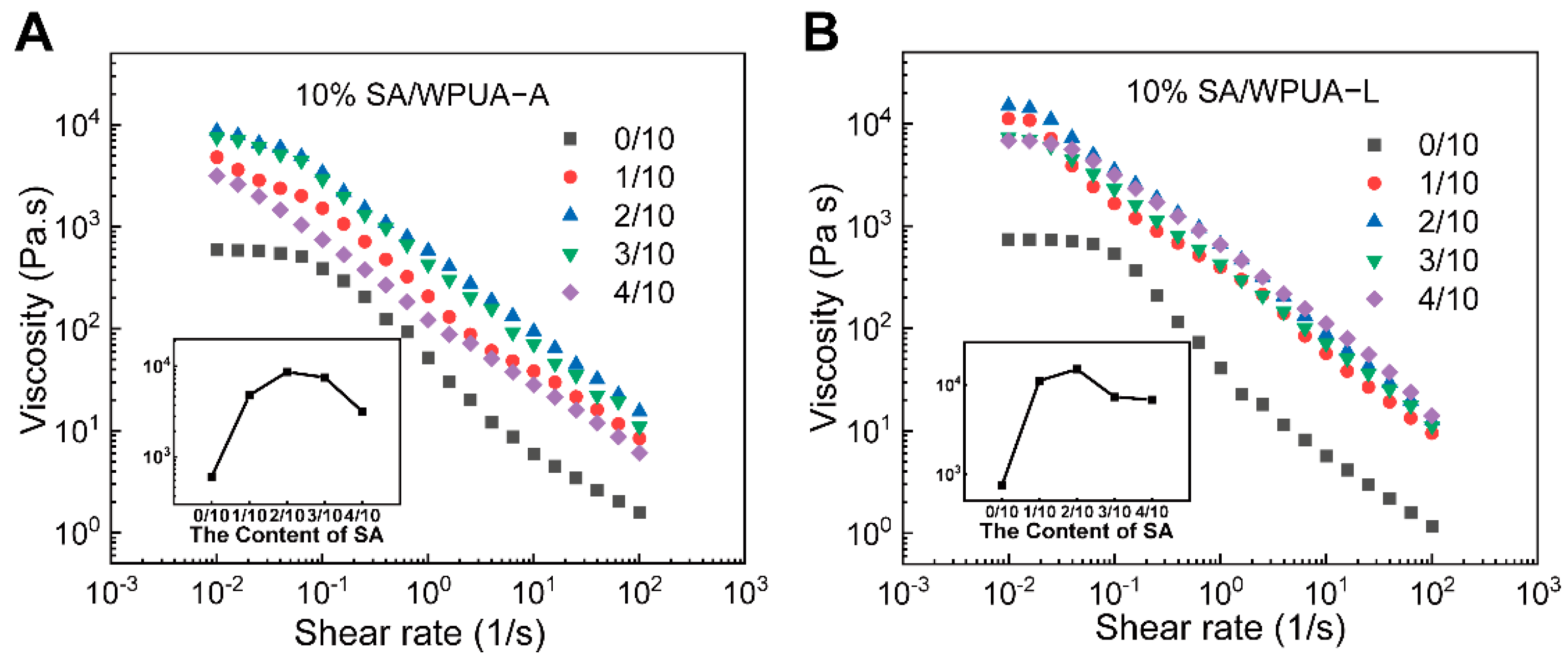
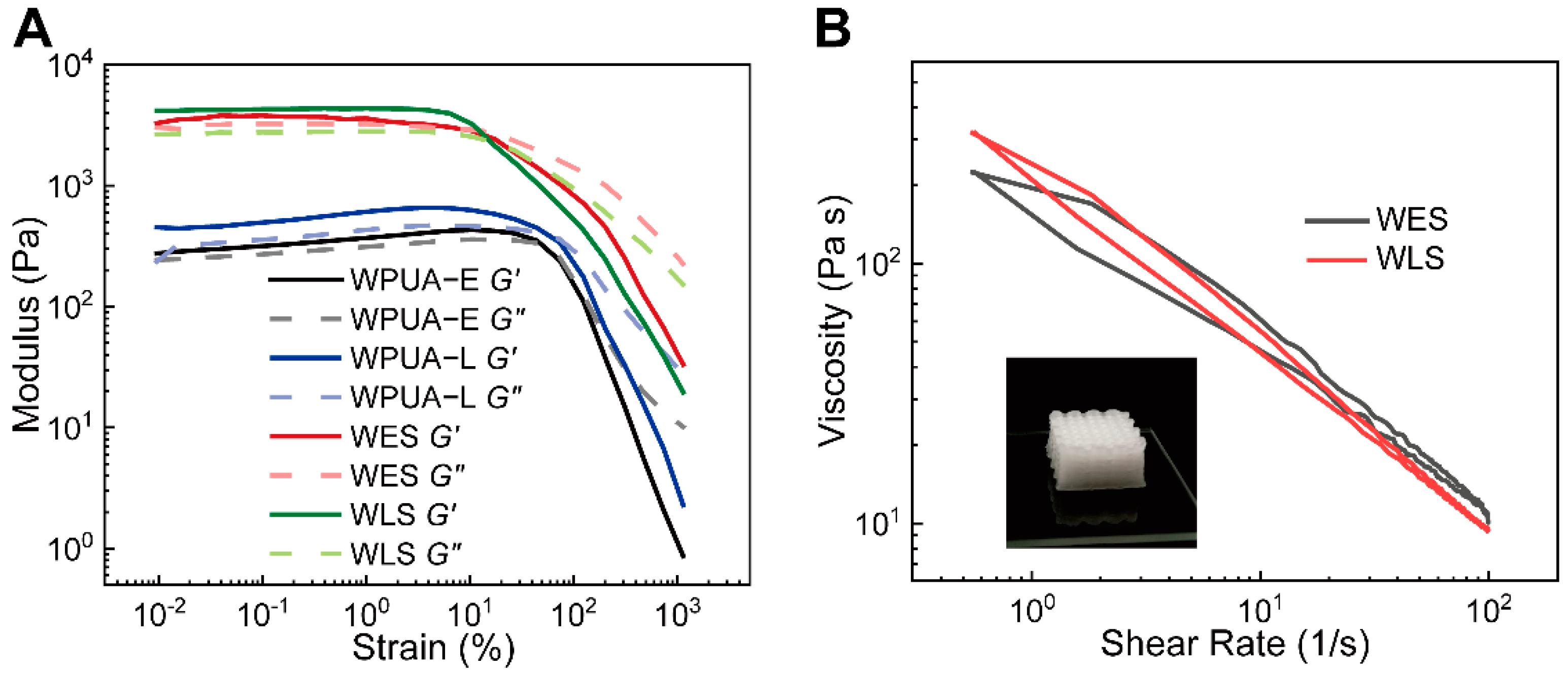
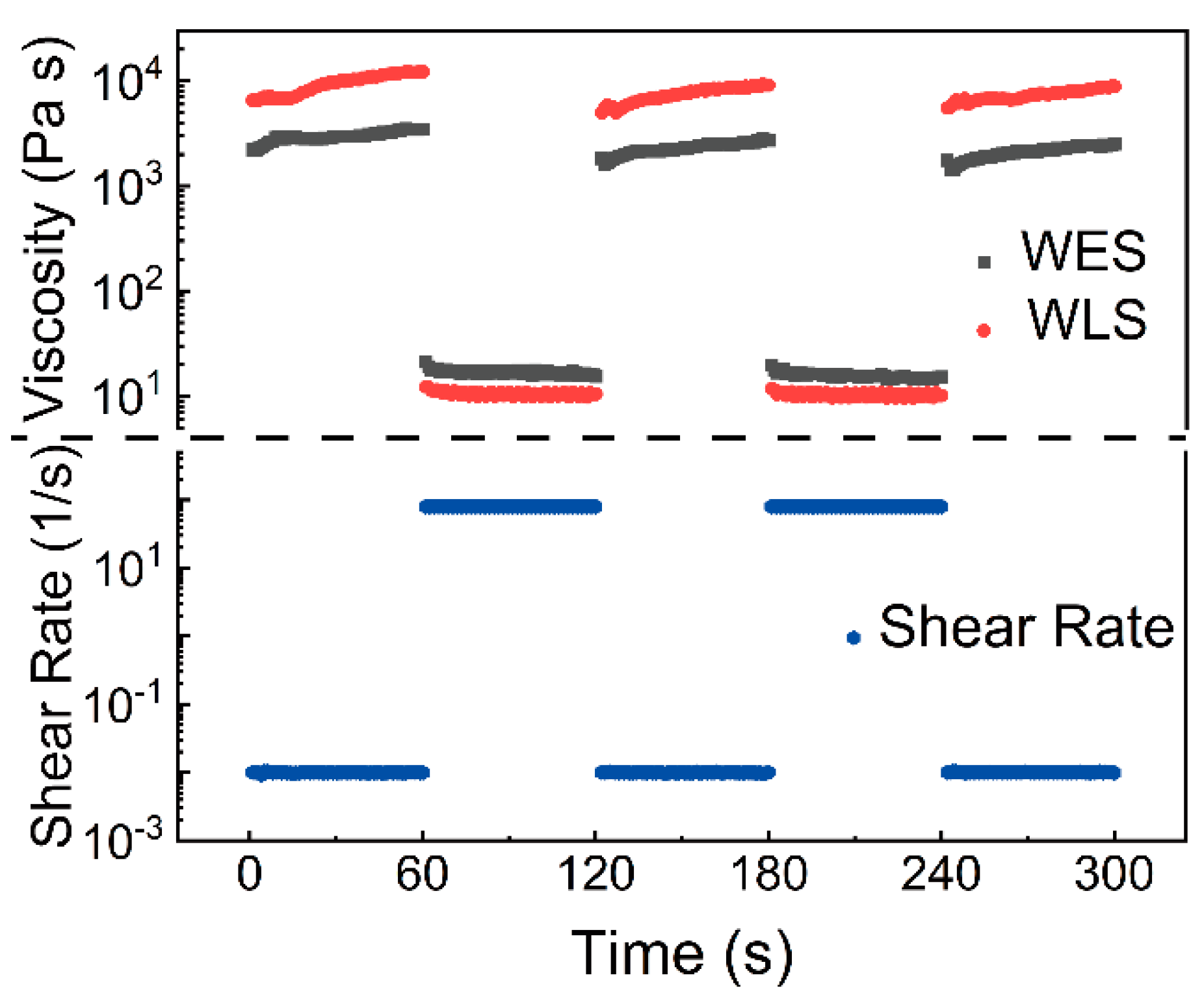
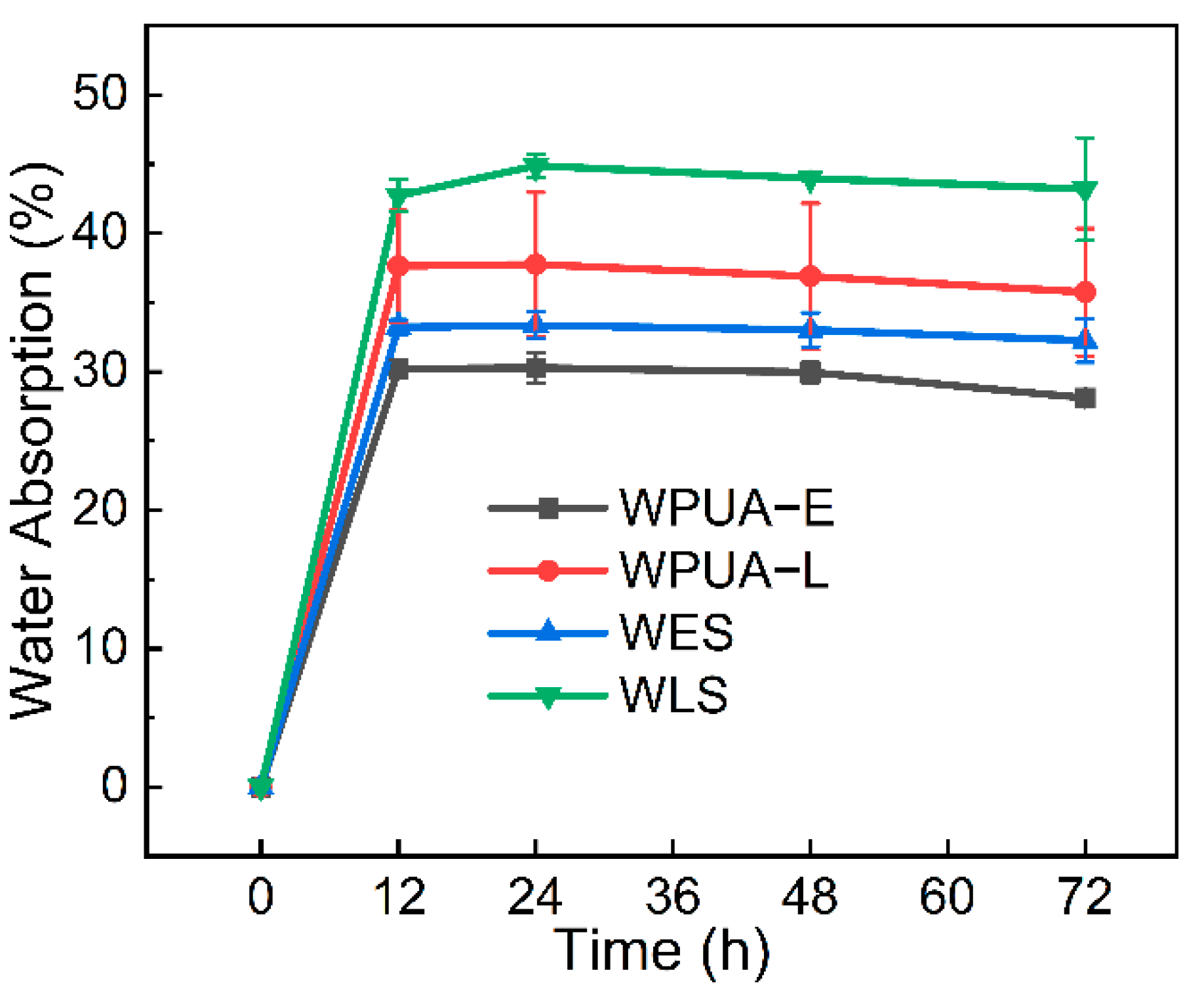
3.2. Rheology and Water Swelling Properties
3.3. Mechanical Properties
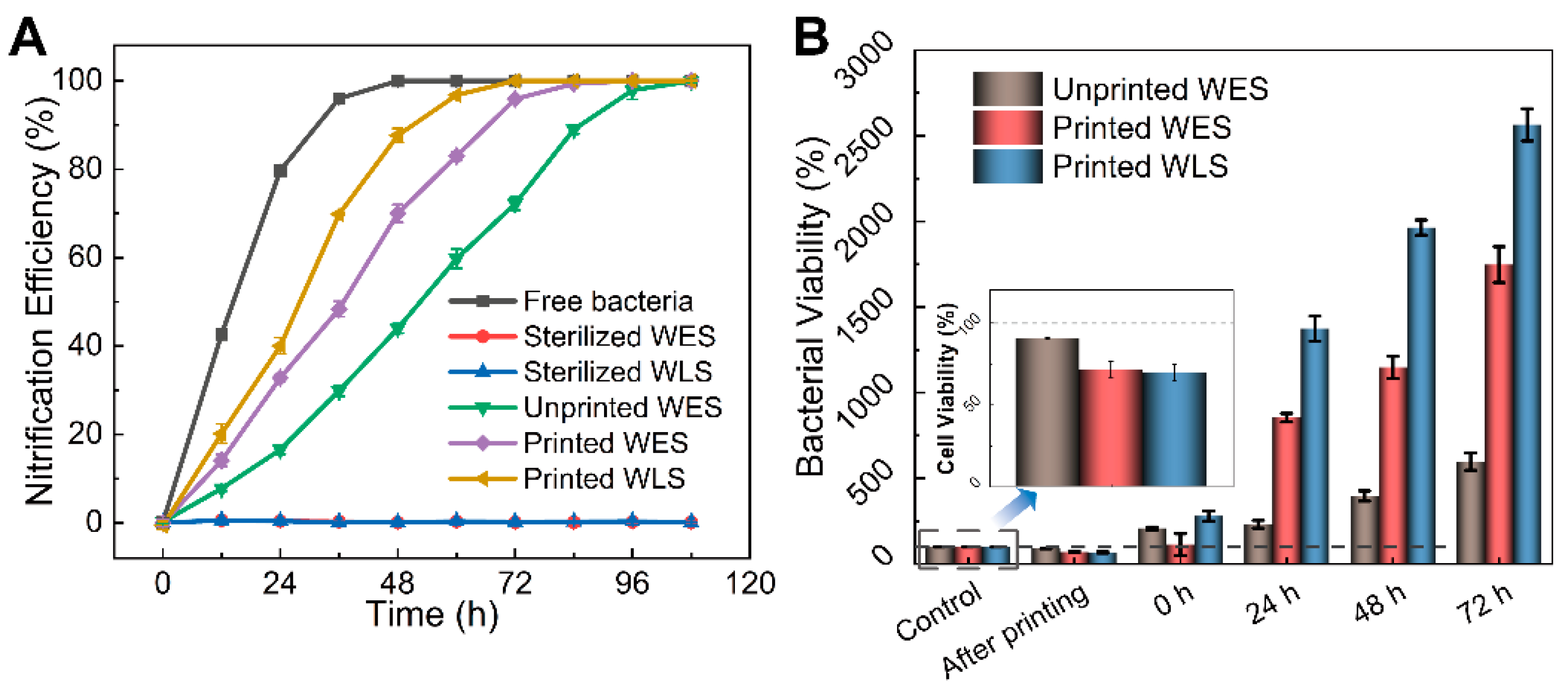
3.4. Biomedical Properties Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Li, Y.; Chen, X. Research progress on enhancing the performance of autotrophic nitrogen removal systems using microbial immobilization technology. Sci. Total Environ. 2021, 774, 145136. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jin, Z.; Wang, B.; Lv, C.; Hu, B.; Shi, D. Treatment of high-strength ammonium wastewater by polyvinyl alcohol–sodium alginate immobilization of activated sludge. Process Biochem. 2017, 63, 214–220. [Google Scholar] [CrossRef]
- Dong, H.; Wang, W.; Song, Z.; Dong, H.; Wang, J.; Sun, S.; Zhang, Z.; Ke, M.; Zhang, Z.; Wu, W.M.; et al. A high-efficiency denitrification bioreactor for the treatment of acrylonitrile wastewater using waterborne polyurethane immobilized activated sludge. Bioresour. Technol. 2017, 239, 472–481. [Google Scholar] [CrossRef]
- Landreau, M.; Byson, S.J.; You, H.; Stahl, D.A.; Winkler, M.K.H. Effective nitrogen removal from ammonium-depleted wastewater by partial nitritation and anammox immobilized in granular and thin layer gel carriers. Water Res. 2020, 183, 116078. [Google Scholar] [CrossRef]
- Jo, B.W.; Song, C.S. Thermoplastics and Photopolymer Desktop 3D Printing System Selection Criteria Based on Technical Specifications and Performances for Instructional Applications. Technologies 2021, 9, 91. Available online: https://www.mdpi.com/2227-7080/9/4/91 (accessed on 29 March 2021). [CrossRef]
- al-Qarni, F.D.; Gad, M.M. Printing Accuracy and Flexural Properties of Different 3D-Printed Denture Base Resins. Materials 2022, 15, 2410. Available online: https://www.mdpi.com/1996-1944/15/7/2410 (accessed on 24 March 2022). [CrossRef]
- Antreas, K.; Piromalis, D. Employing a Low-Cost Desktop 3D Printer: Challenges, and How to Overcome Them by Tuning Key Process Parameters. Int. J. Mech. Appl. 2021, 10, 11–19. [Google Scholar] [CrossRef]
- Weng, Z.X.; Wang, J.L.; Senthil, T.; Wu, L.X. Mechanical and thermal properties of ABS/montmorillonite nanocomposites for fused deposition modeling 3D printing. Mater. Des. 2016, 102, 276–283. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, S.; Wang, Z.; Miao, J.-T.; Zheng, L.; Wu, L.; Weng, Z. UV-Curable, Low-Viscosity Resin with a High Silica Filler Content for Preparing Ultrastiff, 3D-Printed Molds. ACS Appl. Polym. Mater. 2022, 4, 2636–2647. [Google Scholar] [CrossRef]
- Li, Y.; Peng, S.; Miao, J.-T.; Zheng, L.; Zhong, J.; Wu, L.; Weng, Z. Isotropic Stereolithography Resin Toughened by Core-shell Particles. Chem. Eng. J. 2020, 349, 124873. [Google Scholar] [CrossRef]
- Ghosh, U.; Ning, S.; Wang, Y.; Kong, Y.L. Addressing unmet clinical needs with 3D printing technologies. Adv. Healthc. Mater. 2018, 7, 1800417. [Google Scholar] [CrossRef]
- Zhu, Z.; Ng, D.W.H.; Park, H.S.; McAlpine, M.C. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater. 2021, 6, 27–47. [Google Scholar] [CrossRef]
- Hung, K.-S.; Chen, M.-S.; Lan, W.-C.; Cho, Y.-C.; Saito, T.; Huang, B.-H.; Tsai, H.-Y.; Hsieh, C.-C.; Ou, K.-L.; Lin, H.-Y. Three-Dimensional Printing of a Hybrid Bioceramic and Biopolymer Porous Scaffold for Promoting Bone Regeneration Potential. Materials 2022, 15, 1971. Available online: https://www.mdpi.com/1996-1944/15/5/1971 (accessed on 7 March 2022). [CrossRef] [PubMed]
- Qian, F.; Zhu, C.; Knipe, J.M.; Ruelas, S.; Stolaroff, J.K.; DeOtte, J.R.; Duoss, E.B.; Spadaccini, C.M.; Henard, C.A.; Guarnieri, M.T.; et al. Direct Writing of Tunable Living Inks for Bioprocess Intensification. Nano Lett. 2019, 19, 5829–5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogun, H.A.; Sulaiman, R.; Marzouk, S.S.; Giwa, A.; Hasan, S.W. 3D printing and surface imprinting technologies for water treatment: A review. J. Water Process. Eng. 2019, 31, 100786. [Google Scholar] [CrossRef]
- Roach, D.J.; Yuan, C.; Kuang, X.; Li, V.C.; Blake, P.; Romero, M.L.; Hammel, I.; Yu, K.; Qi, H.J. Long Liquid Crystal Elastomer Fibers with Large Reversible Actuation Strains for Smart Textiles and Artificial Muscles. ACS Appl. Mater. Interfaces 2019, 11, 19514–19521. [Google Scholar] [CrossRef]
- Konka, J.; Buxadera-Palomero, J.; Espanol, M.; Ginebra, M.P. 3D printing of hierarchical porous biomimetic hydroxyapatite scaffolds: Adding concavities to the convex filaments. Acta Biomater. 2021, 134, 744–759. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Schiele, N.R.; Corr, D.T.; Huang, Y.; Raof, N.A.; Xie, Y.; Chrisey, D.B. Laser-based direct-write techniques for cell printing. Biofabrication 2010, 2, 032001. [Google Scholar] [CrossRef] [Green Version]
- Mannoor, M.S.; Jiang, Z.; James, T.; Kong, Y.L.; Malatesta, K.A.; Soboyejo, W.O.; Verma, N.; Gracias, D.H.; McAlpine, M.C. 3D printed bionic ears. Nano Lett. 2013, 13, 2634–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hockaday, L.A.; Kang, K.H.; Colangelo, N.W.; Cheung, P.Y.; Duan, B.; Malone, E.; Wu, J.; Girardi, L.N.; Bonassar, L.J.; Lipson, H.; et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 2012, 4, 035005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaffner, M.; Rühs, P.A.; Coulter, F.; Kilcher, S.; Studart, A.R. 3D printing of bacteria into functional complex materials. Sci. Adv. 2017, 3, eaao6804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, C.-T.; Liao, C.-Y.; Dai, N.-T.; Tseng, C.-S.; Yen, B.L.; Hsu, S.-h. 3D printing of tubular scaffolds with elasticity and complex structure from multiple waterborne polyurethanes for tracheal tissue engineering. Appl. Mater. Today 2018, 12, 330–341. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, D.; Zheng, Y.; Zhao, L.; Xu, T.; Guo, Z.; Irfan Hussain, M.; Zeng, J.; Lou, L.; Sun, Y.; et al. A novel waterborne polyurethane with biodegradability and high flexibility for 3D printing. Biofabrication 2020, 12, 035015. [Google Scholar] [CrossRef] [PubMed]
- Larraza, I.; Vadillo, J.; Calvo-Correas, T.; Tejado, A.; Olza, S.; Peña-Rodríguez, C.; Arbelaiz, A.; Eceiza, A. Cellulose and Graphene Based Polyurethane Nanocomposites for FDM 3D Printing: Filament Properties and Printability. Polymers 2021, 13, 839. Available online: https://www.mdpi.com/2073-4360/13/5/839 (accessed on 9 March 2021). [CrossRef]
- Chen, Y.; Yu, Z.; Oguzlu, H.; Jiang, J.; Cho, M.; Karaaslan, M.; Renneckar, S.; Jiang, F. Superelastic and flexible 3D printed waterborne polyurethane/cellulose nanofibrils structures. Addit. Manuf. 2021, 46, 102107. [Google Scholar] [CrossRef]
- Wang, Z.; Ishii, S.; Novak, P.J. Encapsulating microorganisms to enhance biological nitrogen removal in wastewater: Recent advancements and future opportunities. Environ. Sci. Water Res. Technol. 2021, 7, 1402–1416. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. Available online: https://www.mdpi.com/10.3390/polym13071073 (accessed on 29 March 2021). [CrossRef]
- González, L.M.; Mukhitov, N.; Voigt, C.A. Resilient living materials built by printing bacterial spores. Nat. Chem. Biol. 2020, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Chan-Chan, L.H.; González-García, G.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Hernández-Sánchez, F.; Marcos-Fernandez, A.; Cauich-Rodríguez, J.V. Characterization of model compounds and poly(amide-urea) urethanes based on amino acids by FTIR, NMR and other analytical techniques. Eur. Polym. J. 2017, 92, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zheng, Z.; Chen, L.; Tu, X.; Wang, X. Glutathione-responsive biodegradable poly(urea-urethane)s containing L-cystine-based chain extender. J. Biomater. Sci. Polym. Ed. 2013, 24, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Yang, L.; Zhu, Q.; Ma, Q.; Wang, R.; Zhang, C.; Zhang, Z. Indoxacarb-Loaded Anionic Polyurethane Blend with Sodium Alginate Improves pH Sensitivity and Ecological Security for Potential Application in Agriculture. Polymers 2020, 12, 1135. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liang, H.; Zhou, X.; Fang, C.; Zhang, C.; Luo, Y. Synthesis and properties of castor oil-based waterborne polyurethane/sodium alginate composites with tunable properties. Carbohydr. Polym. 2019, 208, 391–397. [Google Scholar] [CrossRef]
- Dunju, W.; Changping, G.; Ruihao, W.; Baohui, Z.; Bing, G.; Fude, N. Additive manufacturing and combustion performance of CL-20 composites. J. Mater. Sci. 2020, 55, 2836–2845. [Google Scholar] [CrossRef]
- Lovecchio, J.; Cortesi, M.; Zani, M.; Govoni, M.; Dallari, D.; Giordano, E. Fiber Thickness and Porosity Control in a Biopolymer Scaffold 3D Printed through a Converted Commercial FDM Device. Materials 2022, 15, 2394. Available online: https://www.mdpi.com/1996-1944/15/7/2394 (accessed on 24 March 2022). [CrossRef]
- Kantaros, A.; Piromalis, D. Fabricating Lattice Structures via 3D Printing: The Case of Porous Bio-Engineered Scaffolds. Appl. Mech. 2021, 2, 289–302. Available online: https://www.mdpi.com/2673-3161/2/2/18 (accessed on 25 May 2021). [CrossRef]
- Gong, Y.; Chen, H.; Li, W.; Zhou, C.; Zhou, R.; Zhao, H.; Shao, H. Gradient Printing Alginate Herero Gel Microspheres for Three-Dimensional Cell Culture. Materials 2022, 15, 2305. Available online: https://www.mdpi.com/1996-1944/15/6/2305 (accessed on 20 March 2022). [CrossRef]
- Choi, D.; Qiu, M.; Hwang, Y.-C.; Oh, W.-M.; Koh, J.-T.; Park, C.; Lee, B.-N. The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells. Materials 2022, 15, 2170. Available online: https://www.mdpi.com/1996-1944/15/6/2170 (accessed on 15 March 2022). [CrossRef]
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D printing-assisted design of scaffold structures. Int. J. Adv. Manuf. Technol. 2016, 82, 559–571. [Google Scholar] [CrossRef]
- Boschetti, F.; Raimondi, M.T.; Migliavacca, F.; Dubini, G. Prediction of the micro-fluid dynamic environment imposed to three-dimensional engineered cell systems in bioreactors. J. Biomech. 2006, 39, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lesman, A.; Blinder, Y.; Levenberg, S. Modeling of flow-induced shear stress applied on 3D cellular scaffolds: Implications for vascular tissue engineering. Biotechnol. Bioeng. 2010, 105, 645–654. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hong, Y.; Guan, F.; Wang, Y.; Tan, Y.; Yue, W.; Wu, M.; Bin, L.; Wang, J.; Wen, J. A rapid and high-throughput microplate spectrophotometric method for field measurement of nitrate in seawater and freshwater. Sci. Rep. 2016, 6, 20165. [Google Scholar] [CrossRef]
- Peng, S.; Li, Y.; Wu, L.; Zhong, J.; Weng, Z.; Zheng, L.; Yang, Z.; Miao, J.T. 3D Printing Mechanically Robust and Transparent Polyurethane Elastomers for Stretchable Electronic Sensors. ACS Appl. Mater. Interfaces 2020, 12, 6479–6488. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M.; Barmar, M. Compatible compositions based on aqueous polyurethane dispersions and sodium alginate. Carbohydr. Polym. 2013, 92, 490–496. [Google Scholar] [CrossRef]
- Banach-Wisniewska, A.; Tomaszewski, M.; Hellal, M.S.; Ziembinska-Buczynska, A. Effect of biomass immobilization and reduced graphene oxide on the microbial community changes and nitrogen removal at low temperatures. Sci. Rep. 2021, 11, 840. [Google Scholar] [CrossRef]
- Jiang, P.; Yan, C.; Guo, Y.; Zhang, X.; Cai, M.; Jia, X.; Wang, X.; Zhou, F. Direct ink writing with high-strength and swelling-resistant biocompatible physically crosslinked hydrogels. Biomater. Sci. 2019, 7, 1805–1814. [Google Scholar] [CrossRef]
- Tuyen, N.V.; Ryu, J.H.; Yae, J.B.; Kim, H.G.; Hong, S.W.; Ahn, D.H. Nitrogen removal performance of anammox process with PVA–SA gel bead crosslinked with sodium sulfate as a biomass carrier. J. Ind. Eng. Chem. 2018, 67, 326–332. [Google Scholar] [CrossRef]
- Skarja, G.; Woodhouse, K. Structure-property relationships of degradable polyurethane elastomers containing an amino acid-based chain extender. J. Appl. Polym. Sci. 2000, 75, 1522–1534. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Deng, H.; Dong, Q.; Zhang, Y.; Zheng, Z.; Hou, A. Study on Anaerobic Ammoniumoxidation (ANAMMOX) Sludge Immobilized in Different Gel Carriers and Its Nitrogen Removal Performance. J. Residuals Sci. Technol. 2015, 12, S47–S54. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, Y.; Hu, J.; Zhang, Y.; Yang, Z.; Peng, S.; Wu, L.; Weng, Z. Waterborne Polyurethane Acrylates Preparation towards 3D Printing for Sewage Treatment. Materials 2022, 15, 3319. https://doi.org/10.3390/ma15093319
Li K, Li Y, Hu J, Zhang Y, Yang Z, Peng S, Wu L, Weng Z. Waterborne Polyurethane Acrylates Preparation towards 3D Printing for Sewage Treatment. Materials. 2022; 15(9):3319. https://doi.org/10.3390/ma15093319
Chicago/Turabian StyleLi, Kunrong, Yan Li, Jiale Hu, Yuanye Zhang, Zhi Yang, Shuqiang Peng, Lixin Wu, and Zixiang Weng. 2022. "Waterborne Polyurethane Acrylates Preparation towards 3D Printing for Sewage Treatment" Materials 15, no. 9: 3319. https://doi.org/10.3390/ma15093319
APA StyleLi, K., Li, Y., Hu, J., Zhang, Y., Yang, Z., Peng, S., Wu, L., & Weng, Z. (2022). Waterborne Polyurethane Acrylates Preparation towards 3D Printing for Sewage Treatment. Materials, 15(9), 3319. https://doi.org/10.3390/ma15093319





