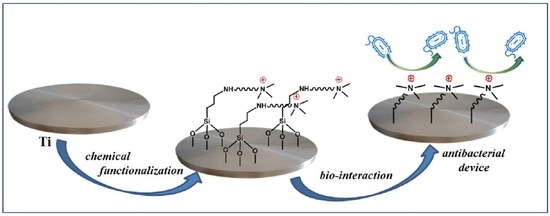
Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instrumentation
2.2. Functionalization of Titanium-Based Samples
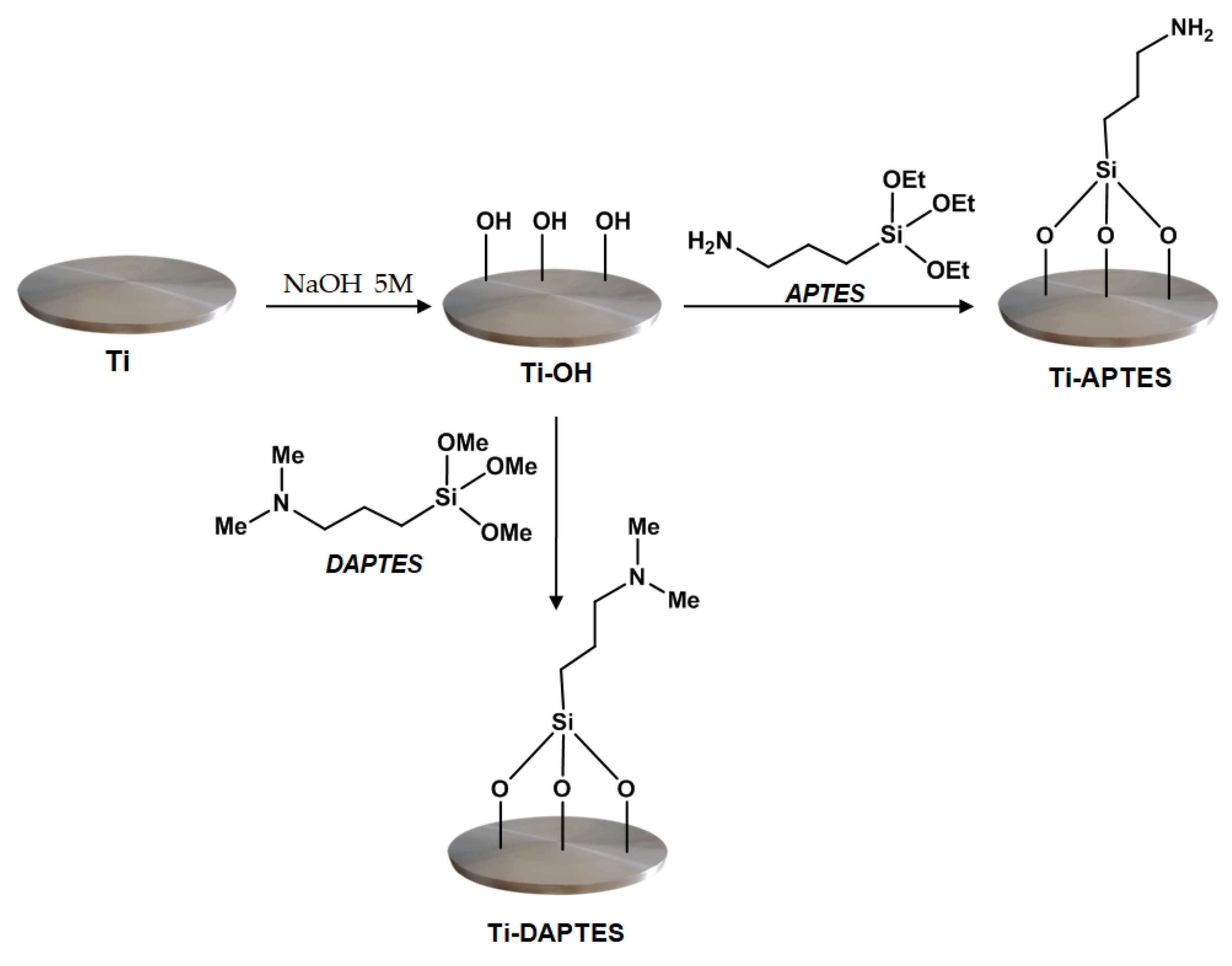
2.2.1. Activation of Titanium Samples
2.2.2. Silanization of Activated Titanium Discs
2.2.3. Synthesis of AEMAC, AUTEAB, and GTAMC Functionalized Titanium Discs
2.2.4. Synthesis of Oleic Acid Salts Functionalized Titanium Disks
2.3. Evaluation of Titanium Discs’ Anti-Bacterial Adhesion Properties
2.3.1. Microbial Strains and Culture Conditions
2.3.2. Adhesion Tests
2.3.3. Statistical Analysis
3. Results and Discussion
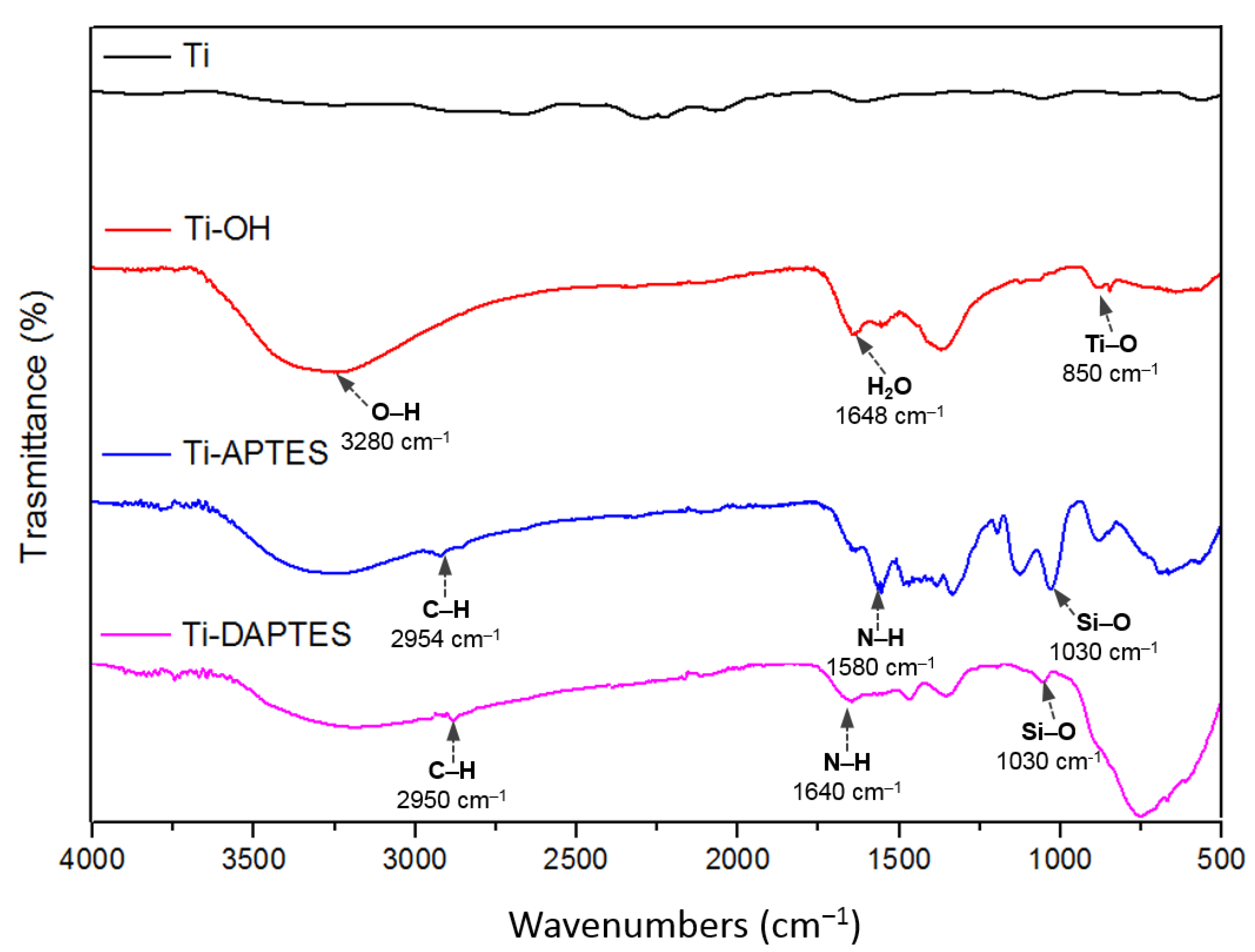
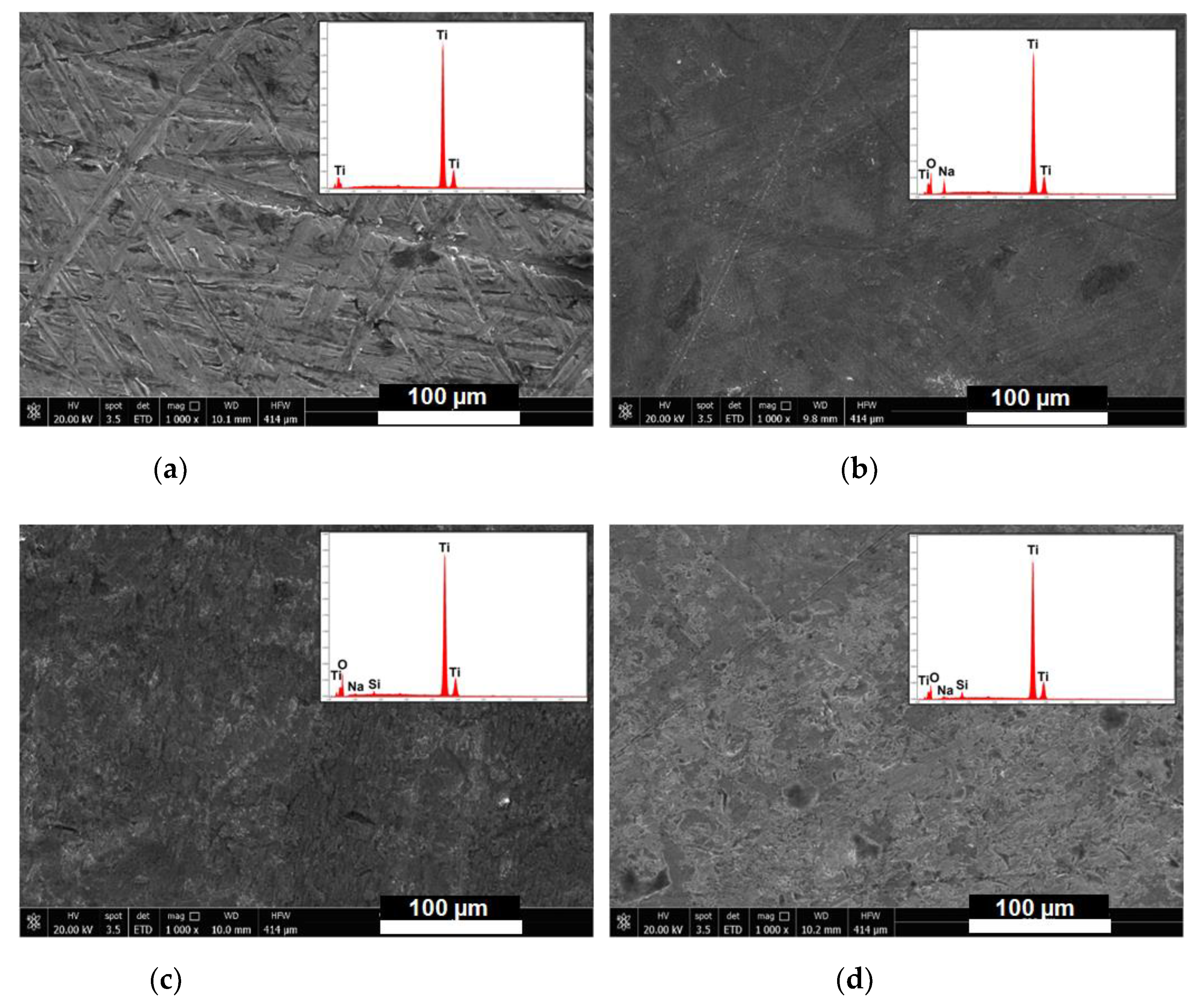
3.1. Titanium Discs Activation
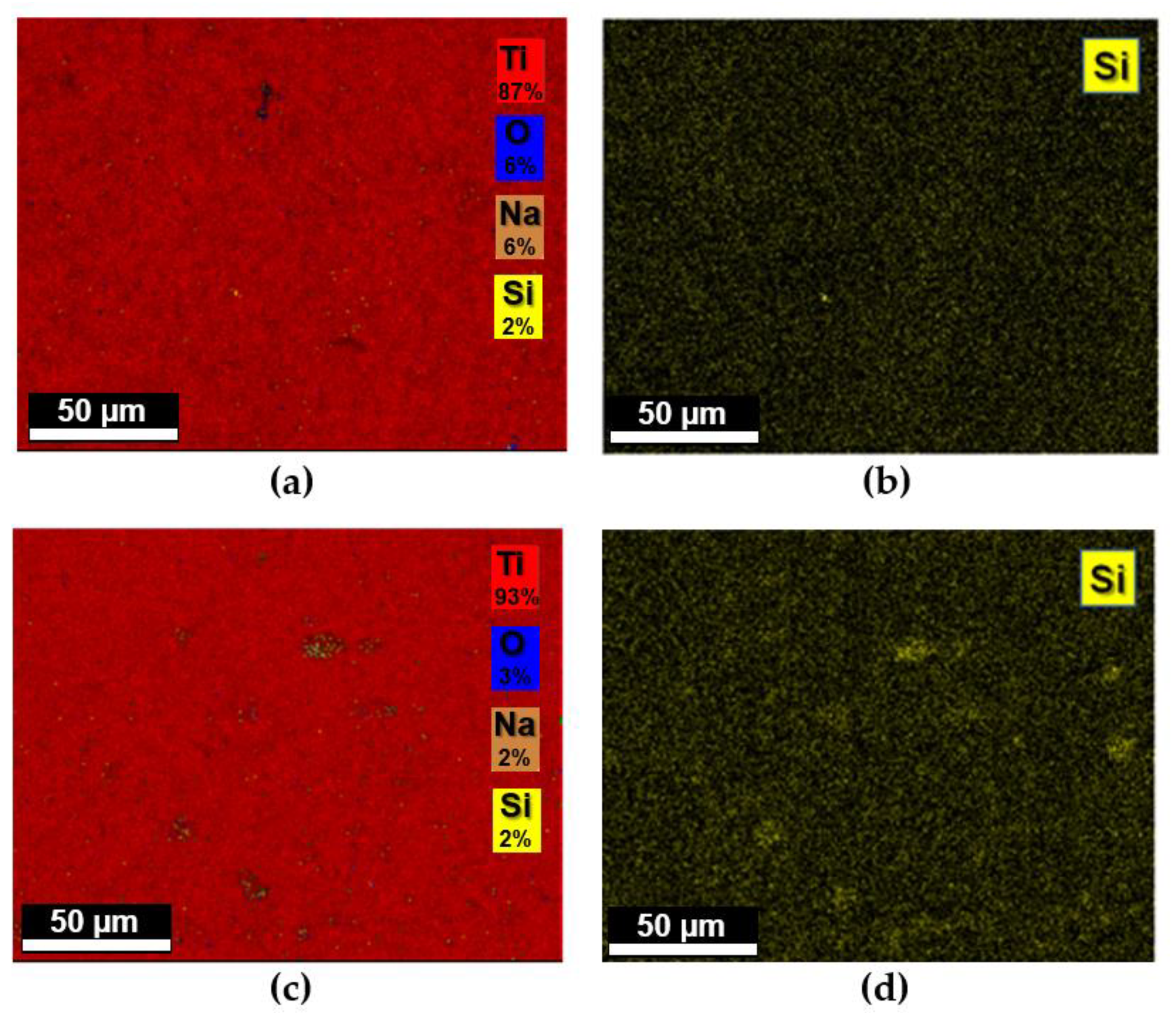
3.2. Titanium Discs Functionalization
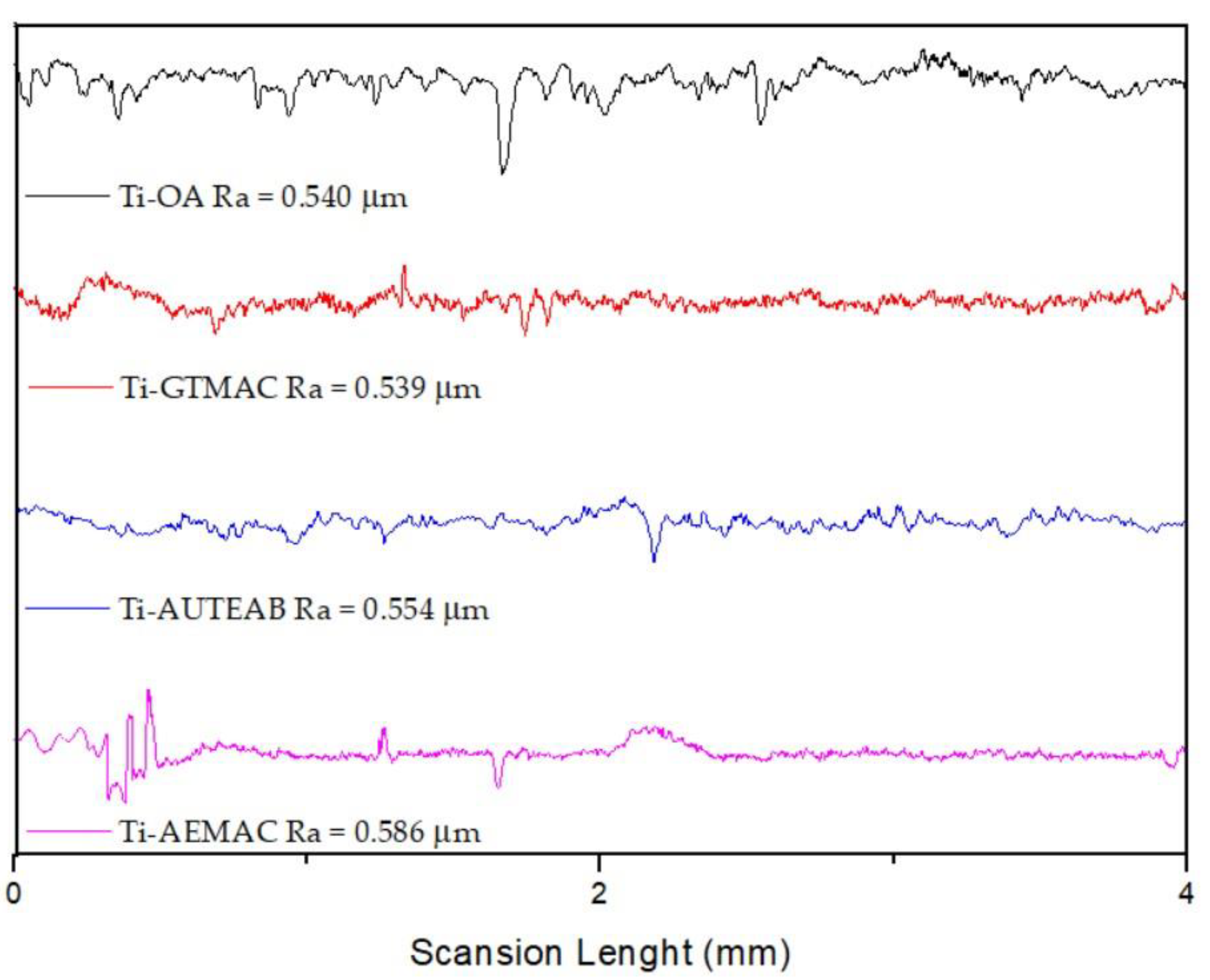
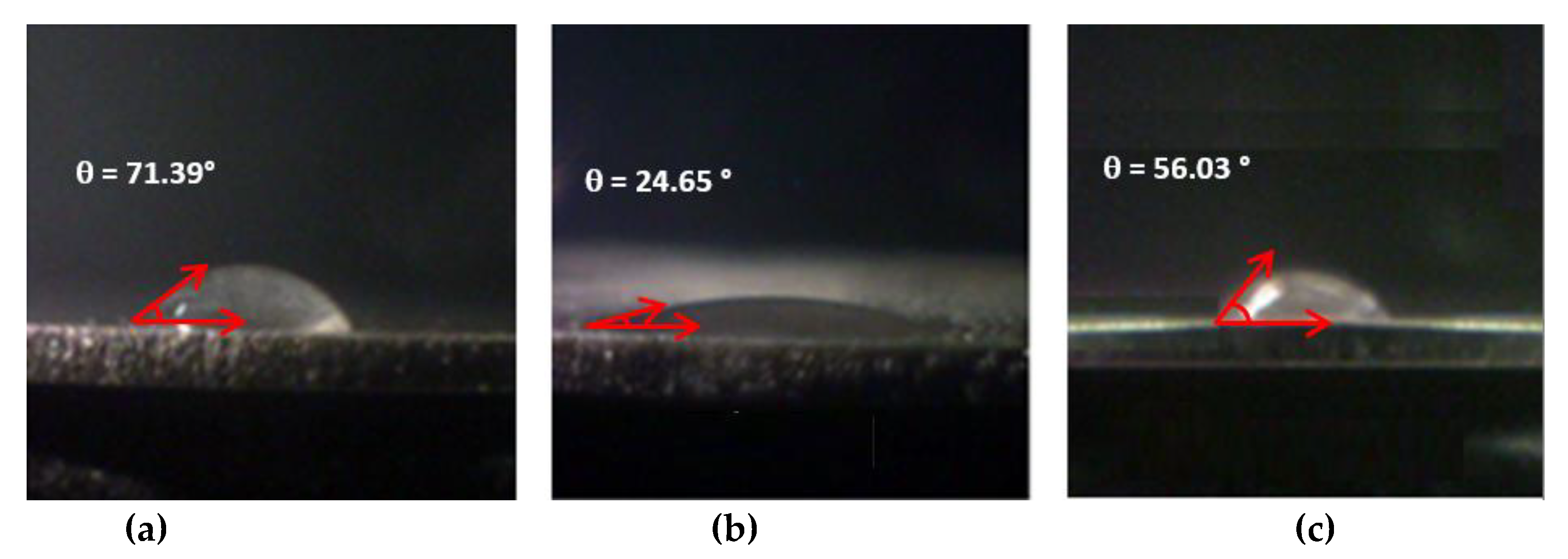
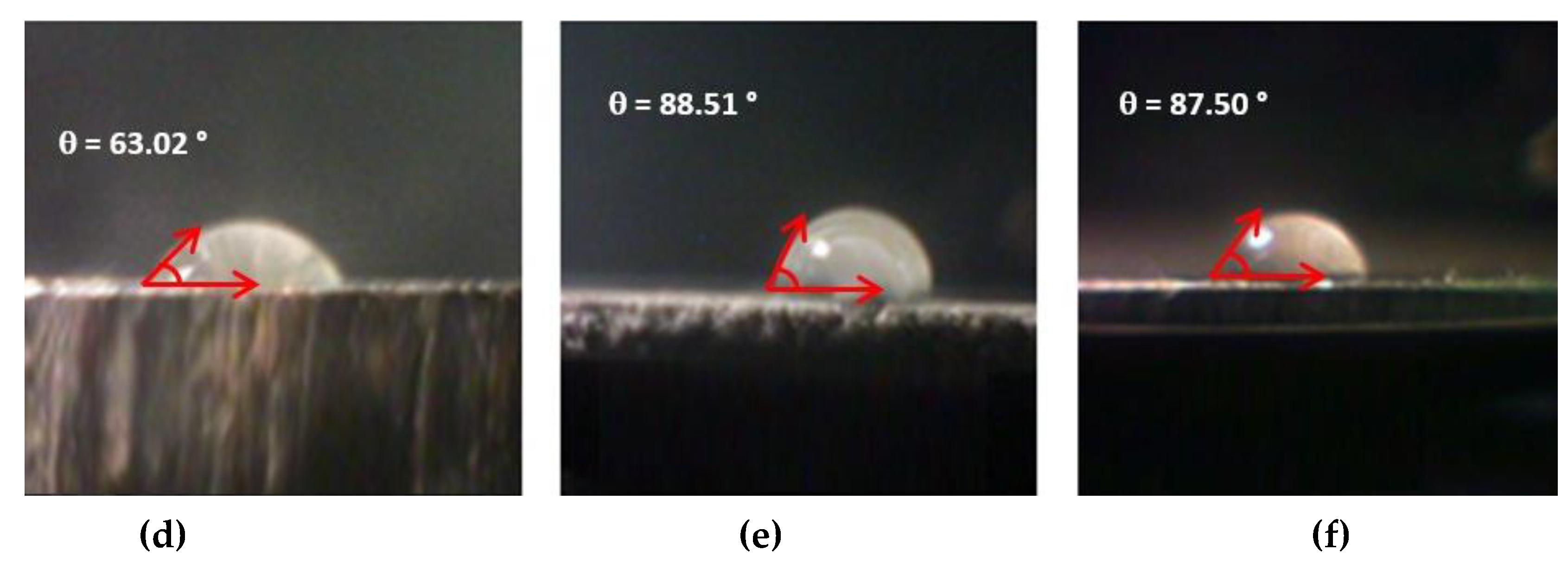
3.3. Roughness and Contact Angle Measurements
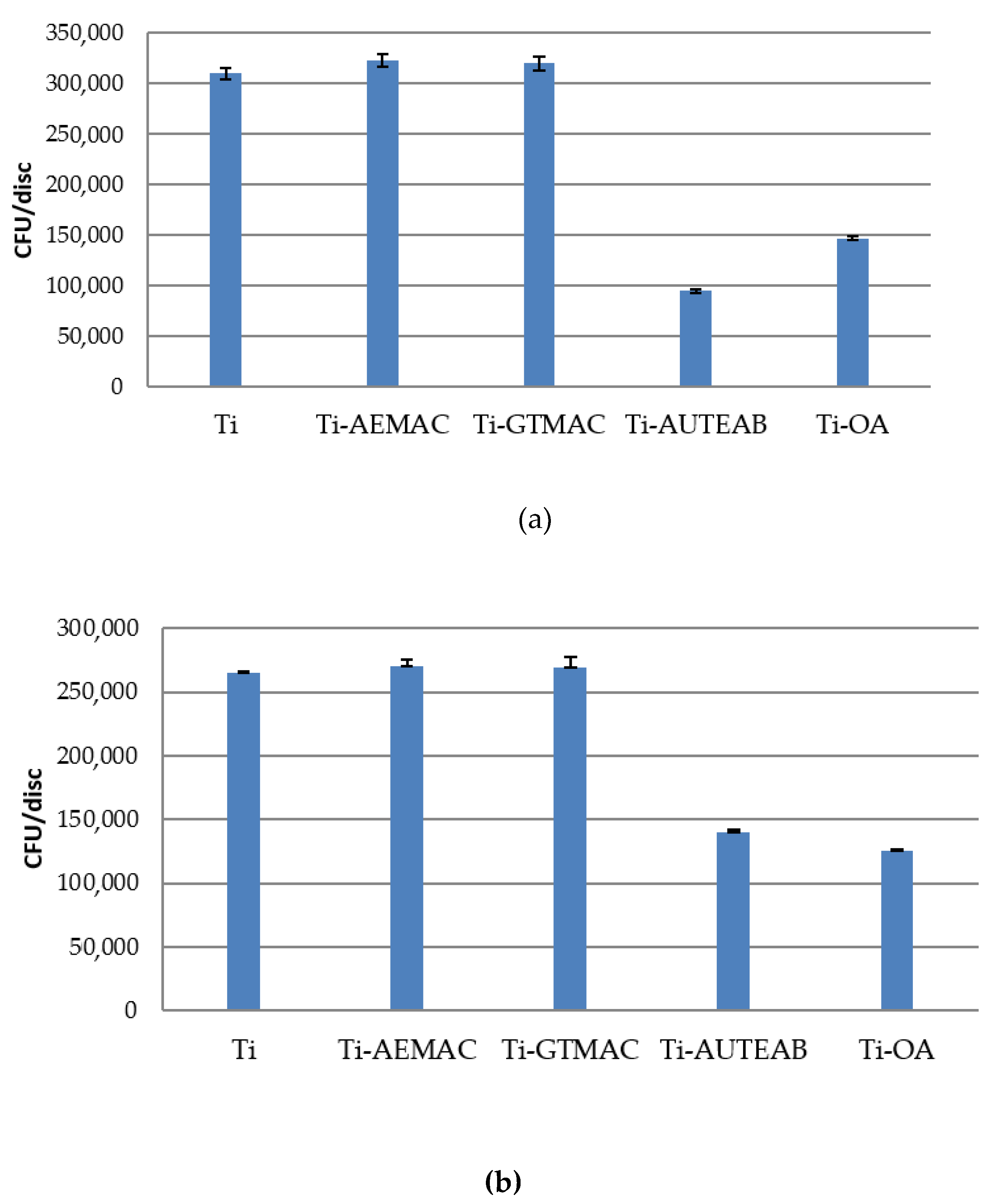
3.4. Antibacterial Activity of Functionalized Titanium Discs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, W.; Yu, C. Wearable and Implantable Devices for Healthcare. Adv. Healthc. Mater. 2021, 10, 2101548. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. Titanium-tissue interface reaction and its control with surface treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legnani, L.; Iannazzo, D.; Pistone, A.; Celesti, C.; Giofrè, S.; Romeo, R.; Di Pietro, A.; Visalli, G.; Fresta, M.; Bottino, P.; et al. Functionalized polyhedral oligosilsesquioxane (POSS) based composites for bone tissue engineering: Synthesis, computational and biological studies. RSC Adv. 2020, 10, 11325–11334. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine. In Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 9783642631191. [Google Scholar]
- Zhao, X.; You, L.; Wang, T.; Zhang, X.; Li, Z.; Ding, L.; Li, J.; Xiao, C.; Han, F.; Li, B. Enhanced osseointegration of titanium implants by surface modification with silicon-doped titania nanotubes. Int. J. Nanomed. 2020, 15, 8583–8594. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef]
- Algraffee, H.; Borumandi, F.; Cascarini, L. Peri-implantitis. Br. J. Oral Maxillofac. Surg. 2012, 50, 689–694. [Google Scholar] [CrossRef]
- Josyula, A.; Parikh, K.S.; Pitha, I.; Ensign, L.M. Engineering biomaterials to prevent post-operative infection and fibrosis. Drug Deliv. Transl. Res. 2021, 11, 1675–1688. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F.; Harris, L.; Douglas, J. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Fernández-Calderón, M.C.; Pérez-Giraldo, C.; Manero, J.M.; Albericio, F.; Gil, F.J.; Rodríguez, D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014, 10, 3522–3534. [Google Scholar] [CrossRef] [PubMed]
- Cheriker, H.; Stern Bauer, T.; Oren, Y.; Nir, S.; Hayouka, Z. Immobilized random peptide mixtures exhibit broad antimicrobial activity with high selectivity. Chem. Commun. 2020, 56, 11022–11025. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Chen, C.; Zhu, J.; Allcock, H.R.; Siedlecki, C.A.; Xu, L.C. Inhibition of bacterial adhesion and biofilm formation by a textured fluorinated alkoxyphosphazene surface. Bioact. Mater. 2021, 6, 447–459. [Google Scholar] [CrossRef]
- Wu, J.; Lin, W.; Wang, Z.; Chen, S.; Chang, Y. Investigation of the hydration of nonfouling material poly(sulfobetaine methacrylate) by low-field nuclear magnetic resonance. Langmuir 2012, 28, 7436–7441. [Google Scholar] [CrossRef]
- Nejadnik, M.R.; van der Mei, H.C.; Norde, W.; Busscher, H.J. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials 2008, 29, 4117–4121. [Google Scholar] [CrossRef]
- Glinel, K.; Thebault, P.; Humblot, V.; Pradier, C.M.; Jouenne, T. Antibacterial surfaces developed from bio-inspired approaches. Acta Biomater. 2012, 8, 1670–1684. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [Green Version]
- Hanawa, T. Research and development of metals for medical devices based on clinical needs. Sci. Technol. Adv. Mater. 2012, 13, 064102. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Zumbuehl, A. Non-leaching surfaces capable of killing microorganisms on contact. J. Mater. Chem. 2009, 19, 7796–7806. [Google Scholar] [CrossRef]
- Chen, R.; Willcox, M.D.P.; Cole, N.; Ho, K.K.K.; Rasul, R.; Denman, J.A.; Kumar, N. Characterization of chemoselective surface attachment of the cationic peptide melimine and its effects on antimicrobial activity. Acta Biomater. 2012, 8, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, S.A.; Leong, S.S.J. Tethering antimicrobial peptides: Current status and potential challenges. Biotechnol. Adv. 2011, 29, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Willcox, M.D.P.; Hume, E.B.H.; Aliwarga, Y.; Kumar, N.; Cole, N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef]
- Fait, M.E.; Bakas, L.; Garrote, G.L.; Morcelle, S.R.; Saparrat, M.C.N. Cationic surfactants as antifungal agents. Appl. Microbiol. Biotechnol. 2019, 103, 97–112. [Google Scholar] [CrossRef]
- Mancuso, R.; Amuso, R.; Armentano, B.; Grasso, G.; Rago, V.; Cappello, A.R.; Galiano, F.; Figoli, A.; De Luca, G.; Hoinkis, J.; et al. Synthesis and Antibacterial Activity of Polymerizable Acryloyloxyalkyltriethyl Ammonium Salts. Chempluschem 2017, 82, 1235–1244. [Google Scholar] [CrossRef]
- Galiano, F.; Figoli, A.; Deowan, S.A.; Johnson, D.; Altinkaya, S.A.; Veltri, L.; De Luca, G.; Mancuso, R.; Hilal, N.; Gabriele, B.; et al. A step forward to a more efficient wastewater treatment by membrane surface modification via polymerizable bicontinuous microemulsion. J. Memb. Sci. 2015, 482, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Gadenne, V.; Lebrun, L.; Jouenne, T.; Thebault, P. Antiadhesive activity of ulvan polysaccharides covalently immobilized onto titanium surface. Colloids Surf. B Biointerfaces 2013, 112, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Galiano, F.; Mancuso, R.; Guzzo, M.G.; Lucente, F.; Gukelberger, E.; Losso, M.A.; Figoli, A.; Hoinkis, J.; Gabriele, B. New polymeric films with antibacterial activity obtained by UV-induced copolymerization of acryloyloxyalkyltriethylammonium salts with 2-hydroethyl methacrylate. Int. J. Mol. Sci. 2019, 20, 2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senna, P.M.; de Almeida Barros Mourão, C.F.; Mello-Machado, R.C.; Javid, K.; Montemezzi, P.; Cury, A.A.D.B.; Meirelles, L. Silane-coating strategy for titanium functionalization does not impair osteogenesis in vivo. Materials 2021, 14, 1814. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Neoh, K.G.; Shi, Z.; Kang, E.T. Assessment of stability of surface anchors for antibacterial coatings and immobilized growth factors on titanium. J. Colloid Interface Sci. 2013, 406, 238–246. [Google Scholar] [CrossRef]
- Das, S.; Goswami, A.; Murali, N.; Asefa, T. Efficient Tertiary Amine/Weak Acid Bifunctional Mesoporous Silica Catalysts for Michael Addition Reactions. ChemCatChem 2013, 5, 910–919. [Google Scholar] [CrossRef]
- Yi, J.H.; Bernard, C.; Variola, F.; Zalzal, S.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Characterization of a bioactive nanotextured surface created by controlled chemical oxidation of titanium. Surf. Sci. 2006, 600, 4613–4621. [Google Scholar] [CrossRef]
- Ehlert, M.; Radtke, A.; Roszek, K.; Jędrzejewski, T.; Piszczek, P. Assessment of titanate nanolayers in terms of their physicochemical and biological properties. Materials 2021, 14, 806. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D. Chemical and biological functionalization of titanium for dental implants. J. Mater. Chem. 2008, 18, 2404–2414. [Google Scholar] [CrossRef]
- Baharloo, B.; Textor, M.; Brunette, D.M. Substratum roughness alters the growth, area, and focal adhesions of epithelial cells, and their proximity to titanium surfaces. J. Biomed. Mater. Res.—Part A 2005, 74, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between Metal Oxides and Biomolecules: From Fundamental Understanding to Applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Lee, B.S.; Jhang, Y.T.; Ma, K.S.K.; Huang, C.P.; Fu, K.L.; Lai, C.H.; Tseng, W.Y.; Kuo, M.Y.P.; Chen, Y.W. Er:YAG laser irradiation enhances bacterial and lipopolysaccharide clearance and human gingival fibroblast adhesion on titanium discs. Sci. Rep. 2021, 11, 23954. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Subh, L.; Correa, W.; Pinkvos, T.J.; Behrens, P.; Brandenburg, K.; Gutsmann, T.; Stiesch, M.; Doll, K.; Winkel, A. Synthetic anti-endotoxin peptides interfere with Gram-positive and Gram-negative bacteria, their adhesion and biofilm formation on titanium. J. Appl. Microbiol. 2020, 129, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lin, X.; Zhao, H.; Chen, Z.; Yang, J.; Li, F.; Liu, C.; Tian, F. Surface functionalization of polyethersulfone membrane with quaternary ammonium salts for contact-active antibacterial and anti-biofouling properties. Materials 2016, 9, 376. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, A.; Ikeda, T.; Endo, T. A novel approach to mode of action of cationic biocides morphological effect on antibacterial activity. J. Appl. Bacteriol. 1995, 78, 55–60. [Google Scholar] [CrossRef]
- De Luca, G.; Amuso, R.; Figoli, A.; Mancuso, R.; Lucadamo, L.; Gabriele, B. Modeling of structure-property relationships of polymerizable surfactants with antimicrobial activity. Appl. Sci. 2018, 8, 1972. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celesti, C.; Gervasi, T.; Cicero, N.; Giofrè, S.V.; Espro, C.; Piperopoulos, E.; Gabriele, B.; Mancuso, R.; Lo Vecchio, G.; Iannazzo, D. Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials 2022, 15, 3283. https://doi.org/10.3390/ma15093283
Celesti C, Gervasi T, Cicero N, Giofrè SV, Espro C, Piperopoulos E, Gabriele B, Mancuso R, Lo Vecchio G, Iannazzo D. Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials. 2022; 15(9):3283. https://doi.org/10.3390/ma15093283
Chicago/Turabian StyleCelesti, Consuelo, Teresa Gervasi, Nicola Cicero, Salvatore Vincenzo Giofrè, Claudia Espro, Elpida Piperopoulos, Bartolo Gabriele, Raffaella Mancuso, Giovanna Lo Vecchio, and Daniela Iannazzo. 2022. "Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties" Materials 15, no. 9: 3283. https://doi.org/10.3390/ma15093283
APA StyleCelesti, C., Gervasi, T., Cicero, N., Giofrè, S. V., Espro, C., Piperopoulos, E., Gabriele, B., Mancuso, R., Lo Vecchio, G., & Iannazzo, D. (2022). Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials, 15(9), 3283. https://doi.org/10.3390/ma15093283