High-Entropy Borides under Extreme Environment of Pressures and Temperatures
Abstract
:1. Introduction
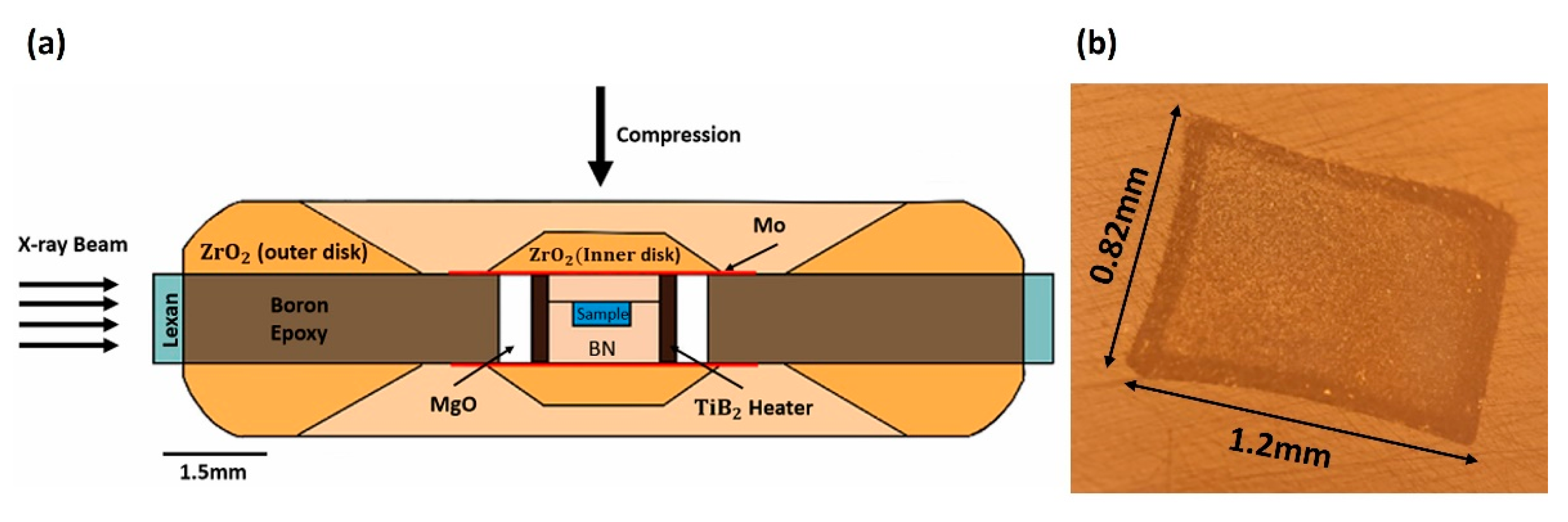
2. Materials and Methods
3. Results
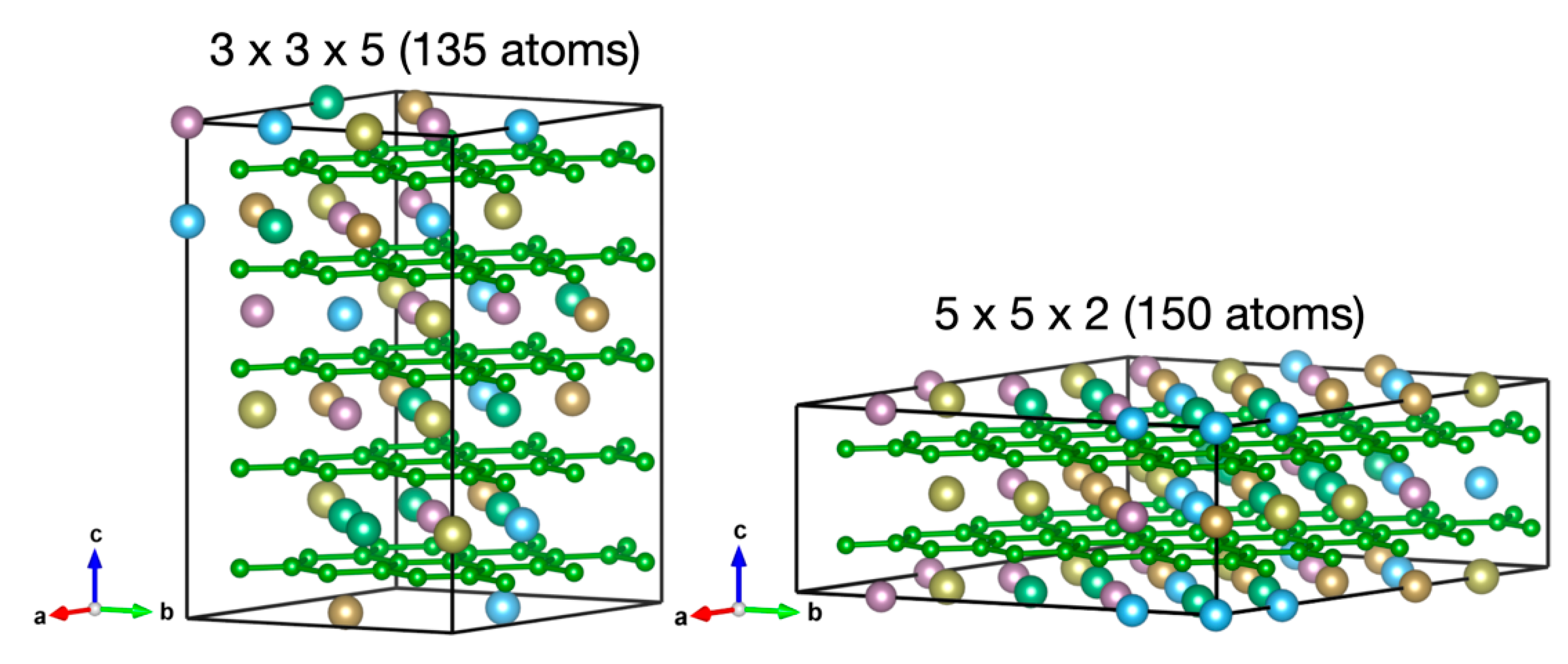
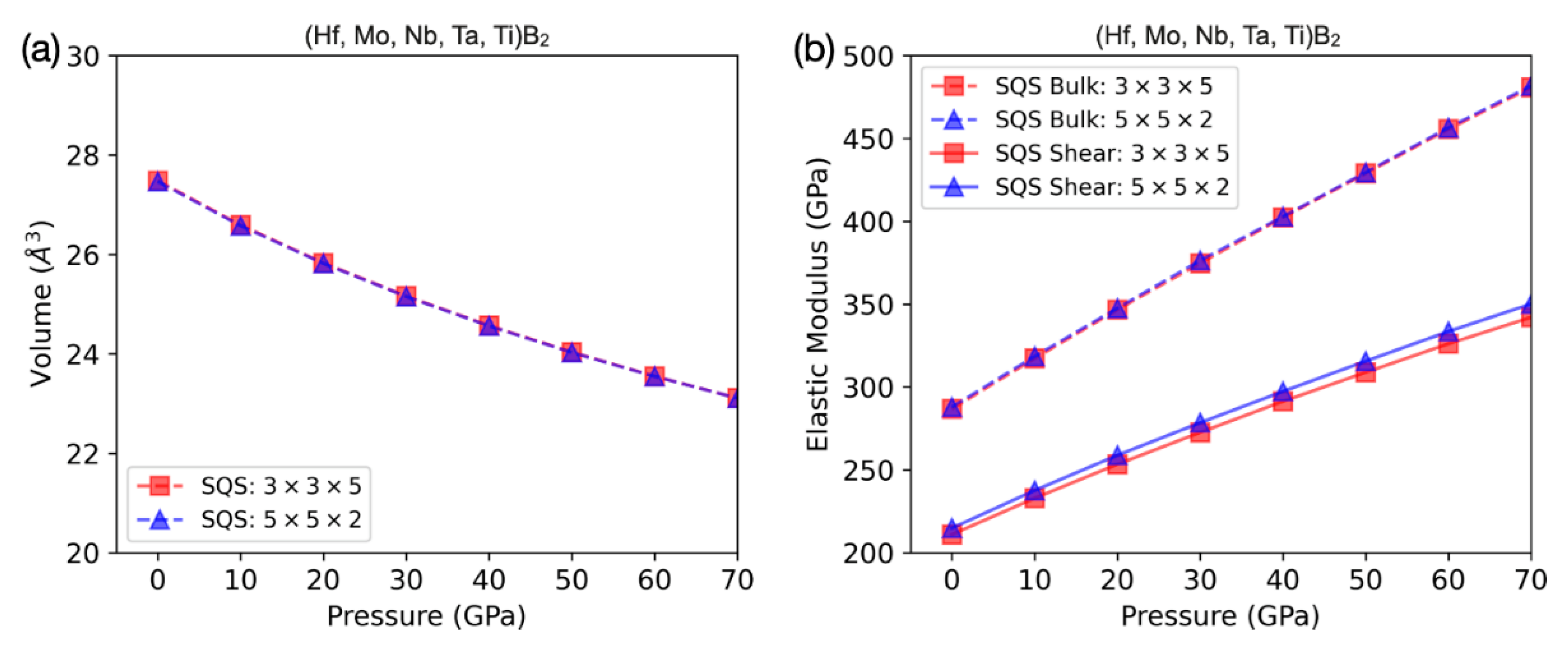
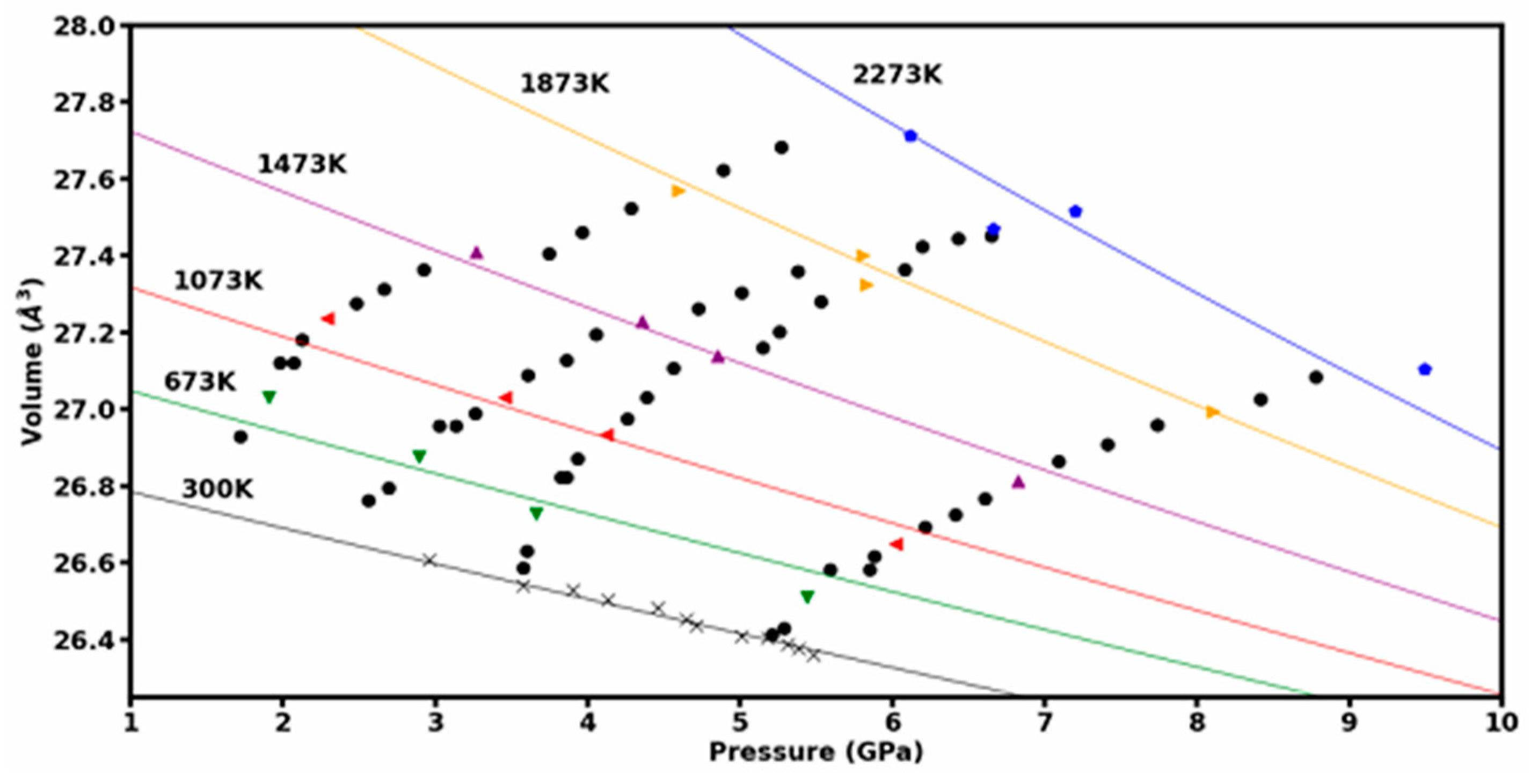
3.1. Theoretical Results
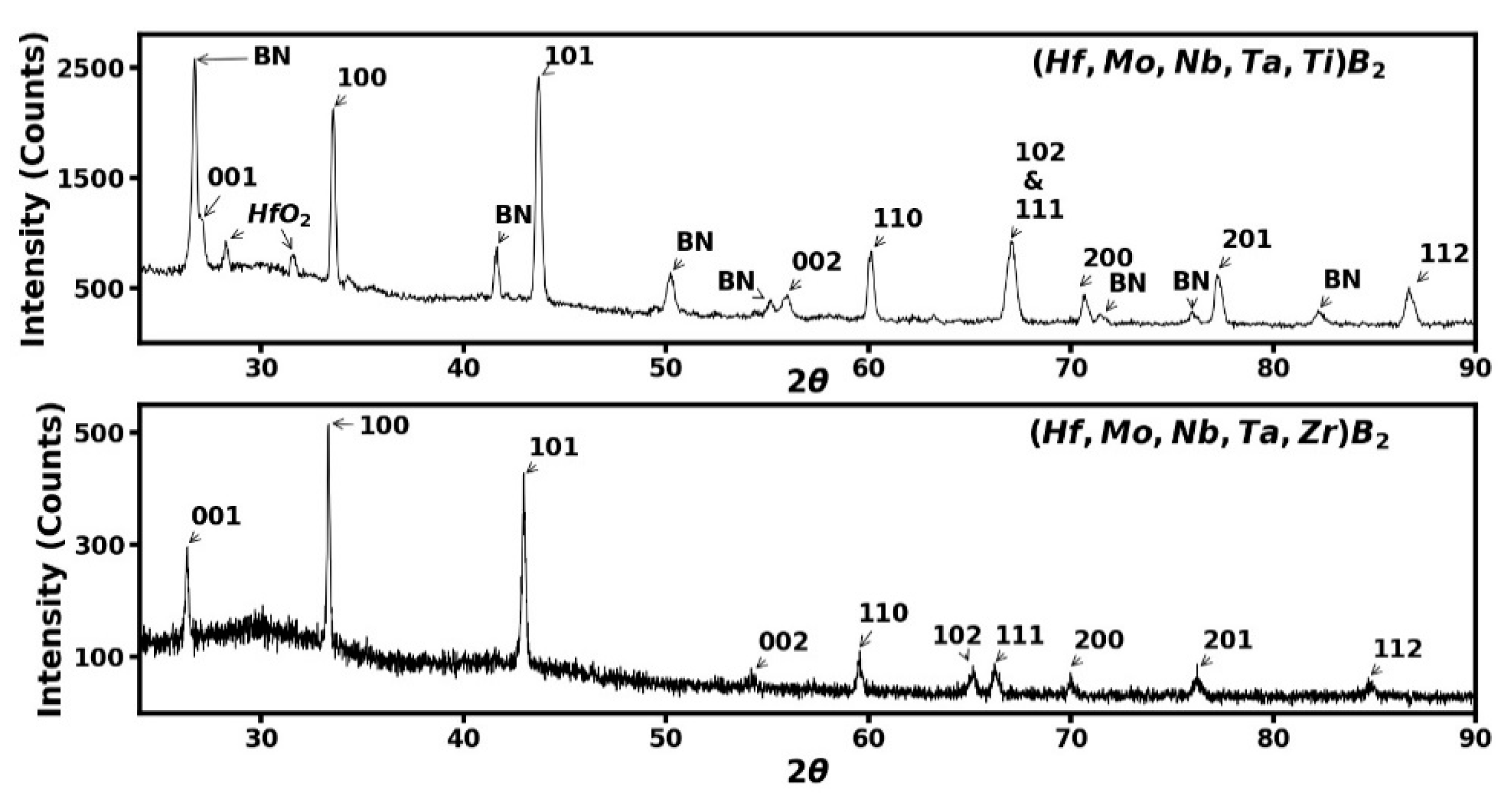
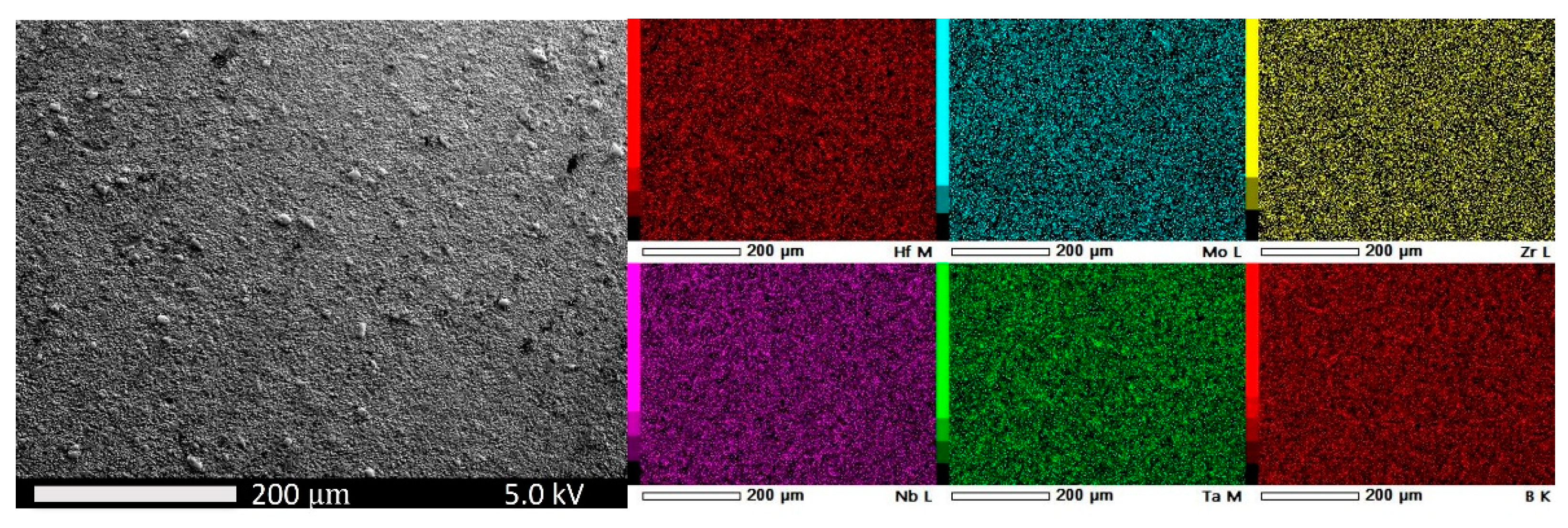
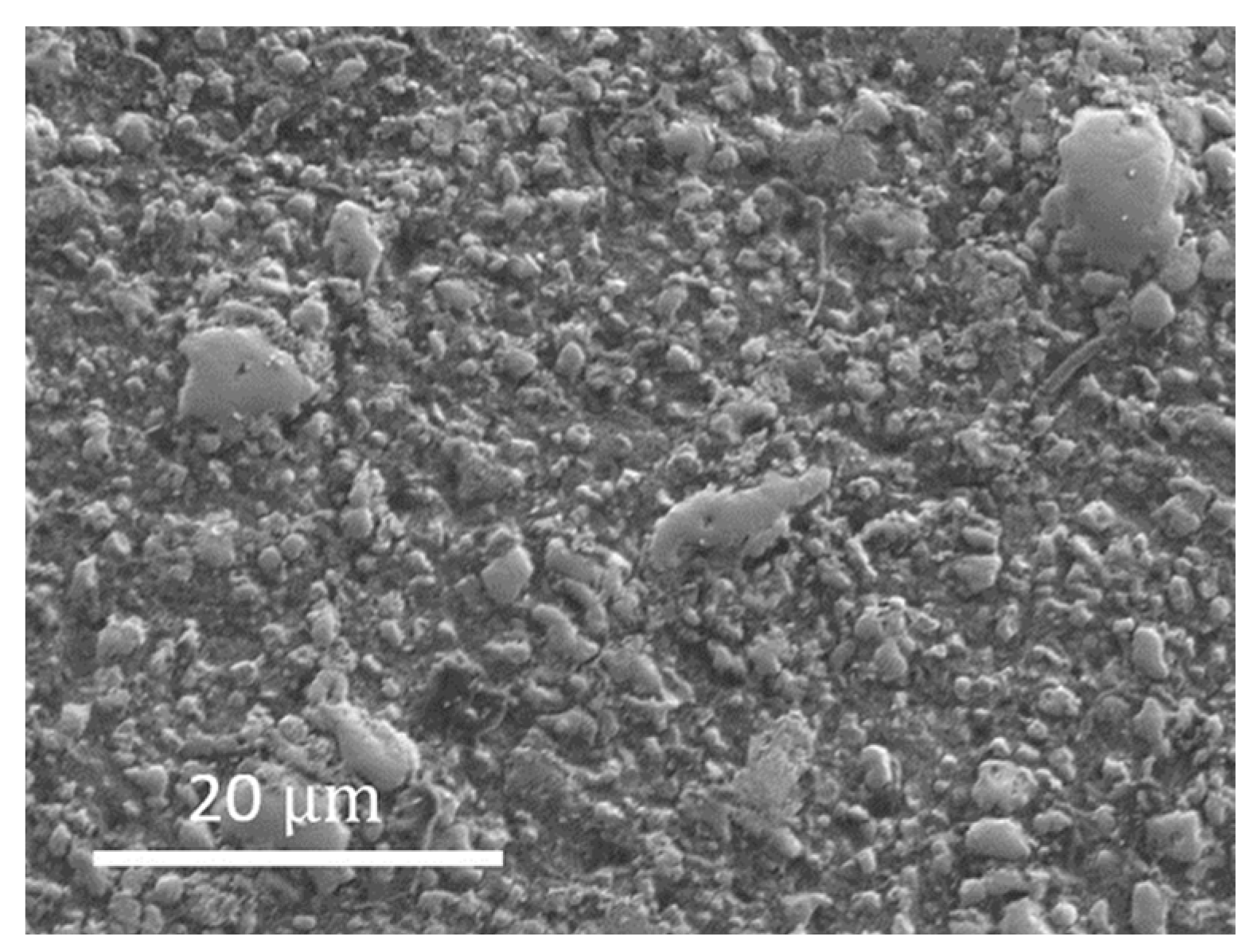
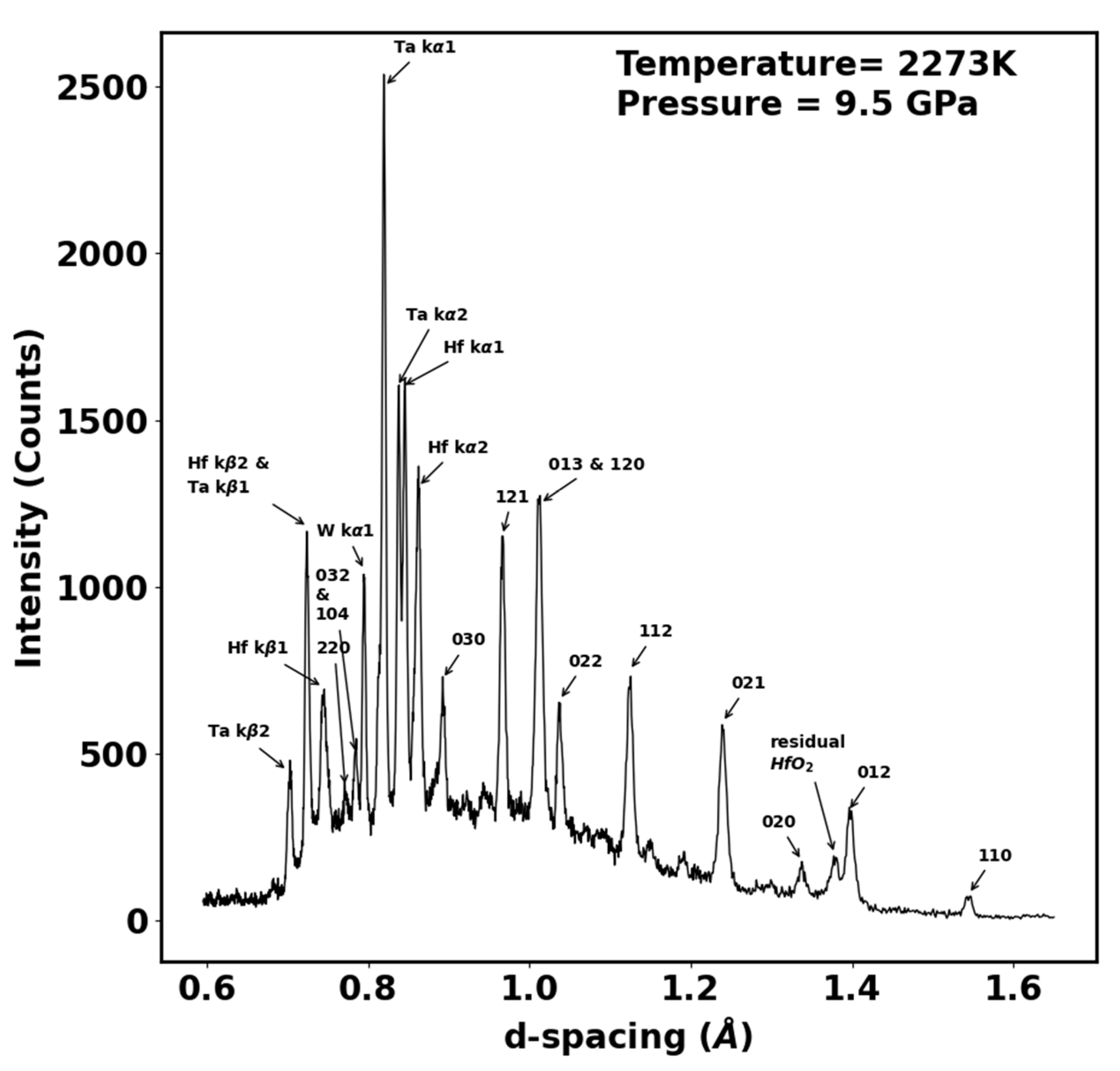
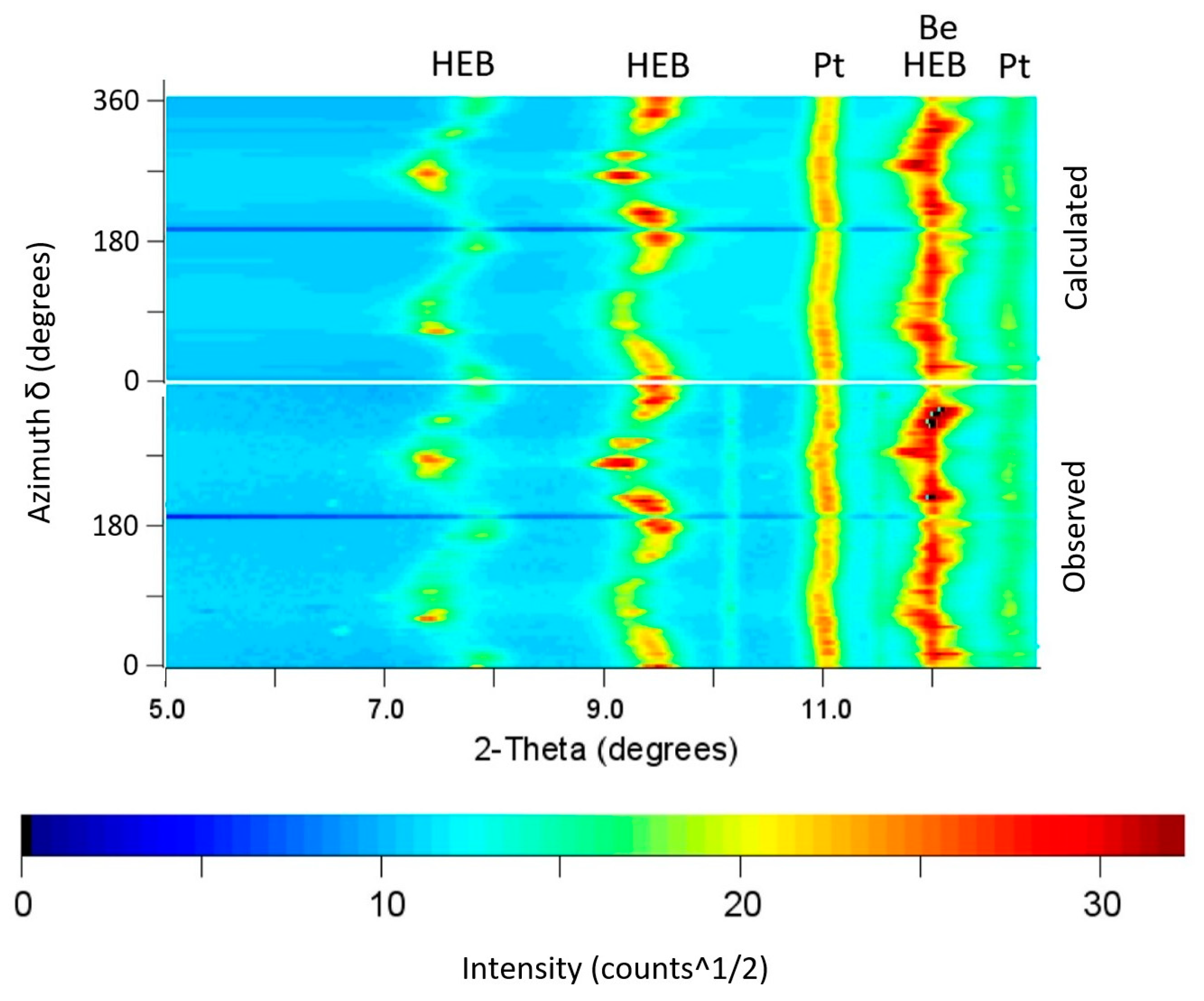
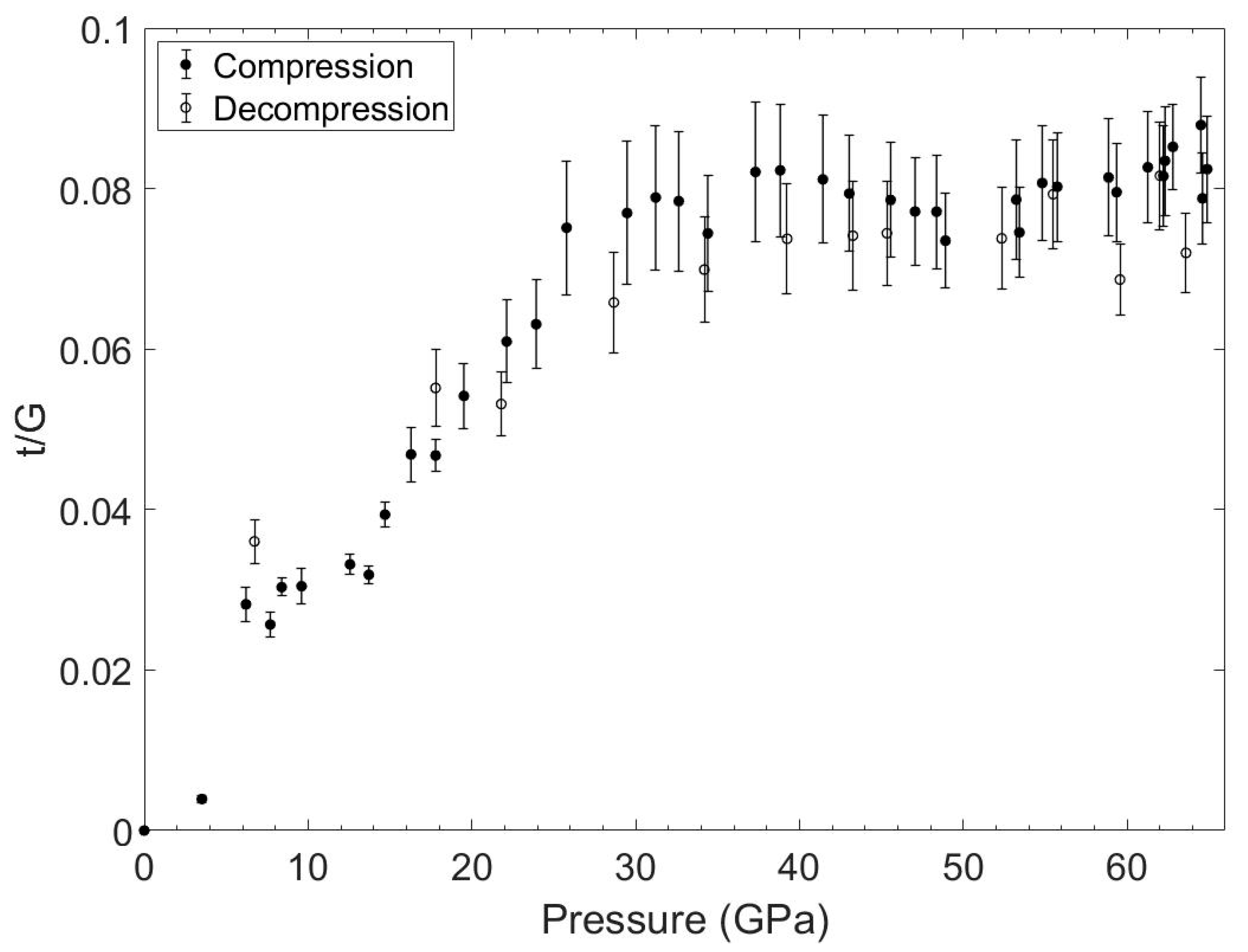
3.2. Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Jothery, H.K.M.; Albarody, T.M.B.; Yusoff, P.S.M.; Abdullah, M.A.; Hussein, A.R. A review of ultra-high temperature materials for thermal protection system. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kuantan, Malaysia, 1–2 October 2019; Volume 863, p. 012003. [Google Scholar]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.; Tang, Z.; Gao, M.; Dahmen, K.; Liaw, P.; Lu, Z. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Ishizu, N.; Kitawaga, J. New high-entropy alloy superconductor Hf21Nb25Ti15V15Zr24. Results Phys. 2019, 13, 102275. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef] [PubMed]
- Iwan, S.; Burrage, K.C.; Storr, B.C.; Catledge, S.A.; Vohra, Y.K.; Hrubiak, R.; Velisavljevic, N. High-pressure high-temperature synthesis, and thermal equation of state of high-entropy transition metal boride. AIP Adv. 2021, 11, 035107. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zunger, A.; Wei, S.-H.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Walle, A.; Tiwary, P.; De Jong, M.; Olmsted, D.L.; Asta, M.; Dick, A.; Shin, D.; Wang, Y.; Chen, L.Q.; Liu, Z.K. Efficient stochastic generation of special quasirandom structures. Calphad 2013, 42, 13–18. [Google Scholar] [CrossRef]
- Le Page, Y. and Saxe, Symmetry-general least-squares extraction of elastic data for strained materials from ab initio calculations of stress. Phys. Rev. B 2002, 65, 104104. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, G.; Zhang, B.; Yan, M.; Fu, Y. A theoretical study of the stability, mechanical and thermal properties of AlNiCuCo equimolar high entropy alloy. Phys. Lett. A 2020, 384, 126797. [Google Scholar] [CrossRef]
- Kono, Y.; Park, C.; Kenney-Benson, C.; Shen, G.; Wang, Y. Toward comprehensive studies of liquids at high pressures and high temperatures: Combined structure, elastic wave velocity, and viscosity measurements in the Paris–Edinburgh cell. Phys. Earth Planet. Inter. 2014, 228, 269. [Google Scholar] [CrossRef]
- Kono, Y.; Irifunea, T.; Higo, Y.; Inoue, T.; Barnhoorn, A. P-V-T relation of MgO derived by simultaneous elastic wave velocity and in situ X-ray measurements: A new pressure scale for the mantle transition region. Phys. Earth Planet. Inter. 2010, 183, 211. [Google Scholar] [CrossRef]
- Burrage, K.C.; Changyong, P.; Vohra, Y.K. Shear strength measurements and hydrostatic compression of rhenium diboride under high pressures. J. Appl. Phys. 2021, 129, 205901. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E. Two-step synthesis process for high-entropy diboride powders. J. Am. Ceram. Soc. 2019, 103, 724. [Google Scholar] [CrossRef]
- Fei, Y. Thermal Expansion. In Mineral Physics & Crystallography; American Geophysical Union (AGU): Washington DC, USA, 2013; pp. 29–44. [Google Scholar]
- Anderson, O.L. Equations of State of Solids for Geophysics and Ceramic Science; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Shen, G.; Mao, H.K. High-pressure studies with X-rays using diamond anvil cells. Rep. Prog. Phys. 2017, 80, 016101. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwan, S.; Lin, C.-M.; Perreault, C.; Chakrabarty, K.; Chen, C.-C.; Vohra, Y.; Hrubiak, R.; Shen, G.; Velisavljevic, N. High-Entropy Borides under Extreme Environment of Pressures and Temperatures. Materials 2022, 15, 3239. https://doi.org/10.3390/ma15093239
Iwan S, Lin C-M, Perreault C, Chakrabarty K, Chen C-C, Vohra Y, Hrubiak R, Shen G, Velisavljevic N. High-Entropy Borides under Extreme Environment of Pressures and Temperatures. Materials. 2022; 15(9):3239. https://doi.org/10.3390/ma15093239
Chicago/Turabian StyleIwan, Seth, Chia-Min Lin, Christopher Perreault, Kallol Chakrabarty, Cheng-Chien Chen, Yogesh Vohra, Rostislav Hrubiak, Guoyin Shen, and Nenad Velisavljevic. 2022. "High-Entropy Borides under Extreme Environment of Pressures and Temperatures" Materials 15, no. 9: 3239. https://doi.org/10.3390/ma15093239
APA StyleIwan, S., Lin, C.-M., Perreault, C., Chakrabarty, K., Chen, C.-C., Vohra, Y., Hrubiak, R., Shen, G., & Velisavljevic, N. (2022). High-Entropy Borides under Extreme Environment of Pressures and Temperatures. Materials, 15(9), 3239. https://doi.org/10.3390/ma15093239





