Dielectric Properties of Bi2/3Cu3Ti4O12 Ceramics Prepared by Mechanical Ball Milling and Low Temperature Conventional Sintering
Abstract
:1. Introduction
2. Experiment Procedure
2.1. Materials
2.2. Synthesis of Bi2/3Cu3Ti4O12 Ceramics
2.3. Characterization Methods
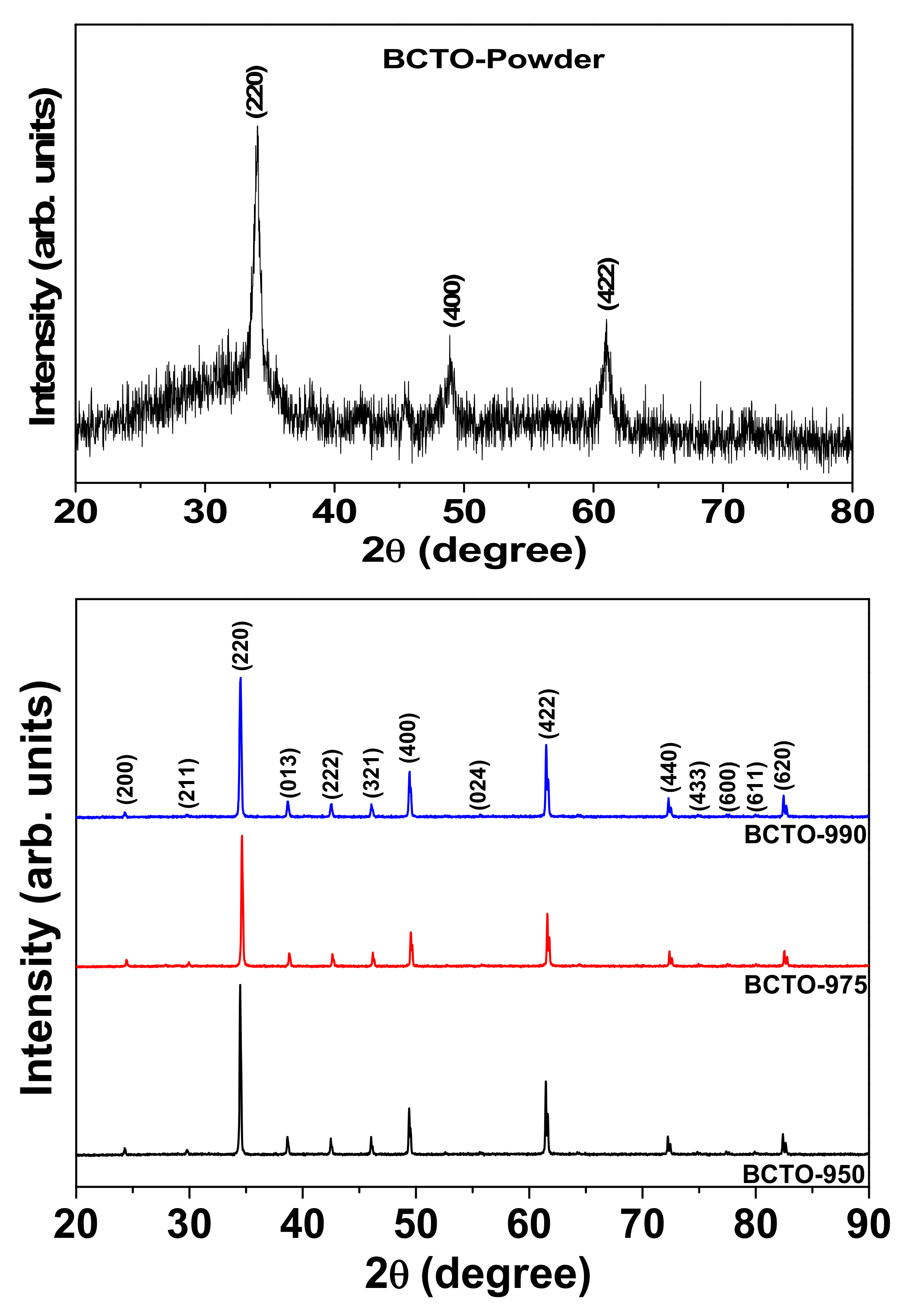
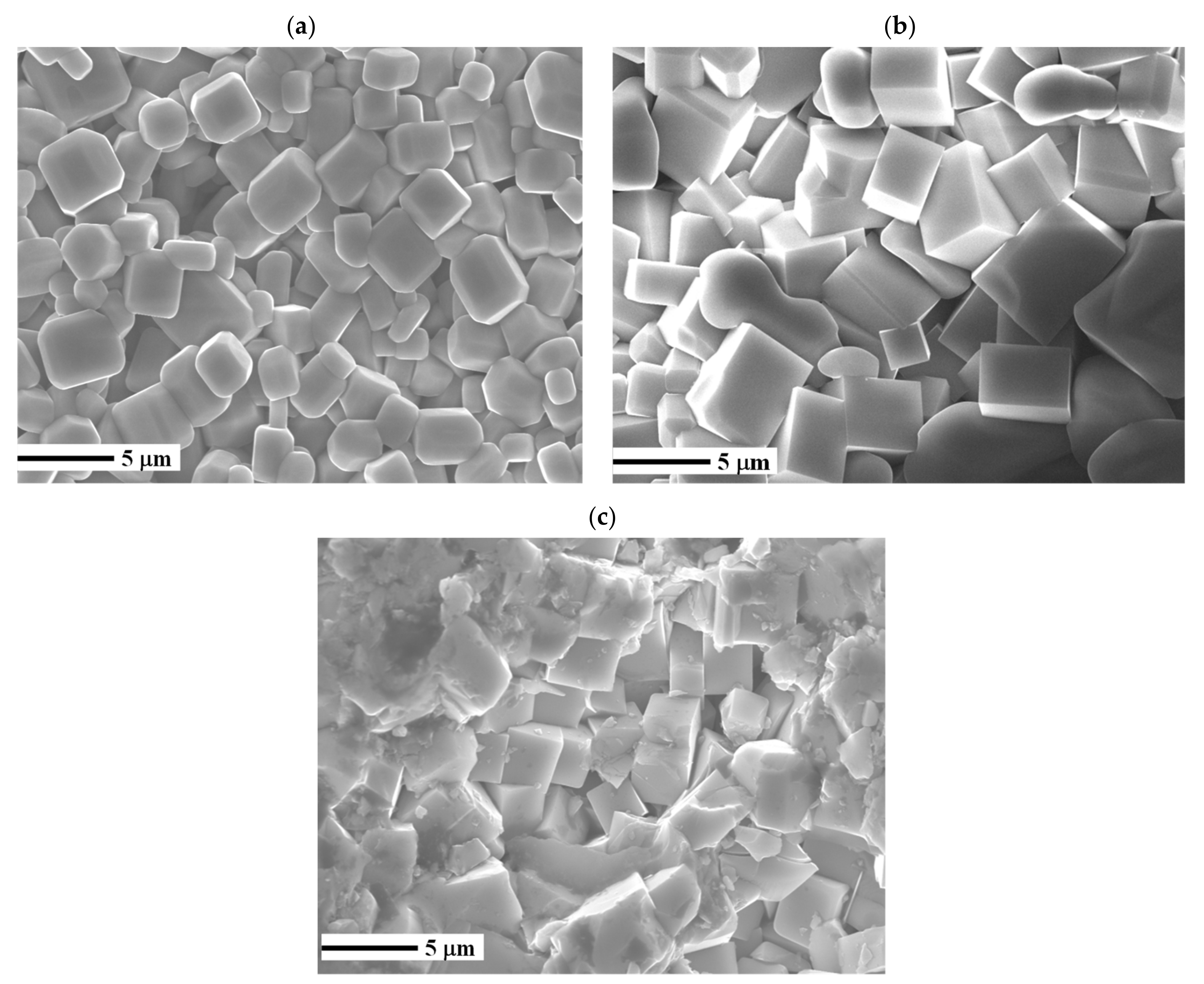
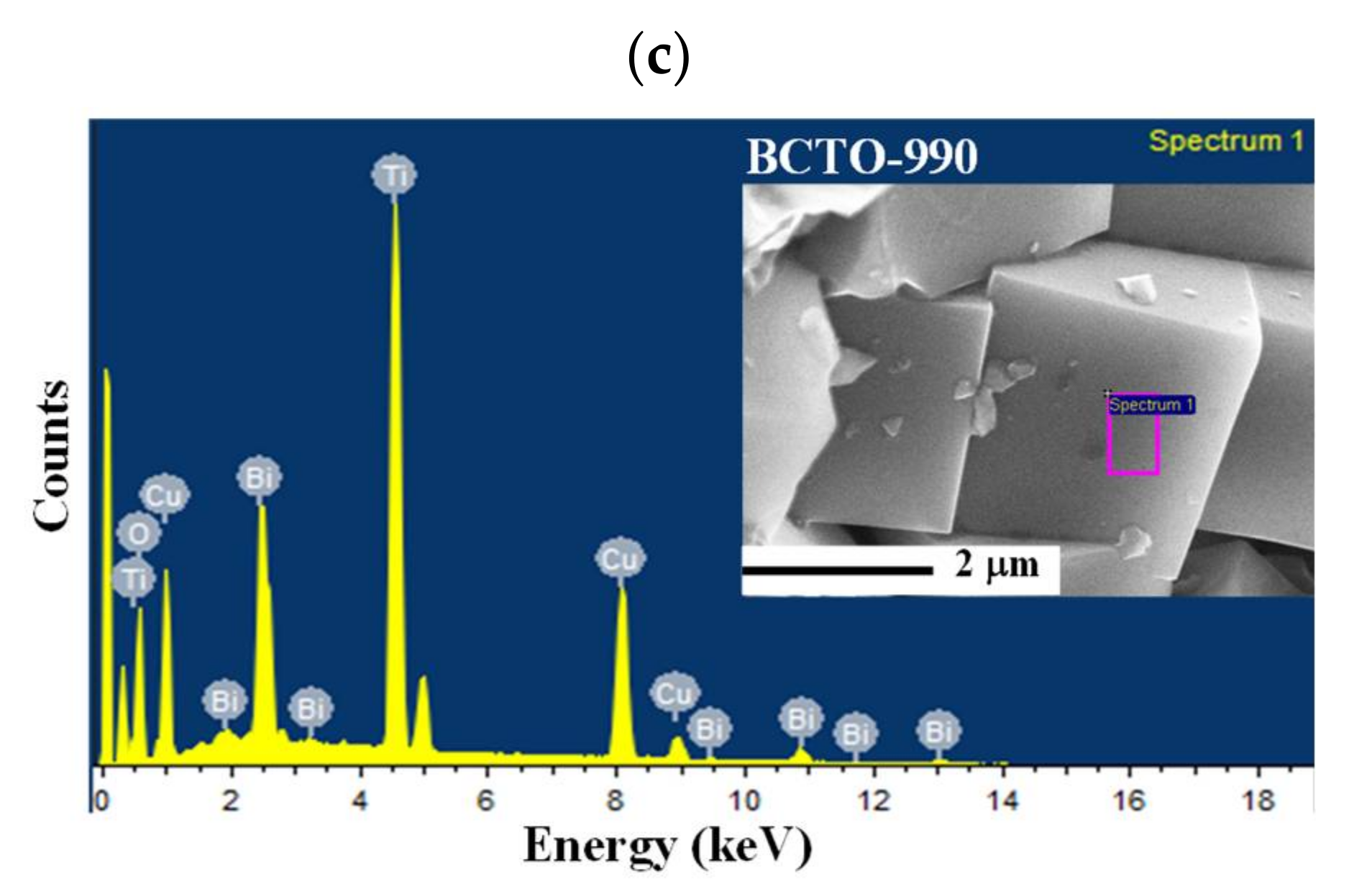
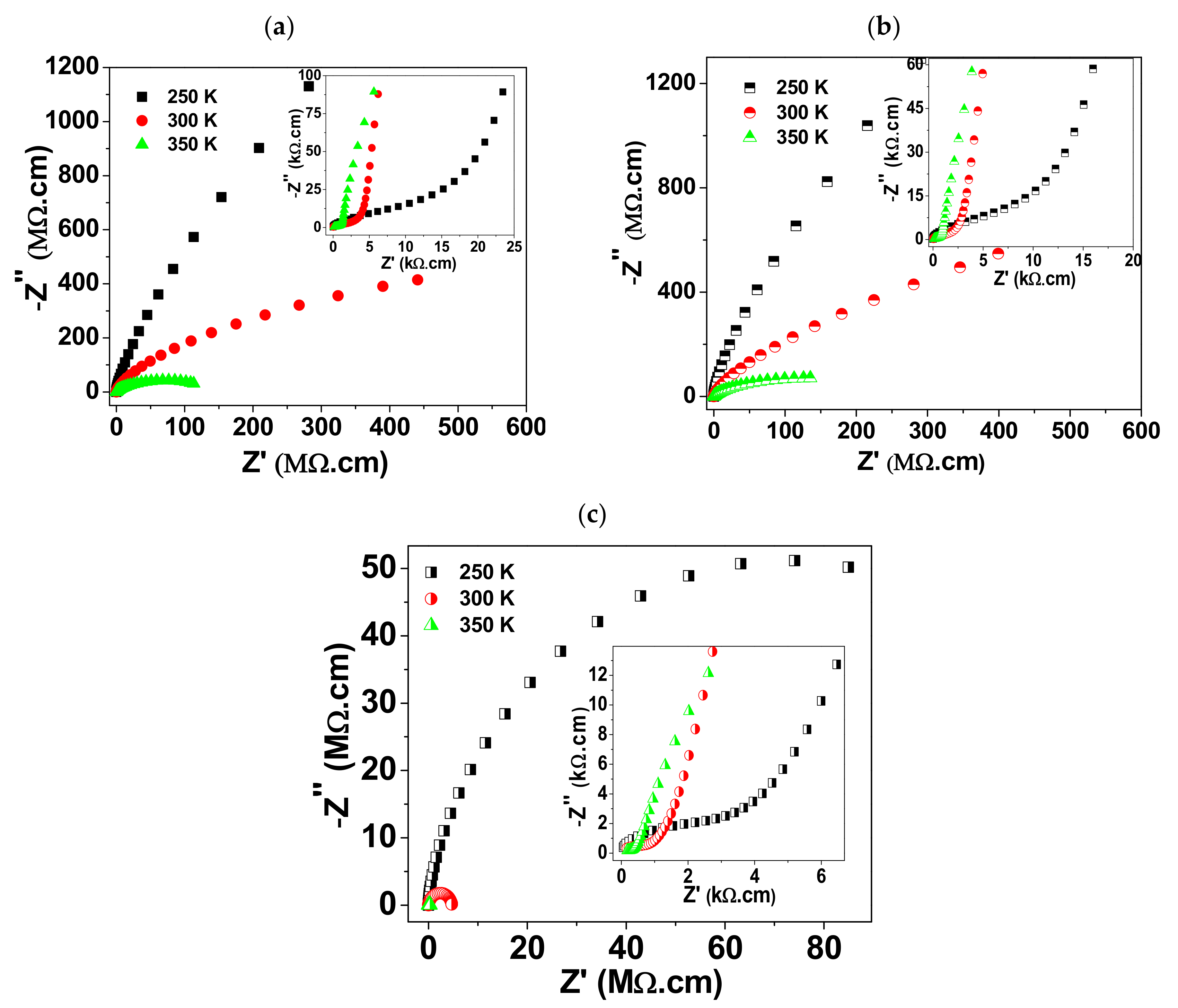
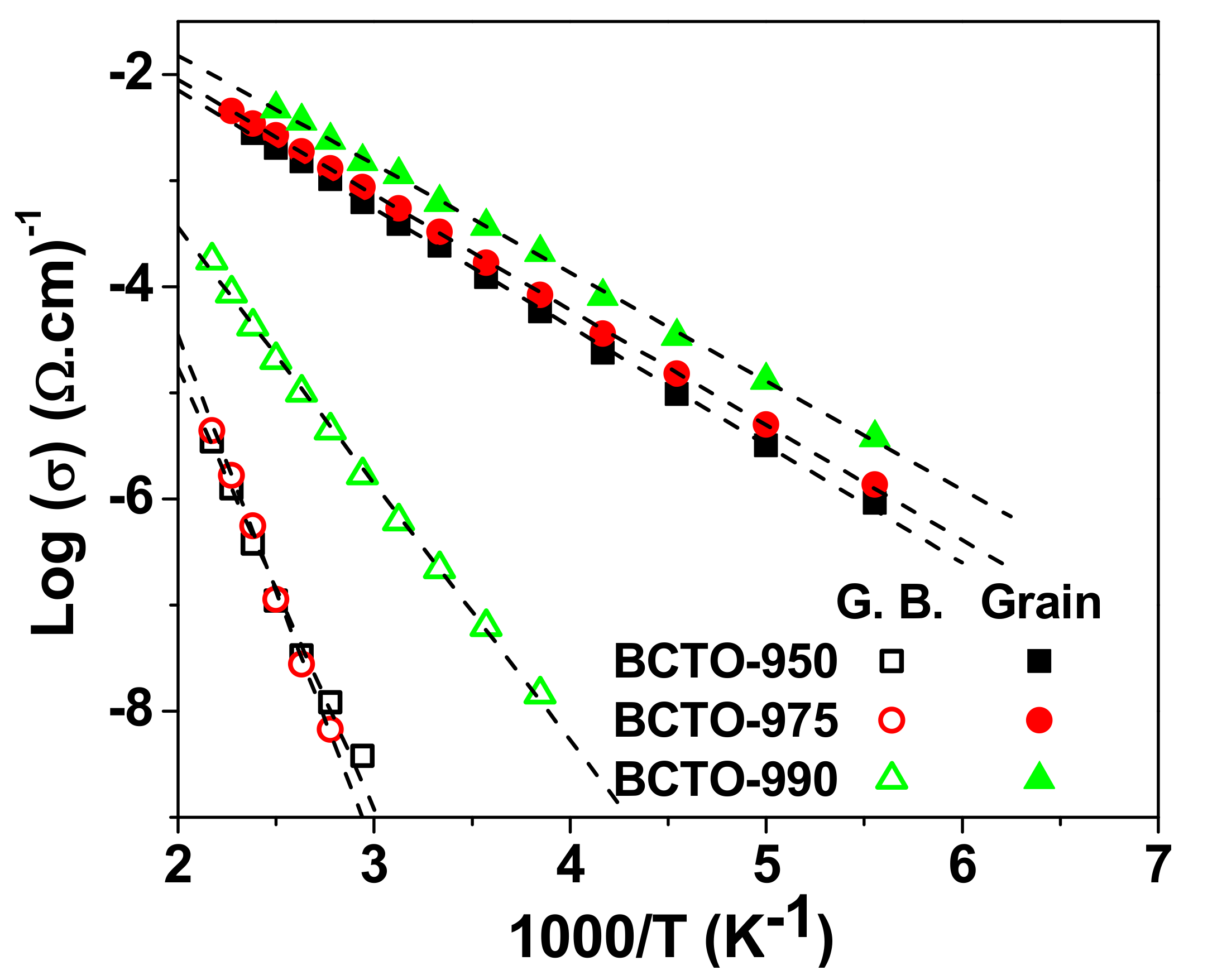
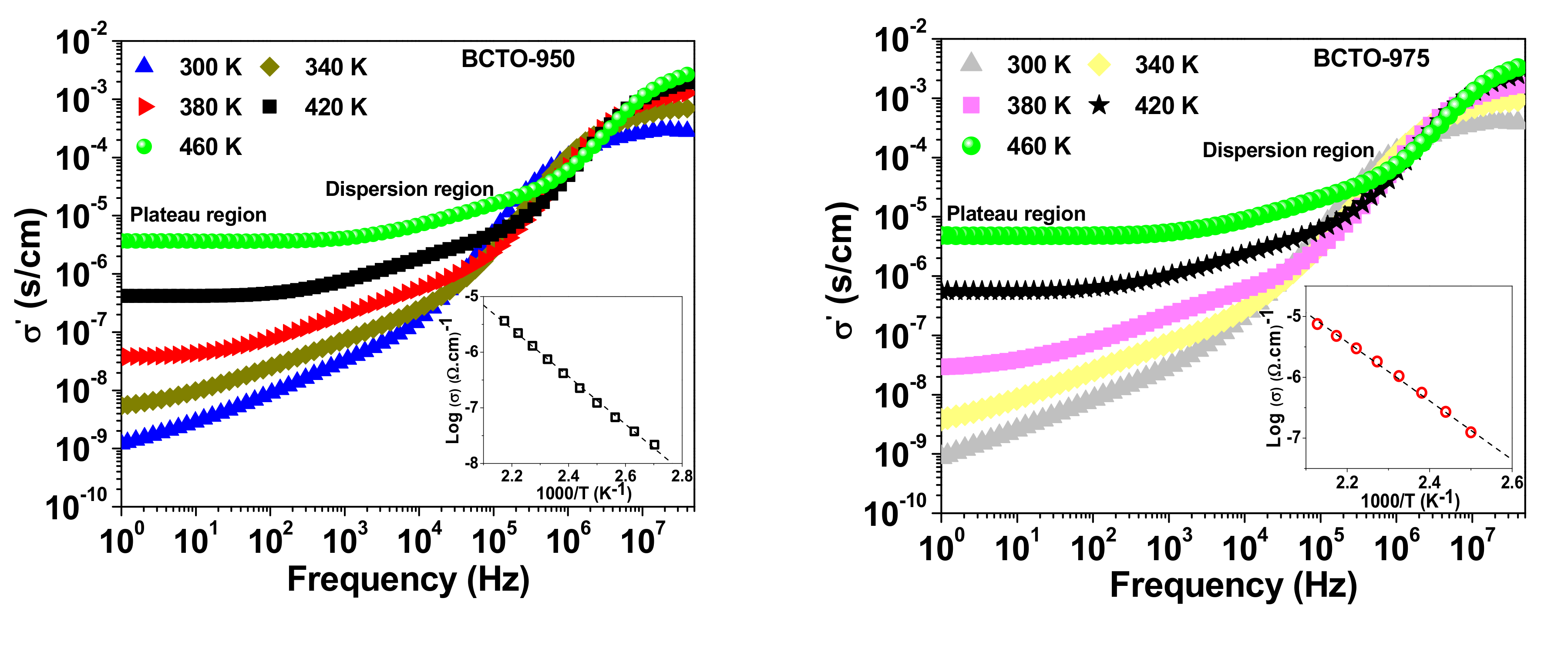
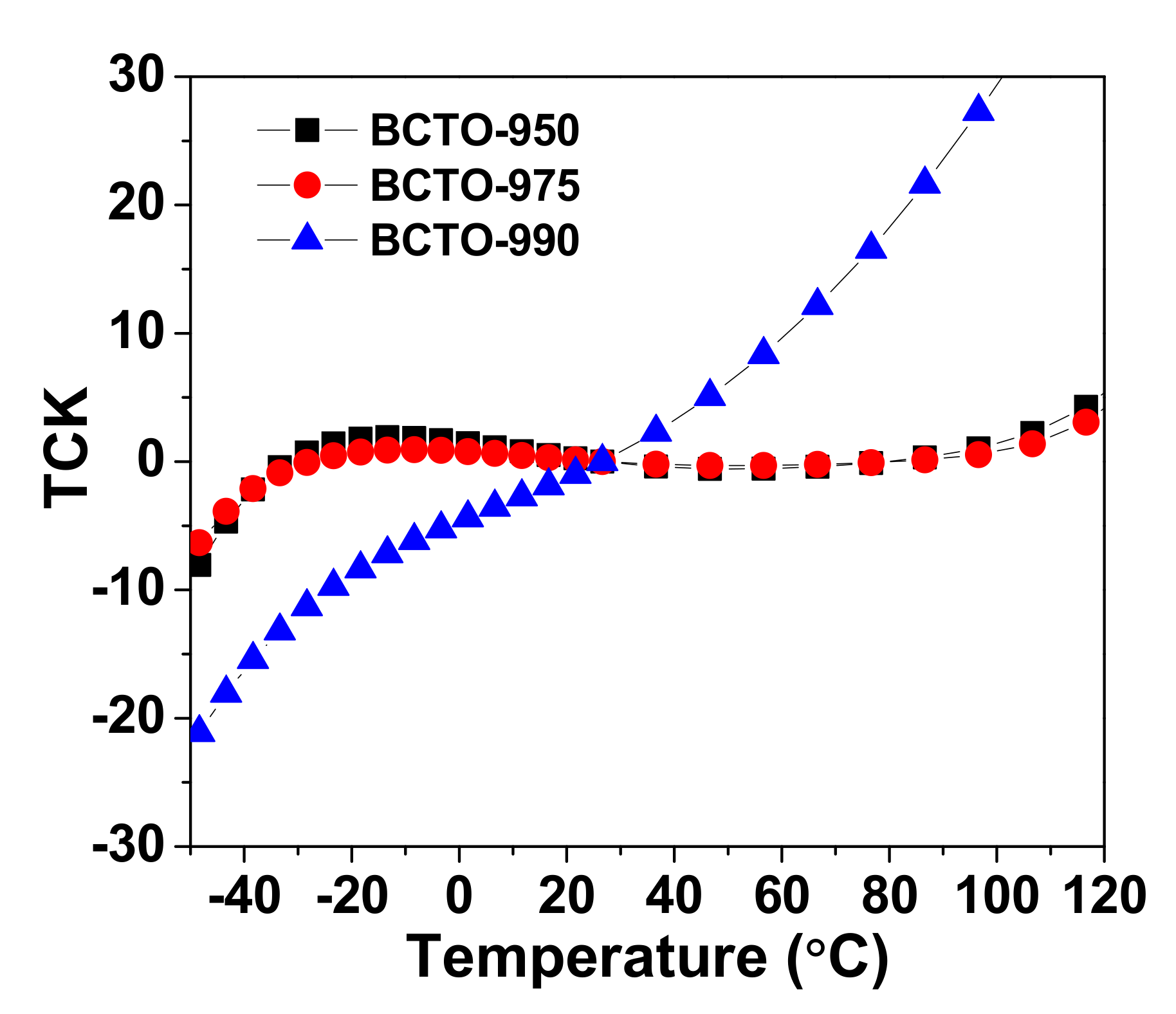
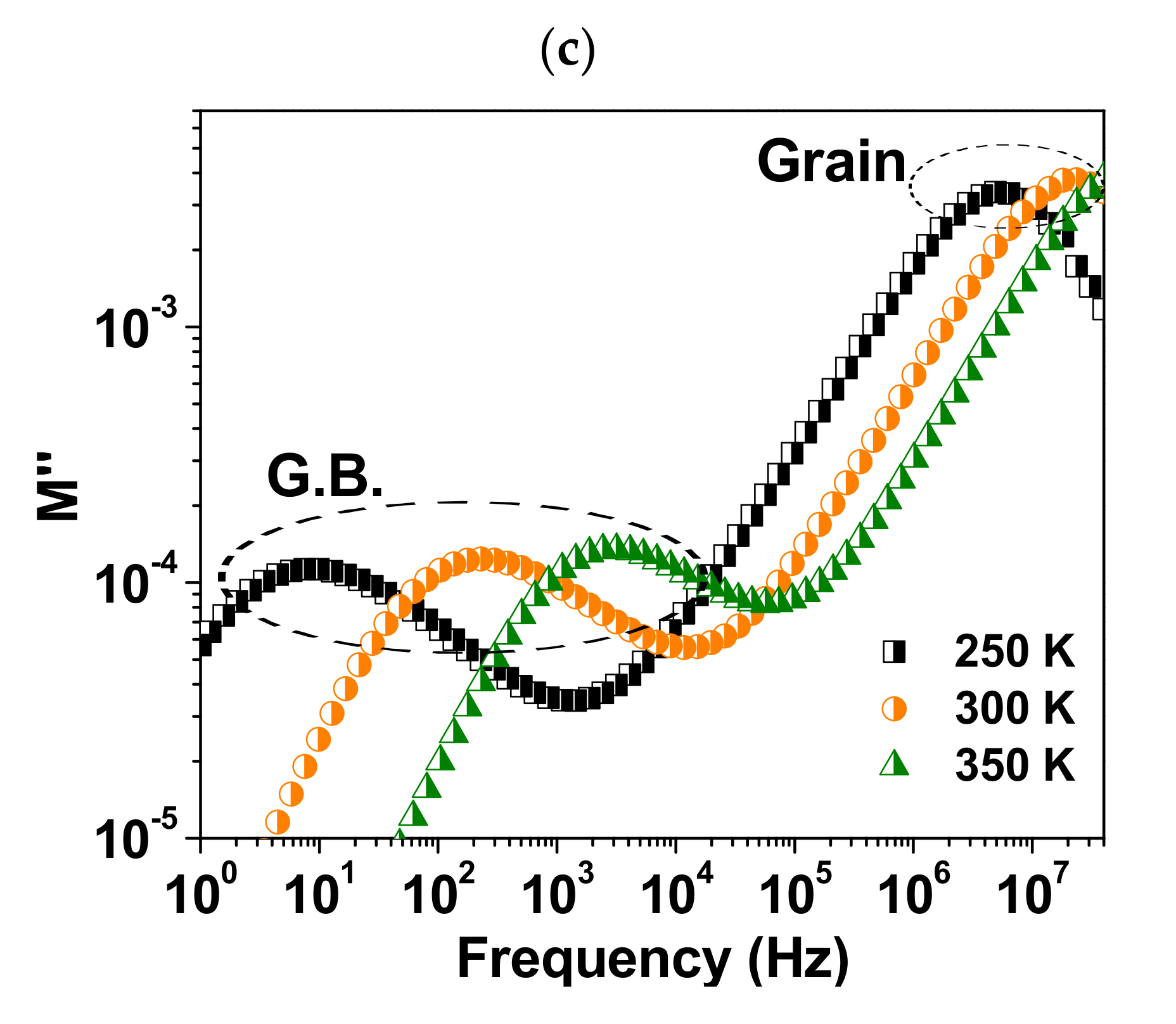
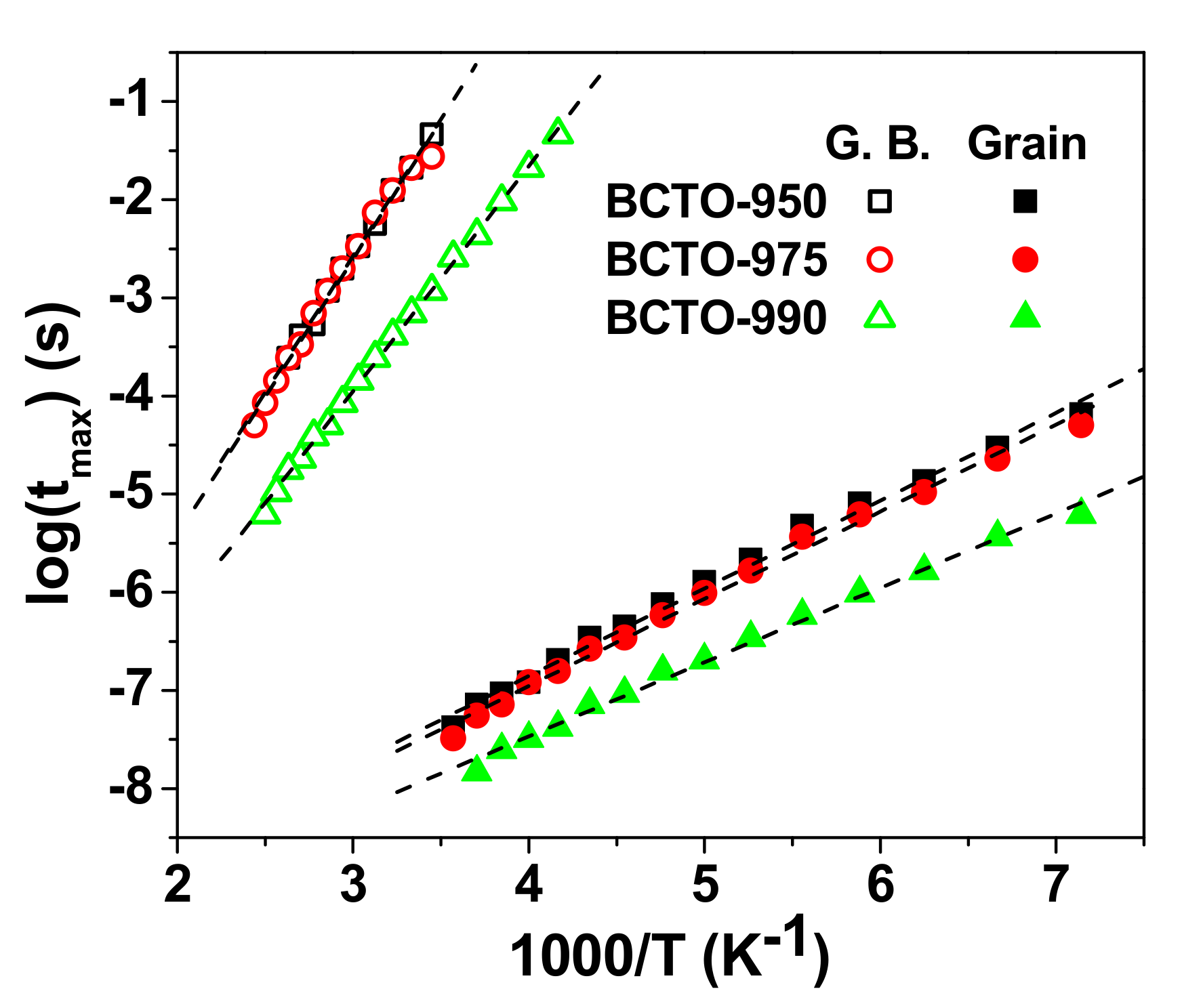
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subramanian, M.A.; Li, D.; Duan, N.; Reisner, B.A.; Sleight, A.W. High Dielectric Constant in ACu3Ti4O12 and ACu3Ti3FeO12 Phases. J. Solid State Chem. 2000, 151, 323–325. [Google Scholar] [CrossRef]
- Chinnathambi, M.; Sakthisabarimoorthi, A.; Jose, M.; Robert, R. Study of the Electrical and Dielectric behaviour of selenium doped CCTO ceramics prepared by a facile sol-gel route. Mater. Chem. Phys. 2021, 272, 124970. [Google Scholar] [CrossRef]
- Boonlakhorn, J.; Kidkhunthod, P.; Thongbai, P. Investigation of the dielectric properties and nonlinear electrical response of CaCu3Ti4O12 ceramics prepared by a chemical combustion method. J. Mater. Sci. Mater. Electron. 2020, 31, 4511–4519. [Google Scholar] [CrossRef]
- Kotb, H.M.; Ahmad, M.M.; Aldabal, S.; Alshoaibi, A.; Aljaafari, A. Structural and dielectric behavior of Al-substituted CaCu3Ti4O12 ceramics with giant dielectric constant by spark plasma sintering. J. Mater. Sci. Mater. Electron. 2019, 30, 18259–18267. [Google Scholar] [CrossRef]
- Kotb, H.M.; Ahmad, M.M.; Alshoaibi, A.; Yamada, K. Dielectric Response and Structural Analysis of (A3+, Nb5+) Cosubstituted CaCu3Ti4O12 Ceramics (A: Al and Bi). Materials 2020, 13, 5822. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M. Giant dielectric constant in CaCu3Ti4O12 nanoceramics. Appl. Phys. Lett. 2013, 102, 232908. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Yamada, K. Grain size effect on the giant dielectric constant of CaCu3Ti4O12 nanoceramics prepared by mechanosynthesis and spark plasma sintering. J. Appl. Phys. 2014, 115, 154103. [Google Scholar] [CrossRef]
- Yue, X.; Long, W.; Liu, J.; Pandey, S.; Zhong, S.; Zhang, L.; Du, S.; Xu, D. Enhancement of dielectric and non-ohmic properties of graded Co doped CaCu3Ti4O12 thin films. J. Alloy. Compd. 2020, 816, 152582. [Google Scholar] [CrossRef]
- Sinclair, D.C.; Adams, T.B.; Morrison, F.; West, A.R. CaCu3Ti4O12: One-step internal barrier layer capacitor. Appl. Phys. Lett. 2002, 80, 2153–2155. [Google Scholar] [CrossRef]
- Adams, T.B.; Sinclair, D.C.; West, A.R. Characterization of grain boundary impedances in fine- and coarse-grained CaCu3Ti4O12 ceramics. Phys. Rev. B 2006, 73, 094124. [Google Scholar] [CrossRef]
- Liu, J.; Duan, C.-G.; Yin, W.-G.; Mei, W.N.; Smith, R.W.; Hardy, J.R. Large dielectric constant and Maxwell-Wagner relaxation in Bi2/3Cu3Ti4O12. Phys. Rev. B 2004, 70, 144106. [Google Scholar] [CrossRef]
- Szwagierczak, D. Dielectric behavior of Bi2/3Cu3Ti4O12 ceramic and thick films. J. Electroceramics 2009, 23, 56–61. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, P.; Yang, L.; Shi, P.; Chao, X.; Yang, Z. Synthesis, dielectric properties of Bi2/3Cu3Ti4O12 ceramics by the sol–gel method. J. Mater. Sci. Mater. Electron. 2015, 26, 1959–1968. [Google Scholar] [CrossRef]
- Gautam, P.; Khare, A.; Sharma, S.; Singh, N.; Mandal, K.D. Characterization of Bi2/3Cu3Ti4O12 ceramics synthesized by semi-wet route. Prog. Nat. Sci. 2016, 26, 567–571. [Google Scholar] [CrossRef]
- Yang, L.; Huang, G.; Wang, T.; Hao, H.; Tian, Y. Colossal dielectric permittivity and relevant mechanism of Bi2/3Cu3Ti4O12 ceramics. Ceram. Int. 2016, 42, 9935–9939. [Google Scholar] [CrossRef]
- Yang, L.; Chao, X.; Yang, Z.; Zhao, N.; Wei, L.; Yang, Z. Dielectric constant versus voltage and non-Ohmic characteristics of Bi2/3Cu3Ti4O12 ceramics prepared by different methods. Ceram. Int. 2016, 42, 2526–2533. [Google Scholar] [CrossRef]
- Jesus, L.M.; Barbosa, L.B.; Ardila, D.R.; Silva, R.S.; M’Peko, J.C. Effect of conventional and laser sintering on the (micro)structural and dielectric properties of Bi2/3Cu3Ti4O12 synthesized through a polymeric precursor route. J. Alloys. Compd. 2018, 735, 2384–2394. [Google Scholar] [CrossRef]
- Jesus, L.; Silva, R.; Raj, R.; M’Peko, J.-C. Electric field-assisted flash sintering of Bi2/3Cu3Ti4O12 starting from a multi-phase precursor powder. J. Eur. Ceram. Soc. 2020, 40, 4004–4009. [Google Scholar] [CrossRef]
- Wurst, J.C.; Nelson, J.A. Lineal intercept technique for measuring grain size in two-phase polycrystalline ceramics. J. Am. Ceram. Soc. 1972, 55, 109. [Google Scholar] [CrossRef]
- Li, M.; Feteira, A.; Sinclair, D.C. Relaxor ferroelectric-like high effective permittivity in leaky dielectrics/oxide semiconductors induced by electrode effects: A case study of CuO ceramics. J. Appl. Phys. 2009, 105, 114109. [Google Scholar] [CrossRef]
- Liu, Z.; Chao, X.; Liang, P.; Yang, Z.; Zhi, L.; Ihlefeld, J. Differentiated electric behaviors of La2/3Cu3Ti4O12 ceramics prepared by different methods. J. Am. Ceram. Soc. 2014, 97, 2154–2163. [Google Scholar] [CrossRef]
- Zhao, N.; Liang, P.; Wei, L.; Yang, L.; Yang, Z. Synthesis and dielectric anomalies of CdCu3Ti4O12 ceramics. Ceram. Int. 2015, 41, 8501–8510. [Google Scholar] [CrossRef]
- Foeller, P.; Dean, J.S.; Reaney, I.M.; Sinclair, D.C. Design of a bilayer ceramic capacitor with low temperature coefficient of capacitance. Appl. Phys. Lett. 2016, 109, 082904. [Google Scholar] [CrossRef] [Green Version]
- Moulson, A.J.; Herbert, J.M. Electroceramics: Materials, Properties, Applications, 2nd ed.; Wiley: New York, NY, USA, 2003; p. 557. [Google Scholar]
- Sinclair, D.C.; West, A.R. Impedance and modulus spectroscopy of semiconducting BaTiO3 showing positive temperature coefficient of resistance. J. Appl. Phys. 1989, 66, 3850–3856. [Google Scholar] [CrossRef]
- Costa, S.I.R.; Li, M.; Frade, J.R.; Sinclair, D.C. Modulus spectroscopy of CaCu3Ti4O12 ceramics: Clues to the internal barrier layer capacitance mechanism. RSC Adv. 2013, 3, 7030–7036. [Google Scholar] [CrossRef]
- Li, J.; Liang, P.; Yi, J.; Chao, X.; Yang, Z. Phase Formation and Enhanced Dielectric Response of Y2/3Cu3Ti4O12 Ceramics Derived from the Sol-Gel Process. J. Am. Ceram. Soc. 2015, 98, 795–803. [Google Scholar] [CrossRef]





| Atomic Percentage (at %) | Bi:Cu:Ti:O | ||||
|---|---|---|---|---|---|
| Bi | Cu | Ti | O | ||
| BCTO-950 | 2.58 ± 0.02 | 11.81 ± 0.03 | 14.83 ± 0.11 | 70.78 ± 0.12 | 1:4.6:5.8:27.4 |
| BCTO-975 | 2.55 ± 0.02 | 11.58 ± 0.02 | 15.66 ± 0.13 | 70.21 ± 0.13 | 1:4.5:6.1:27.5 |
| BCTO-990 | 3.57 ± 0.01 | 16.87 ± 0.06 | 21.83 ± 0.15 | 57.52 ± 0.20 | 1:4.5:6.1:16.1 |
| E(G.) (eV) | E(G.B.) (eV) | ER(G.) (eV) | ER(G.B.) (eV) | RG. (Ω·cm) | CG. (F) | RG.B. (Ω·cm) | CG.B. (F) | |
|---|---|---|---|---|---|---|---|---|
| BCTO-CS950 | 0.231 | 0.848 | 0.176 | 0.557 | 4134 | 8.3 × 10−11 | >109 | 7.2 × 10−10 |
| BCTO-CS975 | 0.214 | 0.931 | 0.176 | 0.553 | 3038 | 8.2 × 10−11 | >109 | 9.3 × 10−10 |
| BCTO-CS990 | 0.197 | 0.513 | 0.150 | 0.454 | 1600 | 10.4 × 10−11 | 4.6 × 106 | 3.1 × 10−9 |
| Ε′ | tan δ | tan δ)min | |
|---|---|---|---|
| at 1.1 kHz | |||
| BCT-CS950 | 660 | 0.06 | 0.04 (at 7 kHz) |
| BCT-CS975 | 792 | 0.08 | 0.05 (at 9 kHz) |
| BCT-CS990 | 3040 | 0.32 | 0.14 (at 15 kHz) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.M.; Alshoaibi, A.; Ansari, S.A.; Kayed, T.S.; Khater, H.A.; Kotb, H.M. Dielectric Properties of Bi2/3Cu3Ti4O12 Ceramics Prepared by Mechanical Ball Milling and Low Temperature Conventional Sintering. Materials 2022, 15, 3173. https://doi.org/10.3390/ma15093173
Ahmad MM, Alshoaibi A, Ansari SA, Kayed TS, Khater HA, Kotb HM. Dielectric Properties of Bi2/3Cu3Ti4O12 Ceramics Prepared by Mechanical Ball Milling and Low Temperature Conventional Sintering. Materials. 2022; 15(9):3173. https://doi.org/10.3390/ma15093173
Chicago/Turabian StyleAhmad, Mohamad M., Adil Alshoaibi, Sajid Ali Ansari, Tarek S. Kayed, Hassan A. Khater, and Hicham Mahfoz Kotb. 2022. "Dielectric Properties of Bi2/3Cu3Ti4O12 Ceramics Prepared by Mechanical Ball Milling and Low Temperature Conventional Sintering" Materials 15, no. 9: 3173. https://doi.org/10.3390/ma15093173
APA StyleAhmad, M. M., Alshoaibi, A., Ansari, S. A., Kayed, T. S., Khater, H. A., & Kotb, H. M. (2022). Dielectric Properties of Bi2/3Cu3Ti4O12 Ceramics Prepared by Mechanical Ball Milling and Low Temperature Conventional Sintering. Materials, 15(9), 3173. https://doi.org/10.3390/ma15093173








