Removal of Fe2+ and Mn2+ from Polluted Groundwater by Insoluble Humic Acid/Tourmaline Composite Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of IHA/TM Composite Particles and Determination of Their Optimum Ratio
2.2. Material Characterisation
2.3. Static Adsorption Test
2.3.1. Effect of IHA/TM Dose on Adsorption
2.3.2. Effect of Initial pH on Adsorption
2.3.3. Adsorption Isotherm Experiment
2.3.4. Adsorption Kinetics
2.3.5. Regeneration Test
3. Results and Discussion
3.1. Optimal Ratio of IHA and TM
3.2. Effect of IHA/TM Dose on the Adsorption of Fe2+ and Mn2+
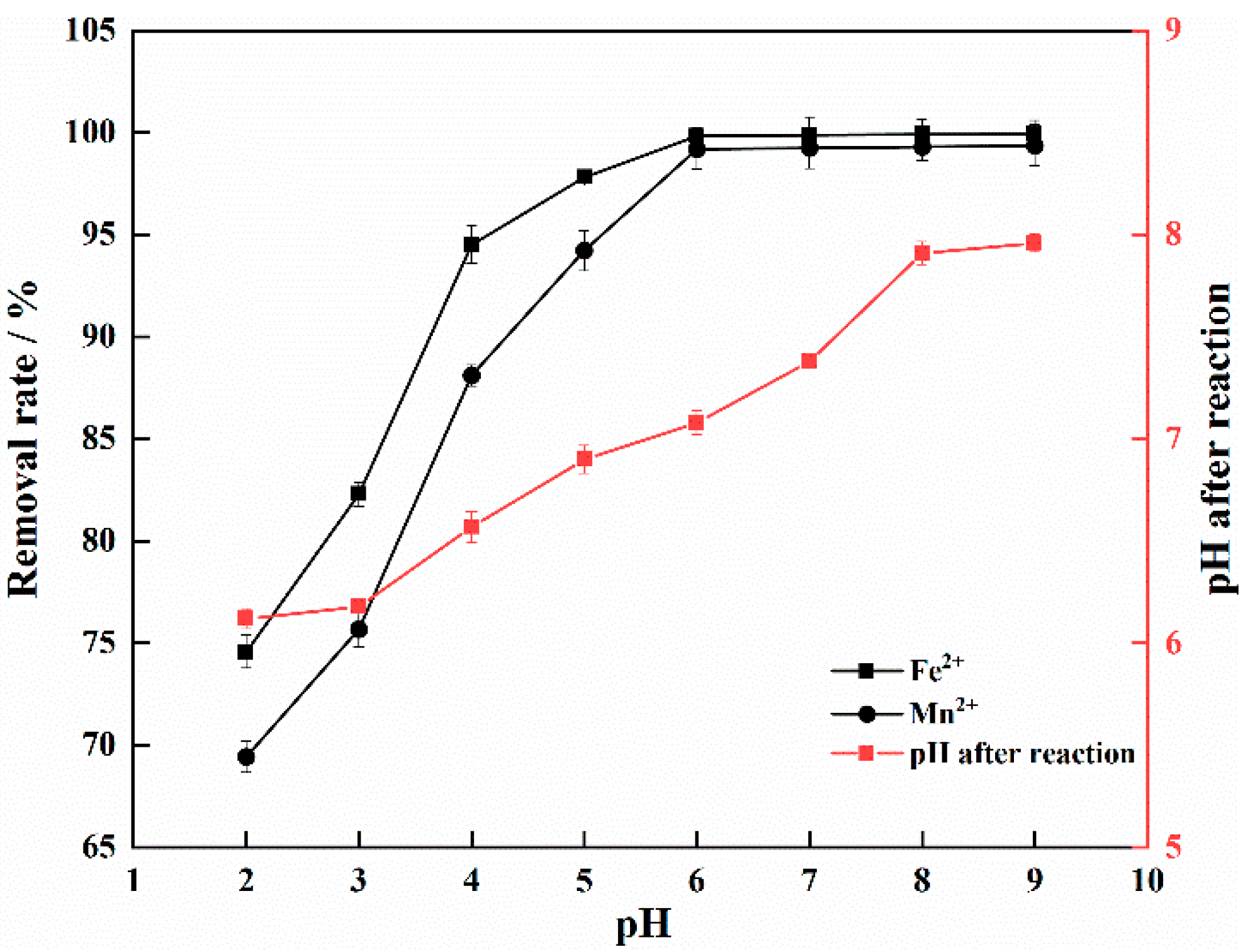
3.3. Effect of Initial pH on Adsorption of Fe2+ and Mn2+
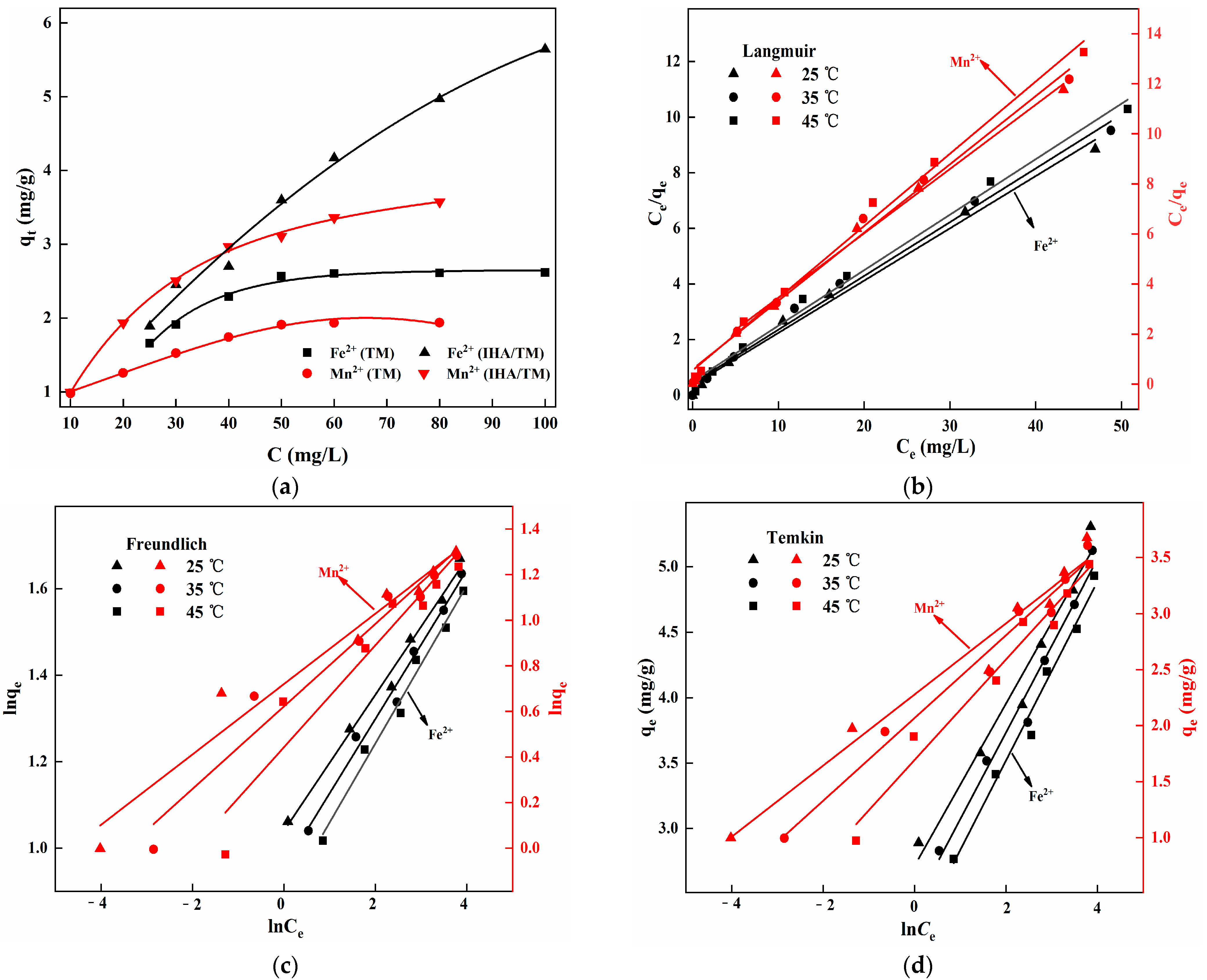
3.4. Isothermal Adsorption Analysis
3.5. Thermodynamic Analysis
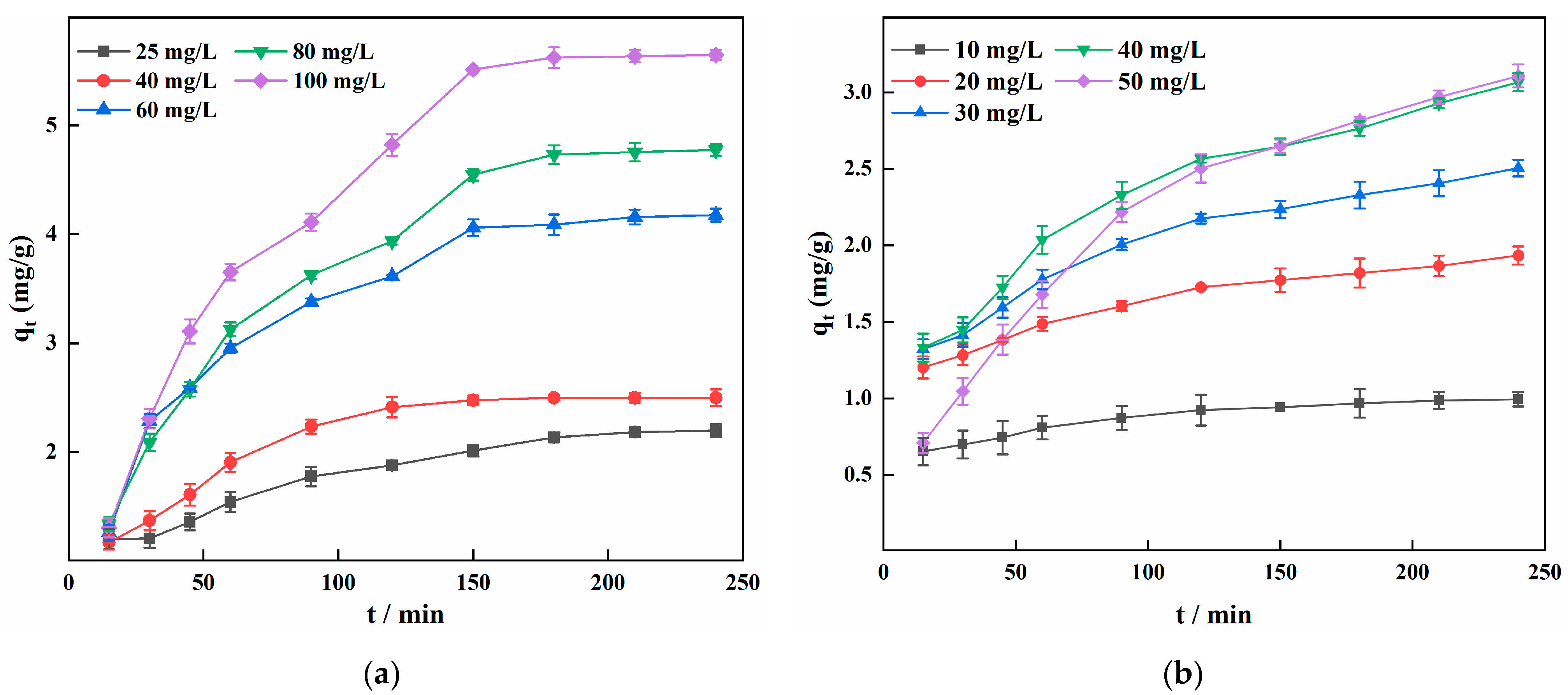
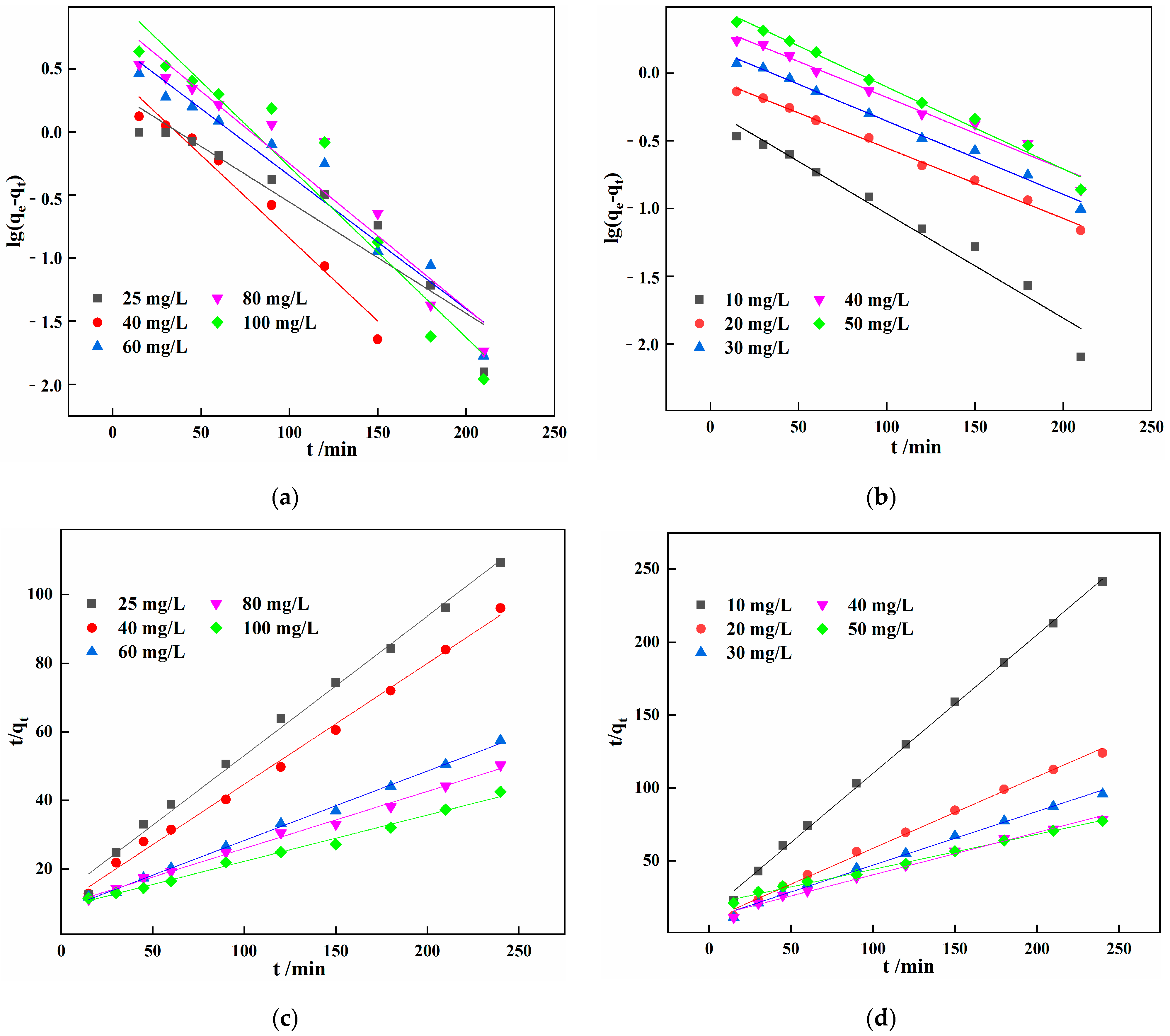
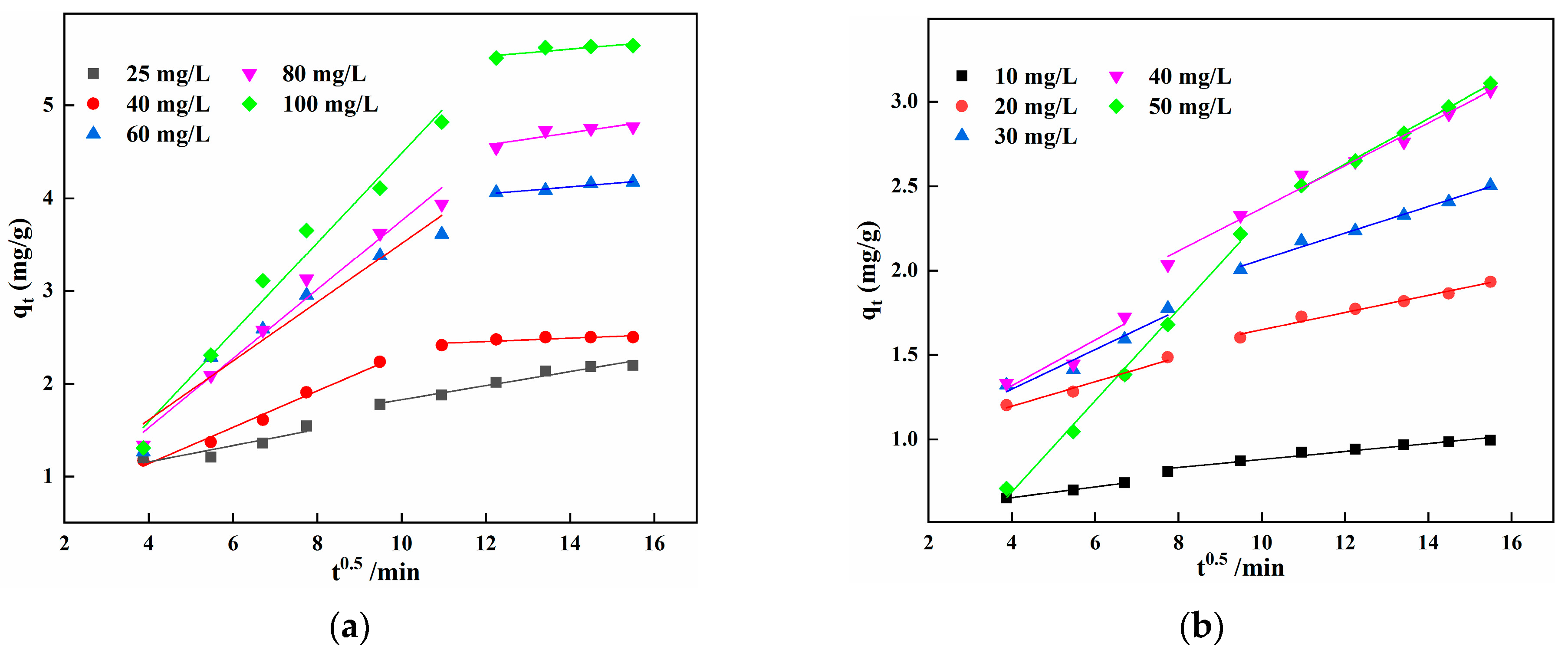
3.6. Adsorption Kinetic Analysis
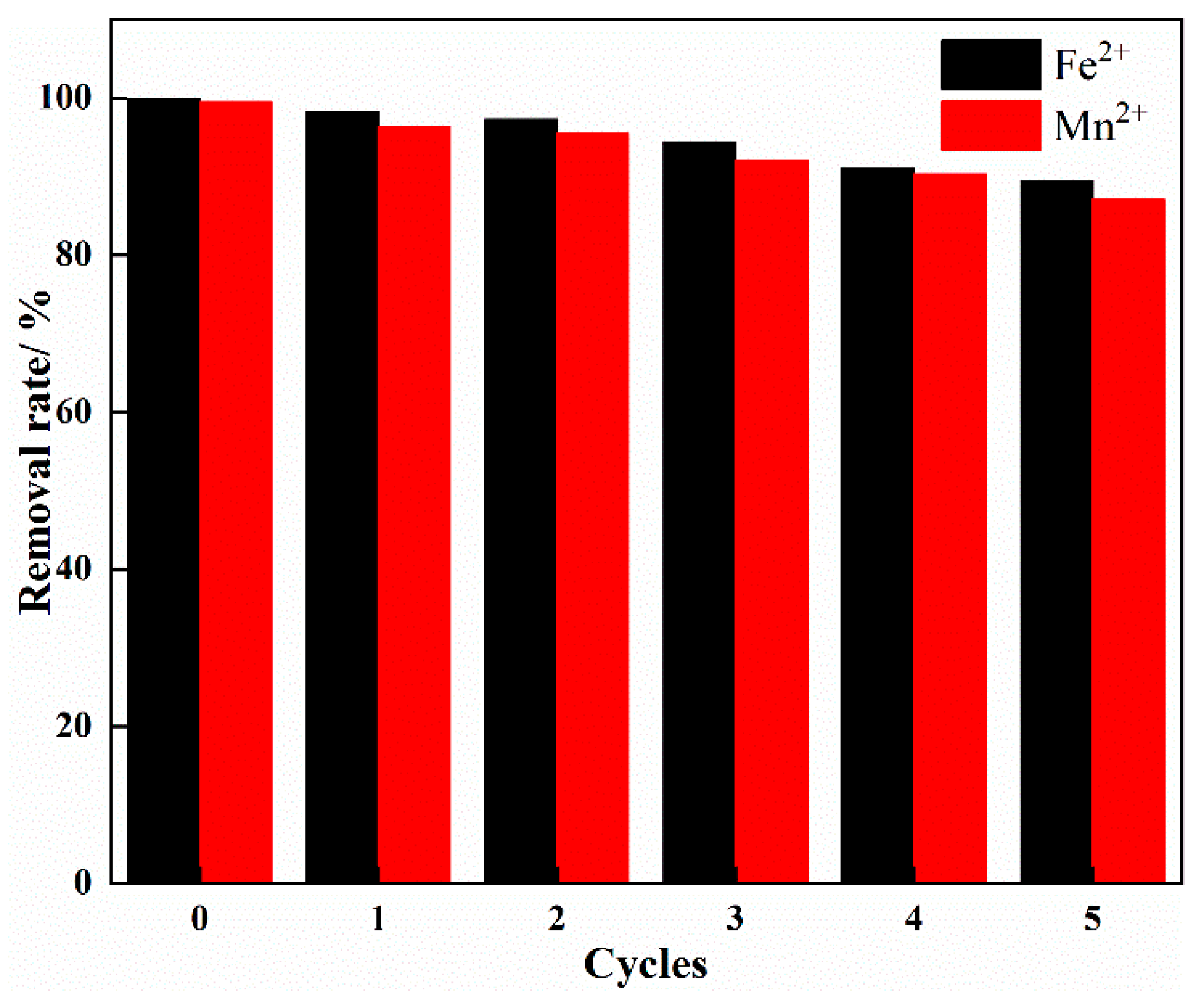
3.7. Desorption and Reusability of IHA/TM
3.8. Comparison of Saturated Adsorption Capacity of Materials
3.9. Microscopic Characterisation of IHA/TM Composite Particles
3.9.1. XRD Analysis
3.9.2. FTIR Analysis
3.9.3. SEM Analysis
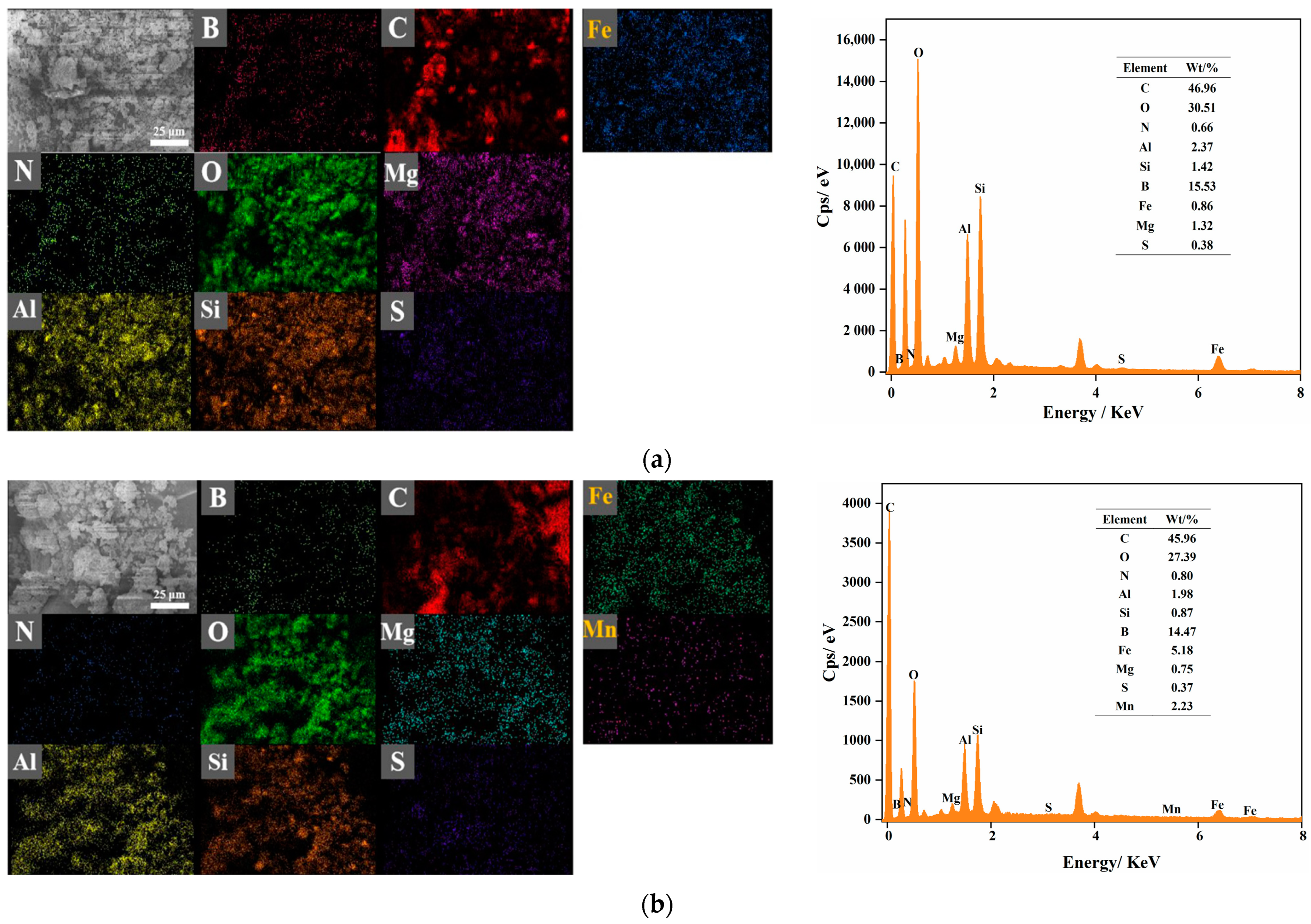
3.9.4. EDS Analysis
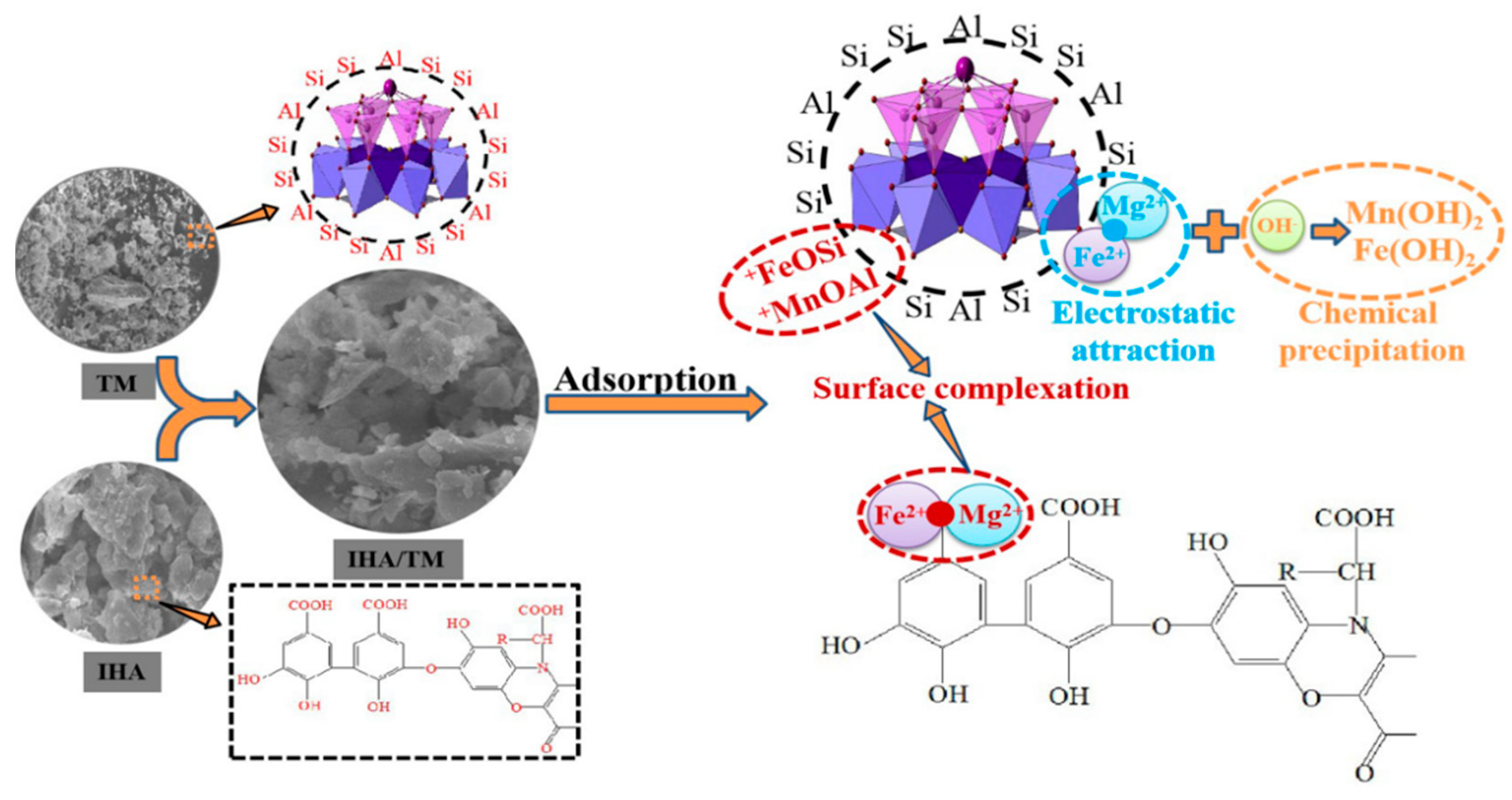
4. Mechanistic Analysis of Iron and Manganese Removal
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chitaranjan, D.; Ramakar, J.; Desai, V. Rice Husk and Sugarcane Baggase Based Activated Carbon for Iron and Manganese Removal. Aquat. Proc. 2015, 4, 1126–1133. [Google Scholar] [CrossRef]
- Akbar, N.; Abdul, A.; Adlan, M. Iron and Manganese Removal from Groundwater Using High Quality Limestone. Appl. Mech. Mater. 2015, 802, 460–465. [Google Scholar] [CrossRef]
- Adeyeye, O.; Xiao, C.; Zhang, Z.; Liang, X. State, source and triggering mechanism of iron and manganese pollution in groundwater of Changchun, Northeastern China. Environ. Monit. Assess. 2020, 192, 619–634. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, H.; Li, W. Migration of hazardous lements in acid coal mine drainage. J. China Coal Soc. 2007, 3, 261–266. [Google Scholar]
- Ren, H.; Zhu, S.; Wang, X.; Liu, Y.; Cao, L. Study on Issues and Countermeasures in Coal Measures Mine Water Resources Exploitation and Utilization. Coal Geol. China 2020, 32, 9–20. [Google Scholar] [CrossRef]
- Ahmad, J.; Cheng, W.; Low, W.; Ali, N.; Megat, M. Study on the removal of iron and manganese in groundwater by granular activated carbon. Desalination 2005, 182, 347–353. [Google Scholar] [CrossRef]
- Shavandi, M.; Haddadian, Z.; Ismail, M.; Abdullah, N.; Abidin, Z. Removal of Fe(III), Mn(II) and Zn(II) from palm oil mill effluent (POME) by natural zeolite. J. Taiwan Inst. Chem. Eng. 2012, 43, 50–759. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, S.; Hu, K.; Wang, W.; Cai, A.; Chang, W.; Li, B. Study on Fluoride, Iron and Manganese Removal from Aqueous Solutions by a Novel Composite Adsorbent. Adv. Mater. Res. 2013, 821–822, 1085–1092. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Liu, L.; Yang, J.; Liu, S.; Zhang, T. Preparation, characterization serpentine-loaded hydroxyapatite and its simultaneous removal performance for fluoride, iron and manganese. RSC Adv. 2021, 11, 16201–16215. [Google Scholar] [CrossRef]
- Tahir, S.S.; Rauf, N. Removal of Fe(II) from the wastewater of a galvanized pipe manufacturing industry by adsorption onto bentonite clay. J. Environ. Manag. 2004, 73, 285–292. [Google Scholar] [CrossRef]
- Geremias, R.; Laus, R.; de Fávere, V.T.; Pedrosa, R.C. Rejeito de mineracao de carvao como adsorvente para remocao da acidz, Fe (III), Al (III) E Mn (II) EM drenagen acida. Quím. Nova 2009, 33, 1677–1681. [Google Scholar] [CrossRef]
- Apkar’yan, A.S.; Gubaidulina, T.A.; Kaminskaya, O.V. Foam-Glass Ceramic Based Filtering Material for Removing Iron and Manganese from Drinking Water. Glass Ceram. 2015, 71, 413–417. [Google Scholar] [CrossRef]
- Aziz, R.A.; Li, C.; Salleh, M. Removal of Iron and Manganese from Palm Oil Mill Effluent (POME) using Activated Clinoptilolite Zeolite. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012029. [Google Scholar] [CrossRef]
- Dhakal, R.; Ghimire, K.; Inoue, K. Adsorptive separation of heavy metals from an aquatic environment using orange waste. Hydrometallurgy 2005, 79, 182–190. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, K.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Unexpectedly High Adsorption Capacity of Esterified Hydroxyapatite for Heavy Metal Removal. Langmuir 2019, 35, 16111–16119. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Terutaro, N. Tourmaline group crystals reaction with water. Ferroelectrics 1992, 137, 13–31. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Zhang, Z.; Wang, B.; Sun, H. Adsorption of Cd(II), Ni(II), and Zn(II) by Tourmaline at Acidic Conditions: Kinetics, Thermodynamics, and Mechanisms. Ind. Eng. Chem. Res. 2012, 51, 4397–4406. [Google Scholar] [CrossRef]
- Leonard, D.; Michael, T.G.R.; Altangerel, A.; Hem, R.P.; Park, C.; Dong, W.K.; Cheol, S.K. Antibacterial and superhydrophilic electrospun polyurethane nanocomposite fibers containing tourmaline nanoparticles. Chem. Eng. J. 2012, 197, 41–48. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X. The surface organic modification of tourmaline powder by span-60 and its composite. Appl. Surf. Sci. 2012, 258, 7540–7545. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Li, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Zhang, J.; Li, H.; Peng, Z.; Xu, R.; et al. Adsorption of Pb(II) by tourmaline-montmorillonite composite in aqueous phase. J. Colloid Interface Sci. 2020, 575, 367–376. [Google Scholar] [CrossRef]
- Liao, G.; Zhao, W.; Li, Q.; Pang, Q.; Xu, Z. Novel Poly(acrylic acid)-modified Tourmaline/Silver Composites for Adsorption Removal of Cu(II) ions and Catalytic Reduction of Methylene Blue in Water. Chem. Lett. 2017, 46, 1631–1634. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Romeo, L. Studies on interactions between membranes (RO and NF) and pollutants (SiO2, NO3−, Mn++ and humic acid) in water. Desalination 2001, 138, 271–281. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, K.; Wei, X.; Liang, Y.; Lu, X. Desorption enhancement of diesel by humic sodium and surfactants in loess soil. J. Saf. Environ. 2004, 4, 522–551. [Google Scholar]
- Zhao, W.; Ren, B.; Andrew, H.; Jiang, F. The adsorption of Mn(II) by insolubilized humic acid. Water Sci. Technol. 2020, 82, 384–396. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, M.; Li, S.; Zhao, H.; Shang, Q. Preparation and adsorption performance of chitosan cross linked insolublized humic acid beads. Ion. Exch. Adsorpt. 2016, 32, 43–53. [Google Scholar] [CrossRef]
- Jin, X.; Zheng, M.; Sarkar, B.; Naidu, R.; Chen, Z. Characterization of bentonite modified with humic acid for the removal of Cu (II) and 2,4-dichlorophenol from aqueous solution. Appl. Clay Sci. 2016, 134, 89–94. [Google Scholar] [CrossRef]
- Laabd, M.; Chafai, H.; Essekri, A.; Elamine, M.; Al-Muhtaseb, S.A.; Lakhmiri, R.; Albourine, A. Single and multi-component adsorption of aromatic acids using an eco-friendly polyaniline-based biocomposite. Sustain. Mater. Technol. 2017, 12, 35–43. [Google Scholar] [CrossRef]
- Li, X.; Shang, C. Role of Humic Acid and Quinone Model Compounds in Bromate Reduction by Zerovalent Iron. Environ. Sci. Technol. 2005, 39, 1092–1100. [Google Scholar] [CrossRef]
- Gao, B.; Li, P.; Yang, R.; Li, A.; Yang, H. Investigation of multiple adsorption mechanisms for efficient removal of ofloxacin from water using lignin-based adsorbents. Sci. Rep. 2019, 9, 637–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, K.; Sun, T.; Sun, L.; Li, H. Adsorption characteristics of copper, lead, zinc and cadmium ions by tourmaline. J. Environ. Sci. 2006, 18, 1221–1225. [Google Scholar] [CrossRef]
- Elmorsi, R.R.; El-Wakeel, S.T.; El-Dein, W.A.S.; Lotfy, H.R.; Rashwan, W.E.; Nagah, M.; Shaaban, S.A.; Sayed Ahmed, S.A.; El-Sherif, I.Y.; Abou-El-Sherbini, K.S. Adsorption of Methylene Blue and Pb2+ by using acid-activated Posidonia oceanica waste. Sci. Rep. 2019, 9, 3356–3368. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Lei, T.; Nie, Y.; Ji, H. Characteristics and interactions of heavy metals with humic acid in gold mining area soil at a upstream of a metropolitan drinking water source. J. Geochem. Explor. 2018, 200, 266–275. [Google Scholar] [CrossRef]
- Samuel, B.; Zhang, J.; Wang, H.; Yin, H.; Chen, H. The influence of pH, co-existing ions, ionic strength, and temperature on the adsorption and reduction of hexavalent chromium by undissolved humic acid. Chemosphere 2018, 212, 209–218. [Google Scholar] [CrossRef]
- Xia, M.; Hu, C.; Zhang, H. Effects of tourmaline addition on the dehydrogenase activity of Rhodopseudomonas palustris. Process Biochem. 2006, 41, 221–225. [Google Scholar] [CrossRef]
- Choi, H.; Yu, S. Application of novel hybrid bioadsorbent, tannin/chitosan/sericite, for the removal of Pb(II) toxic ion from aqueous solution. Korean J. Chem. Eng. 2018, 35, 2198–2206. [Google Scholar] [CrossRef]
- Bagbi, Y.; Sarswat, A.; Mohan, D.; Pandey, A.; Solanki, P. Lead and Chromium Adsorption from Water using L-Cysteine Functionalized Magnetite (Fe3O4) Nanoparticles. Sci. Rep. 2017, 7, 7672–7687. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, P.; Liu, J. Triethylene Tetramine Functionalized Magnetic Graphene Oxide Chitosan Composite with Superior Capacity for the Removal of Phosphate. J. Chem. Eng. Data 2017, 62, 3341–3352. [Google Scholar] [CrossRef]
- Nekouei Marnani, N.; Shahbazi, A. A novel environmental-friendly nanobiocomposite synthesis by EDTA and chitosan functionalized magnetic graphene oxide for high removal of Rhodamine B: Adsorption mechanism and separation property. Chemosphere 2019, 218, 715–725. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Chwastowski, J.; Bradło, D.; Zukowski, W. Adsorption of Cadmium, Manganese and Lead Ions from Aqueous Solutions Using Spent Coffee Grounds and Biochar Produced by Its Pyrolysis in the Fluidized Bed Reactor. Materials 2020, 13, 2782. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 3285–3298. [Google Scholar] [CrossRef]
- Anbalagan, K.; Kumar, P.S.; Gayatri, K.S.; Hameed, S.S.; Sindhuja, M.; Prabhakaran, C.; Karthikeyan, R. Removal and recovery of Ni(II) ions from synthetic wastewater using surface modified Strychnos potatorum seeds: Experimental optimization and mechanism. Desalin. Water Treat. 2015, 53, 171–182. [Google Scholar] [CrossRef]
- Li, X.; Qi, Y.; Li, Y.; Zhang, Y.; He, X.; Wang, Y. Novel magnetic beads based on sodium alginate gel crosslinked by zirconium(IV) and their effective removal for Pb2+ in aqueous solutions by using a batch and continuous systems. Bioresour. Technol. 2013, 142, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Esfandiar, N.; Bahram, N.; Ebadi, T. Removal of Mn(II) from groundwater by sugarcane bagasse and activated carbon (a comparative study): Application of response surface methodology (RSM). J. Ind. Eng. Chem. 2014, 20, 3726–3736. [Google Scholar] [CrossRef]
- Fan, X.; Lin, S.; He, S. Study on Adsorption Characteristics of Fe2+ by Attapulgite. Environ. Sci. Manag. 2010, 35, 77–79. [Google Scholar]
- Fu, H.; Xie, Q. Complexes of fulvic acid on the surface of hematite, goethite, and akaganeite: FTIR observation. Chemosphere 2006, 63, 403–410. [Google Scholar] [CrossRef]
- Wang, D.; Xu, H.; Ma, J.; Lu, X.; Qi, J.; Song, S. Strong promoted catalytic ozonation of atrazine at low temperature using tourmaline as catalyst: Influencing factors, reaction mechanisms and pathways. Chem. Eng. J. 2018, 354, 113–125. [Google Scholar] [CrossRef]
- Adhikari, M.; Hazer, G.C. Humus-metal complex-spectral studies. Indian Chem. Soe. 1976, 53, 513–515. [Google Scholar]
- Sun, N.; Tian, W.; Zhang, Y.; Tian, J. Adsorption properties of Fe2+ and Mn2+ from groundwater in cold regions by carbonated rice husk. Trans. Chin. Soc. Agric. Eng. 2016, 32, 197–205. [Google Scholar] [CrossRef]
- Sangi, M.; Shahmoradi, A.; Zolgharnein, J.; Azimi, G.; Ghorbandoost, M. Removal and recovery of heavy metals from aqueous solution using Ulmus carpinifolia and Fraxinus excelsior tree leaves. J. Hazard. Mater. 2008, 155, 513–522. [Google Scholar] [CrossRef]




| Ions Type | Temperature (°C) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm mg/g | KL | R2 | KF | 1/n | R2 | lnA | B | R2 | ||
| Fe2+ | 25 | 5.010 | 0.399 | 0.992 | 2.402 | 0.189 | 0.982 | 3.145 | 0.684 | 0.978 |
| 35 | 5.175 | 0.459 | 0.991 | 2.597 | 0.172 | 0.983 | 3.635 | 0.663 | 0.970 | |
| 45 | 5.319 | 0.525 | 0.991 | 2.828 | 0.157 | 0.988 | 4.374 | 0.621 | 0.963 | |
| Mn2+ | 25 | 3.465 | 0.534 | 0.992 | 1.556 | 0.224 | 0.900 | 3.772 | 0.449 | 0.964 |
| 35 | 3.596 | 0.640 | 0.990 | 1.861 | 0.181 | 0.951 | 5.594 | 0.370 | 0.972 | |
| 45 | 3.659 | 0.685 | 0.990 | 2.054 | 0.155 | 0.953 | 7.169 | 0.318 | 0.960 | |
| Ion Type | T (°C) | Ce (mg/L) | qe (mg/g) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/(mol k)) |
|---|---|---|---|---|---|---|
| Fe2+ | 25 | 0.54 | 2.46 | −3.74 | 33.64 | 138.24 |
| 35 | 0.47 | 2.47 | −4.12 | |||
| 45 | 0.27 | 2.58 | −5.95 | |||
| Mn2+ | 25 | 0.39 | 0.97 | −2.25 | 22.15 | 86.58 |
| 35 | 0.24 | 0.99 | −3.61 | |||
| 45 | 0.23 | 1.00 | −3.89 |
| Ions Type | Concentration mg/L | Quasi-First Order Dynamics | Quasi-Second Order Dynamics | ||||
|---|---|---|---|---|---|---|---|
| qe mg/g | K1 1/min | R2 | qe mg/g | K2 mg/g/min | R2 | ||
| Fe2+ | 25 | 2.119 | 0.0203 | 0.889 | 2.837 | 0.0131 | 0.996 |
| 40 | 2.973 | 0.0303 | 0.965 | 2.462 | 0.0132 | 0.993 | |
| 60 | 5.159 | 0.0243 | 0.942 | 4.939 | 0.0051 | 0.997 | |
| 80 | 6.912 | 0.0311 | 0.921 | 6.003 | 0.0030 | 0.995 | |
| 100 | 7.853 | 0.0264 | 0.921 | 7.412 | 0.0021 | 0.991 | |
| Mn2+ | 10 | 0.541 | 0.0178 | 0.959 | 1.052 | 0.0602 | 0.999 |
| 20 | 0.924 | 0.0120 | 0.995 | 2.035 | 0.0255 | 0.996 | |
| 30 | 1.543 | 0.0125 | 0.991 | 2.721 | 0.0131 | 0.994 | |
| 40 | 2.445 | 0.0122 | 0.975 | 3.205 | 0.0085 | 0.995 | |
| 50 | 3.188 | 0.0140 | 0.986 | 3.451 | 0.0042 | 0.999 | |
| Ion Type | Concentration mg/L | K1d mg/g·min−1/2 | R12 | K2d mg/g·min−1/2 | R22 |
|---|---|---|---|---|---|
| Fe2+ | 25 | 0.088 | 0.734 | 0.076 | 0.952 |
| 40 | 0.196 | 0.978 | 0.018 | 0.637 | |
| 60 | 0.317 | 0.925 | 0.038 | 0.916 | |
| 80 | 0.372 | 0.974 | 0.066 | 0.674 | |
| 100 | 0.483 | 0.970 | 0.039 | 0.664 | |
| Mn2+ | 10 | 0.032 | 0.991 | 0.024 | 0.950 |
| 20 | 0.073 | 0.962 | 0.051 | 0.972 | |
| 30 | 0.117 | 0.914 | 0.079 | 0.972 | |
| 40 | 0.135 | 0.815 | 0.126 | 0.983 | |
| 50 | 0.270 | 0.991 | 0.135 | 0.997 |
| Adsorbent | pH | qm of Fe2+(mg/g) | qm of Mn2+(mg/g) | Reference |
|---|---|---|---|---|
| Sugarcane bagasse | 4.5 | 0.676 | [44] | |
| Graptolite | 6 | 0.352 | [45] | |
| Limestone | 6.2–6.7 | 0.03 | 0.007 | [2] |
| Granular activated carbon | 7 | 3.601 | 2.545 | [6] |
| Slovakian natural zeolite | 7 | 1.157 | 0.075 | [7] |
| Natural shells | 7.0–9.0 | 4.00 | 3.50 | [8] |
| IHA/TM composite granules | 6.0 | 5.645 | 3.574 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, T.; Yu, X.; Mkandawire, V.; Ma, J.; Li, X. Removal of Fe2+ and Mn2+ from Polluted Groundwater by Insoluble Humic Acid/Tourmaline Composite Particles. Materials 2022, 15, 3130. https://doi.org/10.3390/ma15093130
Liu L, Zhang T, Yu X, Mkandawire V, Ma J, Li X. Removal of Fe2+ and Mn2+ from Polluted Groundwater by Insoluble Humic Acid/Tourmaline Composite Particles. Materials. 2022; 15(9):3130. https://doi.org/10.3390/ma15093130
Chicago/Turabian StyleLiu, Ling, Tianyi Zhang, Xiaowan Yu, Vitumbiko Mkandawire, Jiadi Ma, and Xilin Li. 2022. "Removal of Fe2+ and Mn2+ from Polluted Groundwater by Insoluble Humic Acid/Tourmaline Composite Particles" Materials 15, no. 9: 3130. https://doi.org/10.3390/ma15093130
APA StyleLiu, L., Zhang, T., Yu, X., Mkandawire, V., Ma, J., & Li, X. (2022). Removal of Fe2+ and Mn2+ from Polluted Groundwater by Insoluble Humic Acid/Tourmaline Composite Particles. Materials, 15(9), 3130. https://doi.org/10.3390/ma15093130







