The Effect of Increasing Nickel Content on the Microstructure, Hardness, and Corrosion Resistance of the CuFeTiZrNix High-Entropy Alloys
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of the CuFeTiZrNix Alloys and Analytical Method
2.2. First-Principles Calculations
3. Results and Discussions
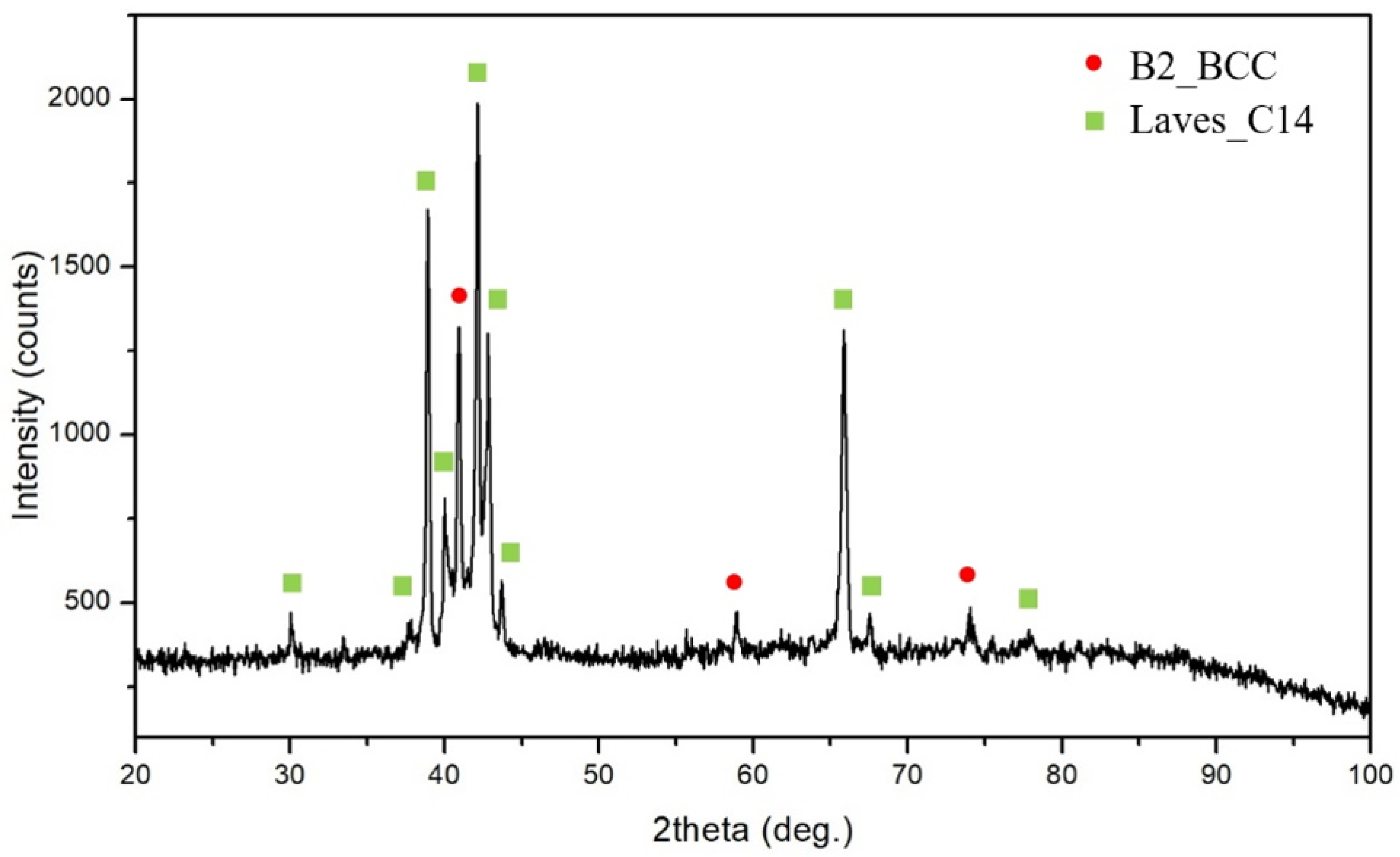
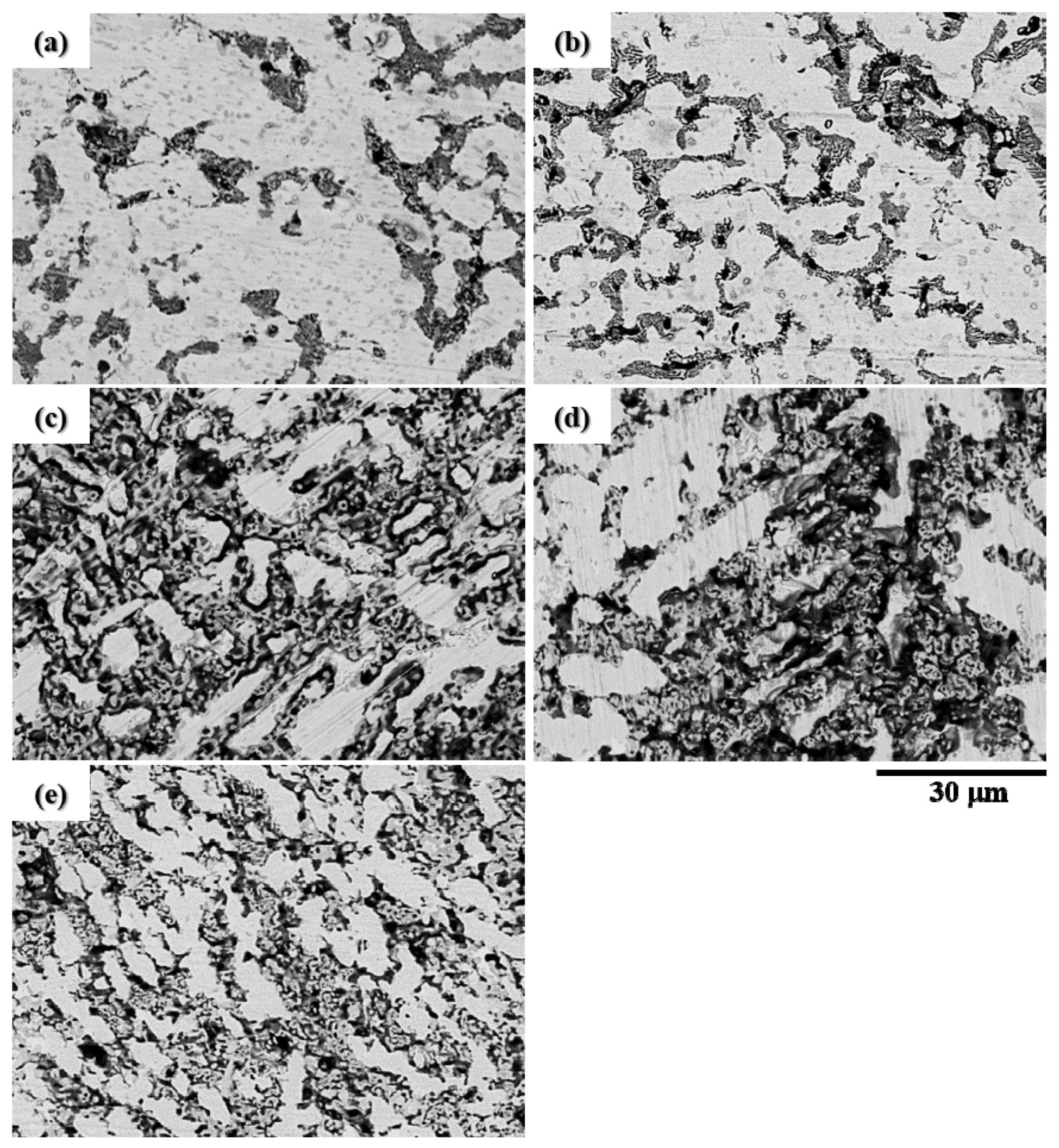
3.1. The Microstructure of the CuFeTiZrNix Alloys
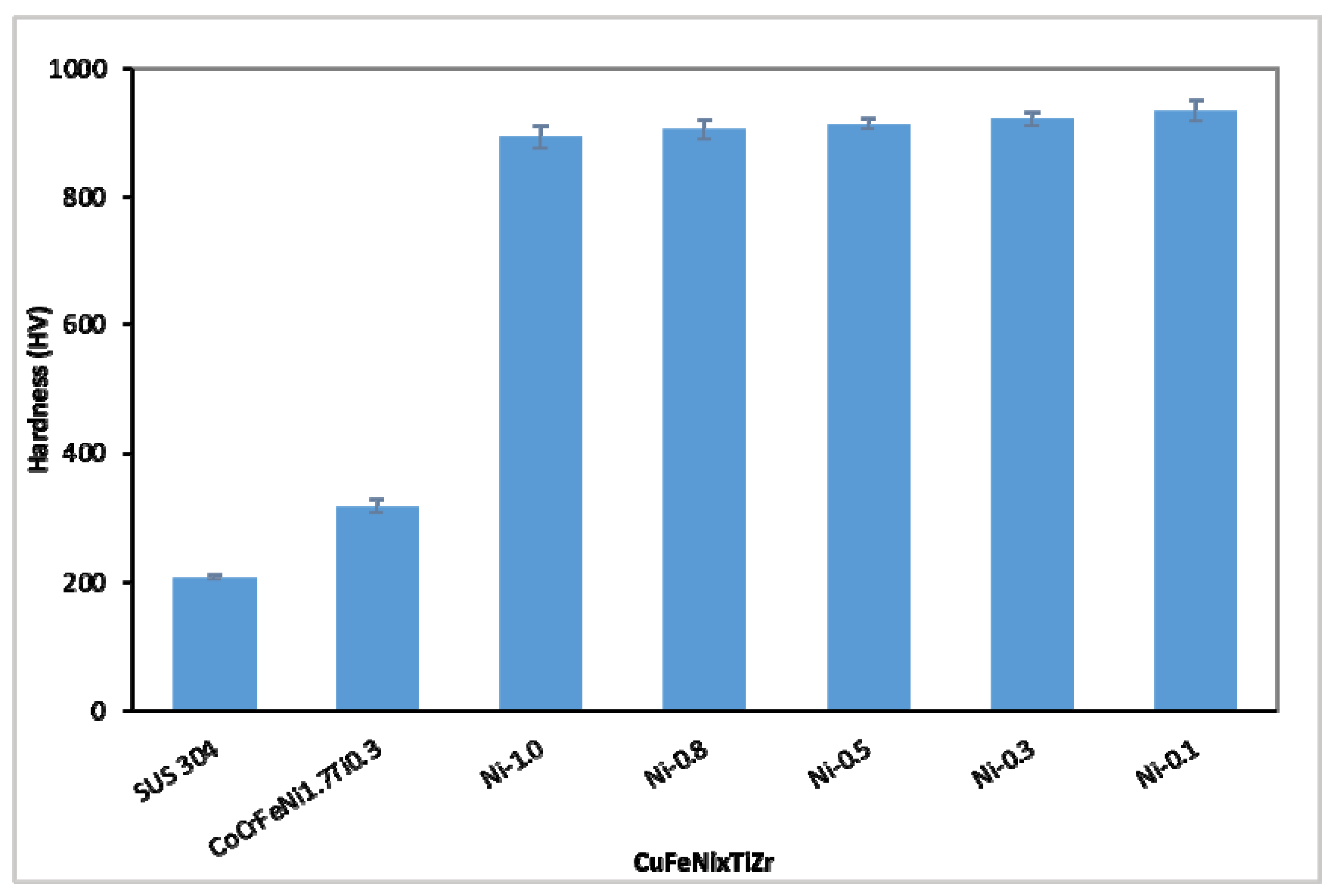
3.2. The Hardness Values of the CuFeTiZrNix Alloys
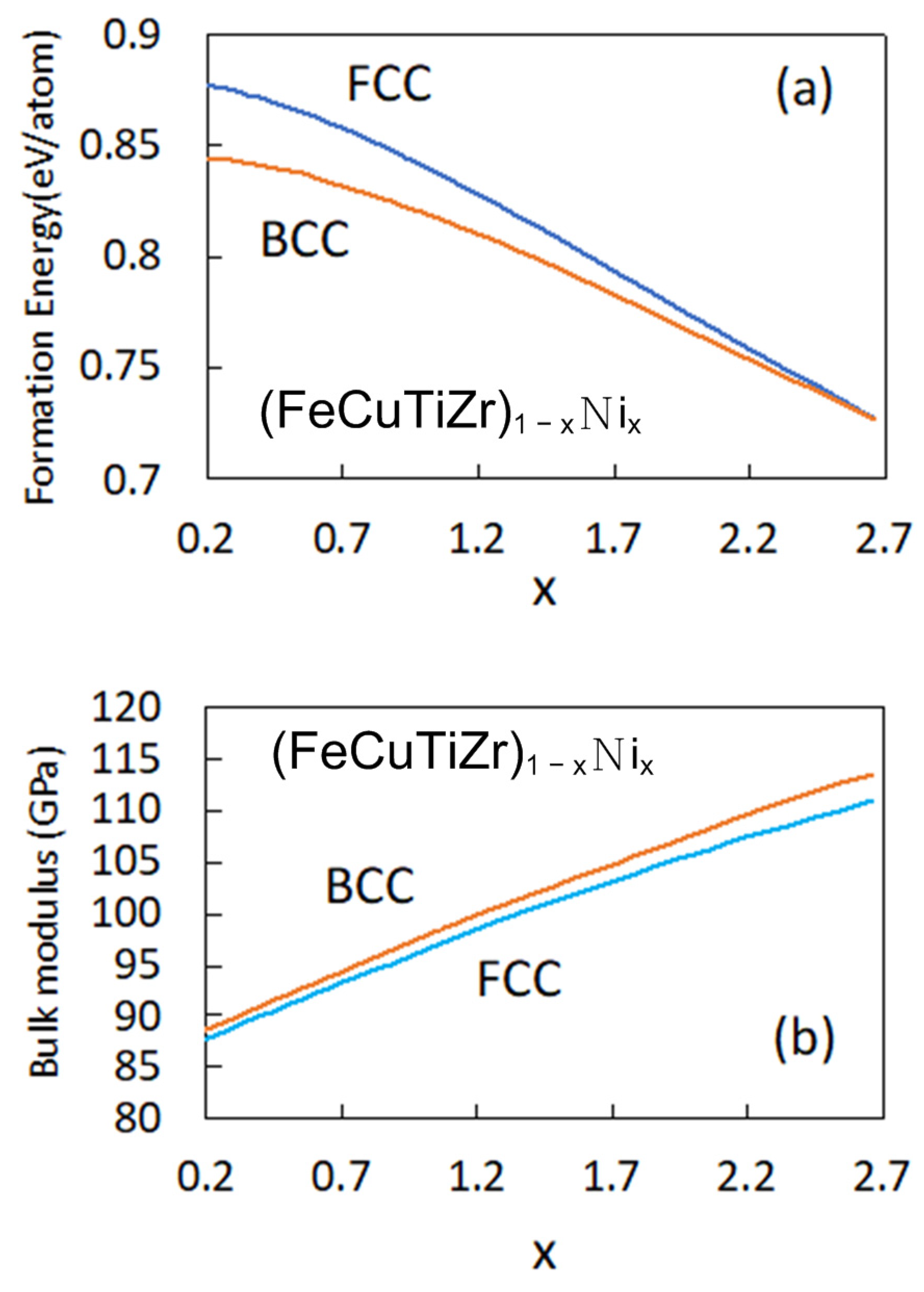
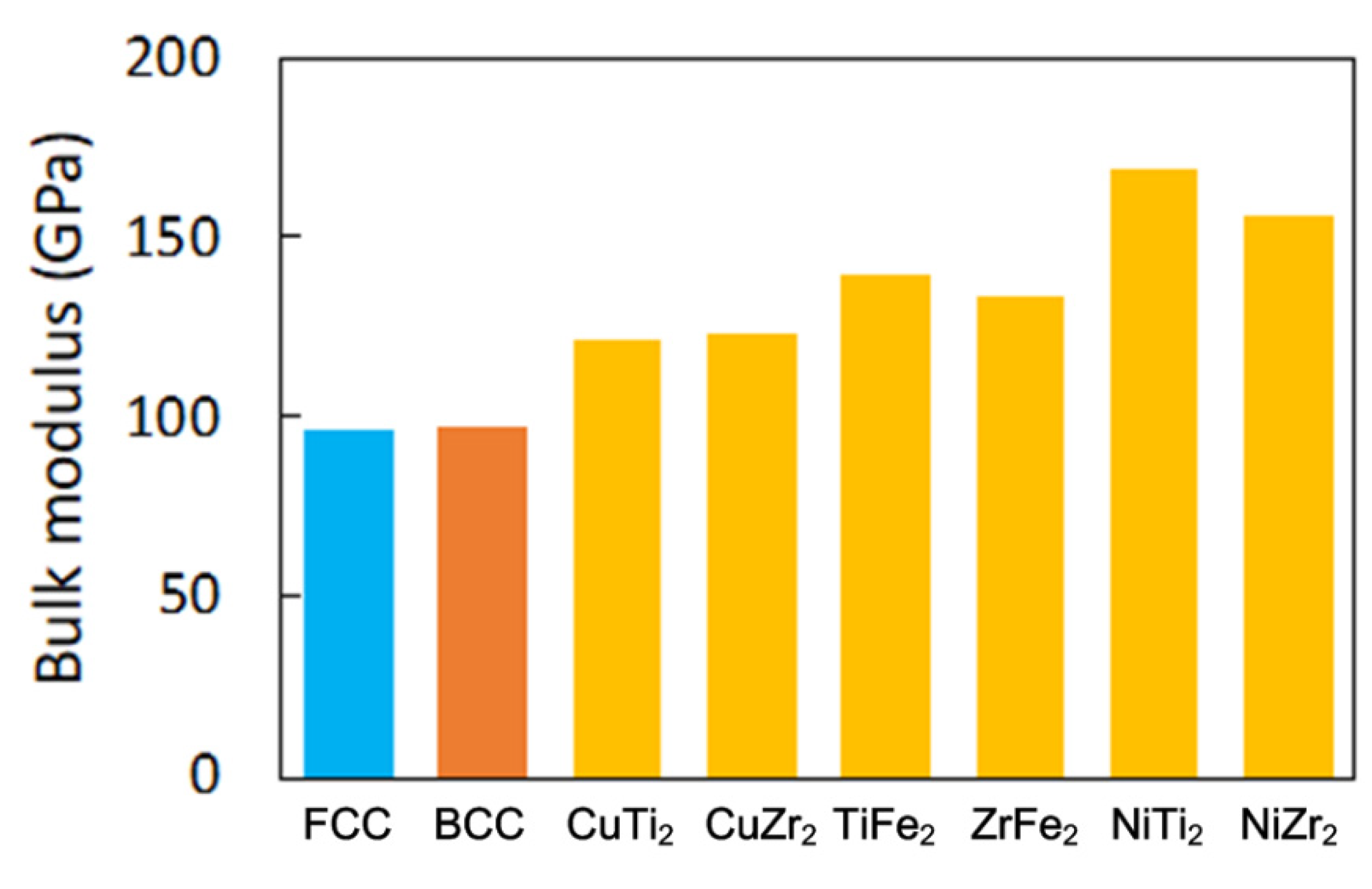
3.3. First-Principles Calculation for the Hardness Properties of the CuFeTiZrNix Alloys
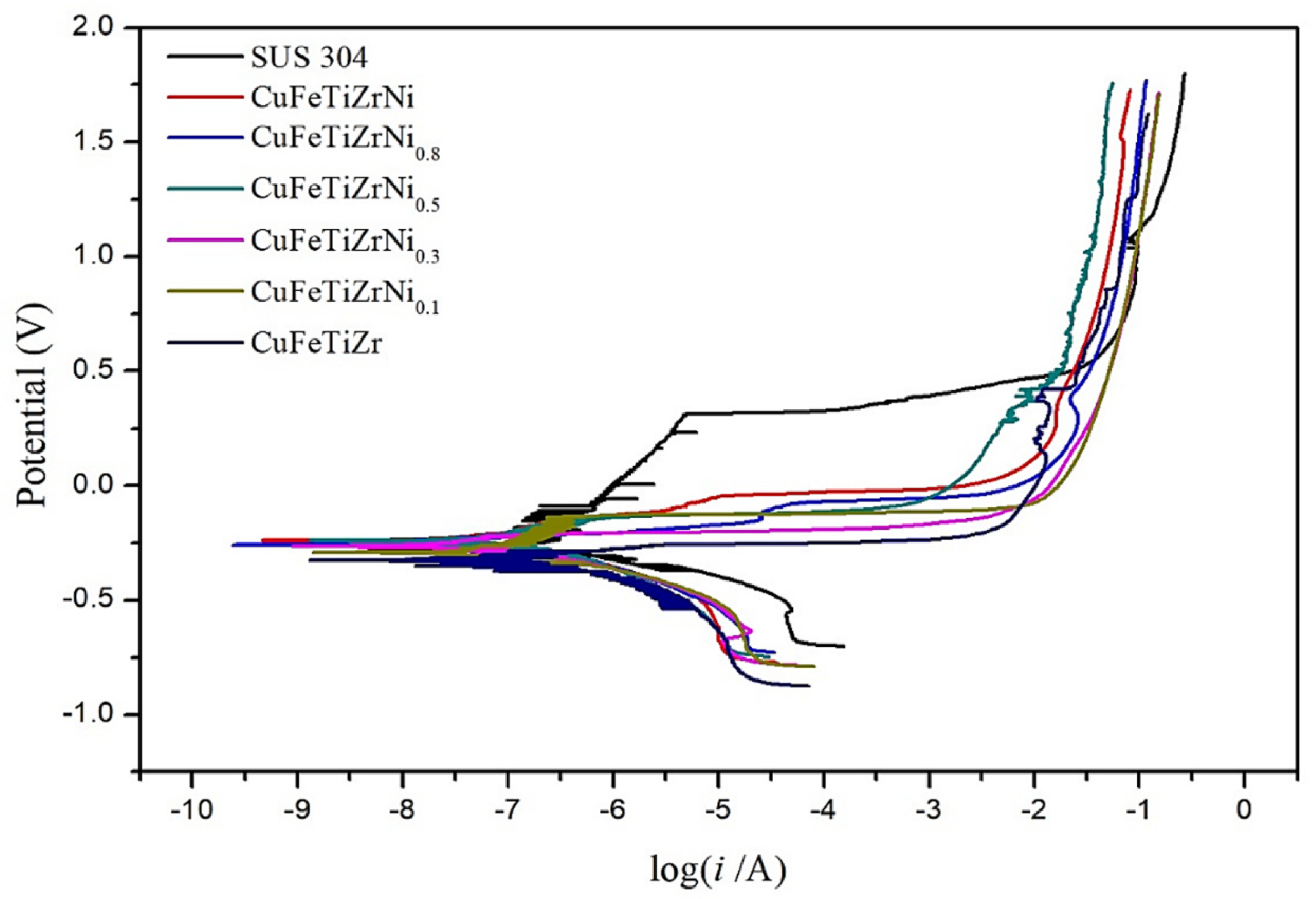
3.4. The Corrosion Resistance of the CuFeTiZrNix Alloys
4. Conclusions
- The B2_BCC and Laves_C14 phases were found in all CuFeTiZrNix alloys. When x was greater than 0.5, the FCC phase was also formed. When x increased to 0.8 and 1.0, the Cu51Zr14 phase was also observed in the CuFeTiZrNix alloys.
- The hardness of the CuFeTiZrNix alloys gradually decreased with increasing Ni content. More of the FCC phase formed in the CuFeTiZrNix alloys as the Ni content increased, and, as shown by first-principles calculations of hardness, the FCC phase had the lowest bulk modulus. Thus, more of the FCC phase forming in the CuFeTiZrNix alloys leads to decreased hardness.
- The corrosion resistance properties of the CuFeTiZrNi0.5, CuFeTiZrNi0.8, and CuFeTiZrNi1.0 alloys were superior to those of the SUS 304 and CoCrFeNi1.7Ti0.3 alloy systems in 3.5 wt.% NaCl solution. An increase in Ni content and the corresponding decrease in Cu content improved the corrosion potential and decreased the corrosion current density, indicating a gradual enhancement in corrosion resistance.
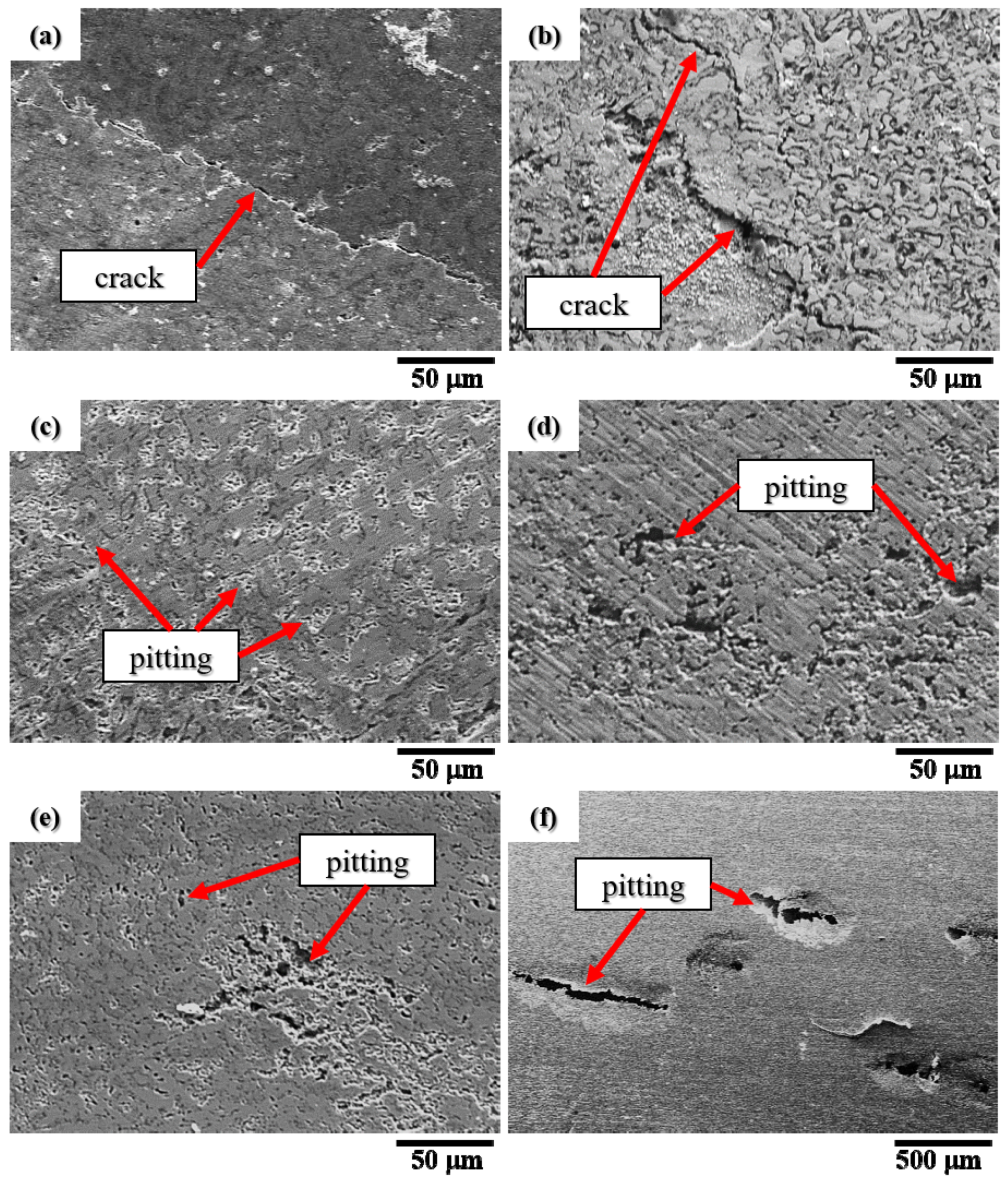
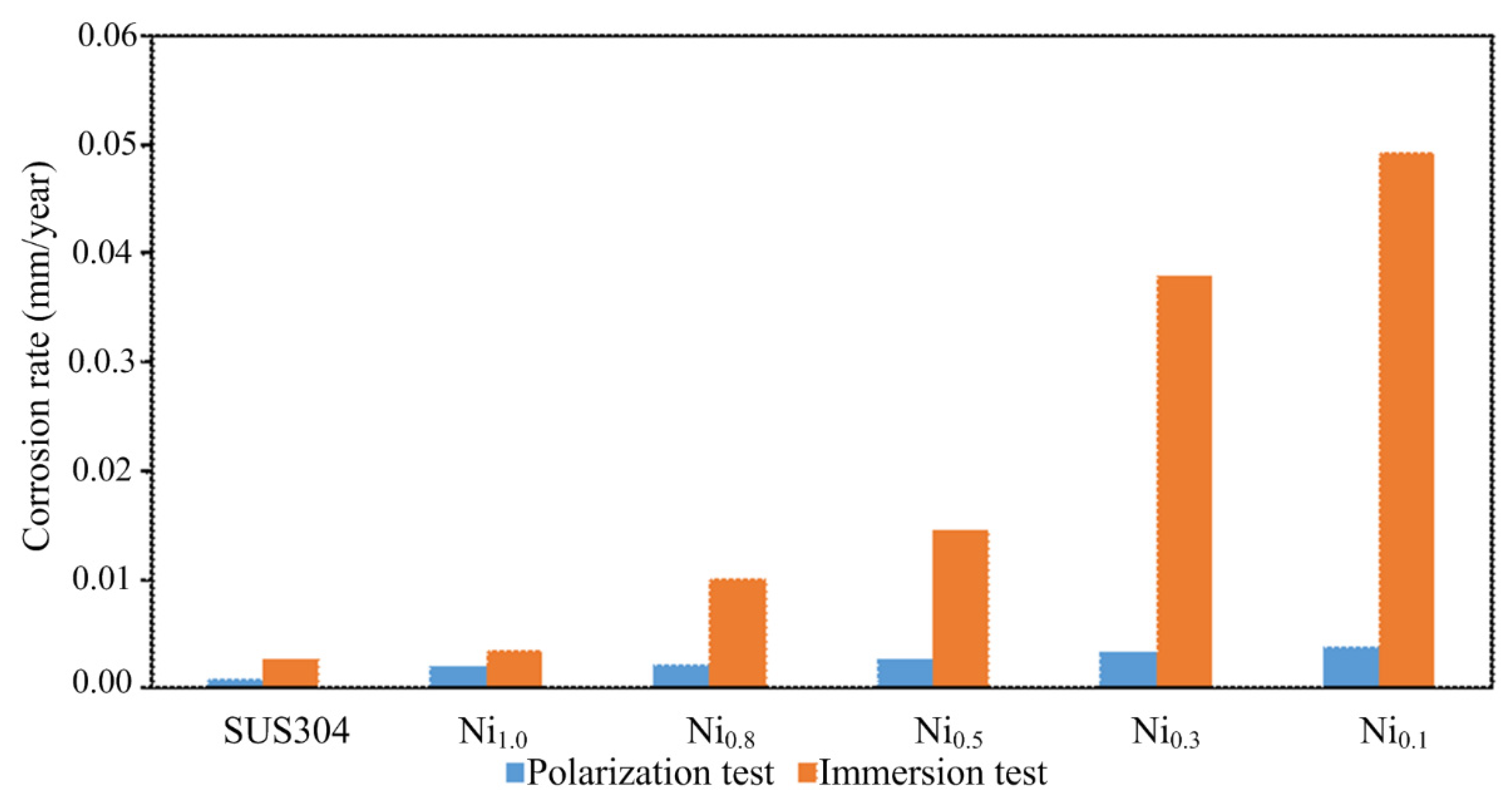
- In the polarization test, stress corrosion creaking (SCC) was observed in the CuFeTiZrNi0.1–0.3 alloys, as well as pitting in the CuFeTiZrNi0.5–1.0 alloys. The corrosion rates in both the polarization and immersion tests decreased slightly with increasing Ni content. In the immersion test, the major type in all CuFeTiZrNix alloys was surface pitting.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J.; Shi, J. Microstructure and mechanical properties of CoCrFeNiTiAlx high-entropy alloys. Mater. Sci. Eng. A 2009, 508, 214–219. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-entropy alloys—A new era of exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Sheikh, S.; Mao, H.; Guo, S. Predicting solid solubility in CoCrFeNiMx (M= 4d transition metal) high-entropy alloys. J. Appl. Phys. 2017, 121, 194903. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-Y.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Jien-Wei, Y. Recent progress in high entropy alloys. Ann. Chim. Sci. Mat 2006, 31, 633–648. [Google Scholar]
- Tsai, M.-H. Three Strategies for the Design of Advanced High-Entropy Alloys. Entropy 2016, 18, 252. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Fan, A.-C.; Wang, H.-A. Effect of atomic size difference on the type of major intermetallic phase in arc-melted CoCrFeNiX high-entropy alloys. J. Alloys Compd. 2017, 695, 1479–1487. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chang, S.-Y.; Hong, Y.-D.; Chen, S.-K.; Lin, S.-J. Anomalous decrease in X-ray diffraction intensities of Cu–Ni–Al–Co–Cr–Fe–Si alloy systems with multi-principal elements. Mater. Chem. Phys. 2007, 103, 41–46. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, M.-R.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Chang, S.-Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Ng, C.; Guo, S.; Luan, J.; Shi, S.; Liu, C.T. Entropy-driven phase stability and slow diffusion kinetics in an Al0.5CoCrCuFeNi high entropy alloy. Intermetallics 2012, 31, 165–172. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Chang, K.-C.; Li, J.-H.; Tsai, R.-C.; Cheng, A.-H. A second criterion for sigma phase formation in high-entropy alloys. Mater. Res. Lett. 2016, 4, 90–95. [Google Scholar] [CrossRef]
- Jiang, H.; Han, K.; Qiao, D.; Lu, Y.; Cao, Z.; Li, T. Effects of Ta addition on the microstructures and mechanical properties of CoCrFeNi high entropy alloy. Mater. Chem. Phys. 2018, 210, 43–48. [Google Scholar] [CrossRef]
- Liu, W.H.; He, J.Y.; Huang, H.L.; Wang, H.; Lu, Z.P.; Liu, C.T. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and V on structure and mechanical properties of high-entropy alloys based on CoCrFeNi system. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, S.; Liu, C.T. Phase Selection in High-Entropy Alloys: From Nonequilibrium to Equilibrium. JOM 2014, 66, 1966–1972. [Google Scholar] [CrossRef]
- Tung, C.-C.; Yeh, J.-W.; Shun, T.-t.; Chen, S.-K.; Huang, Y.-S.; Chen, H.-C. On the elemental effect of AlCoCrCuFeNi high-entropy alloy system. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Chen, M.-R.; Lin, S.-J.; Yeh, J.-W.; Chen, S.-K.; Huang, Y.-S.; Tu, C.-P. Microstructure and properties of Al0.5CoCrCuFeNiTix (x = 0–2.0) high-entropy alloys. Mater. Trans. 2006, 47, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Karpets, M.V.; Myslyvchenko, O.M.; Makarenko, O.S.; Gorban, V.F.; Krapivka, M.O.; Degula, A.I. Effect of nickel on the structure and phase composition of the VCrMnFeCoNixhigh-entropy alloy. J. Superhard Mater. 2015, 37, 182–188. [Google Scholar] [CrossRef]
- Chandan, A.K.; Kishore, K.; Hung, P.T.; Ghosh, M.; Chowdhury, S.G.; Kawasaki, M.; Gubicza, J. Effect of nickel addition on enhancing nano-structuring and suppressing TRIP effect in Fe40Mn40Co10Cr10 high entropy alloy during high-pressure torsion. Int. J. Plast. 2022, 150, 103193. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- López Ríos, M.; Socorro Perdomo, P.P.; Voiculescu, I.; Geanta, V.; Crăciun, V.; Boerasu, I.; Mirza Rosca, J.C. Effects of nickel content on the microstructure, microhardness and corrosion behavior of high-entropy AlCoCrFeNix alloys. Sci. Rep. 2020, 10, 21119. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Duval, T.; Hung, U.D.; Yeh, J.W.; Shih, H.C. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Tsai, K.-Y.; Tsai, C.-W.; Lee, C.; Juan, C.-C.; Yeh, J.-W. Criterion for Sigma Phase Formation in Cr- and V-Containing High-Entropy Alloys. Mater. Res. Lett. 2013, 1, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Yurchenko, N.; Stepanov, N.; Salishchev, G. Laves-phase formation criterion for high-entropy alloys. Mater. Sci. Technol. 2017, 33, 17–22. [Google Scholar] [CrossRef]
- ASTM E92–82; Standard Test Method for Vickers Hardness of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2003.
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of Al xCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM: West Conshohocken, PA, USA, 2004.
- HP. Available online: http://kkr.issp.u-tokyo.ac.jp/jp/ (accessed on 1 April 2022).
- Liu, W.H.; Yang, T.; Liu, C.T. Precipitation hardening in CoCrFeNi-based high entropy alloys. Mater. Chem. Phys. 2018, 210, 2–11. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Kündig, A.A.; Ohnuma, M.; Ping, D.H.; Ohkubo, T.; Hono, K. In situ formed two-phase metallic glass with surface fractal microstructure. Acta Mater. 2004, 52, 2441–2448. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Kong, J.; Zhang, J.; Li, T. Microstructure and mechanical properties of multi-component AlCrFeNiMox high-entropy alloys. J. Alloys Compd. 2013, 573, 96–101. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Hsieh, Y.C. The Studies on Microstructure and Properties of CoCrFeNixTi0.3 High-Entropy Alloys. Master’s Thesis, Feng Chia University, Taichung, Taiwan, 2015. [Google Scholar]
- Dong, Y.; Lu, Y.P.; Zhang, J.J.; Li, T.J. Microstructure and properties of multi-component AlxCoCrFeNiTi0.5 high-entropy alloys. Mater. Sci. Forum 2013, 745, 775–780. [Google Scholar] [CrossRef]
- Praveen, S.; Murty, B.S.; Kottada, R.S. Alloying behavior in multi-component AlCoCrCuFe and NiCoCrCuFe high entropy alloys. Mater. Sci. Eng. A 2012, 534, 83–89. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Fan, J.; Yu, P.; Li, G. Novel Co-free CrFeNiNb0.1Tix high-entropy alloys with ultra high hardness and strength. Mater. Sci. Eng. A. 2019, 764, 138212. [Google Scholar] [CrossRef]
- Chen, Y.C. Study on Microstructure, Hardness and Corrosion of CuZrTiFeCrx High Entropy Alloy. Master’s Thesis, National Taiwan University of Science and Technology, Taipei, Taiwan, 2018. [Google Scholar]
- Kunze, J.; Maurice, V.; Klein, L.H.; Strehblow, H.-H.; Marcus, P. In situ STM study of the effect of chlorides on the initial stages of anodic oxidation of Cu(111) in alkaline solutions. Electrochim. Acta 2003, 48, 1157–1167. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hong, U.T.; Shih, H.C.; Yeh, J.W.; Duval, T. Electrochemical kinetics of the high entropy alloys in aqueous environments—A comparison with type 304 stainless steel. Corros. Sci. 2005, 47, 2679–2699. [Google Scholar] [CrossRef]
- Azarian, N.S.; Ghasemi, H.M.; Monshi, M.R. Synergistic Erosion and Corrosion Behavior of AA5052 Aluminum Alloy in 3.5 wt% NaCl Solution Under Various Impingement Angles. J. Bio- Tribo-Corros. 2015, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Fontana, M.G.; Greene, N.D. Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Hsu, Y.-J.; Chiang, W.-C.; Wu, J.-K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
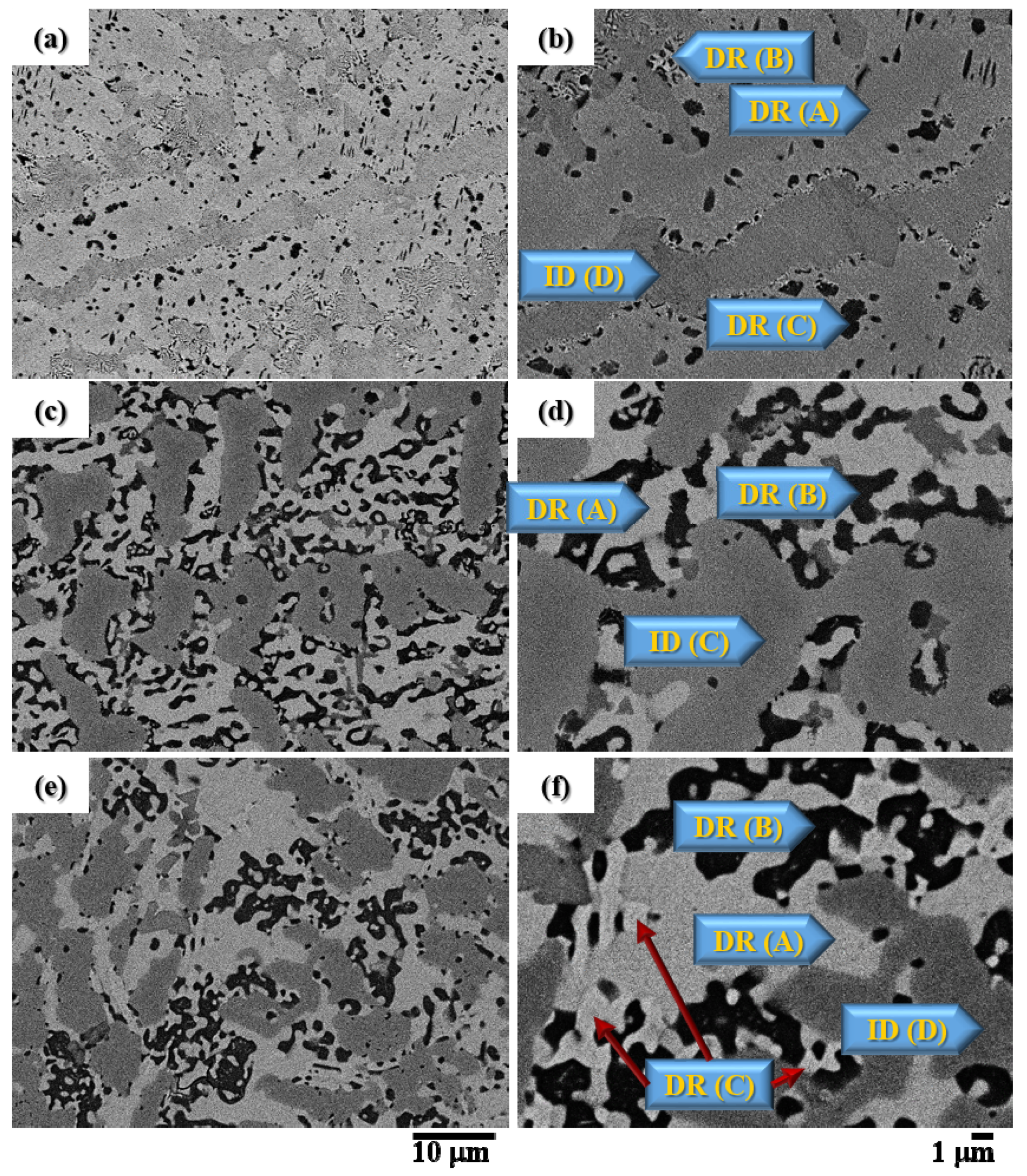



| Alloys | Structure | Phase | Phase Composition (at.%) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Fe | Ni | Ti | Zr | |||
| CuFeTiZrNi0.1 | Dendrite-A | Laves_C14 | 32.5 | 17.2 | 2.0 | 23.8 | 24.5 |
| Dendrite-B | Laves_C14 | 38.0 | 14.6 | 4.0 | 20.2 | 23.2 | |
| Dendrite-C | B2_BCC | 18.5 | 32.5 | 2.6 | 38.5 | 7.9 | |
| Interdendrite-D | Laves_C14 | 25.8 | 27.4 | 2.0 | 21.2 | 23.6 | |
| CuFeTiZrNi0.3 | Dendrite-A | Laves_C14 | 29.2 | 16.3 | 8.0 | 24.3 | 22.2 |
| Dendrite-B | Laves_C14 | 33.0 | 16.2 | 8.5 | 20.7 | 21.6 | |
| Dendrite-C | B2_BCC | 20.6 | 25.5 | 6.4 | 35.0 | 12.5 | |
| Interdendrite-D | Laves_C14 | 26.0 | 25.2 | 6.2 | 19.1 | 23.5 | |
| CuFeTiZrNi0.5 | Dendrite-A | FCC | 43.4 | 4.3 | 14.0 | 9.5 | 28.8 |
| Dendrite-B | B2_BCC | 15.8 | 25.3 | 9.8 | 40.2 | 8.9 | |
| Interdendrite-C | Laves_C14 | 17.4 | 33.0 | 7.5 | 20.6 | 21.5 | |
| CuFeTiZrNi0.8 | Dendrite-A | FCC | 35.2 | 3.1 | 23.1 | 10.8 | 27.8 |
| Dendrite-B | B2_BCC | 13.6 | 21.1 | 17.9 | 38.9 | 8.5 | |
| Dendrite-C | Cu51Zr14 | 51.4 | 10.0 | 11.6 | 10.3 | 16.7 | |
| Interdendrite-D | Laves_C14 | 15.7 | 34.5 | 10.9 | 18.9 | 20.0 | |
| CuFeTiZrNi1.0 | Dendrite-A | FCC | 31.0 | 3.0 | 28.3 | 12.6 | 25.1 |
| Dendrite-B | B2_BCC | 11.2 | 22.3 | 19.7 | 40.0 | 6.8 | |
| Dendrite-C | Cu51Zr14 | 55.1 | 6.9 | 12.0 | 8.3 | 17.7 | |
| Interdendrite-D | Laves_C14 | 13.6 | 35.6 | 14.2 | 17.9 | 18.7 | |
| Alloy | ∆Hmix (kJ/mol) | ∆Smix (J/K mol) | δ | VEC | Ω |
|---|---|---|---|---|---|
| CuFeTiZrNi0.1 | −16.47 | 12.20 | 10.85 | 6.83 | 1.34 |
| CuFeTiZrNi0.3 | −18.51 | 12.82 | 10.77 | 6.97 | 1.25 |
| CuFeTiZrNi0.5 | −20.14 | 13.15 | 10.69 | 7.11 | 1.17 |
| CuFeTiZrNi0.8 | −21.98 | 13.35 | 10.58 | 7.29 | 1.09 |
| CuFeTiZrNi1.0 | −22.88 | 13.38 | 10.51 | 7.4 | 1.05 |
| Alloys | Hardness (HV) | References |
|---|---|---|
| SUS 304 | 207.5 ± 2.7 | This work |
| CoCrFeNi1.7Ti0.3 | 318 | Hsieh [38] |
| CuFeTiZrNi0.1 | 934.8 ± 17.0 | This work |
| CuFeTiZrNi0.3 | 921.9 ± 11.5 | This work |
| CuFeTiZrNi0.5 | 914.2 ± 7.7 | This work |
| CuFeTiZrNi0.8 | 905.9 ± 14.8 | This work |
| CuFeTiZrNi1.0 | 893.8 ± 17.1 | This work |
| Alloys | icorr (A/cm2) | Ecorr (V) | References |
|---|---|---|---|
| CuFeTiZr | 2.04 × 10−7 | −0.328 | Chen [42] |
| SUS 304 | 5.83 × 10−8 | −0.268 | This work |
| CoCrFeNi1.7Ti0.3 | 1.32 × 10−8 | −0.26 | Hsieh [38] |
| CuFeTiZrNi0.1 | 7.04 × 10−8 | −0.292 | This work |
| CuFeTiZrNi0.3 | 5.95 × 10−8 | −0.264 | This work |
| CuFeTiZrNi0.5 | 5.65 × 10−8 | −0.242 | This work |
| CuFeTiZrNi0.8 | 5.32 × 10−8 | −0.240 | This work |
| CuFeTiZrNi1.0 | 5.11 × 10−8 | −0.236 | This work |
| Alloys | Corrosion Rate (mm/year) | |
|---|---|---|
| Polarization Test | Immersion Test | |
| CuFeTiZrNi0.1 | 39.17 × 10−4 | 49.25 × 10−3 |
| CuFeTiZrNi0.3 | 33.83 × 10−4 | 37.86 × 10−3 |
| CuFeTiZrNi0.5 | 26.54 × 10−4 | 14.46 × 10−3 |
| CuFeTiZrNi0.8 | 22.07 × 10−4 | 10.08 × 10−3 |
| CuFeTiZrNi1.0 | 19.43 × 10−4 | 3.58 × 10−3 |
| SUS 304 | 8.03 × 10−4 | 2.74 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.-C.; Chen, S.-Y.; Yu, W.; Okumura, R.; Iikubo, S.; Laksono, A.D.; Yen, Y.-W.; Pasana, A.S. The Effect of Increasing Nickel Content on the Microstructure, Hardness, and Corrosion Resistance of the CuFeTiZrNix High-Entropy Alloys. Materials 2022, 15, 3098. https://doi.org/10.3390/ma15093098
Kuo P-C, Chen S-Y, Yu W, Okumura R, Iikubo S, Laksono AD, Yen Y-W, Pasana AS. The Effect of Increasing Nickel Content on the Microstructure, Hardness, and Corrosion Resistance of the CuFeTiZrNix High-Entropy Alloys. Materials. 2022; 15(9):3098. https://doi.org/10.3390/ma15093098
Chicago/Turabian StyleKuo, Po-Cheng, Sin-Yi Chen, William Yu, Ryo Okumura, Satoshi Iikubo, Andromeda Dwi Laksono, Yee-Wen Yen, and Alberto S. Pasana. 2022. "The Effect of Increasing Nickel Content on the Microstructure, Hardness, and Corrosion Resistance of the CuFeTiZrNix High-Entropy Alloys" Materials 15, no. 9: 3098. https://doi.org/10.3390/ma15093098
APA StyleKuo, P.-C., Chen, S.-Y., Yu, W., Okumura, R., Iikubo, S., Laksono, A. D., Yen, Y.-W., & Pasana, A. S. (2022). The Effect of Increasing Nickel Content on the Microstructure, Hardness, and Corrosion Resistance of the CuFeTiZrNix High-Entropy Alloys. Materials, 15(9), 3098. https://doi.org/10.3390/ma15093098








