Experimental Investigation of Phase Equilibria in the Co-Ta-Si Ternary System
Abstract
:1. Introduction
| System | Phase | Pearson Symbol | Prototype | Space Group | Struktur-bericht | Refs. |
|---|---|---|---|---|---|---|
| Co-Si | (αCo) | cF4 | Cu | Fm-3m | A1 | [26] |
| (εCo) | hP2 | Mg | P63/mmc | A3 | [26] | |
| Co3Si | hP8 | Mg3Cd | P63/mmc | - | [26] | |
| αCo2Si | oP12 | Co2Si | Pnma | C23 | [26] | |
| βCo2Si | - | - | - | - | [26] | |
| CoSi | cP8 | FeSi | P213 | B20 | [26] | |
| CoSi2 | cF12 | CaF2 | Fm-3m | C1 | [26] | |
| (Si) | cF8 | C(diamond) | Fd-3m | A4 | [26] | |
| Co-Ta | Co7Ta2 | hR36 | BaPb3 | R-3m | - | [27] |
| λ1-Co2Ta | hP12 | Zn2Mg | P63/mmc | C14 | [27] | |
| λ2-Co2Ta | cF24 | Cu2Mg | Fd-3m | C15 | [27] | |
| λ3-Co2Ta | hP24 | MgNi2 | P63/mmc | C36 | [27] | |
| Co6Ta7 | hR13 | Fe7W6 | R-3m | D85 | [27] | |
| CoTa2 | tI12 | Al2Cu | I4/mcm | C16 | [27] | |
| (Ta) | cI2 | W | Im-3m | A2 | [27] | |
| Ta-Si | Ta3Si | tP32 | Ti3P | P63/mcm | - | [28] |
| Ta2Si | tI12 | Al2Cu | I4/mcm | C16 | [28] | |
| αTa5Si3 | tI32 | Cr5B3 | I4/mcm | D81 | [28] | |
| βTa5Si3 | hP16 | Mn5Si3 | P63/mcm | D88 | [28] | |
| γTa5Si3 | tI32 | W5Si3 | I4/mcm | D8m | [28] | |
| TaSi2 | hP9 | CrSi2 | P6222 | C40 | [28] | |
| (Si) | cF8 | C(diamond) | Fd-3m | A4 | [28] | |
| Co-Ta-Si | CoTaSi (E) | oP12 | TiNiSi | Pnma | C23 | [34] |
| Co16Ta6Si7 (G) | cF116 | Mg6Cu16Si7 | Fm3m | A1 | [29] | |
| Co4TaSi3 (G″) | hP168 | Y13Pd40Sn31 | P6/mmm | - | [30] | |
| Co3Ta2Si (L) | hP12 | MgZn2 | P63/mmc | C14 | [32] | |
| Co4Ta4Si7 (V) | tI60 | Zr4Co4Ge7 | - | - | [34] |
2. Experimental Procedures
3. Results and Discussion
3.1. Microstructure and Phase Equilibrium
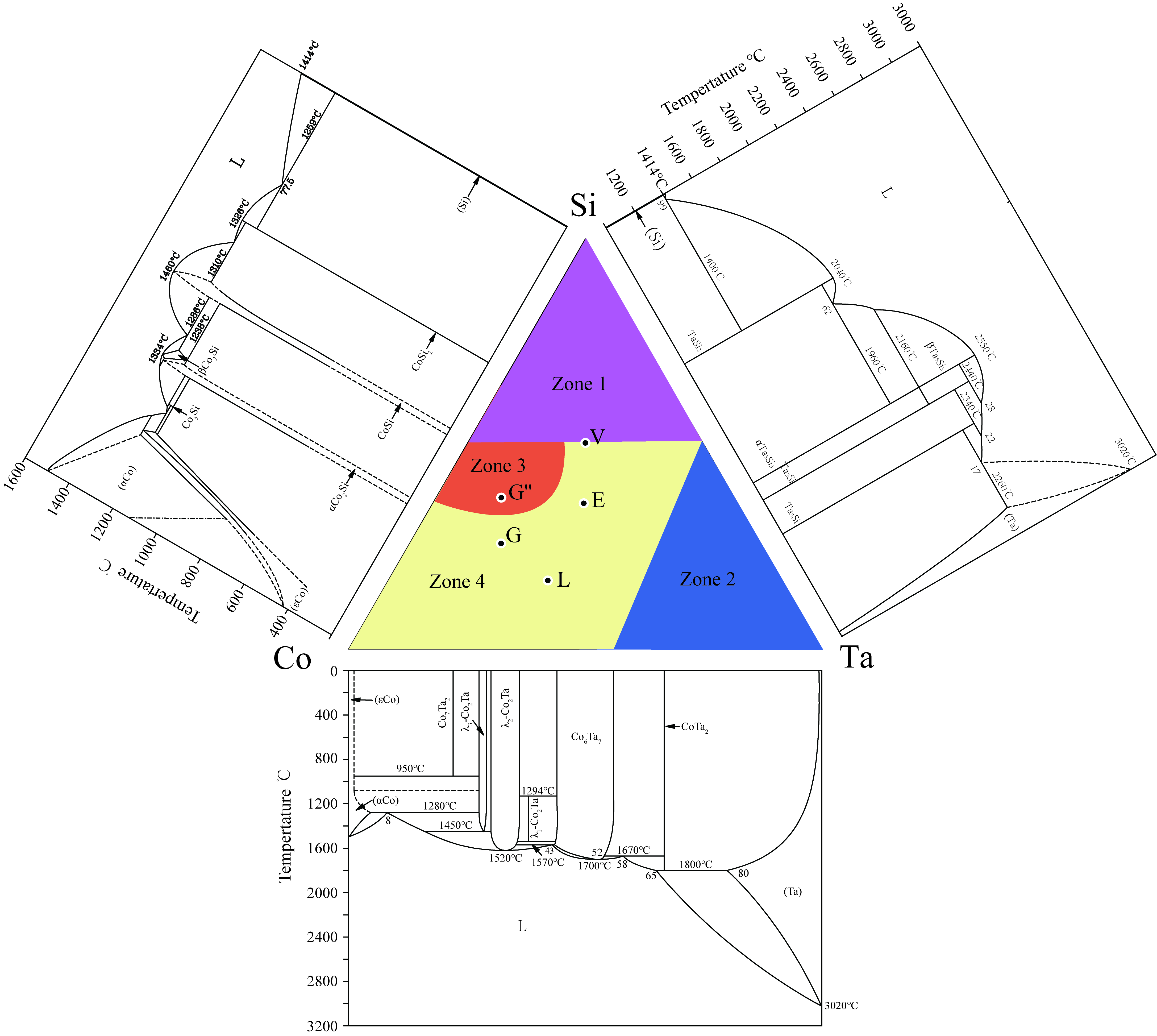
3.1.1. Equilibria at Zone 1
3.1.2. Equilibria at Zone 2
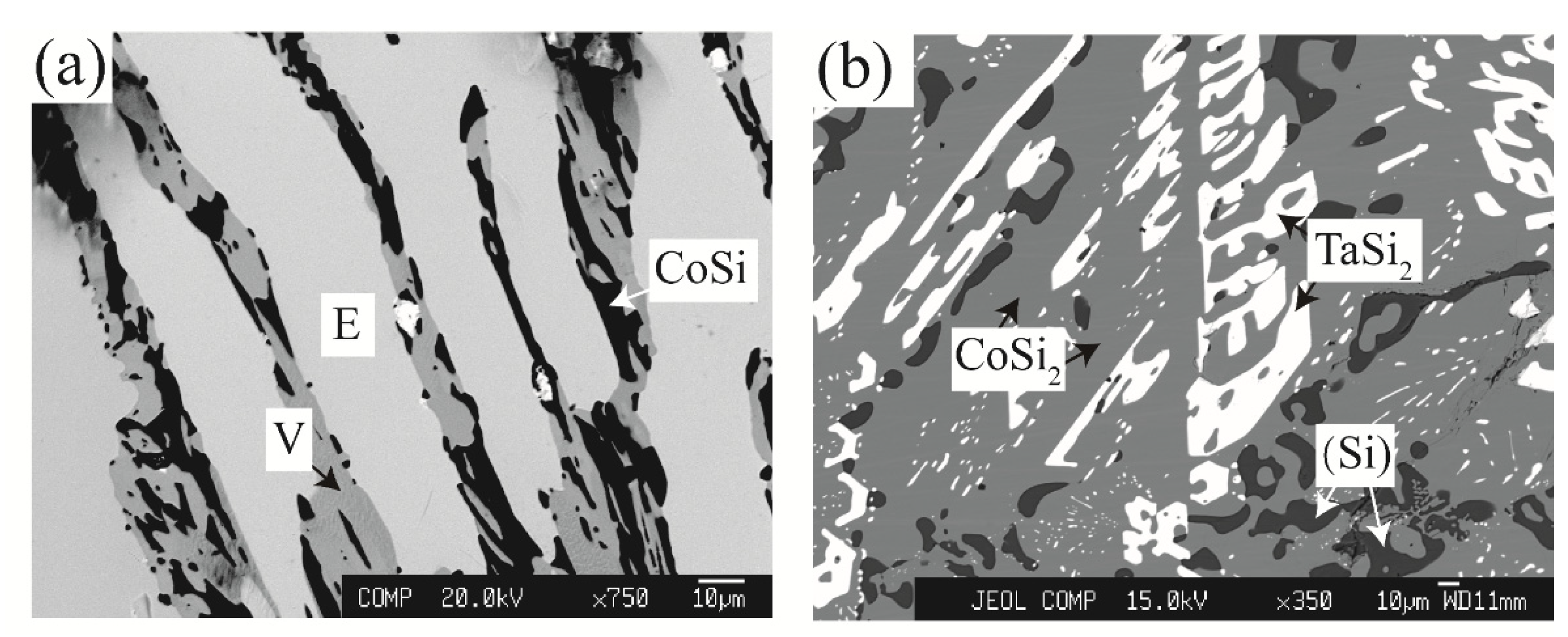
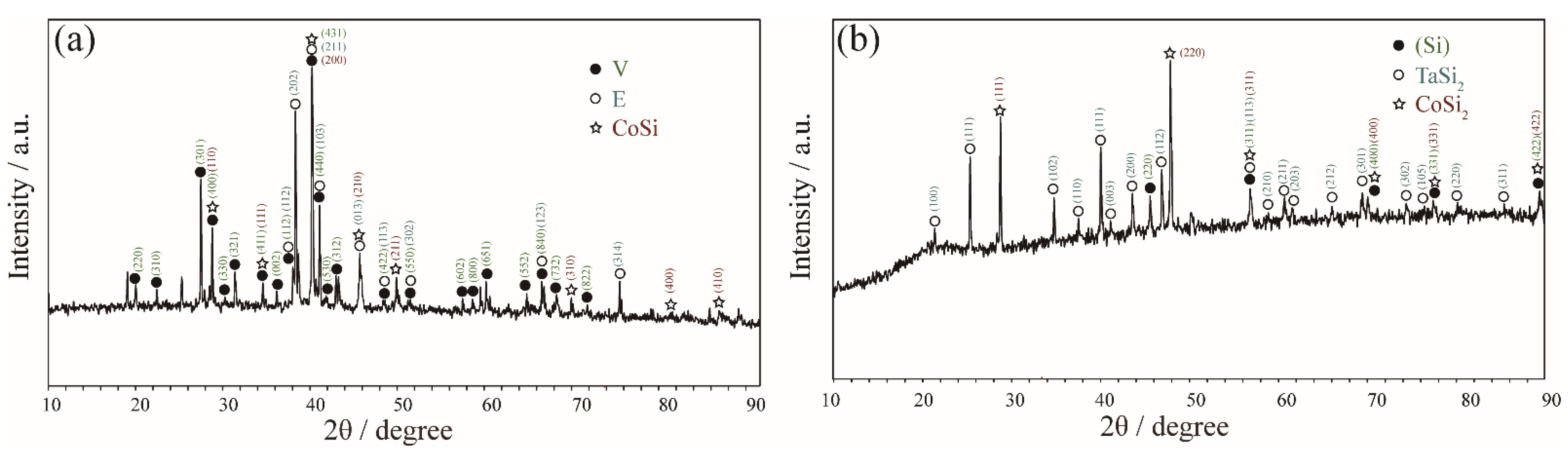
3.1.3. Equilibria at Zone 3
3.1.4. Equilibria at Zone 4
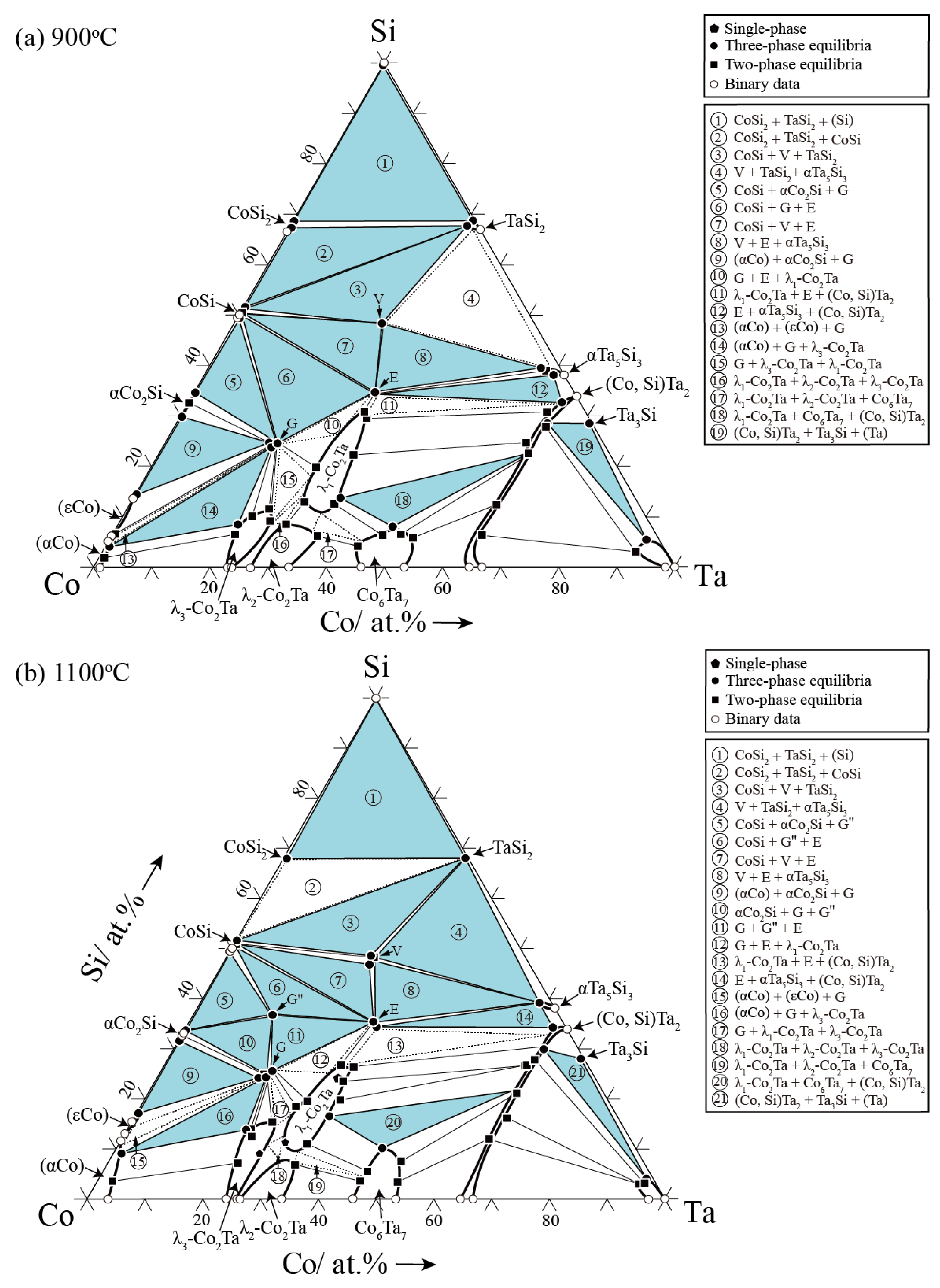
3.2. Isothermal Sections
4. Conclusions
- The four known ternary phases, G (Co16Ta6Si7), E (CoTaSi), G″ (Co4TaSi3) and V (Co4Ta4Si7) have almost no ternary solid solubilities, which could be treated as stoichiometric compounds.
- The high-temperature phase G″ (Co4TaSi3) is only stable at 1100 °C and it disappears when the temperature decreases to 900 °C.
- The addition of Si increases the thermal stability of the binary λ1-Co2Ta (C14 Laves) phase, resulting in the formation of the ternary L phase with the composition of Co32.3–58.8Ta28.2–36.8Si13.0–30.9 at 900 °C and Co39.3–62.3Ta26.8–33.9Si10.9–26.8 at 1100 °C.
- Both the binary CoTa2 and SiTa2 phases crystallize with the same body-centered tetragonal structure (space group: I4/mcm, C16) and they form a continuous solid solution phase (Co, Si)Ta2.
- The maximum solid solubility of Si for the λ3-Co2Ta phase is ~15.3 at.% at 1100 °C and it slightly decreases to be ~11.5 at.% at 900 °C. The solid solubility of Si for the Co6Ta7 phase is always ~12 at.% and does not change with temperature.
- The elemental Ta is hardly dissolved in the CoSi2, CoSi and α-Co2Si phases. Similarly, the elemental Co has negligible solubilities in the TaSi2 and α-Ta5Si3 phases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sato, J.; Omori, T.; Oikawa, K.; Ohnuma, I.; Kainuma, R.; Ishida, K. Cobalt-Base High-Temperature Alloys. Science 2006, 312, 90. [Google Scholar] [CrossRef] [PubMed]
- Mitrica, D.; Badea, I.C.; Serban, B.A.; Olaru, M.T.; Vonica, D.; Burada, M.; Piticescu, R.-R.; Popov, V.V. Complex Concentrated Alloys for Substitution of Critical Raw Materials in Applications for Extreme Conditions. Materials 2021, 14, 1197. [Google Scholar] [CrossRef]
- Bocchini, P.J.; Sudbrack, C.K.; Noebe, R.D.; Dunand, D.C.; Seidman, D.N. Effects of titanium substitutions for aluminum and tungsten in Co-10Ni-9Al-9W (at %) superalloys. Mater. Sci. Eng. A 2017, 705, 122–132. [Google Scholar] [CrossRef]
- Reyes Tirado, F.L.; Perrin Toinin, J.; Dunand, D.C. γ + γ′ microstructures in the Co-Ta-V and Co-Nb-V ternary systems. Acta Mater. 2018, 151, 137–148. [Google Scholar] [CrossRef]
- Berthod, P.; Ozouaki, S.; Aranda, L.; Medjahdi, G.; Etienne, E. Kinetic and Metallography Study of the Oxidation at 1250 °C of {Co + Ni}-Based Superalloys Containing Ti to Form MC Carbides. Metals 2022, 12, 10. [Google Scholar]
- Xu, W.; Wang, Y.; Wang, C.; Liu, X.; Liu, Z.-K. Alloying effects of Ta on the mechanical properties of γ′ Co3 (Al, W): A first-principles study. Scr. Mater. 2015, 100, 5–8. [Google Scholar] [CrossRef]
- Yan, H.; Vorontsov, V.; Dye, D. Effect of alloying on the oxidation behaviour of Co–Al–W superalloys. Corros. Sci. 2014, 83, 382–395. [Google Scholar] [CrossRef]
- Klein, L.; Shen, Y.; Killian, M.S.; Virtanen, S. Effect of B and Cr on the high temperature oxidation behaviour of novel γ/γ′-strengthened Co-base superalloys. Corros. Sci. 2011, 53, 2713–2720. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, C.; Qi, H.; Jiang, L.; Jin, Z.; Zhao, J.-C. Experimental investigation of phase equilibria in the Co-rich part of the Co-Al-X (X = W, Mo, Nb, Ni, Ta) ternary systems using diffusion multiples. J. Alloys Compd. 2017, 691, 110–118. [Google Scholar] [CrossRef]
- Yeh, A.; Wang, S.; Cheng, C.; Chang, Y.; Chang, S. Oxidation Behaviour of Si-Bearing Co-Based Alloys. Oxid. Met. 2016, 86, 99–112. [Google Scholar] [CrossRef]
- Liang, Z.; Göken, M.; Lorenz, U.; Neumeier, S.; Oehring, M.; Pyczak, F.; Stark, A.; Wang, L. Influence of small amounts of Si and Cr on the high temperature oxidation behavior of novel cobalt base superalloys. Corros. Sci. 2021, 184, 109388. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Wang, Y.; Han, J.; Xu, W.; Liu, X. Effects of Transition Elements on the Structural, Elastic Properties and Relative Phase Stability of L12 γ′-Co3Nb from First-Principles Calculations. Metals 2021, 11, 933. [Google Scholar] [CrossRef]
- Lass, E.A. The effects of Fe and Si on the phase equilibria in a γ′-strengthened Co–Al–W-based superalloy. J. Alloys Compd. 2020, 825, 154158. [Google Scholar] [CrossRef]
- Noubary, K.D.; Kellner, M.; Nestler, B. Rotating Directional Solidification of Ternary Eutectic Microstructures in Bi-In-Sn: A Phase-Field Study. Materials 2022, 15, 1160. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Sobolev, S.; Du, Y. Kinetic phase diagrams of ternary Al-Cu-Li system during rapid solidification: A phase-field study. Materials 2018, 11, 260. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; He, W.; Chen, G.; Zeng, W.; Wang, J.; Zeng, L.; Liang, J. The Phase Relations of the Co-Ni-In Ternary System at 673 K and 873 K and Magnetic Properties of Their Compounds. Materials 2020, 13, 3990. [Google Scholar] [CrossRef]
- Ledwig, P.; Kac, M.; Kopia, A.; Falkus, J.; Dubiel, B. Microstructure and Properties of Electrodeposited Nanocrystalline Ni-Co-Fe Coatings. Materials 2021, 14, 3886. [Google Scholar] [CrossRef]
- Yuan, Z.; Kobayashi, S. Determination of Phase Equilibria among δ-Fe, γ-Fe and Fe2M Phases in Fe-Cr-M (M: Hf, Ta) Ternary Systems. Metals 2022, 12, 102. [Google Scholar] [CrossRef]
- Strutynski, C.; Evrard, M.; Le Gendre, A.; Maldonado, A.; Désévédavy, F.; Gadret, G.; Jules, J.-C.; Smektala, F. Physicochemical Properties and Fiber-Drawing Ability of Tellurite Glasses in the TeO2-ZnO-Y2O3 Ternary System. Materials 2022, 15, 1177. [Google Scholar] [CrossRef]
- Uchida, Y.; Watanabe, C.; Tsuruoka, H. Basic Evaluation of Phase Relation in a Phosphorus-Containing System Saturated with CaSiO3 at Elevated Temperatures for the Utilization of Steelmaking Slag and Sewage Sludge as Phosphorus Resources. Minerals 2022, 12, 266. [Google Scholar] [CrossRef]
- Baranauskaite, V.; Belysheva, M.; Pestova, O.; Anufrikov, Y.; Skripkin, M.; Kondratiev, Y.; Khripun, V. Thermodynamic Description of Dilution and Dissolution Processes in the MgCl2-CsCl-H2O Ternary System. Materials 2021, 14, 4047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, C.; Ruan, J.; Yang, S.; Omori, T.; Kainuma, R.; Ishida, K.; Han, J.; Lu, Y.; Liu, X. Development of low-density γ/γ′ Co-Al-Ta-based superalloys with high solvus temperature. Acta Mater. 2020, 188, 652–664. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Ruan, J.; Omori, T.; Kainuma, R.; Ishida, K.; Liu, X. High-strength Co–Al–V-base superalloys strengthened by γ′-Co3 (Al, V) with high solvus temperature. Acta Mater. 2019, 170, 62–74. [Google Scholar] [CrossRef]
- Volz, N.; Xue, F.; Zenk, C.H.; Bezold, A.; Gabel, S.; Subramanyam, A.; Drautz, R.; Hammerschmidt, T.; Makineni, S.K.; Gault, B. Understanding creep of a single-crystalline Co-Al-W-Ta superalloy by studying the deformation mechanism, segregation tendency and stacking fault energy. Acta Mater. 2021, 214, 117019. [Google Scholar] [CrossRef]
- Lenz, M.; Wu, M.; Spiecker, E. Segregation-assisted climb of Frank partial dislocations: An alternative route to superintrinsic stacking faults in L12-hardened superalloys. Acta Mater. 2020, 191, 270–279. [Google Scholar] [CrossRef]
- Zhang, L.; Du, Y.; Xu, H.; Pan, Z. Experimental investigation and thermodynamic description of the Co–Si system. Calphad 2006, 30, 470–481. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, C.; Li, C.; Du, Z. Thermodynamic description of the Co–Ni–Ta system. Calphad 2019, 66, 101649. [Google Scholar] [CrossRef]
- Drouelle, I.; Servant, C. Thermodynamic assessment of the Si–Ta system. J. Alloys Compd. 2013, 551, 293–299. [Google Scholar] [CrossRef]
- Beck, F. Ternary G and E silicides and germanides of transition elements. Trans. Met. Soc. AIME 1963, 27, 575. [Google Scholar]
- Vilasi, M.; Venturini, G.; Steinmetz, J.; Malaman, B. Structure of “TaCo4Si3”, a new silicide closely related to the Y13Pd40Sn31 stannide. J. Alloys Compd. 1995, 227, 32–36. [Google Scholar] [CrossRef]
- Gladyshevsky, E. New ternary compounds with a structure of the Mg6Cu16Si7 type. Dopov. Akad. Nauk Ukr. SSR 1962, 4, 481–483. [Google Scholar]
- Trojko, R.; Blažina, Ž. Metal-metalloid exchange in some Friauf-Laves phases containing two transition metals. J. Less-Common. Met. 1985, 106, 293–300. [Google Scholar] [CrossRef]
- Mittal, R. Si-Stabilised C14 laves phases in the transition metal systems. J. Less-Common. Met. 1978, 60, 75–82. [Google Scholar] [CrossRef]
- Jeitschko, W.; Jordan, A.; Beck, P. V and E phase in ternary systems with transition metals and silicon or germanium. Trans. Met. Soc. AIME 1969, 245, 335–339. [Google Scholar]
- Markiv, V. The crystal structures of the compounds R (M, X) 2 and RMX2 in Zr-Ni-Al, Ti-Fe-Si and related systems. Acta Cryst. 1966, 21, 84–85. [Google Scholar]
- Markiv, B.; Gladyshevskii, E.; Skolozdra, R.; Krypyakevich, P. Ternary compounds RX XX in the systems Ti–V (Fe, Co, Ni)–Si and some related systems. Dopovidi Akad. Nauk. Ukr. Koi RSR SERIYA A Fiz.-Tekhnichni Ta Mat. Nauk. 1967, 3, 266–269. [Google Scholar]
- Beattie, H.J.; Versnyder, F.L. A New Complex Phase in a High-Temperature Alloy. Nature 1956, 178, 208–209. [Google Scholar] [CrossRef]
- Yan, X.; Grytsiv, A.; Rogl, P.; Pomjakushin, V.; Xue, X. On the crystal structure of the Mn–Ni–Si G-phase. J. Alloys Compd. 2009, 469, 152–155. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Takeuchi, T.; Kakubo, Y.; Suzudo, T.; Watanabe, H.; Abe, H.; Toyama, T.; Nagai, Y. The two-step nucleation of G-phase in ferrite. Acta Mater. 2016, 116, 104–113. [Google Scholar] [CrossRef]
- Hu, X. The 1100 °C Isothermal Section of the Ti-Co-Si Ternary System. J. Phase Equilibria 2001, 22, 114–121. [Google Scholar] [CrossRef]
- Gupta, K. The Co-Nb-Si (Cobalt-Niobium-Silicon) System. J. Phase Equilibria Diffus. 2010, 31, 308–312. [Google Scholar] [CrossRef]
- Wang, C.; Huang, L.; Yang, M.; Huang, X.; Zhang, J.; Pan, S.; Yang, S.; Liu, X. Experimental investigation of phase equilibria in the Ni-Ta-Si refractory alloy system. J. Alloys Compd. 2021, 888, 161467. [Google Scholar] [CrossRef]
- Santos, V.; Eleno, L.; Schön, C.; Richter, K. Experimental investigation of phase equilibria in the Nb–Ni–Si refractory alloy system at 1323 K. J. Alloys Compd. 2020, 842, 155373. [Google Scholar] [CrossRef]
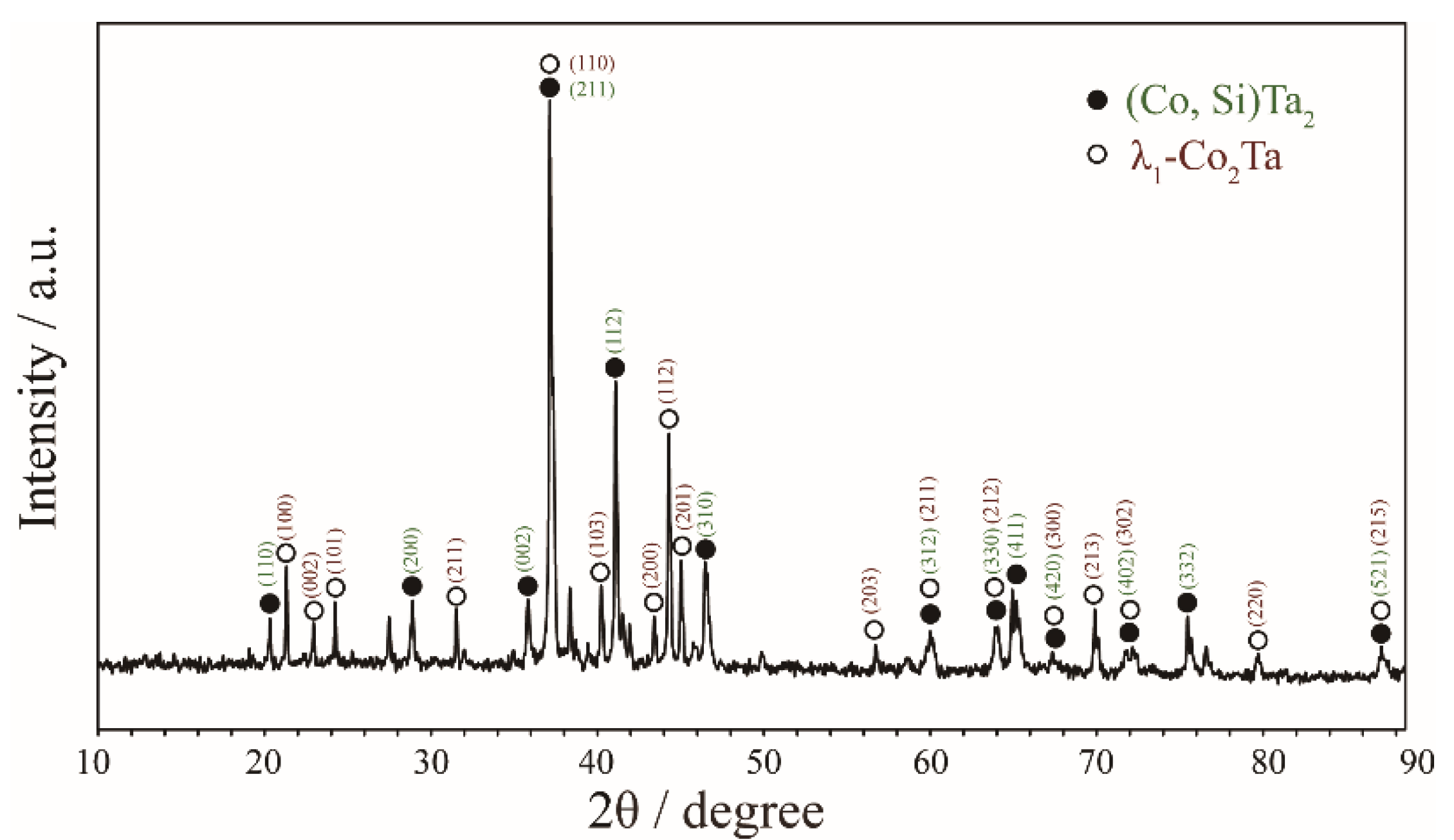
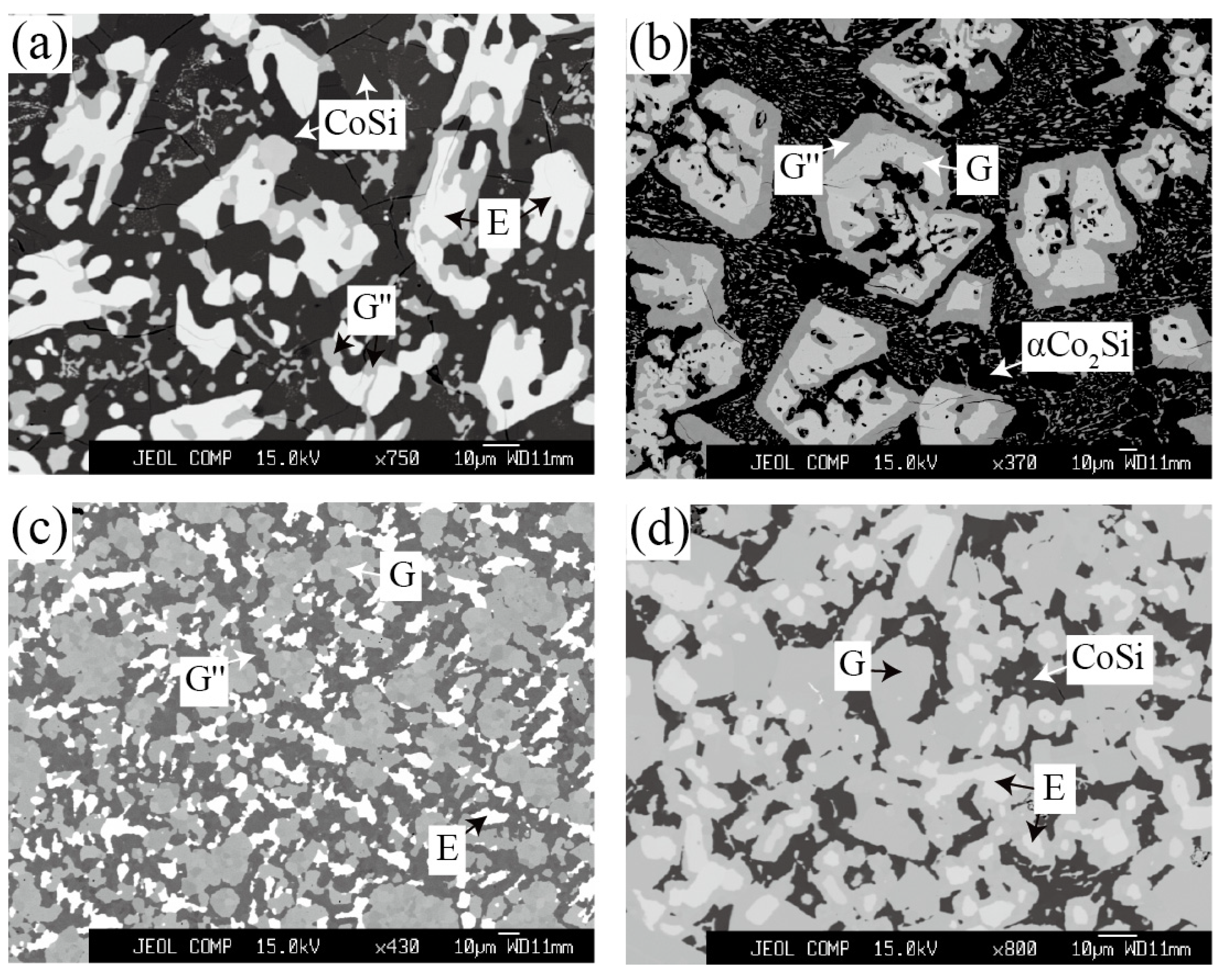
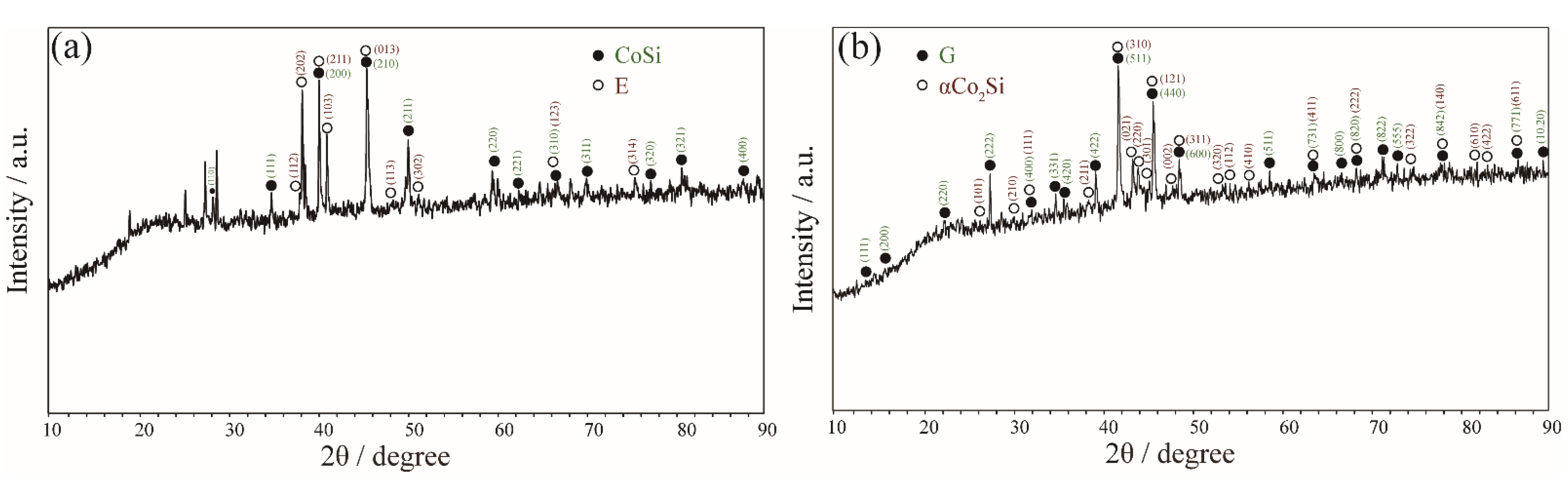


| Alloy (at.%) | Annealed Time | Phase Equilibria | Composition (at.%) | |||||
|---|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Ta | Si | Ta | Si | Ta | Si | |||
| Co10Ta10Si80 | 90 days | TaSi2 / CoSi2 / (Si) | 30.3 | 68.6 | 0.1 | 70.3 | 0.1 | 99.7 |
| Co33Ta14Si53 | 90 days | V / CoSi | 24.8 | 48.4 | 0.1 | 51.6 | ||
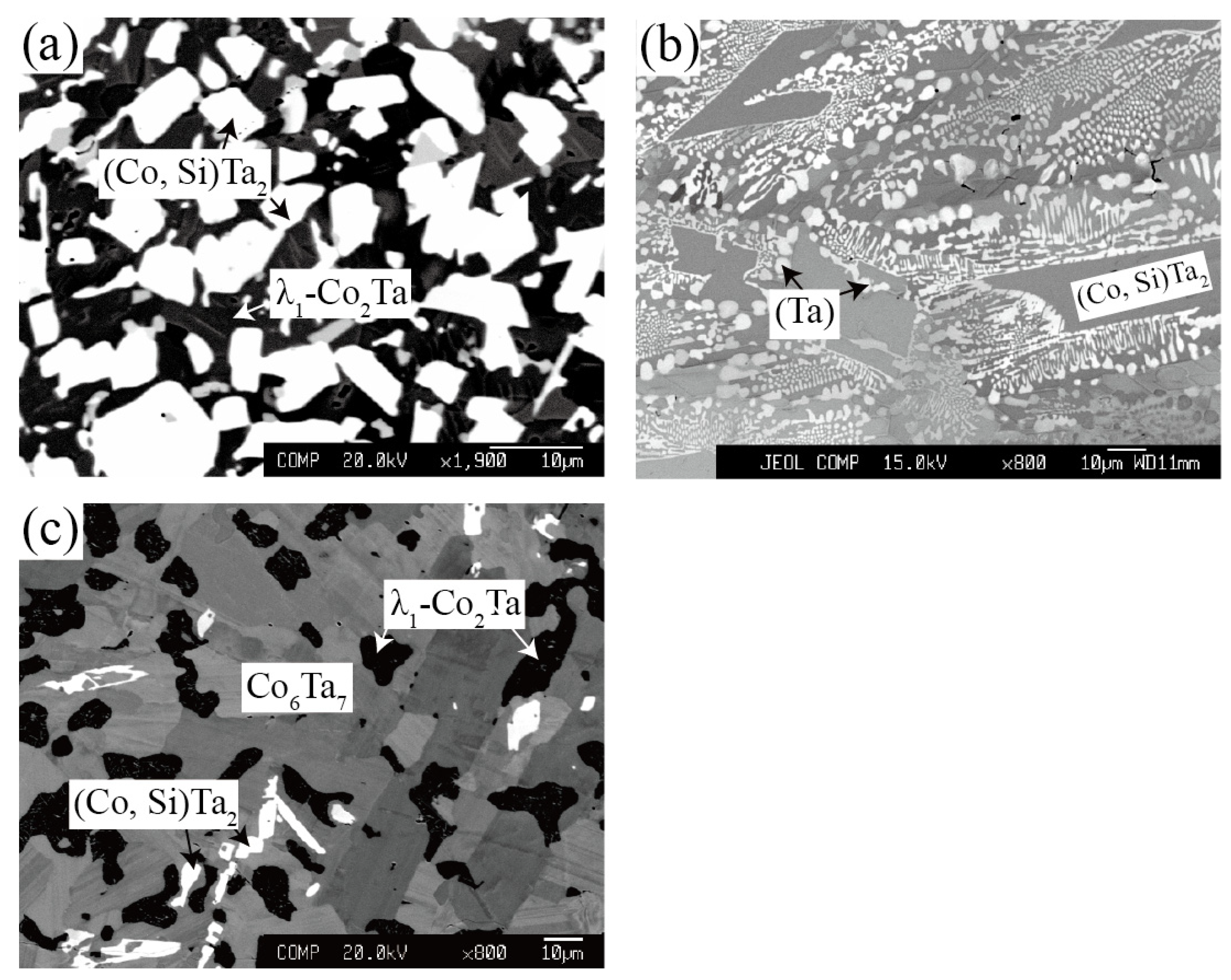
| Co30Ta43Si27 | 90 days | (Co, Si)Ta2 / λ1-Co2Ta | 62.6 | 30.8 | 32.2 | 29.5 | ||
| Co54Ta28Si18 | 90 days | λ1-Co2Ta / G | 28.2 | 18.9 | 19.2 | 25.1 | ||
| Co16Ta18Si66 | 90 days | TaSi2 / CoSi / CoSi2 | 30.4 | 67.8 | 0.2 | 51.8 | 0.2 | 67.2 |
| Co22Ta27Si51 | 90 days | TaSi2 / V / CoSi | 30.6 | 67.8 | 25.3 | 48.2 | 0.5 | 51.6 |
| Co27Ta60Si13 | 90 days | (Co, Si)Ta2 / Co6Ta7 | 63.7 | 22.8 | 49.6 | 6.7 | ||
| Co34Ta26Si40 | 90 days | E / V / CoSi | 31.6 | 34.8 | 22.7 | 46.5 | 0.2 | 50.5 |
| Co25Ta38Si37 | 90 days | αTa5Si3 / E / V | 58.4 | 39.0 | 32.1 | 35.2 | 25.2 | 48.1 |
| Co64.5Ta22.5Si13 | 90 days | λ3-Co2Ta / G | 23.5 | 11.5 | 19.9 | 25.4 | ||
| Co36Ta54Si10 | 90 days | (Co, Si)Ta2 / Co6Ta7 | 64.0 | 20.6 | 49.7 | 9.2 | ||
| Co2Ta75Si23 | 90 days | (Ta) / (Co, Si)Ta2 / Ta3Si | 92.3 | 5.4 | 63.8 | 28.6 | 71.0 | 28.6 |
| Co26Ta7Si67 | 90 days | CoSi2 / TaSi2 | 0.1 | 67.5 | 29.5 | 67.1 | ||
| Co46Ta13Si41 | 90 days | G / CoSi | 18.2 | 25.8 | 0.1 | 51.2 | ||
| Co58Ta10Si32 | 90 days | Co2Si / CoSi / G | 0.1 | 34.6 | 0.1 | 50.6 | 18.0 | 26.2 |
| Co48Ta22Si30 | 90 days | E / G / CoSi | 30.2 | 35.1 | 18.3 | 26.1 | 0.1 | 51.1 |
| Co36Ta33Si31 | 90 days | E / λ1-Co2Ta | 30.9 | 35.2 | 31.1 | 30.9 | ||
| Co59.5Ta22.5Si18 | 90 days | λ3-Co2Ta / G | 22.1 | 11.0 | 18.6 | 24.6 | ||
| Co59Ta31Si10 | 90 days | λ1-Co2Ta / λ2-Co2Ta | 29.7 | 13.0 | 28.8 | 8.5 | ||
| Co11Ta75Si14 | 90 days | (Ta) / (Co, Si)Ta2 | 91.2 | 3.0 | 63.7 | 28.1 | ||
| Co38Ta58Si4 | 90 days | Co6Ta7 / (Co, Si)Ta2 | 63.3 | 12.4 | 52.1 | 5.9 | ||
| Co25Ta33Si42 | 90 days | αTa5Si3 / E / V | 57.2 | 39.6 | 32.4 | 35.6 | 24.4 | 48.9 |
| Co24Ta44Si31 | 90 days | αTa5Si3 / E | 58.1 | 39.4 | 31.6 | 34.8 | ||
| Co65Ta27Si8 | 90 days | G / λ3-Co2Ta | 19.1 | 24.1 | 25.3 | 9.2 | ||
| Co56Ta9Si35 | 90 days | G / αCo2Si / CoSi | 19.0 | 25.0 | 0.4 | 33.6 | 0.3 | 49.2 |
| Co44Ta25Si31 | 90 days | E / G / CoSi | 30.7 | 33.6 | 19.3 | 24.6 | 0.7 | 47.4 |
| Co10Ta59Si31 | 90 days | αTa5Si3 / E / (Co, Si)Ta2 | 58.8 | 39.4 | 32.0 | 29.7 | 63.4 | 33.2 |
| Co61Ta11Si28 | 90 days | G / αCo2Si | 17.7 | 24.8 | 0.1 | 32.6 | ||
| Co70Ta9Si21 | 90 days | G / (εCo) / αCo2Si | 57.7 | 24.3 | 0.1 | 14.4 | 0.2 | 29.7 |
| Co37Ta42Si21 | 90 days | (Co, Si)Ta2 / λ1-Co2Ta | 62.0 | 24.7 | 44.3 | 22.2 | ||
| Co71Ta13Si16 | 90 days | G / (αCo) | 18.8 | 24.4 | 0.5 | 6.7 | ||
| Co78Ta10Si12 | 90 days | λ3-Co2Ta / G / (αCo) | 20.6 | 8.5 | 18.7 | 23.7 | 0.7 | 4.0 |
| Co51Ta37Si12 | 90 days | Co6Ta7 / λ1-Co2Ta | 46.5 | 6.2 | 32.4 | 16.2 | ||
| Co41Ta47Si12 | 90 days | (Co, Si)Ta2 / Co6Ta7 / λ1-Co2Ta | 62.9 | 22.3 | 47.6 | 8.9 | 36.8 | 13.5 |
| Co78Ta17Si5 | 90 days | λ3-Co2Ta / (αCo) | 21.0 | 6.4 | 0.9 | 1.9 | ||
| Co57Ta38Si5 | 90 days | Co6Ta7 / λ2-Co2Ta | 43.2 | 4.1 | 35.5 | 6.1 | ||
| Co27Ta68Si5 | 90 days | (Ta) / (Co, Si)Ta2 | 91.7 | 2.9 | 63.5 | 6.2 | ||
| Alloy (at.%) | Annealed Time | Phase Equilibria | Composition (at.%) | |||||
|---|---|---|---|---|---|---|---|---|
| Phase 1/Phase 2/Phase 3 | Phase 1 | Phase 2 | Phase 3 | |||||
| Ta | Si | Ta | Si | Ta | Si | |||
| Co10Ta10Si80 | 60 days | TaSi2 / CoSi2 / (Si) | 31.0 | 67.3 | 0.7 | 68.0 | 0.1 | 99.0 |
| Co33Ta14Si53 | 60 days | V / CoSi | 25.4 | 48.2 | 0.1 | 50.9 | ||
| Co10Ta38Si52 | 60 days | TaSi2 / αTa5Si3 / V | 31.4 | 68.1 | 58.5 | 39.3 | 25.6 | 48.8 |
| Co46Ta28Si26 | 60 days | E / G | 30.8 | 34.8 | 19.7 | 25.5 | ||
| Co30Ta43Si27 | 60 days | (Co, Si)Ta2 / λ1-Co2Ta | 63.6 | 30.3 | 32.9 | 26.4 | ||
| Co54Ta28Si18 | 60 days | λ1-Co2Ta / G | 28.2 | 19.5 | 20.1 | 25.4 | ||
| Co16Ta18Si66 | 60 days | CoSi / TaSi2 / V | 30.8 | 67.6 | 0.1 | 51.8 | 24.5 | 48.4 |
| Co22Ta27Si51 | 60 days | TaSi2 / V | 31.4 | 68.4 | 25.3 | 48.9 | ||
| Co27Ta60Si13 | 60 days | (Co, Si)Ta2 / Co6Ta7 | 63.8 | 21.1 | 50.7 | 7.5 | ||
| Co35Ta5Si60 | 60 days | V / CoSi | 23.8 | 48.6 | 0.1 | 51.2 | ||
| Co34Ta26Si40 | 60 days | E / V / CoSi | 31.8 | 35.4 | 25.2 | 46.8 | 0.1 | 51.9 |
| Co25Ta38Si37 | 60 days | E / αTa5Si3 / V | 57.7 | 39.6 | 32.0 | 35.6 | 25.7 | 48.3 |
| Co64.5Ta22.5Si13 | 60 days | λ3-Co2Ta / G | 22.1 | 12.6 | 19.5 | 23.8 | ||
| Co2Ta75Si23 | 60 days | Ta / (Co, Si)Ta2 / Ta3Si | 94.8 | 4.0 | 64.0 | 30.0 | 27.9 | 71.6 |
| Co26Ta7Si67 | 60 days | TaSi2 / CoSi2 / (Si) | 30.4 | 68.1 | 0.1 | 67.8 | 0.1 | 99.5 |
| Co46Ta13Si41 | 60 days | E / G″ / CoSi | 30.2 | 35.0 | 13.0 | 37.4 | 0.1 | 50.5 |
| Co58Ta10Si32 | 60 days | αCo2Si / G / G″ | 0.1 | 34.7 | 18.6 | 25.8 | 13.6 | 37.1 |
| Co48Ta22Si30 | 60 days | G / G″ / E | 18.7 | 25.9 | 13.4 | 37.3 | 30.7 | 34.8 |
| Co36Ta33Si31 | 60 days | E / λ1-Co2Ta | 30.7 | 35.4 | 30.5 | 26.8 | ||
| Co59.5Ta22.5Si18 | 60 days | G / λ3-Co2Ta | 18.8 | 25.1 | 24.2 | 15.3 | ||
| Co59Ta31Si10 | 60 days | λ1-Co2Ta | 28.7 | 11.3 | ||||
| Co11Ta75Si14 | 60 days | (Ta) / (Co, Si)Ta2 | 95.0 | 3.1 | 63.3 | 27.9 | ||
| Co38Ta58Si4 | 60 days | Co6Ta7 / (Co, Si)Ta2 | 63.5 | 12.0 | 52.1 | 3.4 | ||
| Co25Ta33Si42 | 60 days | E / V | 31.4 | 35.9 | 24.6 | 48.8 | ||
| Co24Ta44Si31 | 60 days | αTa5Si3 / E | 58.6 | 39.4 | 30.8 | 35.5 | ||
| Co65Ta27Si8 | 60 days | λ3-Co2Ta | 25.1 | 9.1 | ||||
| Co42Ta15Si43 | 60 days | CoSi / E | 0.3 | 50.8 | 30.0 | 35.0 | ||
| Co26Ta33Si41 | 60 days | V / E | 24.9 | 48.5 | 31.3 | 35.1 | ||
| Co56Ta9Si35 | 60 days | G" / αCo2Si / CoSi | 13.6 | 36.5 | 0.1 | 33.9 | 0.1 | 49.3 |
| Co44Ta25Si31 | 60 days | G / G" | 18.9 | 25.6 | 13.9 | 36.4 | ||
| Co10Ta59Si31 | 60 days | αTa5Si3 / Ta2Si / E | 58.7 | 39.1 | 63.4 | 34.1 | 32.9 | 34.4 |
| Co61Ta11Si28 | 60 days | G / αCo2Si | 18.1 | 24.9 | 0.2 | 32.7 | ||
| Co70Ta9Si21 | 60 days | G / (εCo) / αCo2Si | 17.9 | 24.6 | 0.2 | 17.4 | 0.1 | 32.0 |
| Co54Ta25Si21 | 60 days | λ1-Co2Ta / G | 26.8 | 18.5 | 19.2 | 25.6 | ||
| Co54Ta25Si21 | 60 days | λ1-Co2Ta | 31.2 | 24.1 | ||||
| Co37Ta42Si21 | 60 days | (Co, Si)Ta2 / λ1-Co2Ta | 62.4 | 26.6 | 32.5 | 23.5 | ||
| Co26Ta53Si21 | 60 days | (Co, Si)Ta2 / λ1-Co2Ta | 62.7 | 26.9 | 33.9 | 19.9 | ||
| Co60Ta24Si16 | 60 days | λ3-Co2Ta / G | 21.3 | 13.9 | 18.9 | 24.5 | ||
| Co78Ta10Si12 | 60 days | λ3-Co2Ta / G / (αCo) | 20.6 | 13.8 | 18.8 | 24.2 | 1.3 | 8.9 |
| Co51Ta37Si12 | 60 days | Co6Ta7 / λ1-Co2Ta | 45.8 | 5.5 | 32.6 | 10.9 | ||
| Co41Ta47Si12 | 60 days | (Co, Si)Ta2 / Co6Ta7 / λ1-Co2Ta | 62.6 | 21.5 | 45.9 | 10.1 | 33.6 | 16.5 |
| Co21Ta67Si12 | 60 days | (Co, Si)Ta2 / (Ta) | 94.1 | 3.0 | 63.6 | 24.5 | ||
| Co78Ta17Si5 | 60 days | λ3-Co2Ta / (αCo) | 22.4 | 7.1 | 2.6 | 3.5 | ||
| Co57Ta38Si5 | 60 days | Co6Ta7 / λ2-Co2Ta | 45.3 | 3.6 | 32.5 | 6.8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Huang, X.; Huang, L.; Yang, M.; Yang, P.; Cui, Y.; Zhang, J.; Yang, S.; Liu, X. Experimental Investigation of Phase Equilibria in the Co-Ta-Si Ternary System. Materials 2022, 15, 3097. https://doi.org/10.3390/ma15093097
Wang C, Huang X, Huang L, Yang M, Yang P, Cui Y, Zhang J, Yang S, Liu X. Experimental Investigation of Phase Equilibria in the Co-Ta-Si Ternary System. Materials. 2022; 15(9):3097. https://doi.org/10.3390/ma15093097
Chicago/Turabian StyleWang, Cuiping, Xiang Huang, Liangfeng Huang, Mujin Yang, Peng Yang, Yunrui Cui, Jinbin Zhang, Shuiyuan Yang, and Xingjun Liu. 2022. "Experimental Investigation of Phase Equilibria in the Co-Ta-Si Ternary System" Materials 15, no. 9: 3097. https://doi.org/10.3390/ma15093097
APA StyleWang, C., Huang, X., Huang, L., Yang, M., Yang, P., Cui, Y., Zhang, J., Yang, S., & Liu, X. (2022). Experimental Investigation of Phase Equilibria in the Co-Ta-Si Ternary System. Materials, 15(9), 3097. https://doi.org/10.3390/ma15093097