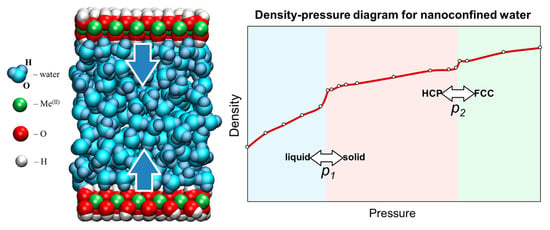
Structure, Properties, and Phase Transformations of Water Nanoconfined between Brucite-like Layers: The Role of Wall Surface Polarity
Abstract
:1. Introduction
2. Materials and Methods
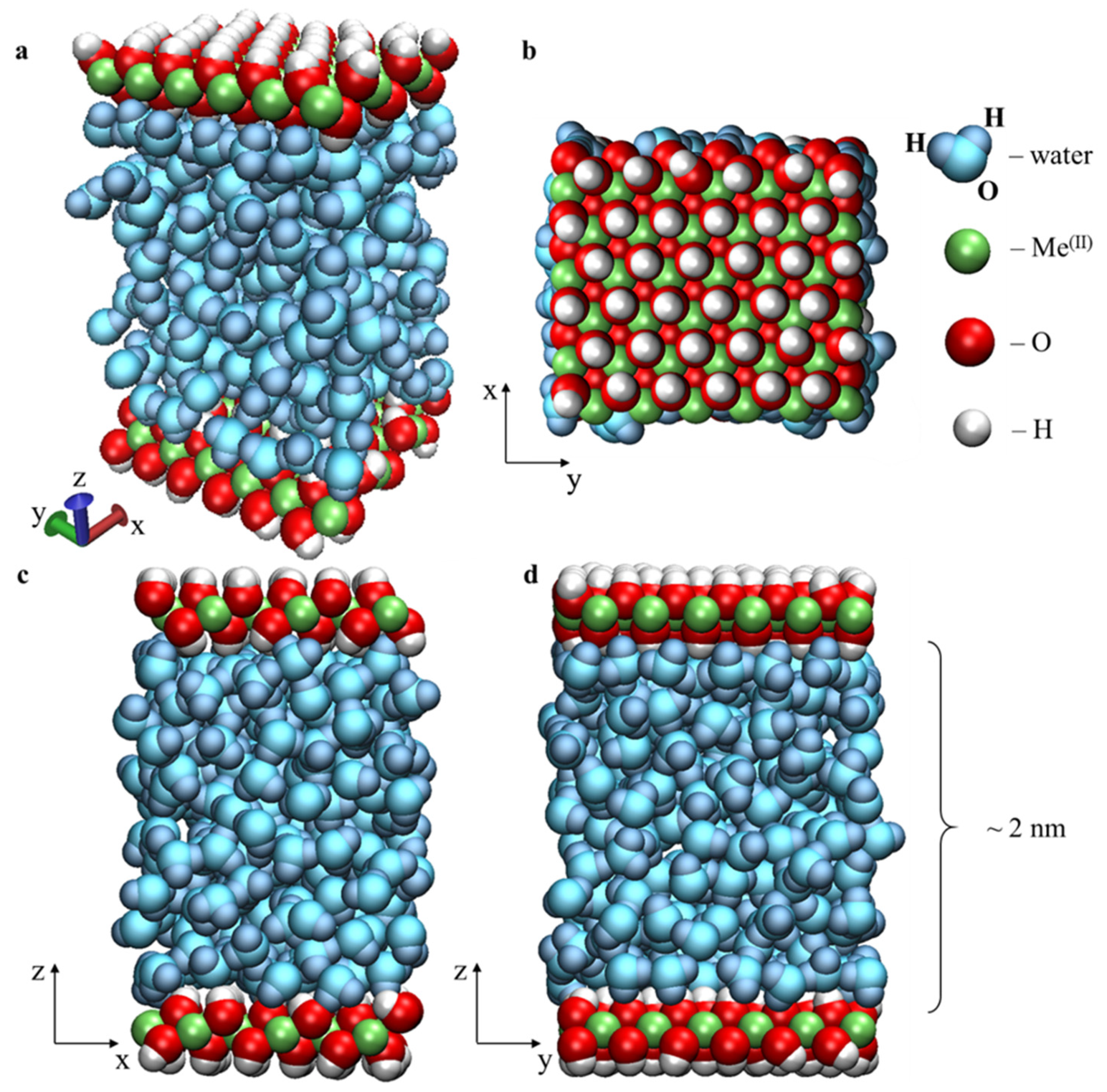
2.1. Molecular Dynamics Model
- Water density and compressibility;
- Spatial arrangement of water molecules, including the radial distribution functions (RDFs);
- The number of hydrogen bonds;
- The self-diffusion coefficient.
2.2. Advantages of the Model
2.3. Assumptions of the Model
2.4. Limitations of the Model
3. Results
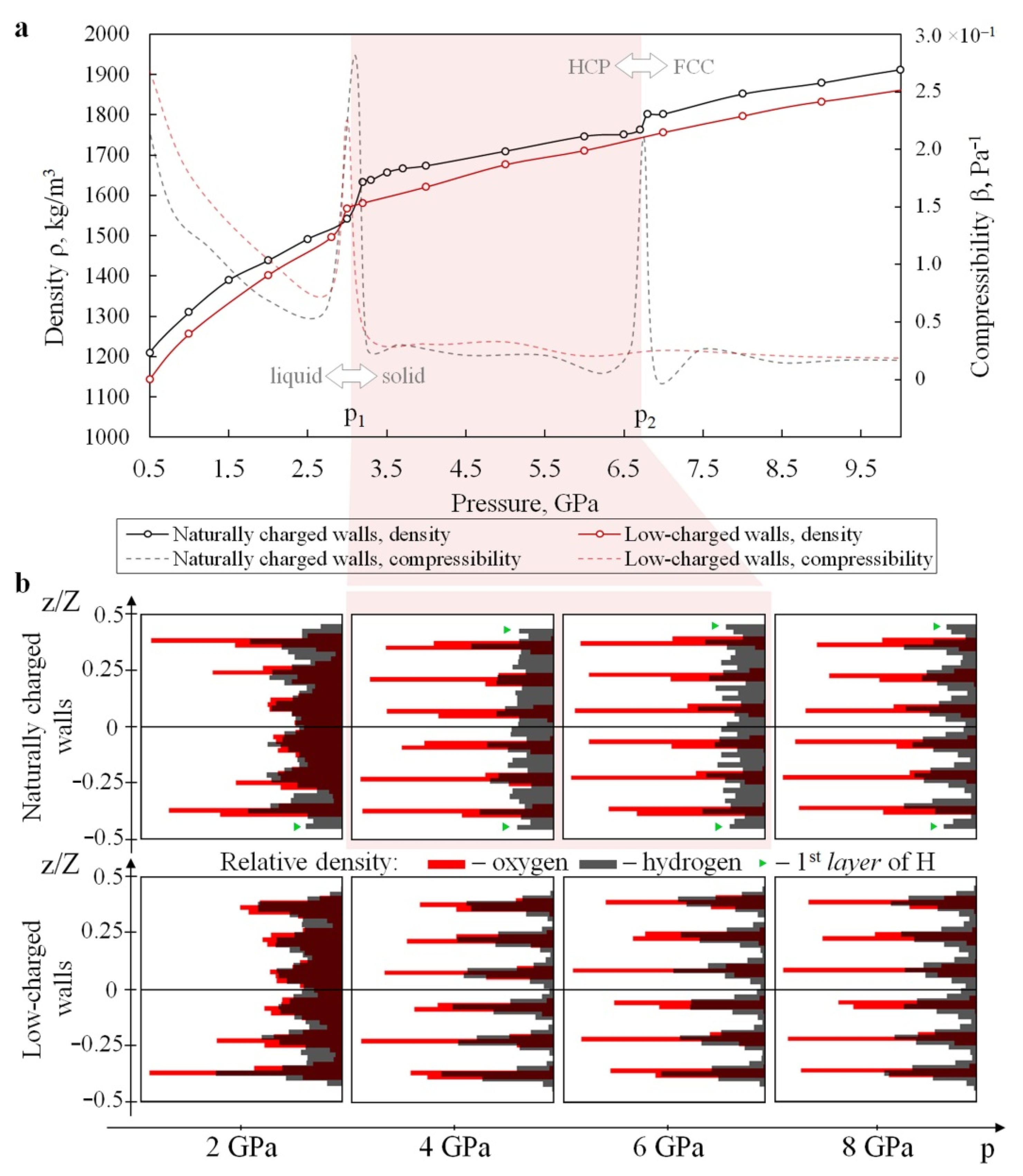
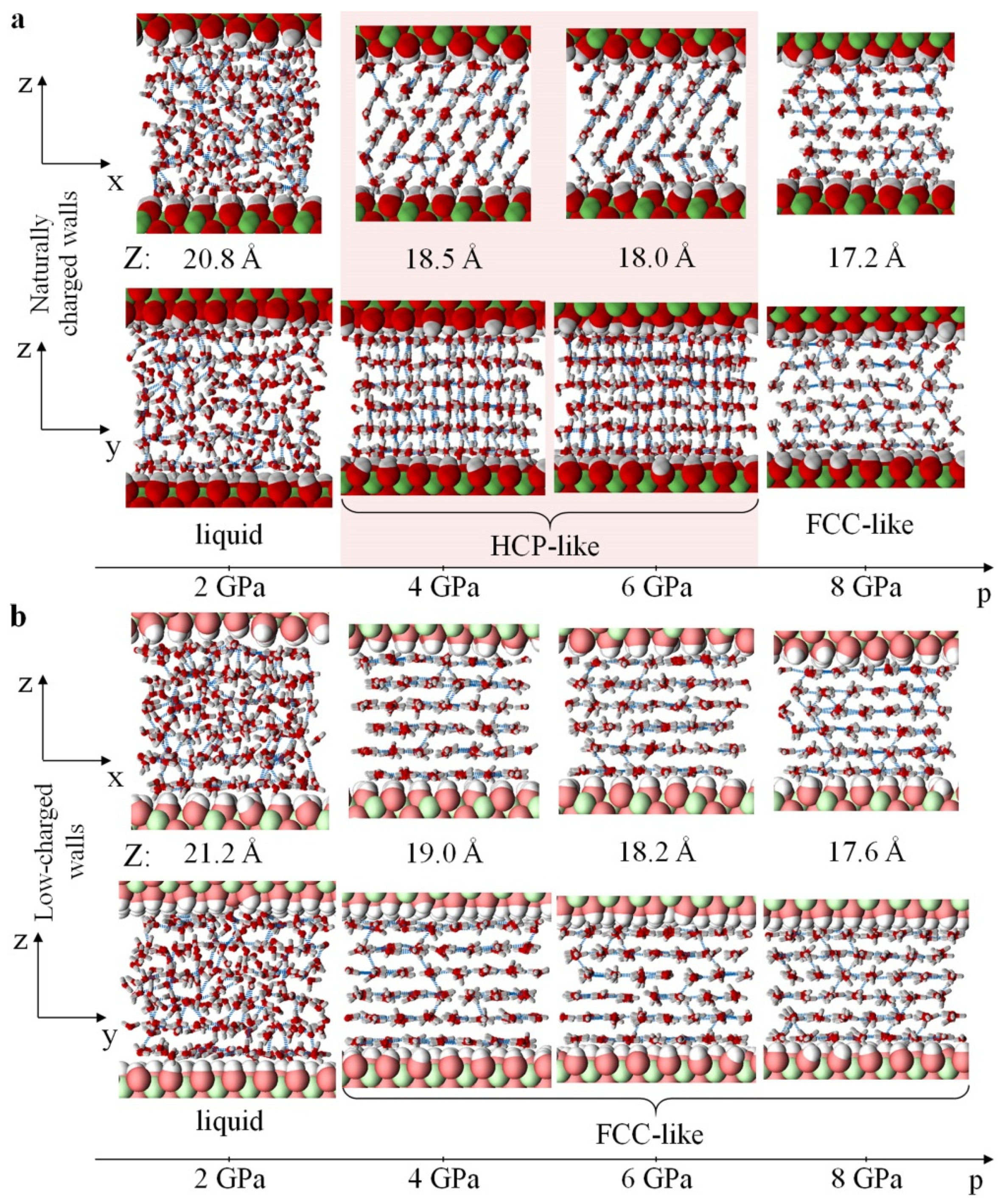
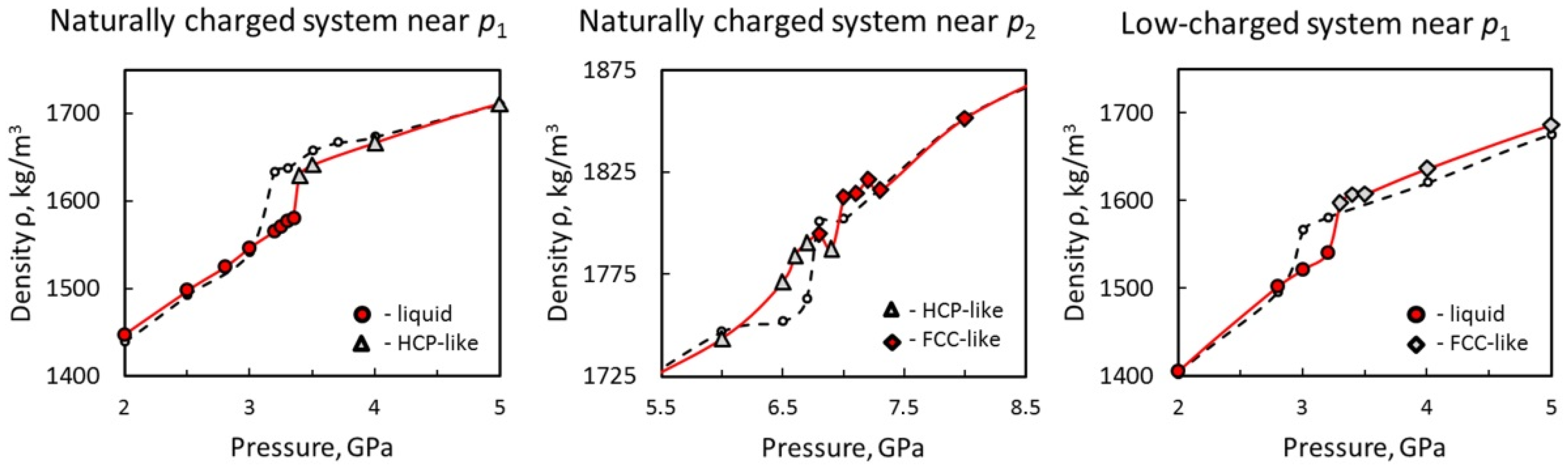
3.1. Structure and Properties of Nanoconfined Water
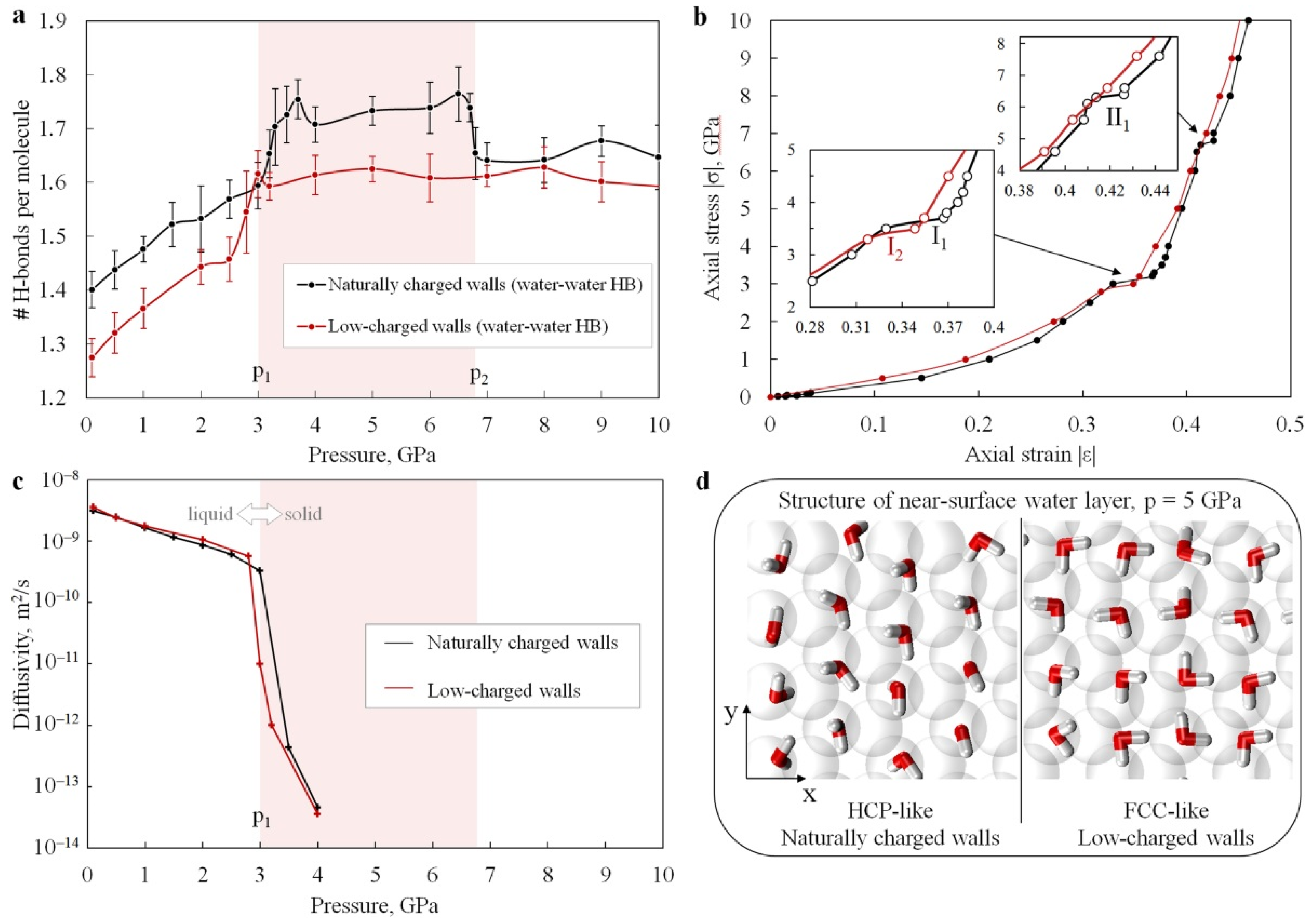
3.2. Hydrogen Bonding
3.3. Mechanical Response of Confined Water to Compression
4. Discussion of Geoscience-Related and Friction-Related Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Zhang, J.; Zhang, Y.; Deng, Y. Nanoconfined ionic liquids. Chem. Rev. 2016, 117, 6755–6833. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Santiburcio, D.; Marx, D. Chemistry in nanoconfined water. Chem. Sci. 2017, 8, 3444–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uskov, A.V.; Nefedov, D.Y.; Charnaya, E.V.; Haase, J.; Michel, D.; Kumzerov, Y.A.; Fokin, A.V.; Bugaev, A.S. Polymorphism of metallic sodium under nanoconfinement. Nano Lett. 2016, 16, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Charnaya, E.V.; Lee, M.K.; Chang, L.J.; Kumzerov, Y.A.; Fokin, A.V.; Samoylovich, M.I.; Bugaev, A.S. Impact of opal nanoconfinement on electronic properties of sodium particles: NMR studies. Phys. Lett. A 2015, 379, 705–709. [Google Scholar] [CrossRef]
- Latysheva, E.N.; Pirozerskii, A.L.; Charnaya, E.V.; Kumzerov, Y.A.; Fokin, A.V.; Nedbai, A.I.; Bugaev, A.S. Polymorphism of Ga-In alloys in nanoconfinement conditions. Phys. Solid State 2015, 57, 131–135. [Google Scholar] [CrossRef]
- Huber, P. Soft matter in hard confinement: Phase transition thermodynamics, structure, texture, diffusion and flow in nanoporous media. J. Phys. Condens. Matter 2015, 27, 103102. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, B.D.; Ha, J.M.; Hillmyer, M.A.; Ward, M.D. Manipulating crystal growth and polymorphism by confinement in nanoscale crystallization chambers. Acc. Chem. Res. 2012, 45, 414–423. [Google Scholar] [CrossRef]
- Yang, Z.C.; Qian, J.S.; Yu, A.Q.; Pan, B.C. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc. Natl. Acad. Sci. USA 2019, 116, 6659–6664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz-Santiburcio, D.; Marx, D. On the complex structural diffusion of proton holes in nanoconfined alkaline solutions within slit pores. Nat. Commun. 2016, 7, 12625. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Bosenberg, U.; Gosalawir, R.; Dornheim, M.; Cerenius, Y.; Besenbaher, F.; Jensen, T.R. A reversible nanoconfined chemical reaction. ACS Nano 2010, 4, 3903–3908. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Besenbacher, F.; Jensen, T.R. Nanoconfined hydrides for energy storage. Nanoscale 2011, 3, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Merlet, C.; Pean, C.; Rotenberg, B.; Madden, P.A.; Daffos, B.; Taberna, P.-L.; Simon, P.; Salanne, M. Highly confined ions store charge more efficiently in supercapacitors. Nat. Commun. 2013, 4, 2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhou, H.; Sheridan, E.; Walmsley, J.C.; Ren, D.; Chen, D. Geometrically confined favourable ion packing for high gravimetric capacitance in carbon–ionic liquid supercapacitors. Energy Environ. Sci. 2016, 9, 232–239. [Google Scholar] [CrossRef]
- Hoang, H.T.; Segers-Nolten, I.M.; Berenschot, J.W.; de Boer, M.J.; Tas, N.R.; Haneveld, J.; Elwenspoek, M.C. Fabrication and interfacing of nanochannel devices for single-molecule studies. J. Micromech. Microeng. 2009, 19, 065017. [Google Scholar] [CrossRef]
- Napoli, M.; Eijkel, J.C.; Pennathur, S. Nanofluidic technology for biomolecule applications: A critical review. Lab. Chip 2010, 10, 957–985. [Google Scholar] [CrossRef] [PubMed]
- Henrich, B.; Cupelli, C.; Santer, M.; Moseler, M. Continuum concepts in nanoscale capillary impregnation. New J. Phys. 2008, 10, 113022. [Google Scholar] [CrossRef]
- Wang, L.; Parsa, E.; Gao, Y.; Ok, J.T.; Neeves, K.; Yin, X.; Ozkan, E. Experimental study and modeling of the effect of nanoconfinement on hydrocarbon phase behavior in unconventional reservoirs. In Proceedings of the SPE Western North American and Rocky Mountain Joint Meeting, Denver, CO, USA, 17–18 April 2014. [Google Scholar]
- Gor, G.Y.; Gurevich, B. Gassmann theory applies to nanoporous media. Geophys. Res. Lett. 2018, 45, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Delville, A. Structure and properties of confined liquids: A molecular model of the clay-water interface. J. Phys. Chem. 1993, 97, 9703–9712. [Google Scholar] [CrossRef]
- Hwang, H.; Seoung, D.; Lee, Y.; Liu, Z.; Liermann, H.-P.; Cynn, H.; Kao, C.-C.; Mao, H.-K. A role for subducted super-hydrated kaolinite in Earth’s deep water cycle. Nat. Geosci. 2017, 10, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Tsukanov, A.; Shilko, E.; Psakhie, S. Structural Transformations of the Nanoconfined Water at High Pressures: A Potential Factor for Dynamic Rupture in the Subduction Zones. In Trigger Effects in Geosystems; Kocharyan, G., Lyakhov, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 297–306. [Google Scholar]
- Popov, V.L. Thermodynamics and kinetics of shear-induced melting of a thin layer of lubricant confined between solids. Tech. Phys. 2001, 46, 605–615. [Google Scholar] [CrossRef]
- Huang, X.; Wu, J.; Zhu, Y.; Zhang, Y.; Feng, X.; Lu, X. Flow-resistance analysis of nano-confined fluids inspired from liquid nano-lubrication: A review. Chin. J. Chem. Eng. 2017, 25, 1552–1562. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Shi, Y.; Lu, X.; Li, J.; Lu, L. Lubrication behavior of water molecules confined in TiO2 nanoslits: A molecular dynamics study. J. Chem. Eng. Data 2016, 61, 4023–4030. [Google Scholar] [CrossRef]
- Xie, G.X.; Li, G.; Luo, J.B.; Liu, S.H. Effects of electric field on characteristics of nano-confined lubricant films with ZDDP additive. Tribol. Int. 2010, 43, 975–980. [Google Scholar] [CrossRef]
- Thompson, P.A.; Robbins, M.O. Origin of stick-slip motion in boundary lubrication. Science 1990, 250, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Capozza, R.; Fasolino, A.; Ferranio, M.; Vanossi, A. Lubricated friction on nanopatterned surfaces via molecular dynamics simulation. Phys. Rev. B 2008, 77, 235432. [Google Scholar] [CrossRef] [Green Version]
- Tsukanov, A.A.; Shilko, E.V. Computer-aided design of boron nitride-based membranes with armchair and zigzag nanopores for efficient water desalination. Materials 2020, 13, 5256. [Google Scholar] [CrossRef]
- Salman, S.; Zhao, Y.; Zhang, X.; Su, J. Effect of temperature on the coupling transport of water and ions through a carbon nanotube in an electric field. J. Chem. Phys. 2020, 153, 184503. [Google Scholar] [CrossRef]
- Fang, C.; Huang, D.; Su, J. Osmotic water permeation through a carbon nanotube. J. Phys. Chem. Lett. 2020, 11, 940–944. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, D.; Su, J.; Gao, S. Coupled transport of water and ions through graphene nanochannels. J. Phys. Chem. C 2020, 124, 17320–17330. [Google Scholar] [CrossRef]
- Hilder, T.A.; Gordon, D.; Chung, S.H. Salt rejection and water transport through boron nitride nanotubes. Small 2009, 5, 2183–2190. [Google Scholar] [CrossRef]
- Mondelo-Martell, M.; Huarte-Larrañaga, F. Diffusion of H2 and D2 confined in single-walled carbon nanotubes: Quantum dynamics and confinement effects. J. Phys. Chem. A 2016, 120, 6501–6512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Yameen, B.; Neumann, R.; Ensinger, W.; Knoll, W.; Azzaroni, O. Biosensing and supramolecular bioconjugation in single conical polymer nanochannels. Facile incorporation of biorecognition elements into nanoconfined geometries. J. Am. Chem. Soc. 2008, 130, 16351–16357. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, G.; Katsov, K.; Sides, S.W.; Wang, J.; Tang, J.; Fredrickson, G.H.; Moskovits, M.; Stucky, G.D. Composite mesostructures by nano-confinement. Nat. Mater. 2004, 3, 816. [Google Scholar] [CrossRef] [PubMed]
- Talyzin, A.V.; Solozhenko, V.L.; Kurakevych, O.O.; Szabo, T.; Dekany, I.; Kurnosov, A.; Dmitriev, A. Colossal pressure-induced lattice expansion of graphite oxide in the presence of water. Angew. Chem. Int. Ed. 2008, 47, 8268–8271. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, I.V.; Glaznev, I.S.; Gubar, A.V.; Aristov, Y.I.; Kirik, S.D. Synthesis and water sorption properties of a new composite “CaCl2 confined into SBA-15 pores”. Micropor. Mesopor. Mat. 2010, 129, 243–250. [Google Scholar] [CrossRef]
- Song, P.; Dai, J.; Chen, G.; Yu, Y.; Fang, Z.; Lei, W.; Fu, S.; Wang, H.; Chen, Z.-G. Bioinspired design of strong, tough, and thermally stable polymeric materials via nanoconfinement. ACS Nano 2018, 12, 9266–9278. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Chen, X.; Wang, G.; Huang, X.; Li, A.; Dong, W. Nanoconfinement effects on thermal properties of nanoporous shape-stabilized composite PCMs: A review. Nano Energy 2018, 53, 769–797. [Google Scholar] [CrossRef]
- Elmahdy, M.M.; Chrissopoulou, K.; Afratis, A.; Floudas, G.; Anastasiadis, S.H. Effect of confinement on polymer segmental motion and ion mobility in PEO/layered silicate nanocomposites. Macromolecules 2006, 39, 5170–5173. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Jiang, L. 1D Nanoconfined ordered-assembly reaction. Adv. Mater. Interfaces 2019, 6, 1900104. [Google Scholar] [CrossRef]
- Qiu, K.; Chai, G.; Jiang, C.; Ling, M.; Tang, J.; Guo, Z. Highly efficient oxygen reduction catalysts by rational synthesis of nanoconfined maghemite in a nitrogen-doped graphene framework. ACS Catal. 2016, 6, 3558–3568. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, W.; Wang, J.; Rozen, I.; He, S.; Chen, C.; Kim, H.G.; Lee, H.-J.; Lee, H.-B.-R.; Kwon, S.H.; et al. Nanoconfined atomic layer deposition of TiO2/Pt nanotubes: Toward ultrasmall highly efficient catalytic nanorockets. Adv. Func. Mater. 2017, 27, 1700598. [Google Scholar] [CrossRef]
- Gordillo, M.C.; Nagy, G.; Marti, J. Structure of water nanoconfined between hydrophobic surfaces. J. Chem. Phys. 2005, 123, 054707. [Google Scholar] [CrossRef] [PubMed]
- Bampoulis, P.; Siekman, M.H.; Kooij, E.S.; Lohse, D.; Zandvliet, H.J.W.; Poelsema, B. Latent heat induced rotation limited aggre-gation in 2D ice nanocrystals. J. Chem. Phys. 2015, 143, 034702. [Google Scholar] [CrossRef] [PubMed]
- Sotthewes, K.; Bampoulis, P.; Zandvliet, H.J.W.; Lohse, D.; Poelsema, B. Pressure-induced melting of confined ice. ACS Nano 2017, 11, 12723–12731. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Kumacheva, E. Simple liquids confined to molecularly thin layers. I. Confinement-induced liquid-to-solid phase transitions. J. Chem. Phys. 1998, 108, 6996–7009. [Google Scholar] [CrossRef]
- Iakovlev, E.; Zhilyaev, P.; Akhatov, I. Atomistic study of the solid state inside graphene nanobubbles. Sci. Rep. 2017, 7, 17906. [Google Scholar] [CrossRef] [Green Version]
- Zheligovskaya, E.A.; Malenkov, G.G. Crystalline water ices. Russ. Chem. Rev. 2006, 75, 57–76. [Google Scholar] [CrossRef]
- Giovambattista, N.; Rossky, P.J.; Debenedetti, P.G. Computational studies of pressure, temperature, and surface effects on the structure and thermodynamics of confined water. Annu. Rev. Phys. Chem. 2012, 63, 179–200. [Google Scholar] [CrossRef]
- Åqvist, J.; Luzhkov, V. Ion permeation mechanism of the potassium channel. Nature 2000, 404, 881–884. [Google Scholar] [CrossRef]
- Manni, L.S.; Assenza, S.; Duss, M.; Vallooran, J.J.; Juranyi, F.; Jurt, S.; Zarbe, O.; Landau, E.M.; Mezzenga, R. Soft biomimetic nanoconfinement promotes amorphous water over ice. Nat. Nanotechnol. 2019, 14, 609–615. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, B.; Tu, Y.; Duan, M.; Xiu, P.; Li, J.; Fang, H. Critical dipole length for the wetting transition due to collective water-dipoles interactions. Sci. Rep. 2012, 2, 358. [Google Scholar] [CrossRef] [PubMed]
- Giovambattista, N.; Debenedetti, P.G.; Rossky, P.J. Effect of surface polarity on water contact angle and interfacial hydration structure. J. Phys. Chem. B 2007, 111, 9581–9587. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Foster, A.S.; Alava, M.J.; Laurson, L. Stick-slip control in nanoscale boundary lubrication by surface wettability. Phys. Rev. Lett. 2015, 114, 095502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, H.R.; Asper, R. Bivalent metal hydroxides. In Preparation and Crystal Growth of Materials with Layered Structures; Lieth, R.M.A., Ed.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 77–140. [Google Scholar]
- Costa, D.G.; Rocha, A.B.; Souza, W.F.; Chiaro, S.S.X.; Leitao, A.A. Structural and energetic analysis of Mg x M1− x (OH) 2 (M= Zn, Cu or Ca) brucite-like compounds by DFT calculations. J. Phys. Chem. C 2008, 112, 10681–10687. [Google Scholar] [CrossRef]
- Parise, J.B.; Marshall, W.G.; Smith, R.I.; Lutz, H.D.; Möller, H. The nuclear and magnetic structure of “white rust”—Fe(OH0.86D0.14)2. Am. Mineral. 2000, 85, 189–193. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Tsukanov, A.A.; Psakhie, S.G. Energy and structure of bonds in the interaction of organic anions with layered double hydroxide nanosheets: A molecular dynamics study. Sci. Rep. 2016, 6, 19986. [Google Scholar] [CrossRef] [Green Version]
- MacKerell, A.D., Jr.; Bashford, D.; Belott, M.; Dunbrack, R.L., Jr.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Hockney, R.W.; Goel, S.P.; Eastwood, J.W. A 10000 particle molecular dynamics model with long range forces. Chem. Phys. Lett. 1973, 21, 589–591. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Docampo-Álvarez, B.; Gomez-Gonzalez, V.; Montes-Campos, H.; Otero-Mato, J.M.; Mendez-Morales, T.; Cabeza, O.; Gallego, L.J.; Lynden-Bell, R.M.; Ivanistsev, V.B.; Fedorov, M.V. Molecular dynamics simulation of the behaviour of water in nano-confined ionic liquid–water mixtures. J. Phys. Condens. Matter 2016, 28, 464001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iakovlev, E.; Zhilyaev, P.; Akhatov, I.S. Modeling of the phase transition inside graphene nanobubbles filled with ethane. Phys. Chem. Chem. Phys. 2019, 21, 18099–18104. [Google Scholar] [CrossRef] [PubMed]
- Voevodin, A.A.; O’neill, J.P.; Zabinski, J.S. Nanocomposite tribological coatings for aerospace applications. Surf. Coat. Technol. 1999, 116, 36–45. [Google Scholar] [CrossRef]
- Hermann, A.; Mookherjee, M. High-pressure phase of brucite stable at Earth’s mantle transition zone and lower mantle conditions. PNAS 2016, 113, 13971–13976. [Google Scholar] [CrossRef] [Green Version]
- Holz, M.; Heil, S.R.; Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740–4742. [Google Scholar] [CrossRef]
- Daucik, K.; Dooley, R.B. Revised Release on the Pressure along the Melting and Sublimation Curves of Ordinary Water Substance, Release R14-08; International Association for the Properties of Water and Steam (IAPWS): Merseyside, UK, 2011. [Google Scholar]
- Lee, G.W.; Evans, W.J.; Yoo, C.S. Crystallization of water in a dynamic diamond-anvil cell: Evidence for ice VII-like local order in supercompressed water. Phys. Rev. B 2006, 74, 134112. [Google Scholar] [CrossRef]
- Dolan, D.H.; Gupta, Y.M. Nanosecond freezing of water under multiple shock wave compression: Optical transmission and imaging measurements. J. Chem. Phys. 2004, 121, 9050. [Google Scholar] [CrossRef]
- Dolan, D.H.; Knudson, M.D.; Hall, C.A.; Deeney, C. Ametastable limit for compressed liquid water. Nat. Phys. 2007, 3, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Stafford, S.J.P.; Chapman, D.J.; Bland, S.N.; Eakins, D.E. Observations on the nucleation of ice vii in compressed water. AIP. Conf. Proc. 2017, 1793, 130005. [Google Scholar] [CrossRef] [Green Version]
- Myint, P.C.; Chernov, A.A.; Sadigh, B.; Benedict, L.X.; Hall, B.M.; Hamel, S.; Belof, J.L. Nanosecond freezing of water at high pressures: Nucleation and growth near the metastability limit. Phys. Rev. Lett. 2018, 121, 155701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodnikova, M.N. A new approach to the mechanism of solvophobic interactions. J. Mol. Liq. 2007, 136, 211–213. [Google Scholar] [CrossRef]
- Honeycutt, J.D.; Andersen, H.C. Molecular dynamics study of melting and freezing of small Lennard-Jones clusters. J. Phys. Chem. 1987, 91, 4950–4963. [Google Scholar] [CrossRef]
- Stukowski, A. Structure identification methods for atomistic simulations of crystalline materials. Modell. Simul. Mater. Sci. Eng. 2012, 20, 045021. [Google Scholar] [CrossRef]
- Han, S.; Choi, M.; Kumar, P.; Stanley, H.E. Phase transitions in confined water nanofilms. Nat. Phys. 2010, 6, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Myint, P.C.; Benedict, L.X.; Belof, J.L. Free energy models for ice VII and liquid water derived from pressure, entropy, and heat capacity relations. J. Chem. Phys. 2017, 147, 084505. [Google Scholar] [CrossRef]
- Goncharov, A.F.; Sanloup, C.; Goldman, N.; Crowhurst, J.C.; Bastea, S.; Howard, W.M.; Fried, L.E.; Guignot, N.; Mezouar, M.; Meng, Y. Dissociative melting of ice VII at high pressure. J. Chem. Phys. 2009, 130, 124514. [Google Scholar] [CrossRef] [PubMed]
- King, D.M. Modeling the Ice VI to VII Phase Transition. 2009 NSF/REU Project; University of Notre Dame: Notre Dame, IN, USA, 31 July 2009; Available online: https://physics.nd.edu/assets/25212/king_dawn_modeling_phase_trans.pdf (accessed on 29 March 2022).
- Gowers, R.J.; Linke, M.; Bamoud, J.; Reddy, T.J.E.; Melo, M.N.; Seyler, S.L.; Dotson, D.L.; Domanski, J.; Buchoux, S.; Kenney, I.M.; et al. MDAnalysis: A Python package for the rapid analysis of molecular dynamics simulations. In Proceedings of the 15th Python in Science Conference, Austin, TX, USA, 11–17 July 2016. [Google Scholar]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [Green Version]
- de Leeuw, N.H.; Cooper, T.G. Surface simulation studies of the hydration of white rust Fe(OH)2, goethite α-FeO(OH) and hematite α-Fe2O3. Geochim. Cosmochim. Acta 2007, 71, 1655–1673. [Google Scholar] [CrossRef]
- Gleason, A.E.; Bolme, C.A.; Galtier, E.; Lee, H.J.; Granados, E.; Dolan, D.H.; Seagle, C.T.; Ao, T.; Ali, S.; Lazicki, A.; et al. Compression freezing kinetics of water to ice VII. Phys. Rev. Lett. 2017, 119, 025701. [Google Scholar] [CrossRef]
- Detournay, E.; Cheng, A.H.-D. Fundamentals of poroelasticity. In Comprehensive Rock Engineering: Principles, Practice and Projects; Fairhurst, C., Ed.; Pergamon Press: Oxford, UK, 1993; Volume 2, pp. 113–171. [Google Scholar]
- Cheng, A.H.-D. Poroelasticity; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Tschauner, O.; Huang, S.; Greenberg, E.; Prakapenka, V.B.; Ma, C.; Rossman, G.R.; Shen, A.H.; Zhang, D.; Newville, M.; Lanzirotti, A.; et al. Ice-VII inclusions in diamonds: Evidence for aqueous fluid in Earth’s deep mantle. Science 2018, 359, 1136–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Israelachvili, J.N.; Landman, U. Nanotribology: Friction, wear and lubrication at the atomic scale. Nature 1995, 374, 607–616. [Google Scholar] [CrossRef]
- Grigoriev, A.S.; Shilko, E.V.; Dmitriev, A.I.; Tarasov, S.Y. Suppression of wear in dry sliding friction induced by negative thermal expansion. Phys. Rev. E. 2020, 102, 042801. [Google Scholar] [CrossRef] [PubMed]
- Hynninen, T.; Heinonen, V.; Dias, C.L.; Karttunen, M.; Foster, A.S.; Ala-Nissila, T. Cutting ice: Nanowire regelation. Phys. Rev. Lett. 2010, 105, 086102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Huang, Y.; Sun, P.; Liu, X.; Ma, Z.; Zhou, Y.; Zhou, J.; Zheng, W.; Sun, C.Q. Ice Regelation: Hydrogen-bond extraordinary recoverability and water quasisolid-phase-boundary dispersivity. Sci. Rep. 2015, 5, 13655. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Schusteritsch, G.; Pickard, C.J.; Salzmann, C.G.; Michaelides, A. Double-layer ice from first principles. Phys. Rev. B 2017, 95, 094121. [Google Scholar] [CrossRef] [Green Version]
- Greaves, C.; Thomas, M.A. Refinement of the structure of deuterated nickel hydroxide, Ni (OD) 2, by powder neutron diffraction and evidence for structural disorder in samples with high surface area. Acta Crystallogr. Sect. B Struct. Sci. 1986, 42, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Zigan, F.; Rothbauer, R. Neutronenbeugungsmessungen am brucit. Neues Jahrb. Für Mineral. Mon. 1967, 1967, 137–143. [Google Scholar]
- Lotmar, W.; Feitknecht, W. Uber Anderungen der Ionenabstande in Hydroxyd-Schichtengittern. Z. Fuer Krist. Krist. Krist. Krist. 1936, 93, 368–378. [Google Scholar] [CrossRef]
- Baneeva, M.I.; Popova, S.V. The analysis of zinc hydroxide under high pressure and temperatures. Geokhimiya 1969, 1014–1016. [Google Scholar]
- Christensen, A.N.; Ollivier, G. Hydrothermal preparation and low temperature magnetic properties of Mn (OH) 2. Solid State Commun. 1972, 10, 609–614. [Google Scholar] [CrossRef]
- Hemmingsen, L.; Bauer, R.; Bjerrum, M.J.; Schwarz, K.; Blaha, P.; Andersen, P. Structure, chemical bonding, and nuclear quadrupole interactions of β-Cd (OH) 2: Experiment and first principles calculations. Inorg. Chem. 1999, 38, 2860–2867. [Google Scholar] [CrossRef] [PubMed]
- Busing, W.R.; Levy, H.A. Neutron diffraction study of calcium hydroxide. J. Chem. Phys. 1957, 26, 563–568. [Google Scholar] [CrossRef]
- Grazulis, S.; Daskevic, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quiros, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef] [PubMed]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.T.; Quiros, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Cryst. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD-visual molecular dynamics. J. Molec. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Kirk, D. NVIDIA CUDA software and GPU parallel computing architecture. In Proceedings of the 6th International Symposium on Memory Management (ISMM’07), Montreal, QC, Canada, 21–22 October 2007. [Google Scholar]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Plimpton, S.; Crozier, P.; Thompson, A. LAMMPS-large-scale atomic/molecular massively parallel simulator. Sandia Natl. Lab. 2007, 18, 43. [Google Scholar]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The open visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.M.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Adinets, A.V.; Bryzgalov, P.A.; Voevodin, V.V.; Zhumatii, S.A.; Nikitenko, D.A.; Stefanov, K.S. Job digest: An approach to dynamic analysis of job characteristics on supercomputers. Numer. Methods Program. 2012, 13, 160–166. [Google Scholar]
- Zacharov, I.; Arslanov, R.; Gunin, M.; Stefonishin, D.; Bykov, A.; Pavlov, S.; Panarin, O.; Maliutin, A.; Rykovanov, S.; Fedorov, M. “Zhores”–Petaflops supercomputer for data-driven modeling, machine learning and artificial intelligence installed in Skolkovo Institute of Science and Technology. Open Eng. 2019, 9, 512–520. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukanov, A.A.; Shilko, E.V.; Popov, M. Structure, Properties, and Phase Transformations of Water Nanoconfined between Brucite-like Layers: The Role of Wall Surface Polarity. Materials 2022, 15, 3043. https://doi.org/10.3390/ma15093043
Tsukanov AA, Shilko EV, Popov M. Structure, Properties, and Phase Transformations of Water Nanoconfined between Brucite-like Layers: The Role of Wall Surface Polarity. Materials. 2022; 15(9):3043. https://doi.org/10.3390/ma15093043
Chicago/Turabian StyleTsukanov, Alexey A., Evgeny V. Shilko, and Mikhail Popov. 2022. "Structure, Properties, and Phase Transformations of Water Nanoconfined between Brucite-like Layers: The Role of Wall Surface Polarity" Materials 15, no. 9: 3043. https://doi.org/10.3390/ma15093043
APA StyleTsukanov, A. A., Shilko, E. V., & Popov, M. (2022). Structure, Properties, and Phase Transformations of Water Nanoconfined between Brucite-like Layers: The Role of Wall Surface Polarity. Materials, 15(9), 3043. https://doi.org/10.3390/ma15093043